Fetal and Infant Effects of Maternal Opioid Use during Pregnancy: A Literature Review including Clinical, Toxicological, Pharmacogenomic, and Epigenetic Aspects for Forensic Evaluation
Abstract
1. Introduction
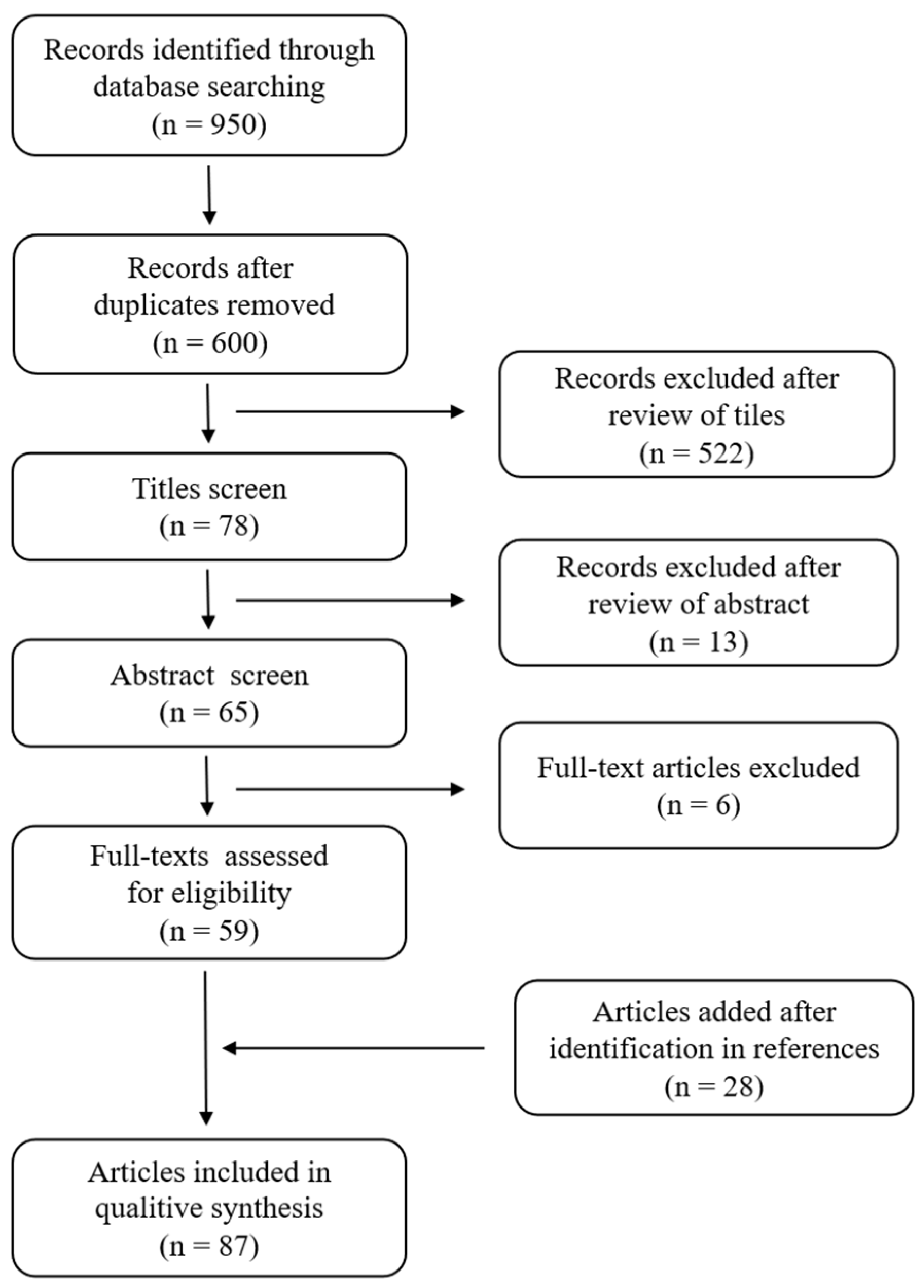
2. Materials and Methods
3. Results
4. Discussion
4.1. Clinical Implications for the Fetus and Infant
4.1.1. Pregnancy Complications
4.1.2. Postnatal Complications
Neonatal Abstinence Syndrome: Neonatal Opioid Withdrawal Syndrome (NOWS)
Complications Due to Direct Opioid Exposure
4.2. Fetus and Infant Cause of Death
4.2.1. Intrauterine Fetal Death
4.2.2. Infant Death
4.3. Toxicological Analyses
4.4. The Pharmacogenetics of Opioids in Pregnancy and Epigenetics Research
4.4.1. Pharmacogenomic of Opioids in Pregnancy: Fetal and Infant Effects
CYP2D6
OPRM1, COMT, and ABCB1
UDP-Glucoronosyl-Transferase (UGT) and Organic Cation Transporter 1 (OCT1)
Methadone Pharmacogenetics and CYP2B6
4.4.2. Epigenetics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaltenbach, K.; Berghella, V.; Finnegan, L. Opioid dependence during pregnancy. Effects and management. Obstet. Gynecol. Clin. N. Am. 1998, 25, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Sithisarn, T.; Granger, D.T.; Bada, H.S. Consequences of prenatal substance use. Int. J. Adolesc. Med. Health 2012, 24, 105–112. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Drug Abuse, Substance Use While Pregnant and Breast Feeding. Available online: https://nida.nih.gov/publications/research-reports/substance-use-in-women/substance-use-while-pregnant-breastfeeding (accessed on 27 December 2022).
- Substance Abuse and Mental Health Services Administration 2020 National Survey on Drug Use and Health: Detailed Tables. Available online: https://www.samhsa.gov/data/report/2020-nsduh-detailed-tables (accessed on 27 December 2022).
- Bertaso, A.; Gottardo, R.; Murari, M.; Mazzola, M.; Porpiglia, N.M.; Taus, F.; Beghini, R.; Gandini, F.; Bortolotti, F. Hair testing applied to the assessment of in utero exposure to drugs: Critical analysis of 51 cases of the University Hospital of Verona. Drug Test. Anal. 2023, 15, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Friguls, B.; Joya, X.; Garcia-Serra, J.; Gómez-Culebras, M.; Pichini, S.; Martinez, S.; Vall, O.; Garcia-Algar, O. Assessment of exposure to drugs of abuse during pregnancy by hair analysis in a Mediterranean island. Addiction 2012, 107, 1471–1479. [Google Scholar] [CrossRef]
- NIDA. Heroin DrugFacts. Available online: https://nida.nih.gov/publications/drugfacts/heroin (accessed on 27 December 2022).
- Huestis, M.A.; Choo, R.E. Drug abuse’s smallest victims: In utero drug exposure. Forensic Sci. Int. 2002, 128, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; To, W.K.; Duthie, S.J.; Ma, H.K. Narcotic addiction in pregnancy with adverse maternal and perinatal outcome. Aust. N. Z. J. Obstet. Gynaecol. 1992, 32, 216–221. [Google Scholar] [CrossRef]
- British Columbia Centre on Substance Use. A Guideline for the Clinical Management of Opioid Use Disorder—Pregnancy Supplement. Available online: https://www.bccsu.ca/wp-content/uploads/2018/06/OUD-Pregnancy.pdf (accessed on 27 December 2022).
- Fishman, B.; Daniel, S.; Koren, G.; Lunenfeld, E.; Levy, A. Pregnancy outcome following opioid exposure: A cohort study. PLoS ONE 2019, 14, e0219061. [Google Scholar] [CrossRef]
- Pötsch, L.; Skopp, G.; Emmerich, T.P.; Becker, J.; Ogbuhui, S. Report on intrauterine drug exposure during second trimester of pregnancy in a heroin-associated death. Ther. Drug Monit. 1999, 21, 593–597. [Google Scholar] [CrossRef]
- Winklbaur, B.; Kopf, N.; Ebner, N.; Jung, E.; Thau, K.; Fischer, G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: A knowledge synthesis for better treatment for women and neonates. Addiction 2008, 103, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Rausgaard, N.L.K.; Broe, A.; Bliddal, M.; Nohr, E.A.; Ibsen, I.O.; Albertsen, T.L.; Ravn, P.; Damkier, P. Use of opioids among pregnant women 1997-2016: A Danish drug utilization study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 289, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bhatt-Mehta, V.; Jing, X.; Wang, X.; Zhu, H.J. Transplacental methadone exposure and risk of Neonatal Opioid Withdrawal Syndrome. Pharmacotherapy 2023, 44, 22–27. [Google Scholar] [CrossRef]
- Bashore, R.A.; Ketchum, J.S.; Staischm, K.J.; Barrett, C.T.; Zimmermann, E.G. Heroin addiction and pregnancy. West. J. Med. 1981, 134, 506–514. [Google Scholar]
- Anbalagan, S.; Mendez, M.D. Neonatal Abstinence Syndrome; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ryan, K.S.; Prewitt, K.C.; Hayer, S.; Hedges, M.A.; Benson, A.E.; Lo, J.O. Opioid Use in Pregnancy: A Review. Obstet. Gynecol. Surv. 2023, 78, 35–49. [Google Scholar] [CrossRef]
- Esposito, D.B.; Bateman, B.; Werler, M.; Straub, L.; Mogun, H.; Hernandez-Diaz, S.; Huybrechts, K. Ischemic Placental Disease, Preterm Delivery, and Their Association With Opioid Use During Pregnancy. Am. J. Epidemiol. 2022, 191, 759–768. [Google Scholar] [CrossRef]
- Borrelli, K.N.; Wachman, E.M.; Beierle, J.A.; Taglauer, E.S.; Jain, M.; Bryant, C.D.; Zhang, H. Effect of Prenatal Opioid Exposure on the Human Placental Methylome. Biomedicines 2022, 10, 1150. [Google Scholar] [CrossRef]
- Little, B.; Snell, L.M.; Klein, V.R.; Gilstrap, L.C.; Knoll, K.A.; Breckenridge, J.D. Maternal and fetal effects of heroin addiction during pregnancy. J. Reprod. Med. 1990, 35, 159–162. [Google Scholar]
- O’Donnell, F.T.; Jackson, D.L. Opioid Use Disorder and Pregnancy. Mo. Med. 2017, 114, 181–186. [Google Scholar] [PubMed]
- Kandall, S.R.; Albin, S.; Lowinson, J.; Berle, B.; Eidelman, A.I.; Gartner, L.M. Differential effects of maternal heroin and methadone use on birthweight. Pediatrics 1976, 58, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Wolff, K.; Perez-Montejano, R. Opioid neonatal abstinence syndrome: Controversies and implications for practice. Curr. Drug Abus. Rev. 2014, 7, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Athanasakis, E.; Karavasiliadou, S.; Styliadis, I. The factors contributing to the risk of sudden infant death syndrome. Hippokratia 2011, 15, 127–131. [Google Scholar] [PubMed]
- Maguire, D.J.; Taylor, S.; Armstrong, K.; Shaffer-Hudkins, E.; Germain, A.M.; Brooks, S.S.; Cline, G.J.; Andersen, J.M.; Høiseth, G.; Nygaard, E. Prenatal exposure to methadone or buprenorphine and long-term outcomes: A meta-analysis. Early Hum. Dev. 2020, 143, 104997. [Google Scholar]
- Benck, K.N.; Seide, K.; Jones, A.K.; Omori, M.; Rubinstein, L.B.; Beckwith, C.; Nowotny, K.M. United States county jail treatment and care of pregnant incarcerated persons with opioid use disorder. Drug Alcohol Depend. 2023, 247, 109863. [Google Scholar] [CrossRef]
- Burns, L.; Conroy, E.; Mattick, R.P. Infant mortality among women on a methadone program during pregnancy. Drug Alcohol Rev. 2010, 29, 551–556. [Google Scholar] [CrossRef]
- Caritis, S.N.; Panigrahy, A. Opioids affect the fetal brain: Reframing the detoxification debate. Am. J. Obstet. Gynecol. 2019, 221, 602–608. [Google Scholar] [CrossRef]
- Cohen, M.C.; Morley, S.R.; Coombs, R.C. Maternal use of methadone and risk of sudden neonatal death. Acta Paediatr. 2015, 104, 883–887. [Google Scholar] [CrossRef]
- Conradt, E.; Flannery, T.; Aschner, J.L.; Annett, R.D.; Croen, L.A.; Duarte, C.S.; Friedman, A.M.; Guille, C.; Hedderson, M.M.; Hofheimer, J.A.; et al. Prenatal Opioid Exposure: Neurodevelopmental Consequences and Future Research Priorities. Pediatrics 2019, 144, e20190128. [Google Scholar] [CrossRef] [PubMed]
- Concheiro, M.; Lendoiro, E.; de Castro, A.; Gónzalez-Colmenero, E.; Concheiro-Guisan, A.; Peñas-Silva, P.; Macias-Cortiña, M.; Cruz-Landeira, A.; López-Rivadulla, M. Bioanalysis for cocaine, opiates, methadone, and amphetamines exposure detection during pregnancy. Drug. Test. Anal. 2017, 9, 898–904. [Google Scholar] [CrossRef]
- de Castro, A.; Jones, H.E.; Johnson, R.E.; Gray, T.R.; Shakleya, D.M.; Huestis, M.A. Methadone, cocaine, opiates, and metabolite disposition in umbilical cord and correlations to maternal methadone dose and neonatal outcomes. Ther. Drug. Monit. 2011, 33, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Dryden, C.; Young, D.; Hepburn, M.; Mactier, H. Maternal methadone use in pregnancy: Factors associated with the development of neonatal abstinence syndrome and implications for healthcare resources. BJOG 2009, 116, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Duska, M.; Goodman, D. Implementation of a prenatal naloxone distribution program to decrease maternal mortality from opioid overdose. Matern. Child. Health J. 2022, 26, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Epstein, R.A.; Bobo, W.V.; Martin, P.R.; Morrow, J.A.; Wang, W.; Chandrasekhar, R. Increasing pregnancy-related use of prescribed opioid analgesics. Ann. Epidemiol. 2013, 23, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Galli, J.; Loi, E.; Franzoni, A.; Accorsi, P.; Micheletti, S.; Pansera, L.; Fazzi, E. Long-Term Visual and Neurodevelopmental Outcomes in Two Children with Congenital Nystagmus Secondary to Methadone Exposure In utero. Neuropediatrics 2023, 54, 412–416. [Google Scholar] [CrossRef]
- Garrison, L.; Leeman, L.; Savich, R.D.; Gutierrez, H.; Rayburn, W.F.; Bakhireva, L.N. Fetal Growth Outcomes in a Cohort of Polydrug- and Opioid-Dependent Patients. J. Reprod. Med. 2016, 61, 311–319. [Google Scholar]
- Irnes, E.; Oltedal, L.; Bartsch, H.; Eide, G.E.; Elgen, I.B.; Aukland, S.M. Brain morphology in school-aged children with prenatal opioid exposure: A structural MRI study. Early Hum. Dev. 2017, 106–107, 33–39. [Google Scholar] [CrossRef]
- Kandall, S.R.; Gaines, J. Maternal substance use and subsequent sudden infant death syndrome (SIDS) in offspring. Neurotoxicol. Teratol. 1991, 13, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Kandall, S.R.; Gaines, J.; Habel, L.; Davidson, G.; Jessop, D. Relationship of maternal substance abuse to subsequent sudden infant death syndrome in offspring. J. Pediatr. 1993, 123, 120–126. [Google Scholar] [CrossRef]
- Kushnir, A.; Bhavsar, R.; Hanna, E.; Hegyi, T. Neonatal Abstinence Syndrome in Infants with Prenatal Exposure to Methadone versus Buprenorphine. Children 2023, 10, 1030. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.J. Buprenorphine versus Methadone in Pregnancy. N. Engl. J. Med. 2023, 388, 957. [Google Scholar]
- López, P.; Bermejo, A.M.; Tabernero, M.J.; Cabarcos, P.; Alvarez, I.; Fernández, P. Cocaine and opiates use in pregnancy: Detection of drugs in neonatal meconium and urine. J. Anal. Toxicol. 2009, 33, 351–355. [Google Scholar] [CrossRef][Green Version]
- McGlone, L.; Hamilton, R.; McCulloch, D.L.; MacKinnon, J.R.; Bradnam, M.; Mactier, H. Visual outcome in infants born to drug-misusing mothers prescribed Methadone in pregnancy. Br. J. Ophthalmol. 2014, 98, 238–245. [Google Scholar] [CrossRef]
- Monnelly, V.J.; Hamilton, R.; Chappell, F.M.; Mactier, H.; Boardman, J.P. Childhood neurodevelopment after prescription of maintenance methadone for opioid dependency in pregnancy: A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2019, 61, 750–760. [Google Scholar] [CrossRef]
- Montanari, E.; Bonasoni, M.P.; Licata, M.; Salomone, A.; Gerace, E.; Vivarelli, M.; Giorgetti, R.; Tagliabracci, A. Toxicological and histological analyses for a stillborn delivered by a mother under methadone maintenance therapy. Forensic Toxicol. 2018, 36, 514–524. [Google Scholar] [CrossRef]
- Montanari, E.; Bonasoni, M.P.; Alessandrini, F.; Frazzi, R.; Mocchegiani, F.; Busardò, F.P.; Giorgetti, R.; Tagliabracci, A. CYP2B6, ABCB1 and OPRM1 profile in a stillborn affected by chronic methadone intoxication. Forensic Toxicol. 2019, 37, 507–516. [Google Scholar] [CrossRef]
- Newbury, J.; Sargayoos, M.; Bora, S.; Henderson, J. Associations between social adversity, caregiver psychological factors, and language outcomes in 9.5-year-old children born to women with opioid use disorder. Child. Neuropsychol. 2023, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pandya, V.; Wilker, C.; Johnson-Davis, K.L. Longitudinal trends in meconium drug detection in 46 US states between the years 2015 and 2020. J. Anal. Toxicol. 2023, 47, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ordean, A.; Tubman-Broeren, M. Safety and Efficacy of Buprenorphine-Naloxone in Pregnancy: A Systematic Review of the Literature. Pathophysiology 2023, 30, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ostrea, E.M.; Knapp, D.K.; Tannenbaum, L.; Ostrea, A.R.; Romero, A.; Salari, V. Estimates of illicit drug use during pregnancy by maternal interview, hair analysis, and meconium analysis. J. Pediatr. 2001, 138, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Hussain, T.; Holder, G.; Bhoyar, A.; Ewer, A.K. Maternal methadone therapy increases QTc interval in newborn infants. Arch. Dis. Child Fetal Neonatal Ed. 2011, 96, F141–F143. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Cacho, W.A.; Flores, S.; Schrader, R.M.; McKay, J.; Rayburn, W.F. Effect of chronic maternal methadone therapy on intrapartum fetal heart rate patterns. J. Soc. Gynecol. Investig. 2006, 13, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Goodwin, D.; Wilkins, G.O.; Averin, R.; Choo, E.; Schroeder, J.R.; Jasinski, D.R.; Rolley, E.J.; Hendrée, E.J.; Huestis, M.A. Buprenorphine and Norbuprenorphine in Hair of Pregnant Women and Their Infants after Controlled Buprenorphine Administration. Clin. Chem. 2007, 53, 2136–2143. [Google Scholar]
- Ross, E.; Graham, D.; Money, K. Developmental Consequences of Fetal Exposure to Drugs: What We Know and What We Still Must Learn. Neuropsychopharmacology 2015, 40, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.E.; Lemon, L.S.; Mokhtari, N.B.; Parks, W.T.; Catov, J.M.; Venkataramanan, R.; Caritis, S.N. Delayed villous maturation in term placentas exposed to opioid maintenance therapy: A retrospective cohort study. Am. J. Obstet. Gynecol. 2017, 216, e1–e418. [Google Scholar] [CrossRef] [PubMed]
- Spowart, K.M.; Reilly, K.; Mactier, H.; Hamilton, R. Executive functioning, behavioural, emotional, and cognitive difficulties in school-aged children prenatally exposed to methadone. Front. Pediatr. 2023, 11, 1118634. [Google Scholar] [CrossRef]
- Ward, S.L.; Bautista, D.; Chan, L.; Derry, M.; Lisbin, A.; Durfee, M.J.; Mills, K.S.; Keens, T.G. Sudden infant death syndrome in infants of substance-abusing mothers. J. Pediatr. 1990, 117, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.V.; Hyatt, B.W.; Visconti, K.C.; Chernicky, L.; Chattin, K.; Fortner, K.B. Neonatal head circumference in newborns with neonatal abstinence syndrome. Pediatrics 2019, 143, e20180541. [Google Scholar] [CrossRef]
- Whiteman, V.E.; Salemi, J.L.; Mogos, M.F.; Cain, M.A.; Aliyu, M.H.; Salihu, H.M. Maternal opioid drug use during pregnancy and its impact on perinatal morbidity, mortality, and the costs of medical care in the United States. J. Pregnancy 2014, 2014, 906723. [Google Scholar] [CrossRef]
- Walhovd, K.B.; Westlye, L.T.; Moe, V. White matter characteristics and cognition in prenatally opiate- and polysubstance-exposed children: A diffusion tensor imaging study. Am. J. Neuroradiol. 2010, 31, 894–900. [Google Scholar] [CrossRef]
- Wurst, K.E.; Zedler, B.K.; Joyce, A.R.; Sasinowski, M.; Murrelle, E.L. A Swedish Population-based Study of Adverse Birth Outcomes among Pregnant Women Treated with Buprenorphine or methadone: Preliminary Findings. Subst. Abus. 2016, 10, 89–97. [Google Scholar] [CrossRef]
- Zedler, B.K.; Mann, A.L.; Kim, M.M.; Amick, H.R.; Joyce, A.R.; Murrelle, E.L.; Jones, H.E. Buprenorphine compared with methadone to treat pregnant women with opioid use disorder: A systematic review and meta-analysis of safety in the mother, fetus and child. Addiction 2016, 111, 2115–2128. [Google Scholar] [CrossRef]
- Zipursky, J.; Juurlink, D.N. Opioid use in pregnancy: An emerging health crisis. Obstet. Med. 2021, 14, 211–219. [Google Scholar] [CrossRef]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin. Pharmacol. Ther. 2021, 110, 888–896. [Google Scholar] [CrossRef] [PubMed]
- CPIC Guideline for Opioids and CYP2D6, OPRM1, and COMT. Available online: https://cpicpgx.org/guidelines/guideline-for-codeine-and-cyp2d6/ (accessed on 28 December 2022).
- Yalçin, N.; Flint, R.B.; van Schaik, R.H.N.; Simons, S.H.P.; Allegaert, K. The Impact of Pharmacogenetics on Pharmacokinetics and Pharmacodynamics in Neonates and Infants: A Systematic Review. Pharmgenom. Pers. Med. 2022, 15, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Madadi, P.; Avard, D.; Koren, G. Pharmacogenetics of opioids for the treatment of acute maternal pain during pregnancy and lactation. Curr. Drug Metab. 2012, 13, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Mactier, H.; McLaughlin, P.; Gillis, C.; Osselton, M.D. Variations in Infant CYP2B6 Genotype Associated with the Need for Pharmacological Treatment for Neonatal Abstinence Syndrome in Infants of Methadone-Maintained Opioid-Dependent Mothers. Am. J. Perinatol. 2017, 34, 918–921. [Google Scholar] [PubMed]
- McPhail, B.T.; Emoto, C.; Butler, D.; Fukuda, T.; Akinbi, H.; Vinks, A.A. Opioid Treatment for Neonatal Opioid Withdrawal Syndrome: Current Challenges and Future Approaches. J. Clin. Pharmacol. 2021, 61, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lewis, T.; Gauda, E.; Gobburu, J.; Ivaturi, V. Mechanistic population pharmacokinetics of morphine in neonates with abstinence syndrome after oral administration of diluted tincture of opium. J. Clin. Pharmacol. 2016, 56, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- van Hoogdalem, M.W.; McPhail, B.T.; Hahn, D.; Wexelblatt, S.L.; Akinbi, H.T.; Vinks, A.A.; Mizuno, T. Pharmacotherapy of neonatal opioid withdrawal syndrome: A review of pharmacokinetics and pharmacodynamics. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 87–103. [Google Scholar] [CrossRef]
- Wachman, E.M. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA 2013, 309, 1821–1827. [Google Scholar] [CrossRef]
- Albano, G.D.; La Spina, C.; Pitingaro, W.; Milazzo, V.; Triolo, V.; Argo, A.; Malta, G.; Zerbo, S. Intrauterine and Neonatal Exposure to Opioids: Toxicological, Clinical, and Medico-Legal Issues. Toxics 2023, 11, 62. [Google Scholar] [CrossRef]
- Kintz, P. Contribution of in utero drug exposure when interpreting hair results in young children. Forensic Sci. Int. 2015, 249, 314–317. [Google Scholar] [CrossRef]
- Koren, G.; Cairns, J.; Chitayat, D.; Gaedigk, A.; Leeder, S.J. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 2006, 368, 704. [Google Scholar] [CrossRef] [PubMed]
- Madadi, P.; Ross, C.J.; Hayden, M.R.; Carleton, B.C.; Gaedigk, A.; Leeder, J.S.; Koren, G. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: A case-control study. Clin. Pharmacol. Ther. 2009, 85, 31–35. [Google Scholar] [CrossRef]
- Friedrichsdorf, S.J.; Nugent, A.P.; Strobl, A.Q. Codeine-associated pediatric deaths despite using recommended dosing guidelines: Three case reports. J. Opioid. Manag. 2013, 9, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Madadi, P. Safety of codeine during breastfeeding: Fatal morphine poisoning in the breastfed neonate of a mother prescribed codeine. Can. Fam. Physician 2007, 53, 33–35. [Google Scholar] [PubMed]
- Sistonen, J. Prediction of codeine toxicity in infants and their mothers using a novel combination of maternal genetic markers. Clin. Pharmacol. Ther. 2012, 91, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Claessens, A.J.; Risler, L.J.; Eyal, S.; Shen, D.D.; Easterling, T.R.; Hebert, M.F. CYP2D6 mediates 4-hydroxylation of clonidine in vitro: Implication for pregnancy-induced changes in clonidine clearance. Drug Metab. Dispos. 2010, 38, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Badaoui, S.; Hopkins, A.M.; Rodrigues, A.D.; Miners, J.O.; Sorich, M.J.; Rowland, A. Application of Model Informed Precision Dosing to Address the Impact of Pregnancy Stage and CYP2D6 Phenotype on Foetal Morphine Exposure. AAPS J. 2021, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Pogliani, L.; Mameli, C.; Cattaneo, D.; Clementi, E.; Meneghin, F.; Radice, S.; Bruno, S.; Zuccotti, G.V. Acute kidney injury in a preterm infant homozygous for the C3435T polymorphism in the ABCB1 gene given oral morphine. Clin. Kidney J. 2012, 5, 431–433. [Google Scholar] [CrossRef]
- Elens, L.; Norman, E.; Matic, M.; Rane, A.; Fellman, V.; van Schaik, R.H. Genetic Predisposition to Poor Opioid Response in Preterm Infants: Impact of KCNJ6 and COMT Polymorphisms on Pain Relief After Endotracheal Intubation. Ther. Drug Monit. 2016, 38, 525–533. [Google Scholar] [CrossRef]
- Matic, M.; Simons, S.H.; A van Lingen, R.; van Rosmalen, J.; Elens, L.; de Wildt, S.N.; Tibboel, D.; van Schaik, R.H.; Xie, S.; Ma, W.; et al. Rescue morphine in mechanically ventilated newborns associated with combined OPRM1 and COMT genotype. Pharmacogenomics 2014, 15, 1287–1295. [Google Scholar] [CrossRef]
- Hronová, K.; Pokorná, P.; Posch, L.; Slanař, O. Sufentanil and midazolam dosing and pharmacogenetic factors in pediatric analgosedation and withdrawal syndrome. Physiol. Res. 2016, 65 (Suppl. 4), S463–S472. [Google Scholar] [CrossRef] [PubMed]
- Matic, M.; Norman, E.; Rane, A.; Beck, O.; Andersson, M.; Elens, L.; Tibboel, D.; Fellman, V.; van Schaik, R.H. Effect of UGT2B7 −900G>A (−842G>A; rs7438135) on morphine glucuronidation in preterm newborns: Results from a pilot cohort. Pharmacogenomics 2014, 15, 1589–1597. [Google Scholar] [CrossRef]
- Matic, M.; de Wildt, S.N.; Elens, L.; de Hoon, J.N.; Annaert, P.; Tibboel, D.; van Schaik, R.H.; Allegaert, K. SLC22A1/OCT1 Genotype Affects O-desmethyltramadol Exposure in Newborn Infants. Ther. Drug Monit. 2016, 38, 487–492. [Google Scholar] [CrossRef]
- Baldo, B.A. Neonatal opioid toxicity: Opioid withdrawal (abstinence) syndrome with emphasis on pharmacogenomics and respiratory depression. Arch. Toxicol. 2023, 97, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Dennis, B.B.; Bawor, M.; Thabane, L. Impact of ABCB1 and CYP2B6 genetic polymorphisms on methadone metabolism, dose and treatment response in patients with opioid addiction: A systematic review and meta-analysis. PLoS ONE 2014, 9, e86114. [Google Scholar] [CrossRef]
- Wachman, E.M.; Hayes, M.J.; Lester, B.M. Epigenetic variation in the mu-opioid receptor gene in infants with neonatal abstinence syndrome. J. Pediatr. 2014, 165, 472–478. [Google Scholar] [CrossRef]
- Wachman, E.M.; Hayes, M.J.; Shreatha, J. Epigenetic variation in OPRM1 gene in opioid-exposed mother-infant dyads. Genes Brain Behav. 2018, 17, e12476. [Google Scholar] [CrossRef]
- Committee Opinion No. Opioid Use and Opioid Use Disorder in Pregnancy. Obstet. Gynecol. 2017, 130, 488–489. [Google Scholar] [CrossRef]
- Cook, J.L. Epidemiology of opioid use in pregnancy. Best Pract. Res Clin. Obstet. Gynaecol. 2022, 85, 12–17. [Google Scholar] [CrossRef]
- Martin, C.E.; Shadowen, C.; Thakkar, B.; Oakes, T.; Gal, T.S.; Moeller, F.G. Buprenorphine dosing for the treatment of opioid use disorder through pregnancy and postpartum. Curr. Treat. Options Psychiatry 2020, 7, 375–399. [Google Scholar] [CrossRef]
- Sanjanwala, A.R.; Lim, G.; Krans, E.E. Opioids and Opioid Use Disorder in Pregnancy. Obstet. Gynecol. Clin. N. Am. 2023, 50, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Smid, M.C.; Stone, N.M.; Baksh, L.; Debbink, M.P.; Einerson, B.D.; Varner, M.W.; Gordon, A.J.; Clark, E.A.S. Pregnancy-Associated Death in Utah: Contribution of Drug-Induced Deaths. Obstet. Gynecol. 2019, 133, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, J.; Langman, E.; Mansell, D.; Dol, J.; West, C.; Benoit, B. Procedural pain assessment in neonates at risk of neonatal opioid withdrawal syndrome: A scoping review protocol. JBI Evid. Synth. 2023, 21, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Vella, A.; Savona-Ventura, C.; Mahmood, T. Harmful effects of opioid use in pregnancy: A scientific review commissioned by the European Board and College of obstetrics and gynaecology (EBCOG). Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 286, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Kassis, A.; Malone, J.; Yang, J.; Zamzami, E.; Lin, A.H.; Gordon, S.M.; Gong, M.C.; Bardo, M.; Dalmasso, C.; et al. Prenatal Morphine Exposure Increases Cardiovascular Disease Risk and Programs Neurogenic Hypertension in the Adult Offspring. Hypertension 2023, 80, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Alaee, E.; Pachenari, N.; Khani, F.; Semnanian, S.; Shojaei, A.; Azizi, H. Enhancement of neuronal excitability in the medial prefrontal cortex following prenatal morphine exposure. Brain Res. Bull. 2023, 204, 110803. [Google Scholar] [CrossRef] [PubMed]
- Jullien, S. Sudden infant death syndrome prevention. BMC Pediatr. 2021, 21, 320. [Google Scholar] [CrossRef]
- Goldberg, N.; Rodriguez-Prado, Y.; Tillery, R.; Chua, C. Sudden Infant Death Syndrome: A Review. Pediatr. Ann. 2018, 47, e118–e123. [Google Scholar] [CrossRef]
- Van Niekerk, C.; Van Deventer, B.S.; du Toit-Prinsloo, L. Long QT syndrome and sudden unexpected infant death. J. Clin. Pathol. 2017, 70, 808–813. [Google Scholar] [CrossRef]
- Ioakeimidis, N.S.; Papamitsou, T.; Meditskou, S.; Iakovidou-Kritsi, Z. Sudden infant death syndrome due to long QT syndrome: A brief review of the genetic substrate and prevalence. J. Biol. Res. 2017, 24, 6. [Google Scholar] [CrossRef]
- Hahn, D.; Emoto, C.; Euteneuer, J.C.; Mizuno, T.; Vinks, A.A.; Fukuda, T. Influence of OCT1 Ontogeny and Genetic Variation on Morphine Disposition in Critically Ill Neonates: Lessons From PBPK Modeling and Clinical Study. Clin. Pharmacol. Ther. 2019, 105, 761–768. [Google Scholar] [CrossRef]
- Willmann, S.; Edginton, A.N.; Coboeken, K.; Ahr, G.; Lippert, J. Risk to the breast-fed neonate from codeine treatment to the mother: A quantitative mechanistic modeling study. Clin. Pharmacol. Ther. 2009, 86, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, M.J. Oral clonidine in the management of acquired opioid dependency. Neonatal Netw. 2013, 32, 419–424. [Google Scholar] [CrossRef]
- Stevens, J.C.; Marsh, S.A.; Zaya, M.J. Developmental changes in human liver CYP2D6 expression. Drug Metab. Dispos. 2008, 36, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.; Fukuda, T.; Euteneuer, J.C.; Mizuno, T.; Vinks, A.A.; Sadhasivam, S.; Emoto, C. Influence of MRP3 Genetics and Hepatic Expression Ontogeny for Morphine Disposition in Neonatal and Pediatric Patients. J. Clin. Pharmacol. 2020, 60, 992–998. [Google Scholar] [CrossRef]
- Dobrinas, M.; Crettol, S.; Oneda, B.; Lahyani, R.; Rotger, M.; Choong, E.; Lubomirov, R.; Csajka, C.; Eap, C.B. Contribution of CYP2B6 alleles in explaining extreme (S)- methadone plasma levels: A CYP2B6 gene resequencing study. Pharmacogenet. Genom. 2013, 23, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Gadel, S.; Crafford, A.; Regina, K.; Kharasch, E.D. Methadone N-demethylation by the common CYP2B6 allelic variant CYP2B6.6. Drug Metab. Dispos. 2013, 41, 709–713. [Google Scholar] [CrossRef]
- Kharasch, E.D.; Regina, K.J.; Blood, J.; Friedel, C. Methadone pharmacogenetics: CYP2B6 polymorphisms determine plasma concentrations, clearance, and metabolism. Anesthesiology 2015, 123, 1142–1153. [Google Scholar] [CrossRef]
- Kringen, M.K.; Chalabianloo, F.; Bernard, J.P.; Bramness, J.; Molden, E.; Høiseth, G. Combined effect of CYP2B6 genotype and other candidate genes on a steady-state serum concentration of methadone in opioid maintenance treatment. Ther. Drug Monit. 2017, 39, 550–555. [Google Scholar] [CrossRef]
- Bunten, H.; Liang, W.J.; Pounder, D.J.; Seneviratne, C.; Osselton, D. OPRM1 and CYP2B6 gene variants as risk factors in methadone -related deaths. Clin. Pharmacol. Ther. 2010, 88, 383–389. [Google Scholar] [CrossRef]
- Bunten, H.; Liang, W.J.; Pounder, D.J.; Seneviratne, C.; Osselton, D. Interindividual variability in the prevalence of OPRM1 and CYP2B6 gene variations may identify drug-susceptible populations. J. Anal. Toxicol. 2011, 35, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.A.; Yuferov, V.; Hamon, S. Increased OPRM1 DNA methylation in lymphocytes of methadone -maintained former heroin addicts. Neuropsychopharmacology 2009, 34, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Vassoler, F.M.; Byrnes, E.M.; Pierce, R.C. The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology 2014, 76, 269–275. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, E.; Bonasoni, M.P.; Pascali, J.P.; Bini, C.; Pelletti, G.; Gualandi, A.; Dal Lago, G.; Mercati, A.; Mariotti, B.; Pasini, G.P.; et al. Fetal and Infant Effects of Maternal Opioid Use during Pregnancy: A Literature Review including Clinical, Toxicological, Pharmacogenomic, and Epigenetic Aspects for Forensic Evaluation. Children 2024, 11, 278. https://doi.org/10.3390/children11030278
Giovannini E, Bonasoni MP, Pascali JP, Bini C, Pelletti G, Gualandi A, Dal Lago G, Mercati A, Mariotti B, Pasini GP, et al. Fetal and Infant Effects of Maternal Opioid Use during Pregnancy: A Literature Review including Clinical, Toxicological, Pharmacogenomic, and Epigenetic Aspects for Forensic Evaluation. Children. 2024; 11(3):278. https://doi.org/10.3390/children11030278
Chicago/Turabian StyleGiovannini, Elena, Maria Paola Bonasoni, Jennifer Paola Pascali, Carla Bini, Guido Pelletti, Alberto Gualandi, Giovanni Dal Lago, Andrea Mercati, Beatrice Mariotti, Giulia Paola Pasini, and et al. 2024. "Fetal and Infant Effects of Maternal Opioid Use during Pregnancy: A Literature Review including Clinical, Toxicological, Pharmacogenomic, and Epigenetic Aspects for Forensic Evaluation" Children 11, no. 3: 278. https://doi.org/10.3390/children11030278
APA StyleGiovannini, E., Bonasoni, M. P., Pascali, J. P., Bini, C., Pelletti, G., Gualandi, A., Dal Lago, G., Mercati, A., Mariotti, B., Pasini, G. P., Poll, I. A., & Fais, P. (2024). Fetal and Infant Effects of Maternal Opioid Use during Pregnancy: A Literature Review including Clinical, Toxicological, Pharmacogenomic, and Epigenetic Aspects for Forensic Evaluation. Children, 11(3), 278. https://doi.org/10.3390/children11030278






