The Evolution of Scientific Knowledge in Childhood Asthma over Time: A Surprising History
Abstract
1. Introduction
2. Asthma: An Ancient Disease
3. The New Concept of Different Types of Asthma
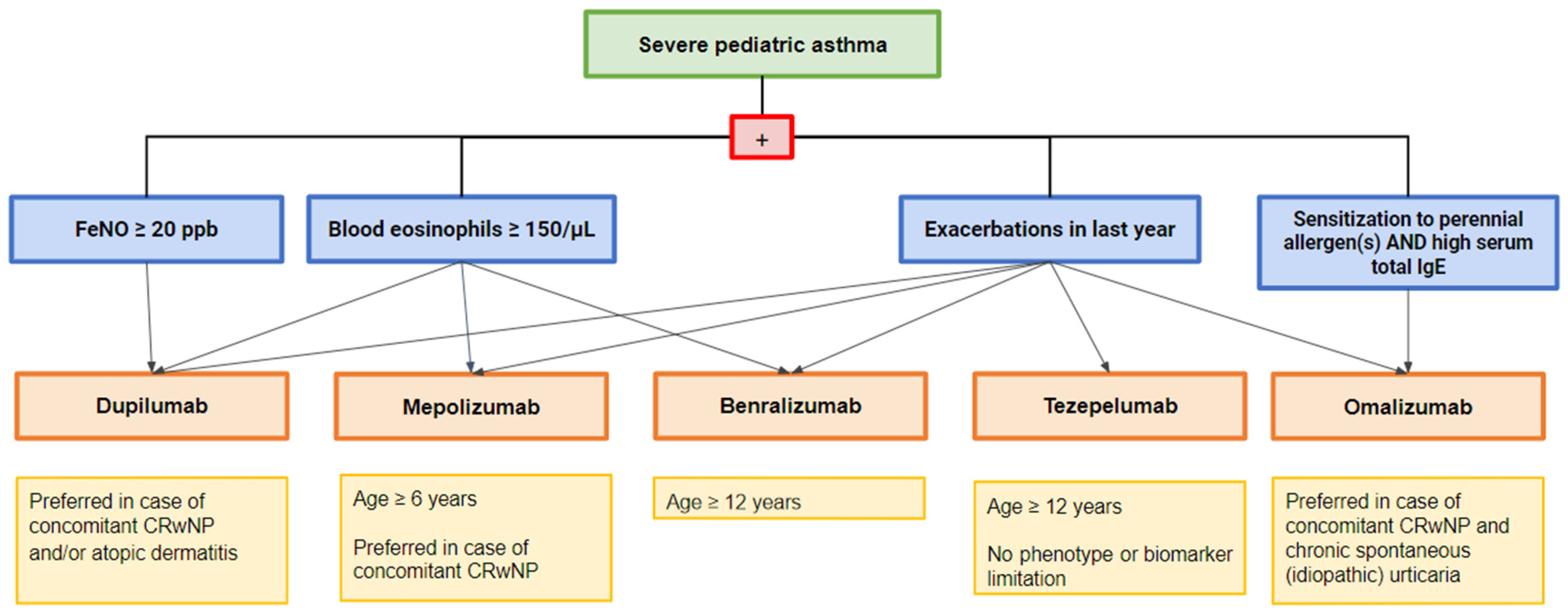
4. Different Endotypes for Different Therapies in Severe Asthma
5. Omalizumab
Safety
6. Mepolizumab
Safety
7. Benralizumab
Safety
8. Dupilumab
Safety
9. Tezepelumab
Safety
10. A Tailored Biologic Therapy for Each Pediatric-Specific Severe Asthma
11. Evolution of Childhood Asthma after Prolonged Biological Treatment
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Iniziative for Asthma. Global Strategy for asthma Management and Prevention. 2023. Available online: https://ginasthma.org/ (accessed on 30 November 2023).
- Azmeh, R.; Greydanus, D.E.; Agana, M.G.; Dickson, C.A.; Patel, D.R.; Ischander, M.M.; Lloyd, R.D. Update in Pediatric Asthma: Selected Issues. Dis.-a-Mon. 2020, 66, 100886. [Google Scholar] [CrossRef] [PubMed]
- Szefler, S.J. Asthma across the lifespan: Time for a paradigm shift. J. Allergy Clin. Immunol. 2018, 142, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Conrad, L.A.; Cabana, M.D.; Rastogi, D. Defining pediatric asthma: Phenotypes to endotypes and beyond. Pediatr. Res. 2021, 90, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Castagnoli, R.; Brambilla, I.; Marseglia, A.; Tosca, M.A.; Marseglia, G.L.; Ciprandi, G. Asthma Endotyping and Biomarkers in Childhood Asthma. Pediatr. Allergy Immunol. Pulmonol. 2018, 31, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, R.; Licari, A.; Manti, S.; Chiappini, E.; Marseglia, G.L. Type-2 inflammatory mediators as targets for precision medicine in children. Pediatr. Allergy Immunol. 2020, 31, 17–19. [Google Scholar] [CrossRef]
- Eyerich, S.; Metz, M.; Bossios, A.; Eyerich, K. New biological treatments for asthma and skin allergies. Allergy 2020, 75, 546–560. [Google Scholar] [CrossRef]
- Bush, A. Which Child with Asthma is a Candidate for Biological Therapies? J. Clin. Med. 2020, 9, 1237. [Google Scholar] [CrossRef]
- Pavord, I.D.; Korn, S.; Howarth, P.; Bleecker, E.R.; Buhl, R.; Keene, O.N.; Ortega, H.; Chanez, P. Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double-blind, placebo-controlled trial. Lancet 2012, 380, 651–659. [Google Scholar] [CrossRef]
- Sze, E.; Bhalla, A.; Nair, P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy 2020, 75, 311–325. [Google Scholar] [CrossRef]
- Andrenacci, B.; De Filippo, M.; Votto, M.; Prevedoni Gorone, M.S.; De Amici, M.; La Grutta, S.; Marseglia, G.L.; Licari, A. Severe pediatric asthma endotypes: Current limits and future perspectives. Expert. Rev. Respir. Med. 2023, 17, 675–690. [Google Scholar] [CrossRef]
- Pijnenburg, M.W.; Fleming, L. Advances in understanding and reducing the burden of severe asthma in children. Lancet Respir. Med. 2020, 8, 1032–1044. [Google Scholar] [CrossRef]
- Licari, A.; Manti, S.; Castagnoli, R.; Parisi, G.F.; Salpietro, C.; Leonardi, S.; Marseglia, G.L. Targeted Therapy for Severe Asthma in Children and Adolescents: Current and Future Perspectives. Pediatr. Drugs 2019, 21, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma. Difficult-to-Treat Asthma in Adolescents and Adult Patients. 2023. Available online: https://ginasthma.org/ (accessed on 30 November 2023).
- Licari, A.; Manti, S.; Castagnoli, R.; Marseglia, A.; Foiadelli, T.; Brambilla, I.; Marseglia, G.L. Immunomodulation in Pediatric Asthma. Front. Pediatr. 2019, 7, 289. [Google Scholar] [CrossRef]
- Lovinsky-Desir, S. The use of biologic therapies for the management of pediatric asthma. Pediatr. Pulmonol. 2020, 55, 803–808. [Google Scholar] [CrossRef]
- Sardon-Prado, O.; Diaz-Garcia, C.; Corcuera-Elosegui, P.; Korta-Murua, J.; Valverde-Molina, J.; Sanchez-Solis, M. Severe Asthma and Biological Therapies: Now and the Future. J. Clin. Med. 2023, 12, 5846. [Google Scholar] [CrossRef]
- Sesé, L.; Schneider, M.; Bourgoin, M.; Saint-Pierre, P.; Lambert, N.; Guiddir, T.; Couderc, R.; Amat, F.; Just, J. Asthma with multiple allergic comorbidities is associated with complete response to omalizumab. Clin. Exp. Allergy 2019, 49, 733–735. [Google Scholar] [CrossRef]
- Sorkness, C.A.; Wildfire, J.J.; Calatroni, A.; Mitchell, H.E.; Busse, W.W.; O’Connor, G.T.; Pongracic, J.A.; Ross, K.; Gill, M.A.; Kattan, M.; et al. Reassessment of Omalizumab-Dosing Strategies and Pharmacodynamics in Inner-City Children and Adolescents. J. Allergy Clin. Immunol. Pract. 2013, 1, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Votto, M.; De Filippo, M.; Licari, A.; Marseglia, A.; De Amici, M.; Marseglia, G.L. Biological Therapies in Children and Adolescents with Severe Uncontrolled Asthma: A Practical Review. Biol. Targets Ther. 2021, 15, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Nieto García, A.; Garriga-Baraut, T.; Plaza Martín, A.M.; Nieto Cid, M.; Torres Borrego, J.; Folqué Giménez, M.D.M.; Lozano Blasco, J.; Bosque García, M.; Moreno-Galarraga, L.; Tortajada-Girbés, M.; et al. Omalizumab outcomes for up to 6 years in pediatric patients with severe persistent allergic asthma. Pediatr. Allergy Immunol. 2021, 32, 980–991. [Google Scholar] [CrossRef]
- Brodlie, M.; McKean, M.C.; Moss, S.; Spencer, D.A. The oral corticosteroid-sparing effect of omalizumab in children with severe asthma. Arch. Dis. Child. 2012, 97, 604–609. [Google Scholar] [CrossRef]
- Busse, W.W.; Morgan, W.J.; Gergen, P.J.; Mitchell, H.E.; Gern, J.E.; Liu, A.H.; Gruchalla, R.S.; Kattan, M.; Teach, S.J.; Pongracic, J.A.; et al. Randomized Trial of Omalizumab (Anti-IgE) for Asthma in Inner-City Children. N. Engl. J. Med. 2011, 364, 1005–1015. [Google Scholar] [CrossRef]
- Lanier, B.; Bridges, T.; Kulus, M.; Taylor, A.F.; Berhane, I.; Vidaurre, C.F. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J. Allergy Clin. Immunol. 2009, 124, 1210–1216. [Google Scholar] [CrossRef]
- Sheehan, W.J.; Krouse, R.Z.; Calatroni, A.; Gergen, P.J.; Gern, J.E.; Gill, M.A.; Gruchalla, R.S.; Khurana Hershey, G.K.; Kattan, M.; Kercsmar, C.M.; et al. Aeroallergen Sensitization, Serum IgE, and Eosinophilia as Predictors of Response to Omalizumab Therapy During the Fall Season Among Children with Persistent Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 3021–3028.e2. [Google Scholar] [CrossRef]
- Bożek, A.; Fischer, A.; Bogacz-Piaseczynska, A.; Canonica, G.W. Adding a biologic to allergen immunotherapy increases treatment efficacy. ERJ Open Res. 2023, 9, 00639–02022. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, V.A.; Licari, A.; Volpini, A.; Fenu, G.; Patria, M.F.; Seminara, M.; Spada, E.; Rusconi, F. Prospective follow up after omalizumab discontinuation in a cohort of children with severe asthma. Pediatr. Pulmonol. 2023, 58, 2424–2426. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Kashitani, Y.; Yoshisue, H.; Nagasaki, M.; Sasajima, T. Real-life long-term safety and effectiveness of omalizumab in Japanese pediatric patients with severe allergic asthma: A post-marketing surveillance. Allergol. Int. 2021, 70, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Normansell, R.; Walker, S.; Milan, S.J.; Walters, E.H.; Nair, P. Omalizumab for asthma in adults and children. Cochrane Database Syst. Rev. 2014, 13, CD003559. [Google Scholar] [CrossRef]
- Kline GS. Nucala® (Mepolizumab) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125526Orig1s000Lbl.pdf (accessed on 27 April 2021).
- Comberiati, P.; McCormack, K.; Malka-Rais, J.; Spahn, J.D. Proportion of Severe Asthma Patients Eligible for Mepolizumab Therapy by Age and Age of Onset of Asthma. J. Allergy Clin. Immunol. Pract. 2019, 7, 2689–2696.e2. [Google Scholar] [CrossRef]
- Farne, H.A.; Wilson, A.; Powell, C.; Bax, L.; Milan, S.J. Anti-IL5 therapies for asthma. Cochrane Database Syst. Rev. 2017, 12, CD010834. [Google Scholar] [CrossRef]
- Ortega, H.G.; Yancey, S.W.; Mayer, B.; Gunsoy, N.B.; Keene, O.N.; Bleecker, E.R.; Brightling, C.E.; Pavord, I.D. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: A secondary analysis of the DREAM and MENSA studies. Lancet Respir. Med. 2016, 4, 549–556. [Google Scholar] [CrossRef]
- Gupta, A.; Ikeda, M.; Geng, B.; Azmi, J.; Price, R.G.; Bradford, E.S.; Yancey, S.W.; Steinfeld, J. Long-term safety and pharmacodynamics of mepolizumab in children with severe asthma with an eosinophilic phenotype. J. Allergy Clin. Immunol. 2019, 144, 1336–1342.e7. [Google Scholar] [CrossRef]
- Gupta, A.; Pouliquen, I.; Austin, D.; Price, R.G.; Kempsford, R.; Steinfeld, J.; Bradford, E.S.; Yancey, S.W. Subcutaneous mepolizumab in children aged 6 to 11 years with severe eosinophilic asthma. Pediatr. Pulmonol. 2019, 54, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, D.P.; Bodtger, U.; Sidenius, K.; Maltbaek, N.; Pedersen, L.; Madsen, H.; Andersson, E.A.; Norgaard, O.; Madsen, L.K.; Chawes, B.L. Efficacy, adverse events, and inter-drug comparison of mepolizumab and reslizumab anti-IL-5 treatments of severe asthma—A systematic review and meta-analysis. Eur. Clin. Respir. J. 2018, 5, 1536097. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Bacharier, L.B.; Gergen, P.J.; Gagalis, L.; Calatroni, A.; Wellford, S.; Gill, M.A.; Stokes, J.; Liu, A.H.; Gruchalla, R.S.; et al. Mepolizumab for urban children with exacerbation-prone eosinophilic asthma in the USA (MUPPITS-2): A randomised, double-blind, placebo-controlled, parallel-group trial. Lancet 2022, 400, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Drick, N.; Seeliger, B.; Welte, T.; Fuge, J.; Suhling, H. Anti-IL-5 therapy in patients with severe eosinophilic asthma—Clinical efficacy and possible criteria for treatment response. BMC Pulm. Med. 2018, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Albers, F.C.; Licskai, C.; Chanez, P.; Bratton, D.J.; Bradford, E.S.; Yancey, S.W.; Kwon, N.; Quirce, S. Baseline blood eosinophil count as a predictor of treatment response to the licensed dose of mepolizumab in severe eosinophilic asthma. Respir. Med. 2019, 159, 105806. [Google Scholar] [CrossRef]
- Kavanagh, J.E.; Hearn, A.P.; Jackson, D.J. A pragmatic guide to choosing biologic therapies in severe asthma. Breathe 2021, 17, 210144. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Fasenra. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/fasenra (accessed on 30 November 2023).
- Ghazi, A.; Trikha, A.; Calhoun, W.J. Benralizumab—A humanized mAb to IL-5Rα with enhanced antibody-dependent cell-mediated cytotoxicity—A novel approach for the treatment of asthma. Expert. Opin. Biol. Ther. 2012, 12, 113–118. [Google Scholar] [CrossRef]
- Kolbeck, R.; Kozhich, A.; Koike, M.; Peng, L.; Andersson, C.K.; Damschroder, M.M.; Reed, J.L.; Woods, R.; Dall’Acqua, W.W.; Stephens, G.L.; et al. MEDI-563, a humanized anti–IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 2010, 125, 1344–1353.e2. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Busse, W.W.; Bleecker, E.R.; FitzGerald, J.M.; Ferguson, G.T.; Barker, P.; Brooks, L.; Olsson, R.F.; Martin, U.J.; Goldman, M. Benralizumab for adolescent patients with severe, eosinophilic asthma: Safety and efficacy after 3 years of treatment. J. Allergy Clin. Immunol. 2021, 148, 266–271.e2. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral Glucocorticoid–Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Tenero, L.; Rossignoli, S.; Piacentini, G. Severe asthma: When to resort to biological agents. Pediatr. Allergy Immunol. 2020, 31, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Just, J.; Deschildre, A.; Lejeune, S.; Amat, F. New perspectives of childhood asthma treatment with biologics. Pediatr. Allergy Immunol. 2019, 30, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Bleecker, E.R.; FitzGerald, J.M.; Ferguson, G.T.; Barker, P.; Sproule, S.; Olsson, R.F.; Martin, U.J.; Goldman, M.; Yañez, A.; et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir. Med. 2019, 7, 46–59. [Google Scholar] [CrossRef]
- Korn, S.; Bourdin, A.; Chupp, G.; Cosio, B.G.; Arbetter, D.; Shah, M.; Gil, E.G. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J. Allergy Clin. Immunol. Pract. 2021, 9, 4381–4392.e4. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Korn, S.; Mathur, S.K.; Barker, P.; Meka, V.G.; Martin, U.J.; Zangrilli, J.G. Safety of Eosinophil-Depleting Therapy for Severe, Eosinophilic Asthma: Focus on Benralizumab. Drug Saf. 2020, 43, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Bacharier, L.B.; Maspero, J.F.; Katelaris, C.H.; Fiocchi, A.G.; Gagnon, R.; De Mir, I.; Jain, N.; Sher, L.D.; Mao, X.; Liu, D.; et al. Dupilumab in Children with Uncontrolled Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 2230–2240. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Dupixent. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent (accessed on 6 December 2023).
- Busse, W.W.; Maspero, J.F.; Rabe, K.F.; Papi, A.; Wenzel, S.E.; Ford, L.B.; Pavord, I.D.; Zhang, B.; Staudinger, H.; Pirozzi, G.; et al. Liberty Asthma QUEST: Phase 3 Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate Dupilumab Efficacy/Safety in Patients with Uncontrolled, Moderate-to-Severe Asthma. Adv. Ther. 2018, 35, 737–748. [Google Scholar] [CrossRef]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; FitzGerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N. Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef]
- Papadopoulos, N.G.; Szefler, S.J.; Bacharier, L.B.; Maspero, J.F.; Domingo, C.; Fiocchi, A.; Lee, J.K.; Daizadeh, N.; Lederer, D.J.; Hardin, M.; et al. Assessment of dupilumab in children with moderate-to-severe type 2 asthma with or without evidence of allergic asthma. Allergy 2023, 78, 2157–2167. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- Agache, I.; Beltran, J.; Akdis, C.; Akdis, M.; Canelo-Aybar, C.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Del Giacco, S.; et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines—Recommendations on the use of biologicals in severe asthma. Allergy 2020, 75, 1023–1042. [Google Scholar] [CrossRef]
- Ferrante, G.; Tenero, L.; Piazza, M.; Piacentini, G. Severe pediatric asthma therapy: Dupilumab. Front. Pediatr. 2022, 10, 963610. [Google Scholar] [CrossRef]
- Bacharier, L.B.; Maspero, J.F.; Katelaris, C.H.; Fiocchi, A.G.; Gagnon, R.; De Mir, I.; Guilbert, T.W.; Jackson, D.J.; Staudinger, H.W.; Laws, E.; et al. Assessment of long-term safety and efficacy of dupilumab in children with asthma (LIBERTY ASTHMA EXCURSION): An open-label extension study. Lancet Respir. Med. 2023, 12, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Maspero, J.; Fitzgerald, M.; Pavord, I.; Wenzel, S.; Zhang, B.; Maroni, J.; Rowe, P.; Amin, N.; Pirozzi, G.; Ruddy, M.; et al. Dupilumab reduces severe exacerbation rate and improves lung function in adolescent patients with uncontrolled, moderate-to-severe asthma: From the liberty asthma quest study. Chest 2018, 154, 25A–27A. [Google Scholar] [CrossRef]
- Menzella, F.; Montanari, G.; Patricelli, G.; Cavazza, A.; Galeone, C.; Ruggiero, P.; Bagnasco, D.; Facciolongo, N. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther. Clin. Risk Manag. 2019, 15, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Tezepelumab: First Approval. Drugs 2022, 82, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Van Dijk, Y.E.; Rutjes, N.W.; Golebski, K.; Şahin, H.; Hashimoto, S.; Maitland-van Der Zee, A.-H.; Vijverberg, S.J.H. Developments in the Management of Severe Asthma in Children and Adolescents: Focus on Dupilumab and Tezepelumab. Pediatr. Drugs 2023, 25, 677–693. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Wechsler, M.E.; Brightling, C.E.; Korn, S.; Corren, J.; Israel, E.; Chupp, G.; Bednarczyk, A.; Ponnarambil, S.; Caveney, S.; et al. Long-term safety and efficacy of tezepelumab in people with severe, uncontrolled asthma (DESTINATION): A randomised, placebo-controlled extension study. Lancet Respir. Med. 2023, 11, 425–438. [Google Scholar] [CrossRef]
- Corren, J.; Menzies-Gow, A.; Bimmel, J.; McGuinness, A.; Almqvist, G.; Bowen, K.; Griffiths, J.M.; Ponnarambil, S.; Bourdin, A.; Israel, E.; et al. Tezepelumab for the treatment of severe asthma: A plain language summary of the PATHWAY and NAVIGATOR studies. Immunotherapy 2023, 15, 1327–1340. [Google Scholar] [CrossRef]
- Hashimoto, S.; Bel, E.H. Current treatment of severe asthma. Clin. Exp. Allergy 2012, 42, 693–705. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2022, 60, 2101499. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Blasi, F.; Carpagnano, G.E.; Guida, G.; Heffler, E.; Paggiaro, P.; Allegrini, C.; Antonelli, A.; Aruanno, A.; Bacci, E.; et al. Severe Asthma Network Italy Definition of Clinical Remission in Severe Asthma: A Delphi Consensus. J. Allergy Clin. Immunol. Pract. 2023, 11, 3629–3637. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; McDonald, V.M.; Pavord, I.D.; Gibson, P.G. Asthma remission: What is it and how can it be achieved? Eur. Respir. J. 2022, 60, 2102583. [Google Scholar] [CrossRef]
- Menzies-Gow, A.N.; Price, D.B. Clinical Remission in Severe Asthma. CHEST 2023, 164, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Ohtawa, J. Effects of adding omalizumab, an anti-immunoglobulin E antibody, on airway wall thickening in asthma. Respiration 2012, 83, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, T.; Niimi, A.; Matsumoto, H.; Ito, I.; Oguma, T.; Otsuka, K.; Takeda, T.; Nakaji, H.; Inoue, H.; Iwata, T.; et al. Comprehensive efficacy of omalizumab for severe refractory asthma: A time-series observational study. Ann. Allergy Asthma Immunol. 2014, 113, 470–475.e2. [Google Scholar] [CrossRef] [PubMed]
- Przybyszowski, M.; Paciorek, K.; Zastrzeżyńska, W.; Gawlewicz-Mroczka, A.; Trojan-Królikowska, A.; Orłowska, A.; Soja, J.; Pawlik, W.; Sładek, K. Influence of Omalizumab Therapy on Airway Remodeling Assessed with High-Resolution Computed Tomography (HRCT) in Severe Allergic Asthma Patients. Adv. Respir. Med. 2018, 86, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and Exacerbations of Refractory Eosinophilic Asthma. N. Engl. J. Med. 2009, 360, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.M.; Dal Negro, R.W.; Micheletto, C.; De Ferrari, L.; Folli, C.; Chiappori, A.; Canonica, G.W. Omalizumab Modulates Bronchial Reticular Basement Membrane Thickness and Eosinophil Infiltration in Severe Persistent Allergic Asthma Patients. Int. J. Immunopathol. Pharmacol. 2012, 25, 475–484. [Google Scholar] [CrossRef]
- Riccio, A.M.; Mauri, P.; De Ferrari, L.; Rossi, R.; Di Silvestre, D.; Benazzi, L.; Chiappori, A.; Dal Negro, R.W.; Micheletto, C.; Canonica, G.W. Galectin-3: An early predictive biomarker of modulation of airway remodeling in patients with severe asthma treated with omalizumab for 36 months. Clin. Transl. Allergy 2017, 7, 6. [Google Scholar] [CrossRef]
- Zastrzeżyńska, W.; Przybyszowski, M.; Bazan-Socha, S.; Gawlewicz-Mroczka, A.; Sadowski, P.; Okoń, K.; Jakieła, B.; Plutecka, H.; Ćmiel, A.; Sładek, K.; et al. Omalizumab may decrease the thickness of the reticular basement membrane and fibronectin deposit in the bronchial mucosa of severe allergic asthmatics. J. Asthma 2020, 57, 468–477. [Google Scholar] [CrossRef]
- Chachi, L.; Diver, S.; Kaul, H.; Rebelatto, M.C.; Boutrin, A.; Nisa, P.; Newbold, P.; Brightling, C. Computational modelling prediction and clinical validation of impact of benralizumab on airway smooth muscle mass in asthma. Eur. Respir. J. 2019, 54, 1900930. [Google Scholar] [CrossRef]
- Tsuge, M.; Ikeda, M.; Tsukahara, H. Novel Lung Growth Strategy with Biological Therapy Targeting Airway Remodeling in Childhood Bronchial Asthma. Children 2022, 9, 1253. [Google Scholar] [CrossRef]
- Jeffery, M.M.; Inselman, J.W.; Maddux, J.T.; Lam, R.W.; Shah, N.D.; Rank, M.A. Asthma Patients Who Stop Asthma Biologics Have a Similar Risk of Asthma Exacerbations as Those Who Continue Asthma Biologics. J. Allergy Clin. Immunol. Pract. 2021, 9, 2742–2750.e1. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Bourdin, A.; Taillé, C.; Kamar, D.; Thonnelier, C.; Lajoinie, A.; Rigault, A.; Deschildre, A.; Molimard, M. Real-life omalizumab exposure and discontinuation in a large nationwide population-based study of paediatric and adult asthma patients. Eur. Respir. J. 2022, 60, 2103130. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Čustović, A.; Cabana, M.D.; Dell, S.D.; Deschildre, A.; Hedlin, G.; Hossny, E.; Le Souëf, P.; Matricardi, P.M.; Nieto, A.; et al. Pediatric asthma: An unmet need for more effective, focused treatments. Pediatr. Allergy Immunol. 2019, 30, 7–16. [Google Scholar] [CrossRef] [PubMed]
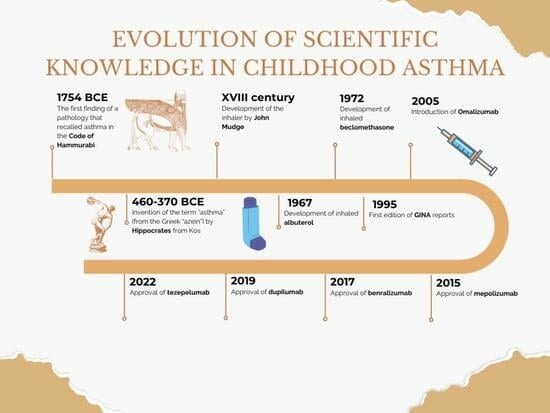
| Year | Main Advancement of Knowledge Related to Asthma |
|---|---|
| 1754 BCE | The first finding of a pathology that recalled asthma in the Code of Hammurabi |
| 460–370 BCE | Invention of the term “asthma” (from the Greek “azein”) by Hippocrates from Kos |
| XVIII century | Development of the inhaler by John Mudge |
| 1967 | Development of inhaled albuterol |
| 1972 | Development of inhaled beclomethasone |
| 1995 | First edition of GINA * recommendations |
| 2000s | The concept of “asthma control” became the main guide in asthma treatment |
| 2005 | Introduction of biologics (omalizumab) as targeted therapy in asthma in Europe |
| 2015 | Approval of mepolizumab for severe eosinophilic asthma |
| 2017 | Approval of benralizumab for severe eosinophilic asthma |
| 2019 | Approval of dupilumab for severe asthma with type 2 inflammation |
| 2022 | Approval of tezepelumab for inadequately controlled severe asthma |
| Biological Drug | Molecular Target | Approved Age for Use | Indication | Administration Dose for Asthma |
|---|---|---|---|---|
| Omalizumab | IgE | ≥6 years | Severe allergic asthma | 0.016 mg/kg/IgE (I.U./mL) every 2–4 w° |
| Mepolizumab | IL5 | ≥6 years | Severe eosinophilic asthma |
|
| Benralizumab | IL 5Rα | ≥12 years (USA) | Severe eosinophilic asthma | 30 mg every 4 w for the first three doses, every 8 w afterwards |
| Dupilumab | IL 4Rα | ≥6 years | Severe T2-high asthma |
|
| Tezepelumab | TSLP * | ≥12 years | Severe asthma | 210 mg every 4 w |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venditto, L.; Morano, S.; Ferrante, G.; Piazza, M.; Tenero, L.; Piacentini, G.; Pecoraro, L. The Evolution of Scientific Knowledge in Childhood Asthma over Time: A Surprising History. Children 2024, 11, 262. https://doi.org/10.3390/children11020262
Venditto L, Morano S, Ferrante G, Piazza M, Tenero L, Piacentini G, Pecoraro L. The Evolution of Scientific Knowledge in Childhood Asthma over Time: A Surprising History. Children. 2024; 11(2):262. https://doi.org/10.3390/children11020262
Chicago/Turabian StyleVenditto, Laura, Sonia Morano, Giuliana Ferrante, Michele Piazza, Laura Tenero, Giorgio Piacentini, and Luca Pecoraro. 2024. "The Evolution of Scientific Knowledge in Childhood Asthma over Time: A Surprising History" Children 11, no. 2: 262. https://doi.org/10.3390/children11020262
APA StyleVenditto, L., Morano, S., Ferrante, G., Piazza, M., Tenero, L., Piacentini, G., & Pecoraro, L. (2024). The Evolution of Scientific Knowledge in Childhood Asthma over Time: A Surprising History. Children, 11(2), 262. https://doi.org/10.3390/children11020262