Design and Evaluation of a Spoke-Based Double-Lumen Pediatric Gastrostomy Tube
Abstract
1. Introduction
1.1. Device and Testing Rig
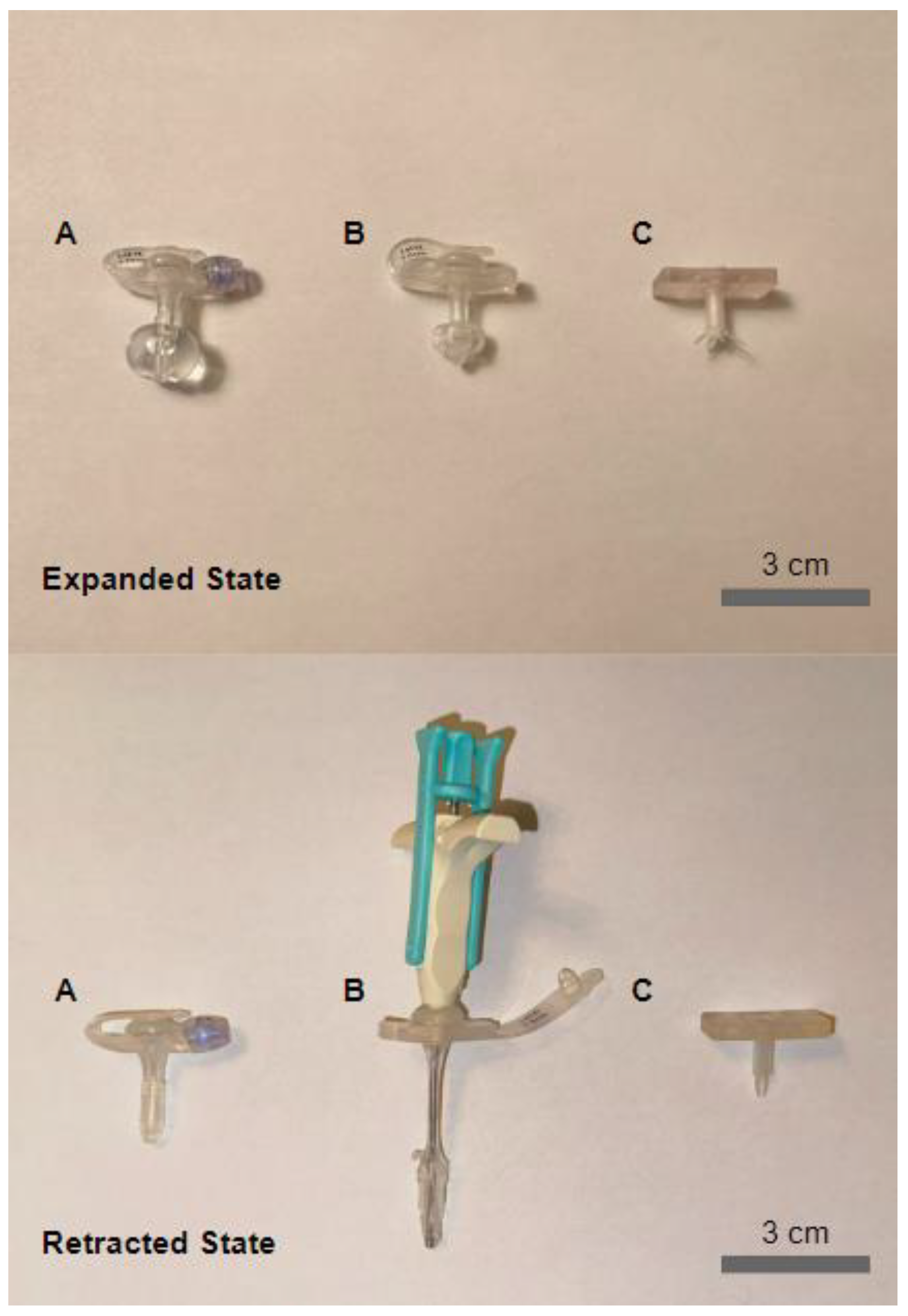
1.1.1. G-Tube Design
1.1.2. Expansion-Retraction Mechanism
1.1.3. Testing Rig: Biofidelic Stomach Model
2. Materials and Methods
2.1. G-Tube Manufacturing
2.2. Biofidelic Stomach Model: Development and Validation
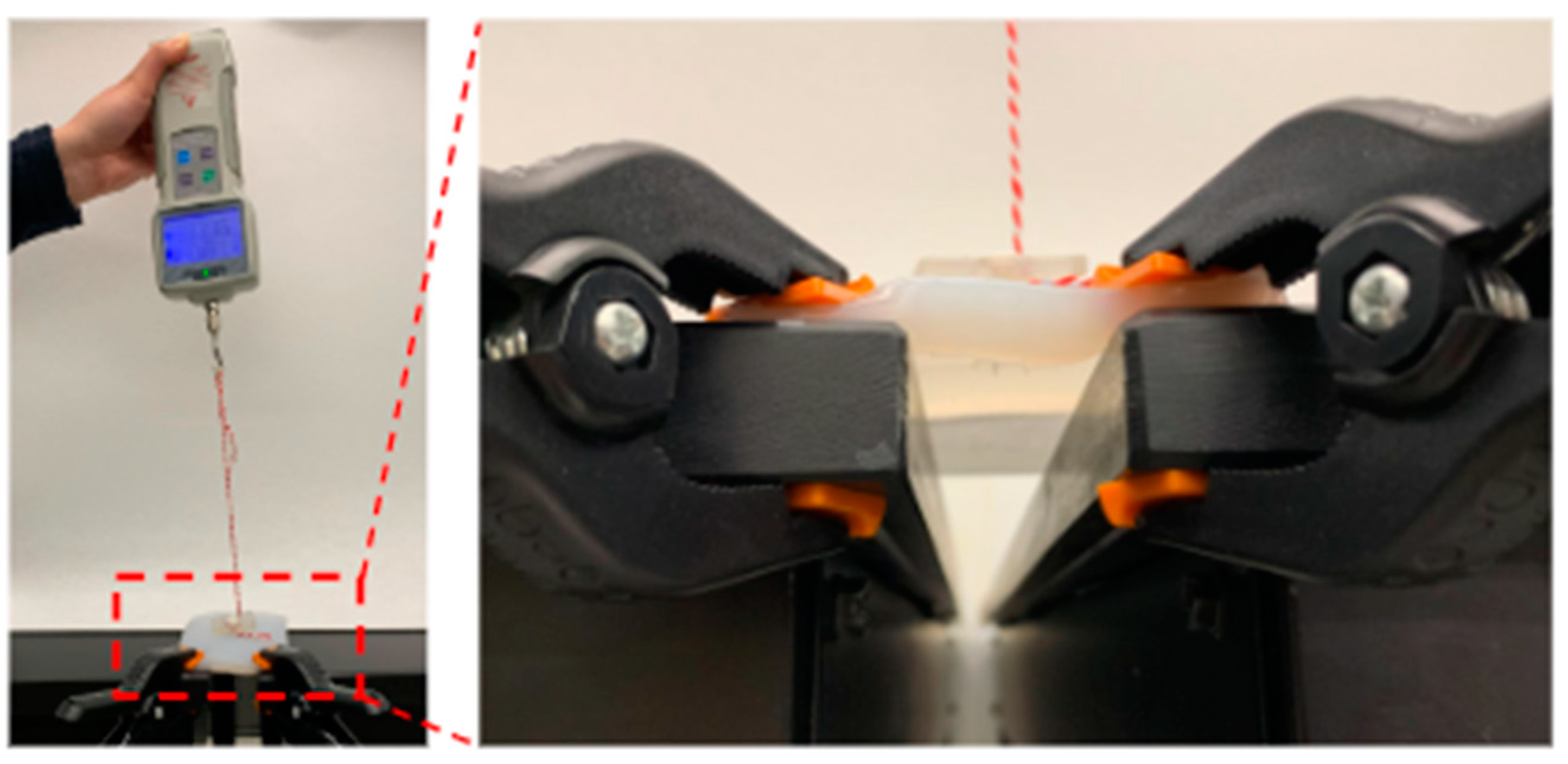
2.3. Expanded State Pull Test
2.4. Retracted State Pull Test
2.5. Usability Test
3. Results
3.1. Expanded State Pull Test
3.2. Retracted State Pull Test
3.3. Usability Test
4. Discussion
4.1. Manufacturing
4.2. Pull Force
4.3. Usability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bankhead, R.; Boullata, J.; Brantley, S.; Corkins, M.; Guenter, P.; Krenitsky, J.; Lyman, B.; Metheny, N.A.; Mueller, C.; Robbins, S.; et al. Enteral Nutrition Practice Recommendations. J. Parenter. Enter. Nutr. 2009, 33, 122–167. [Google Scholar] [CrossRef] [PubMed]
- Villares, J.M.M. The practice of home artificial nutrition in Europe. Nutr. Hosp. 2004, 19, 59–67. [Google Scholar]
- Wicks, C.; Gimson, A.; Vlavianos, P.; Lombard, M.; Panos, M.; Macmathuna, P.; Tudor, M.; Andrews, K.; Westaby, D. Assessment of the Percutaneous Endoscopic Gastrostomy Feeding Tube as Part of an Integrated Approach to Enteral Feeding. Gut 1992, 33, 613–616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aedla, M.; Zhou, A.; Sompel, K.; Hu, K.; Cheng, C.; Hsu, J.; Qian, J.; Zhang, S.; Ho, A.; Slagle, J.; et al. A Study of Postoperative Complications Occurring at Home with Pediatric Gastrostomy Feeding Tubes. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, J.P.; De Roo, A.C.; Nielsen, J.; Ambeba, E.; Cooper, J.N.; Hogan, M.J.; Erdman, S.; Deans, K.J.; Minneci, P.C.; Kenney, B. A Comparison of Pediatric Gastrostomy Tube Placement Techniques. Pediatr. Surg. Int. 2015, 32, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ley, D.; Saha, S. Everything That You Always Wanted to Know about the Management of Percutaneous Endoscopic Gastrostomy (PEG) Tubes (but Were Afraid to Ask). Dig. Dis. Sci. 2023, 68, 2221–2225. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S. Gastrostomy Tubes. Pediatr. Emerg. Care 2017, 33, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Bentley, V.L.; Seemann, N.M.; Blackmore, C. A Comparison of Balloon and Nonballoon Gastrostomy Tubes in Children. J. Pediatr. Surg. 2020, 55, 852–854. [Google Scholar] [CrossRef]
- Rahnemai-Azar, A.A. Percutaneous Endoscopic Gastrostomy: Indications, Technique, Complications and Management. World J. Gastroenterol. 2014, 20, 7739. [Google Scholar] [CrossRef] [PubMed]
- Swindle, M.M.; Smith, A.C.; Helke, K.L. Recommendations for medical device implantation in swine. Isr. J. Vet. Med. 2013, 68, 3. [Google Scholar]
- Ranamukhaarachchi, S.A.; Lehnert, S.; Ranamukhaarachchi, S.L.; Sprenger, L.; Schneider, T.; Mansoor, I.; Rai, K.; Häfeli, U.O.; Stoeber, B. A Micromechanical Comparison of Human and Porcine Skin before and after Preservation by Freezing for Medical Device Development. Sci. Rep. 2016, 6, 32074. [Google Scholar] [CrossRef] [PubMed]
- Kenngott, H.G.; Wünscher, J.J.; Wagner, M.; Preukschas, A.; Wekerle, A.L.; Neher, P.; Suwelack, S.; Speidel, S.; Nickel, F.; Oladokun, D.; et al. OpenHELP (Heidelberg Laparoscopy Phantom): Development of an Open-Source Surgical Evaluation and Training Tool. Surg. Endosc. 2015, 29, 3338–3347. [Google Scholar] [CrossRef] [PubMed]
- Hashem, R.; Kazemi, S.; Stommel, M.; Cheng, L.K.; Xu, W. SoRSS: A Soft Robot for Bio-Mimicking Stomach Anatomy and Motility. Soft Robot. 2023, 10, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Bang, C.S.; Kim, S.O.; Park, D.H. A Novel Human Peristalsis-Inspired 3D-Printed Gastroduodenal Simulator to Evaluate Intragastric/Duodenal Metabolic Devices: A Proof-of-Concept Study. J. Transl. Med. 2022, 20, 149. [Google Scholar] [CrossRef] [PubMed]


| Tube | Size | Classification | Method of Manufacture | Material | Pull Test—Expanded (N) | Pull Test—Retracted (N) | Delta |
|---|---|---|---|---|---|---|---|
| Spoke | 14 F | Soft | Silicone Additive Manufacturing | Medical Grade Silicone | 4.66 | 3.76 | 0.90 |
| Spoke | 24 F | Soft | Silicone Additive Manufacturing | Medical Grade Silicone | 8.84 | 6.70 | 2.14 |
| Spoke | 36 F | Soft | Silicone Additive Manufacturing | Medical Grade Silicone | N/A | N/A | N/A |
| Spoke | 14 F | Hard Modification | Electrical Discharge Machining and Assembly | Acrylonitrile Butadiene Styrene and Aluminum | 6.16 | 4.12 | 2.04 |
| Spoke | 24 F | Hard Modification | Silicone Additive Manufacturing and Aluminum Attachment | Silicone and Aluminum | 10.66 | 6.62 | 4.04 |
| Spoke | 36 F | Hard Modification | Selective Laser Sintering | Thermoplastic Polyurethane | 20.14 | 7.74 | 12.40 |
| Balloon | 14 F | Standard | Extrusion, Dip Coating, and Bonding | Medical Grade Silicone | 41.97 | 6.90 | 35.07 |
| Balloon | 24 F | Standard | Extrusion, Dip Coating, and Bonding | Medical Grade Silicone | 53.17 | 14.03 | 39.13 |
| Non- balloon | 14 F | Standard | Extrusion, Dip Coating, and Bonding | Medical Grade Silicone | 15.70 | 13.40 | 2.30 |
| Non- balloon | 24 F | Standard | Extrusion, Dip Coating, and Bonding | Medical Grade Silicone | 20.77 | 19.03 | 1.73 |
| G-Tube | Trial | Average Time (s) | Number Trials Incomplete | Time Difference between Trials (s) |
|---|---|---|---|---|
| [A] Balloon | Trial 1 Total | 47.2 ± 14.2 | 0 | 18.3 * |
| Trial 2 Total | 28.9 ± 8.8 | 0 | ||
| [B] Non-balloon | Trial 1 Total | 45.1 ± 6.9 | 2 | 15.1 * |
| Trial 2 Total | 30.0 ± 9.2 | 3 | ||
| [C] Spoke | Trial 1 Total | 24.2 ± 13.7 | 0 | 11.9 * |
| Trial 2 Total | 12.3 ± 4.7 | 0 |
| Question | Balloon | Non-Balloon | Spoke |
|---|---|---|---|
| Easiest to use (pre-test) | 40% | 10% | 50% |
| Easiest to use (post-test) | 70% | 0% | 30% |
| Top choice for at-home use (post-test) | 70% | 0% | 30% |
| Median difficulty (1–10) | 2.5 | 6 | 3 |
| Median confidence with at-home placement (1–10) | 6.5 | 3.5 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aedla, M.; Cheng, C.J.; Zhou, A.Y.; Zhang, S.; Hsu, J.; Hu, K.; Qian, J.C.; Sompel, K.V.d.; Ho, A.; Sharma, K.V.; et al. Design and Evaluation of a Spoke-Based Double-Lumen Pediatric Gastrostomy Tube. Children 2024, 11, 263. https://doi.org/10.3390/children11020263
Aedla M, Cheng CJ, Zhou AY, Zhang S, Hsu J, Hu K, Qian JC, Sompel KVd, Ho A, Sharma KV, et al. Design and Evaluation of a Spoke-Based Double-Lumen Pediatric Gastrostomy Tube. Children. 2024; 11(2):263. https://doi.org/10.3390/children11020263
Chicago/Turabian StyleAedla, Mihika, Charlotte J. Cheng, Anson Y. Zhou, Siya Zhang, Jocelyn Hsu, Katherine Hu, Jason C. Qian, Kevin Van de Sompel, Anthony Ho, Karun V. Sharma, and et al. 2024. "Design and Evaluation of a Spoke-Based Double-Lumen Pediatric Gastrostomy Tube" Children 11, no. 2: 263. https://doi.org/10.3390/children11020263
APA StyleAedla, M., Cheng, C. J., Zhou, A. Y., Zhang, S., Hsu, J., Hu, K., Qian, J. C., Sompel, K. V. d., Ho, A., Sharma, K. V., & Logsdon, E. A. (2024). Design and Evaluation of a Spoke-Based Double-Lumen Pediatric Gastrostomy Tube. Children, 11(2), 263. https://doi.org/10.3390/children11020263






