Lactoferrin in the Prevention of Recurrent Respiratory Infections in Preschool Children: A Prospective Randomized Study
Abstract
1. Introduction
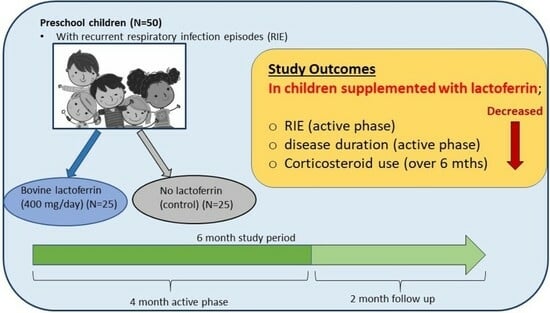
2. Methods
2.1. Study Design
2.2. Patients
2.3. Calculation of Sample Size
2.4. Treatment
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Effect of bLf on Frequency of Respiratory Infections
3.3. Secondary Outcome Measures and Follow Up
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaad, U.B.; Esposito, S.; Razi, C.H. Diagnosis and Management of Recurrent Respiratory Tract Infections in Children: A Practical Guide. Arch. Pediatr. Infect. Dis. 2015, 4, e31039. [Google Scholar] [CrossRef]
- Fiore, M.; Napoleone, E.; Careddu, D.; Meglio, P.; Fiocchi, A.; Cardinale, F.; Rossi, G. Le Infezioni Respiratorie Ricorrenti. I Consigli Della FIMP. Il Med. Pediatra 2010, 3, 9–19. [Google Scholar]
- Chiappini, E.; Santamaria, F.; Marseglia, G.L.; Marchisio, P.; Galli, L.; Cutrera, R.; de Martino, M.; Antonini, S.; Becherucci, P.; Biasci, P.; et al. Prevention of Recurrent Respiratory Infections: Inter-Society Consensus. Ital. J. Pediatr. 2021, 47, 211. [Google Scholar] [CrossRef] [PubMed]
- Albar, A.H.; Almehdar, H.A.; Uversky, V.N.; Redwan, E.M. Structural Heterogeneity and Multifunctionality of Lactoferrin. Curr. Protein Pept. Sci. 2014, 15, 778–797. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.; Adelman, A.S.; Zhuang, W.; Rai, G.P.; Boettcher, J.; Lönnerdal, B. Longitudinal Changes in Lactoferrin Concentrations in Human Milk: A Global Systematic Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1539–1547. [Google Scholar] [CrossRef]
- Rascón-Cruz, Q.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Nakamura-Bencomo, S.I.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A Glycoprotein Involved in Immunomodulation, Anticancer, and Antimicrobial Processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef] [PubMed]
- Zimecki, M.; Actor, J.K.; Kruzel, M.L. The Potential for Lactoferrin to Reduce SARS-CoV-2 Induced Cytokine Storm. Int. Immunopharmacol. 2021, 95, 107571. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ren, Y.; Lu, Q.; Wang, K.; Wu, Y.; Wang, Y.; Zhang, Y.; Cui, X.-S.; Yang, Z.; Chen, Z. Lactoferrin: A Glycoprotein That Plays an Active Role in Human Health. Front. Nutr. 2022, 9, 1018336. [Google Scholar] [CrossRef]
- Rosa, L.; Tripepi, G.; Naldi, E.; Aimati, M.; Santangeli, S.; Venditto, F.; Caldarelli, M.; Valenti, P. Ambulatory COVID-19 Patients Treated with Lactoferrin as a Supplementary Antiviral Agent: A Preliminary Study. J. Clin. Med. 2021, 10, 4276. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin against SARS-CoV-2: In Vitro and In Silico Evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Hasan, S.S.; Kow, C.S.; Merchant, H.A. Lactoferrin Reduces the Risk of Respiratory Tract Infections: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. ESPEN 2021, 45, 26–32. [Google Scholar] [CrossRef] [PubMed]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a Multiple Bioactive Protein: An Overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Mattia, D.A. Generally Recognized as Safe (GRAS) Notification 000423 for Cow’s Milk-Derived Lactoferrin; FDA: Silverspring, MD, USA, 2011. [Google Scholar]
- Miyakawa, M.; Oda, H.; Tanaka, M. Clinical Research Review: Usefulness of Bovine Lactoferrin in Child Health. Biometals 2023, 36, 473–489. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.S.; Williams, L.M.; Williams, E.J.; Wood, L.G. Effect of Lactoferrin Supplementation on Inflammation, Immune Function, and Prevention of Respiratory Tract Infections in Humans: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 1799–1819. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.V.; Trabattoni, D.; Morelli, M.; Borgonovo, S.; Schneider, L.; Clerici, M. Immune Modulation by Lactoferrin and Curcumin in Children with Recurrent Respiratory Infections. J. Biol. Regul. Homeost. Agents 2009, 23, 119–123. [Google Scholar] [PubMed]
- Li, F.; Wu, S.S.; Berseth, C.L.; Harris, C.L.; Richards, J.D.; Wampler, J.L.; Zhuang, W.; Cleghorn, G.; Rudolph, C.D.; Liu, B.; et al. Improved Neurodevelopmental Outcomes Associated with Bovine Milk Fat Globule Membrane and Lactoferrin in Infant Formula: A Randomized, Controlled Trial. J. Pediatr. 2019, 215, 24–31.e8. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Chai, L.; Li, H.; Zhang, Y.; Xie, H.-M.; Shang, J.; Tian, W.; Yang, P.; Jiang, A.C. Effect of Bovine Lactoferrin from Iron-Fortified Formulas on Diarrhea and Respiratory Tract Infections of Weaned Infants in a Randomized Controlled Trial. Nutrition 2016, 32, 222–227. [Google Scholar] [CrossRef]
- King, J.C.; Cummings, G.E.; Guo, N.; Trivedi, L.; Readmond, B.X.; Keane, V.; Feigelman, S.; de Waard, R. A Double-Blind, Placebo-Controlled, Pilot Study of Bovine Lactoferrin Supplementation in Bottle-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 245–251. [Google Scholar] [CrossRef]
- Motoki, N.; Mizuki, M.; Tsukahara, T.; Miyakawa, M.; Kubo, S.; Oda, H.; Tanaka, M.; Yamauchi, K.; Abe, F.; Nomiyama, T. Effects of Lactoferrin-Fortified Formula on Acute Gastrointestinal Symptoms in Children Aged 12–32 Months: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pediatr. 2020, 8, 233. [Google Scholar] [CrossRef]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulatory Effects of Lactoferrin on Antigen Presenting Cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, G.; Yang, D.; Tewary, P.; Varadhachary, A.; Oppenheim, J.J. Lactoferrin Acts as an Alarmin to Promote the Recruitment and Activation of APCs and Antigen-Specific Immune Responses. J. Immunol. 2008, 180, 6868–6876. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-A.; Kruzel, M.L.; Actor, J.K. CHO Expressed Recombinant Human Lactoferrin as an Adjuvant for BCG. Int. J. Immunopathol. Pharmacol. 2015, 28, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Siqueiros-Cendón, T.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F.; García-Montoya, I.A.; Salazar-Martínez, J.; Rascón-Cruz, Q. Immunomodulatory Effects of Lactoferrin. Acta Pharmacol. Sin. 2014, 35, 557–566. [Google Scholar] [CrossRef] [PubMed]
- McCusker, C.; Upton, J.; Warrington, R. Primary Immunodeficiency. Allergy Asthma Clin. Immunol. 2018, 14, 61. [Google Scholar] [CrossRef]
- Eldeniz, F.C.; Gul, Y.; Yorulmaz, A.; Guner, S.N.; Keles, S.; Reisli, I. Evaluation of the 10 Warning Signs in Primary and Secondary Immunodeficient Patients. Front. Immunol. 2022, 13, 900055. [Google Scholar] [CrossRef]
| Whole Group (n = 50) | Control Group (n = 25) | Active Group (n = 25) | p-Value | |
|---|---|---|---|---|
| Age (years) | 4.2 ± 0.1 | 4.4 ± 0.2 | 4.0 ± 0.2 | 0.14 |
| Male gender, n (%) | 32 (64) | 14 (56) | 18 (72) | 0.24 |
| Number of families with at least one member who smokes, n (%) | 14 (28) | 7 (28) | 7 (28) | 1.00 |
| Number of family members, n | 4 (3–4) | 4 (3–4) | 4 (3–4) | 0.17 |
| Symptoms | ||||
| respiratory, n (%) | 39 (78) | 20 (80) | 19 (76) | 0.73 |
| fever, n (%) | 7 (14) | 5 (20) | 2 (8) | 0.22 |
| cough, n (%) | 32 (64) | 16(64) | 16(64) | 1.00 |
| sore throat, n (%) | 15 (30) | 8(32) | 7(28) | 0.76 |
| ear pain, n (%) | 4 (8) | 3(12) | 2(4) | 0.30 |
| nasal congestion n (%) | 26 (52) | 12 (48) | 14 (56) | 0.57 |
| runny nose, n (%) | 18 (36) | 10 (40) | 8 (32) | 0.56 |
| bronchospasm, n (%) | 3 (6) | 1 (4) | 2 (8) | 0.55 |
| Treatment | ||||
| antibiotic, n (%) | 9 (18) | 4 (16) | 5 (20) | 0.71 |
| anti-pyretic, n (%) | 16 (32) | 9 (36) | 7 (28) | 0.54 |
| mucolytic, n (%) | 3 (6) | 2 (8) | 1 (4) | 0.55 |
| corticosteroids (aerosol), n (%) | 16 (32) | 7(28) | 9 (36) | 0.54 |
| Oral corticosteroids, n (%) | 6 (12) | 4(16) | 2(8) | 0.38 |
| bronchodilators n (%) | 5 (10) | 2 (8) | 3 (12) | 0.64 |
| Duration of symptoms (days) | 4 (2–6) | 4 (3–7) | 4 (1–5) | 0.29 |
| Number of days of absence from school | 3 (0–5) | 3 (2–5) | 1 (0–5) | 0.37 |
| Control Group (n = 25) | Active Group (n = 25) | p-Value | |
|---|---|---|---|
| 4-month period (active phase) | |||
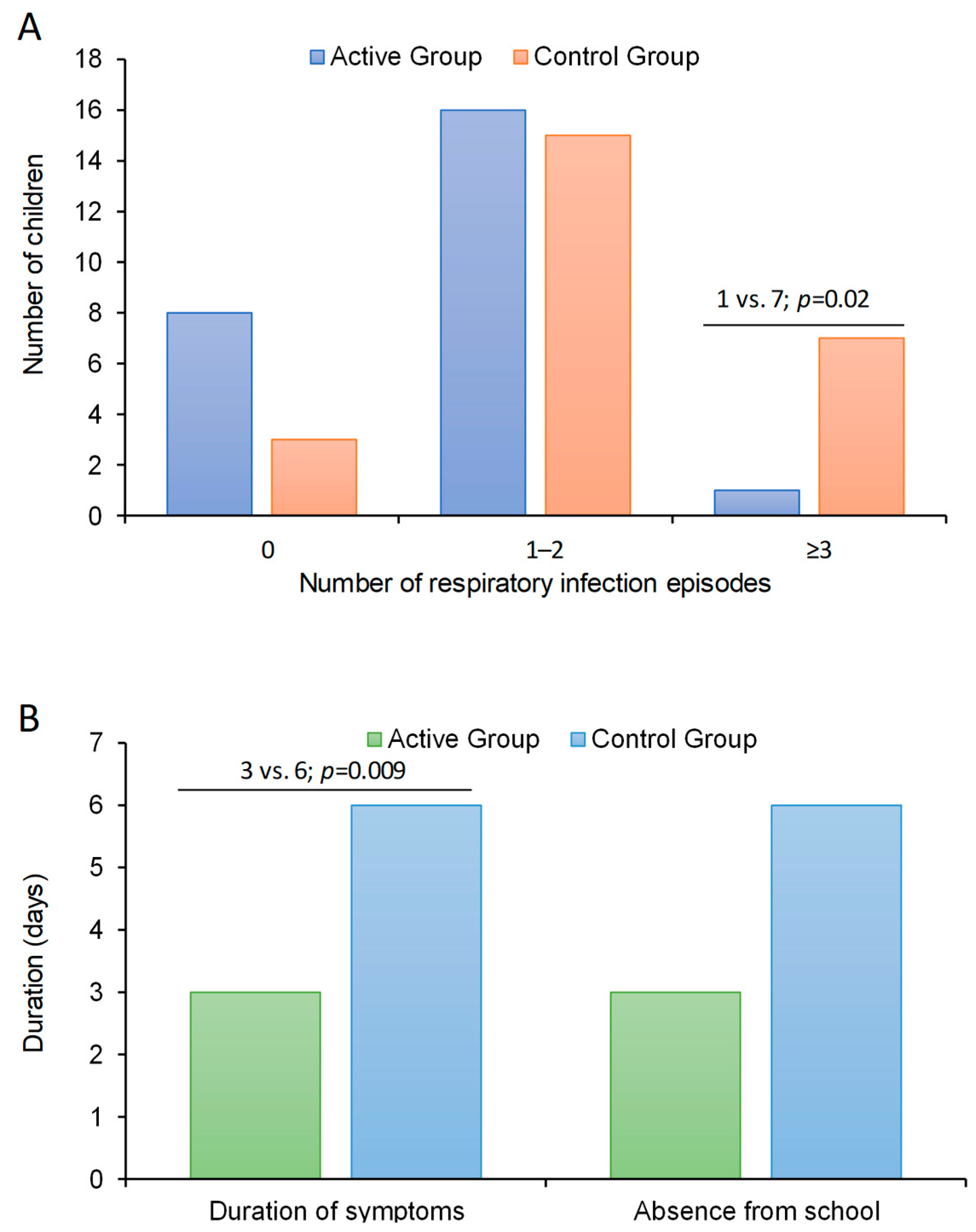
| Episodes of respiratory infections (n) | 2 (1–3) | 1 (0–2) | 0.02 |
| Duration of symptoms (days) | 6 (5–9) | 3 (0–6) | 0.01 |
| Absence from school (days) | 6 (2–8) | 3 (0–7) | 0.15 |
| 2-month period (follow-up) | |||
| Episodes of respiratory infections (n) | 0 (0–1) | 1 (0–1) | 0.23 |
| Duration of symptoms (days) | 0 (0–4) | 0 (0–3) | 0.84 |
| Absence from school (days) | 0 (0–4) | 0 (0–0) | 0.60 |
| Total period | |||
| Treated with antibiotics, n (%) | 9 (36) | 12 (48) | 0.39 |
| Treated with cortisone, n (%) | 15 (60) | 8 (32) | 0.047 |
| Patients with fever, n (%) | 16 (64) | 12 (48) | 0.25 |
| Patients with COVID, n (%) | 2 (8) | 1 (4) | 1.00 |
| Patients with flu vaccine, n (%) | 9 (36) | 9 (36) | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasinato, A.; Fama, M.; Tripepi, G.; Egan, C.G.; Baraldi, E., on behalf of the LIRAR Study Group. Lactoferrin in the Prevention of Recurrent Respiratory Infections in Preschool Children: A Prospective Randomized Study. Children 2024, 11, 249. https://doi.org/10.3390/children11020249
Pasinato A, Fama M, Tripepi G, Egan CG, Baraldi E on behalf of the LIRAR Study Group. Lactoferrin in the Prevention of Recurrent Respiratory Infections in Preschool Children: A Prospective Randomized Study. Children. 2024; 11(2):249. https://doi.org/10.3390/children11020249
Chicago/Turabian StylePasinato, Angela, Mario Fama, Giovanni Tripepi, Colin Gerard Egan, and Eugenio Baraldi on behalf of the LIRAR Study Group. 2024. "Lactoferrin in the Prevention of Recurrent Respiratory Infections in Preschool Children: A Prospective Randomized Study" Children 11, no. 2: 249. https://doi.org/10.3390/children11020249
APA StylePasinato, A., Fama, M., Tripepi, G., Egan, C. G., & Baraldi, E., on behalf of the LIRAR Study Group. (2024). Lactoferrin in the Prevention of Recurrent Respiratory Infections in Preschool Children: A Prospective Randomized Study. Children, 11(2), 249. https://doi.org/10.3390/children11020249