Soluble Receptor for Advanced Glycation End Products (sRAGE) Level and Its Prognostic Significance in Children with Acute Lymphoblastic Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of Complete Blood Count and ESR
2.2. Measurement of Serum Biochemical Parameters
2.3. Serum sRAGE Level Measurement
2.4. Statistical Analysis
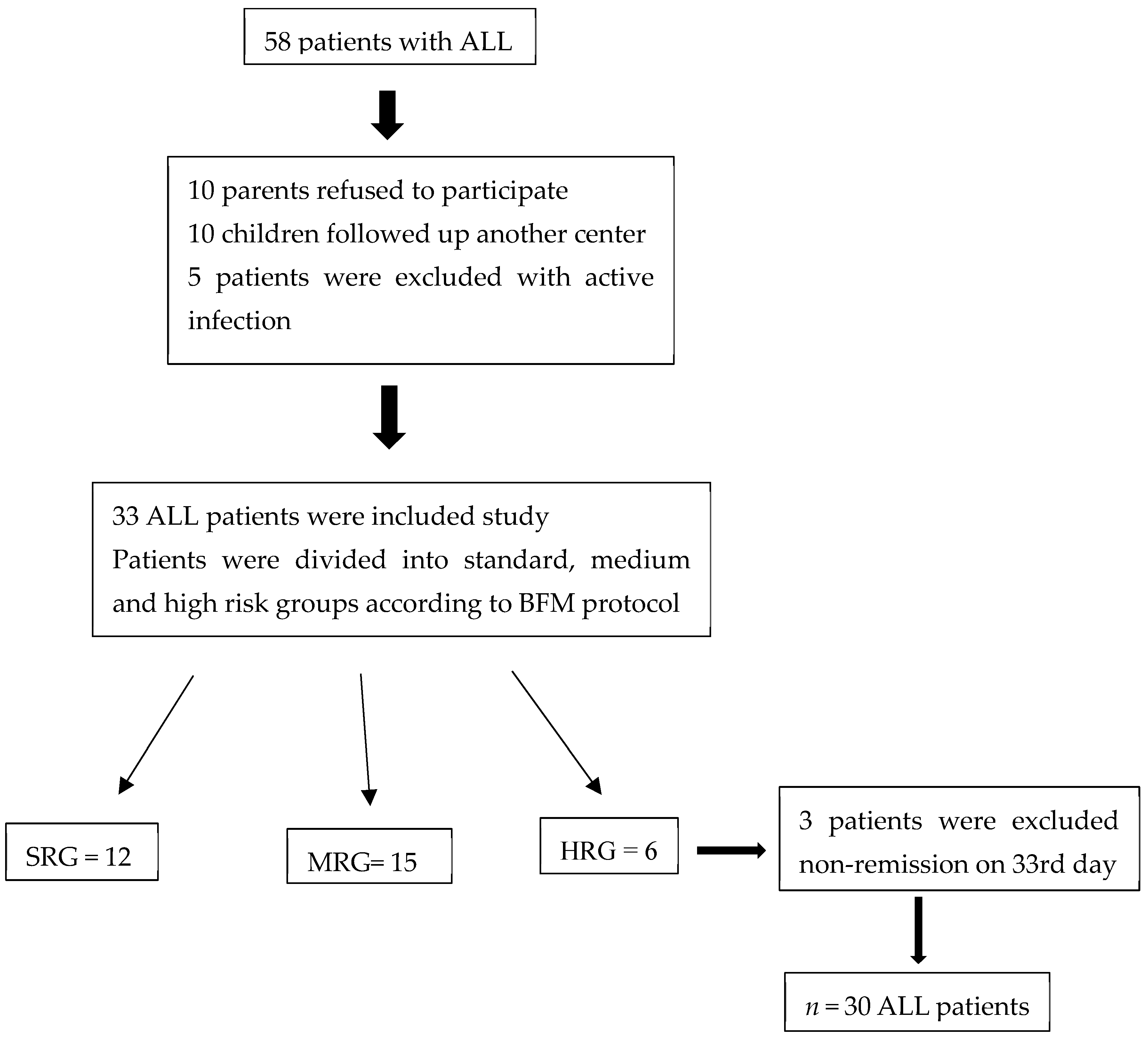
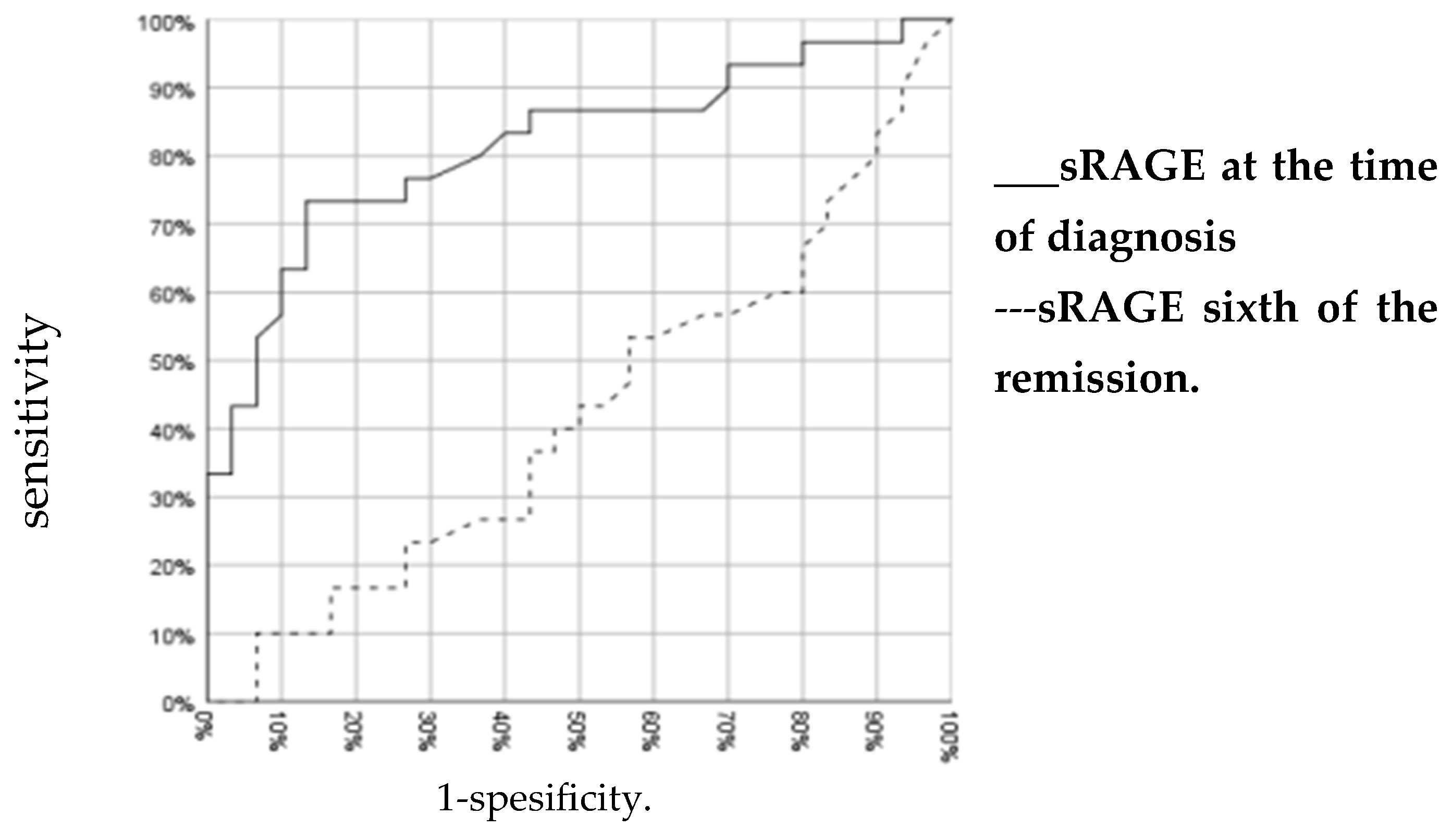
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sinnett, D.; Labuda, D.; Krajinovic, M. Challenges identifying genetic determinants of pediatric cancers the childhood leukemia experience. Fam. Cancer 2006, 5, 35–47. [Google Scholar] [CrossRef]
- Pillai, M.P.; Carroll, W.L. Acute Lymphoblastic Leukemia. In Lanzkowsky’s Manual of Pediatric Hematology and Oncology, 7th ed.; Lanskowsky, P., Lipton, J.M., Fish, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 413–439. [Google Scholar]
- Gaynon, P.S. Childhood acute lymphoblastic leukaemia and relapse. Br. J. Haematol. 2005, 131, 579–587. [Google Scholar] [CrossRef]
- Downing, J.R.; Shannon, K.M. Acute leukemia: A pediatric perspective. Cancer Cell 2002, 2, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, M.B.; Modarressi, M.H.; Shabani, M.; Asgarian, H.; Sharifian, R.A.; Vossough, P.; Shokri, F. Expression of the testisspecific gene, TSGA10, in Iranian patients with acute lymphoblastic leukemia (ALL). Leuk. Res. 2006, 30, 883–889. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.; Bagatini, M.D.; Santos, K.F.; Spanevello, R.M.; Maldonado, P.A.; Brulé, A.O.; Araújo Mdo, C.; Schetinger, M.R.; Morsch, V.M. Measurement of oxidative stress and antioxidant status in acute lymphoblastic leukemia patients. Clin. Biochem. 2008, 41, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.; Silverman, L.B. Acute lymphoblastic leukemia. In Nathan and Oski’s Hematology and Oncology of Infancy and Childhood; Orkin, S.H., Fisher, D.E., Giruburg, D., Look, A.T., Lux, S.E., Northon, D.G., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2015; pp. 1527–1551. [Google Scholar]
- Udensi, U.K.; Tchounwou, P.B. Dual effect of oxidative stress on leukemia cancer induction and treatment. J. Exp. Clin. Cancer Res. 2014, 33, 106. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, L.B.; Mdhaffar, M.; Ghozzi, H.; Ammar, M.; Hakim, A.; Atheymen, R.; Sahnoun, Z.; Elloumi, M.; Zeghal, K. Oxidative stress in Tunisian patients with acute lymphoblastic leukemia and its involvement in leukaemic relapse. Pediatr. Hematol. Oncol. 2017, 39, e124–e130. [Google Scholar] [CrossRef]
- Kumar, S.; Yedjou, C.G.; Tchounwou, P.B. Arsenic trioxide induces oxidative stress, DNA damage, and mitochondrial pathway of apoptosis in human leukemia (HL-60) cells. J. Exp. Clin. Cancer Res. 2014, 33, 42. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, H.; Siddiqui, Z.; Khan, M.Y.; Rehman, S.; Shahab, U.; Godovikova, T.; Silnikov, V.; Moinuddin. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin. Cancer Biol. 2018, 49, 44–55. [Google Scholar] [CrossRef]
- Hyogo, H.; Yamagishi, S. Advanced glycation end products (AGEs) and their involvement in liver disease. Curr. Pharm. Des. 2008, 14, 969–972. [Google Scholar] [CrossRef]
- Chao, C.H.; Cha, J. Analysis of neutrophil gelatinase-associated lipocalin, vascular endothelial growth factor, and soluble receptor for advanced glycation end-products in bone marrow supernatant in hematologic malignancies. Clin. Biochem. 2020, 80, 19–24. [Google Scholar] [CrossRef]
- Leclerc, E.; Vetter, S.W. The role of S100 proteins and their receptor RAGE in pancreatic cancer. Biochim. Biophys. Acta 2015, 1852, 2706–2711. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, F.; Qiao, Y.; Wang, P.; Du, H.; Si, C.; Wang, X.; Chen, K.; Song, F. Genetically Modified Circulating Levels of Advanced Glycation End-Products and Their Soluble Receptor (AGEs-RAGE Axis) with Risk and Mortality of Breast Cancer. Cancers 2022, 14, 6124. [Google Scholar] [CrossRef]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Sorci, G.; Riuzzi, F.; Giambanco, I.; Donato, R. RAGE in tissue homeostasis, repair and regeneration. Biochim. Biophys. Acta 2013, 1833, 101–109. [Google Scholar] [CrossRef]
- Erusalimsky, J.D. The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol. 2021, 42, 101958. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Carroll, W.I. Acute Lymphoblastic Leukemia. In Lanzkowsky’s Manual of Pediatric Hematology and Oncology, 6th ed.; Lipton, J.M., Fish, J.D., Eds.; Haley M: London, UK, 2016; pp. 367–389. [Google Scholar]
- Whitlock, J.A.; Gaynon, P.S. Acute lymphoblastic leukemia in children. In Wintrobe’s Clinical Hematology, 12th ed.; Greer, J.P., Foerster, J., Rodgers, G.M., Paraskevas, F., Glader, B., Arber, D.A., Means, R.T., Eds.; Lippincott Williams and Wilkins: New York, NY, USA, 2009; pp. 1889–1917. [Google Scholar]
- Perez, J.; Arellano, G.; Garza, J.; Rivera, L.; Almaguer, D. Revisiting the complete blood count and clinical findings at diagnosis of childhood acute lymphoblastic leukemia: 10-year experience at a single center. Hematol. Transfus. Cell Ther. 2019, 41, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, C.D.; Fuentes, M.K.; Huang, E.H.; Arumugam, T. RAGE and RAGE ligands in cancer. Curr. Mol. Med. 2007, 7, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Yan, S.F.; Herold, K.; Clynes, R.; Schmidt, A.M. Receptor for advanced glycation end products: Fundamental roles in the inflammatory response: Winding the way to the pathogenesis of endothelial dysfunction and atherosclerosis. Ann. N. Y. Acad. Sci. 2008, 1126, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Grote, V.A.; Nieters, A.; Kaaks, R.; Tjønneland, A.; Roswall, N.; Overvad, K.; Nielsen, M.R.S.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Racine, A.; et al. The associations of advanced glycation end products and its soluble receptor with pancreatic cancer risk: A case-control study within the prospective EPIC Cohort. Cancer Epidemiol. Biomark. Prev. 2012, 21, 19–28. [Google Scholar] [CrossRef]
- Tesarova, P.; Kalousova, M.; Jachymova, M.; Mestek, O.; Petruzelka, L.; Zima, T. Receptor for advanced glycation end products (RAGE) soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Investig. 2007, 25, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Krechler, T.; Jachymova, M.; Mestek, O.; Zak, A.; Zima, T.; Kalousova, M. Soluble receptor for advanced glycation end-products (sRAGE) and polymorphisms of RAGE and glyoxalase I genes in patients with pancreas cancer. Clin. Biochem. 2010, 43, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.; Cui, M.; Wang, J.; Wang, H. Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): A new biomarker for lung cancer. Neoplasma 2010, 57, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, S.J.; Choi, H.; Shin, T.R.; Sim, Y.S. Diagnostic Utility and Tendency of Bronchial and Serum Soluble Receptor for Advanced Glycation EndProducts (sRAGE) in Lung Cancer. Cancers 2023, 15, 2819. [Google Scholar] [CrossRef] [PubMed]
- Healey, G.D.; Pan-Castillo, B.; Garcia-Parra, J.; Davies, J.; Roberts, S.; Jones, E.; Dhar, K.; Nandanan, S.; Tofazzal, N.; Piggott, L.; et al. Antibody drug conjugates against the receptor for advanced glycation end products (RAGE), a novel therapeutic target in endometrial cancer. J. Immunother. Cancer 2019, 7, 280. [Google Scholar] [CrossRef]
- Malik, P.; Chaudhry, N.; Mittal, R.; Mukherjee, T.K. Role of receptor for advanced glycation end products in the complication and progression of various types of cancers. Biochim. Biophys. Acta 2015, 1850, 1898–1904. [Google Scholar] [CrossRef]
| Parameters (103/μL) | Patient at Diagnosis | Patient during the Sixth Month of Remission | Control Group | p * |
|---|---|---|---|---|
| WBC | 33.5 ± 60.5 | 5.6 ± 2.5 | 7.4 ± 2.5 | >0.05 |
| p = 0.004 | ||||
| Neutrophil | 1.3 ± 1.5 | 3.3 ± 2.1 | 3.4 ± 1.4 | 0.000 |
| p = 0.000 | ||||
| Lymphocytes | 19.5 ± 40.5 | 1.7 ± 1.0 | 3.2 ± 1.5 | >0.05 |
| p = 0.000 | ||||
| Eosinophils | 0.0627 ± 0.0871 | 0.1225 ± 0.1026 | 0.1657 ± 0.1617 | 0.000 |
| p = 0.036 | ||||
| Platelets | 89.3 ± 68.5 | 240.3 ± 60.9 | 329.8 ± 106.6 | 0.000 |
| p = 0.000 | ||||
| Hemoglobin (gr/dL) | 7.7 ± 2.4 | 13.8 ± 1.0 | 13.5 ± 1.3 | 0.000 |
| p = 0.000 | ||||
| Parameters | Patient at Diagnosis | Patient during the Sixth Month of Remission | Control Group | p * |
|---|---|---|---|---|
| Albumin (g/L) | 3.77 ± 0.57 | 4.58 ± 0.30 | 4.29 ± 0.44 | 0.000 |
| p = 0.000 | ||||
| LDH (U/L) | 1023.4 ± 1386.5 | 242.1 ± 8.4 | 233.1 ± 39.5 | 0.000 |
| p = 0.003 | ||||
| AST (U/L) | 61.0 ± 78.2 | 27.5 ± 8.4 | 25.0 ± 12.9 | 0.001 |
| p = 0.003 | ||||
| ALT (U/L) | 50.2 ± 91.0 | 33.6 ± 28.1 | 14.2 ± 9.8 | 0.000 |
| p > 0.05 | ||||
| ALP (U/L) | 157.8 ± 93.2 | 267.4 ± 279.9 | 189.5 ± 95.7 | 0.095 |
| p = 0.002 | ||||
| Kreatinin (mg/dL) | 0.42 ± 0.21 | 0.39 ± 0.14 | 0.50 ± 0.14 | 0.006 |
| p > 0.05 | ||||
| Ürik Asit (mg/dL) | 5.80 ± 3.73 | 3.51 ± 1.35 | 3.36 ± 1.0 | 0.002 |
| p = 0.000 | ||||
| BUN (mg/dL) | 12.29 ± 3.61 | 10.53 ± 2.77 | 10.13 ± 1.70 | 0.016 |
| p > 0.05 | ||||
| ESR (mm/h) | 25.27 ± 16.43 | 6.36 ± 5.44 | 5.93 ± 3.86 | |
| p = 0.000 | 0.000 | |||
| B-ALL | T-ALL | Control Group | p | |
|---|---|---|---|---|
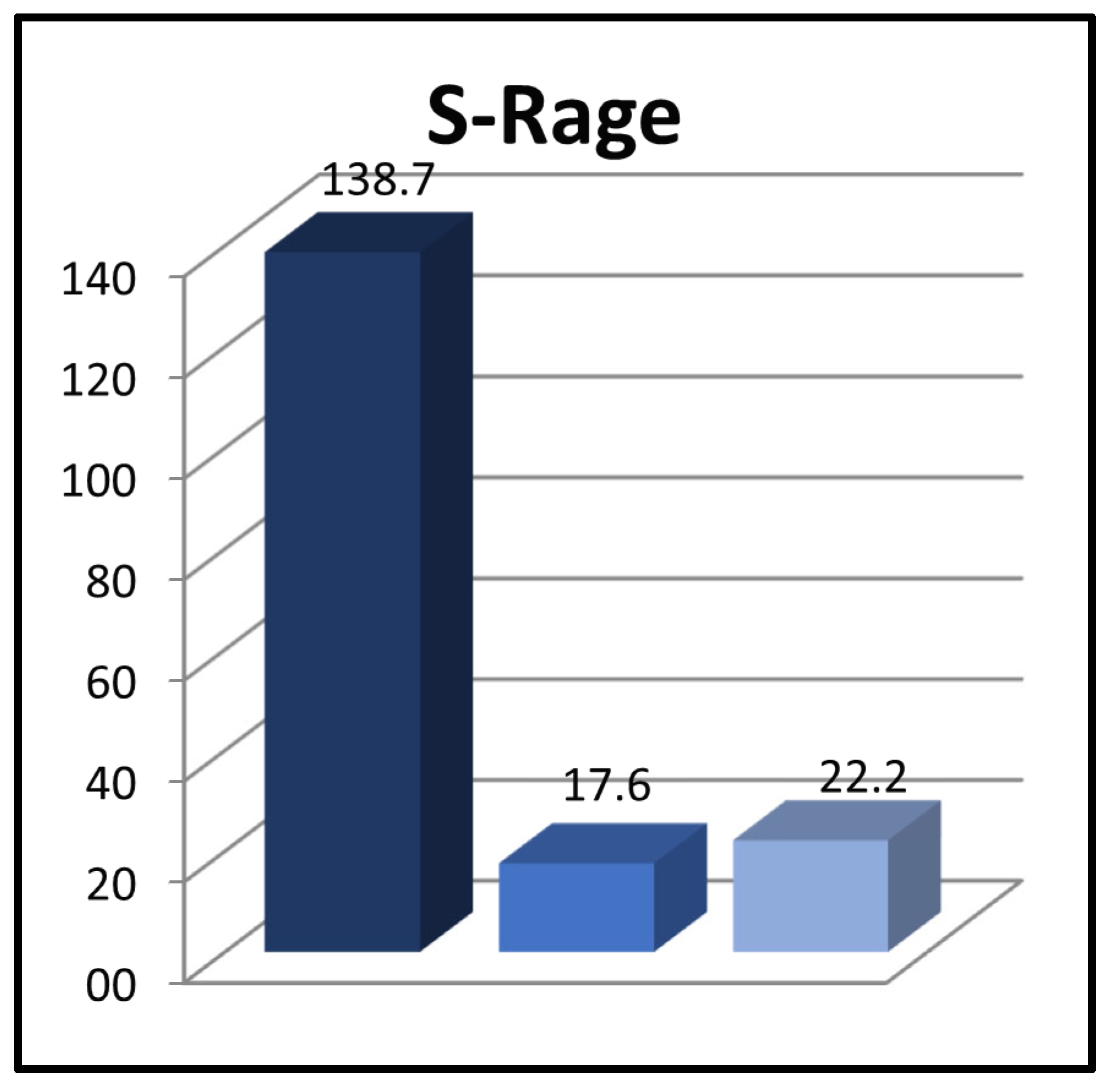
| sRAGE at the time of diagnosis (pg/mL) | 84.5 ± 82.7 * | 490.9 ± 236.9 ** | 22.2 ± 23.7 | * 0.001 ** 0.000 |
| 0.003 | ||||
| sRAGE during the sixth month of remission (pg/mL) | 11.9 ± 12.0 | 55.0 ± 30.9 | ||
| 0.013 | ||||
| SRG | MRG | HRG | p | |
|---|---|---|---|---|
| sRAGE at diagnosis (pg/mL) | 45.8 ± 33.1 | 212 ± 222.1 | 143.9 ± 111.5 | >0.05 |
| 0.025 | ||||
| sRAGE during the sixth month of remission (pg/mL) | 7.8 ± 8.4 | 26.6 ± 25.9 | 11.8 ± 11 | >0.05 |
| 0.017 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkan, B.; Altuner Torun, Y.; Karakukcu, C.; Celik, B. Soluble Receptor for Advanced Glycation End Products (sRAGE) Level and Its Prognostic Significance in Children with Acute Lymphoblastic Leukemia. Children 2024, 11, 176. https://doi.org/10.3390/children11020176
Ozkan B, Altuner Torun Y, Karakukcu C, Celik B. Soluble Receptor for Advanced Glycation End Products (sRAGE) Level and Its Prognostic Significance in Children with Acute Lymphoblastic Leukemia. Children. 2024; 11(2):176. https://doi.org/10.3390/children11020176
Chicago/Turabian StyleOzkan, Busra, Yasemin Altuner Torun, Cigdem Karakukcu, and Binnaz Celik. 2024. "Soluble Receptor for Advanced Glycation End Products (sRAGE) Level and Its Prognostic Significance in Children with Acute Lymphoblastic Leukemia" Children 11, no. 2: 176. https://doi.org/10.3390/children11020176
APA StyleOzkan, B., Altuner Torun, Y., Karakukcu, C., & Celik, B. (2024). Soluble Receptor for Advanced Glycation End Products (sRAGE) Level and Its Prognostic Significance in Children with Acute Lymphoblastic Leukemia. Children, 11(2), 176. https://doi.org/10.3390/children11020176





