3D Back Contour Metrics in Predicting Idiopathic Scoliosis Progression: Retrospective Cohort Analysis, Case Series Report and Proof of Concept
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics
3.2. Predictive Modeling
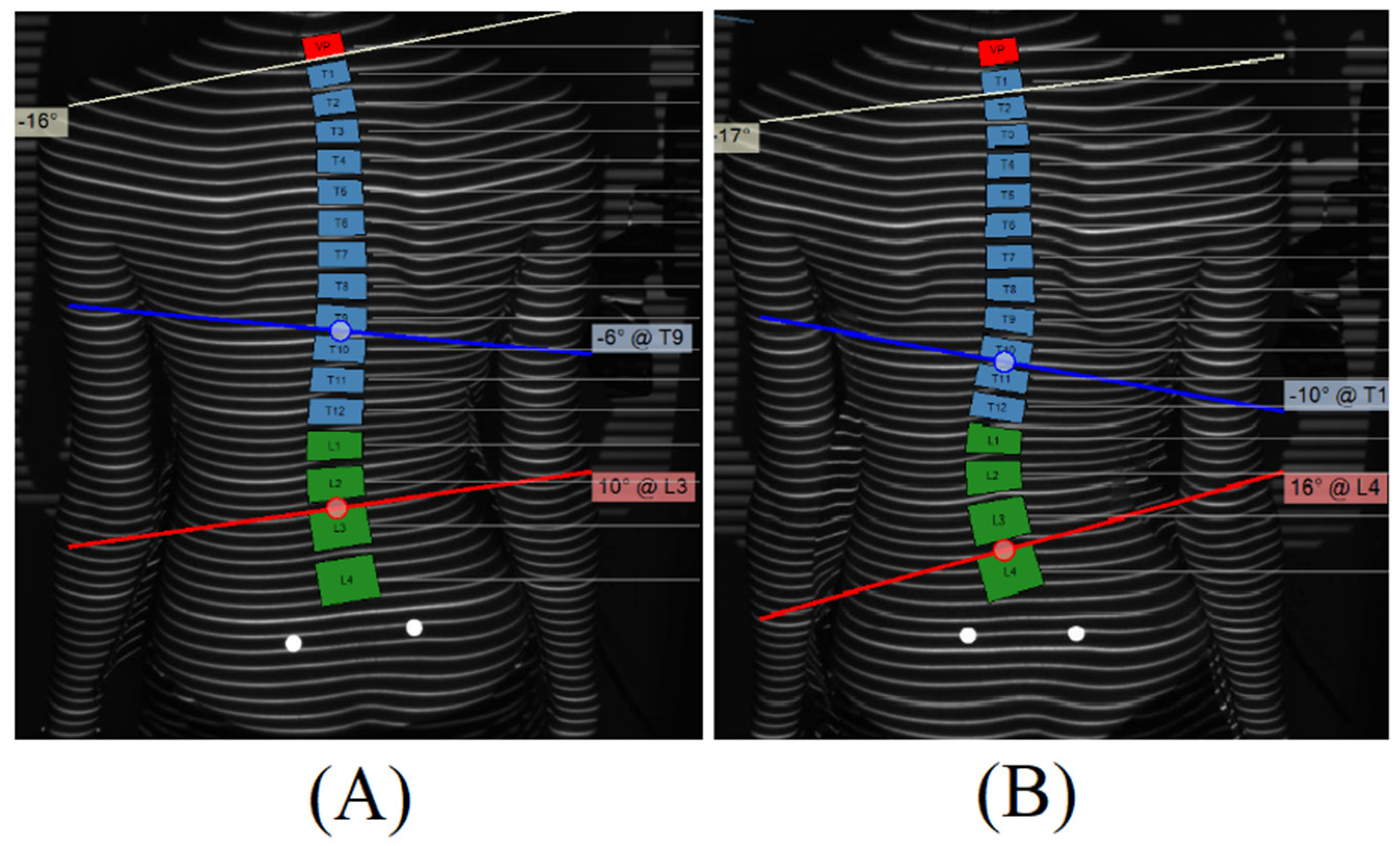
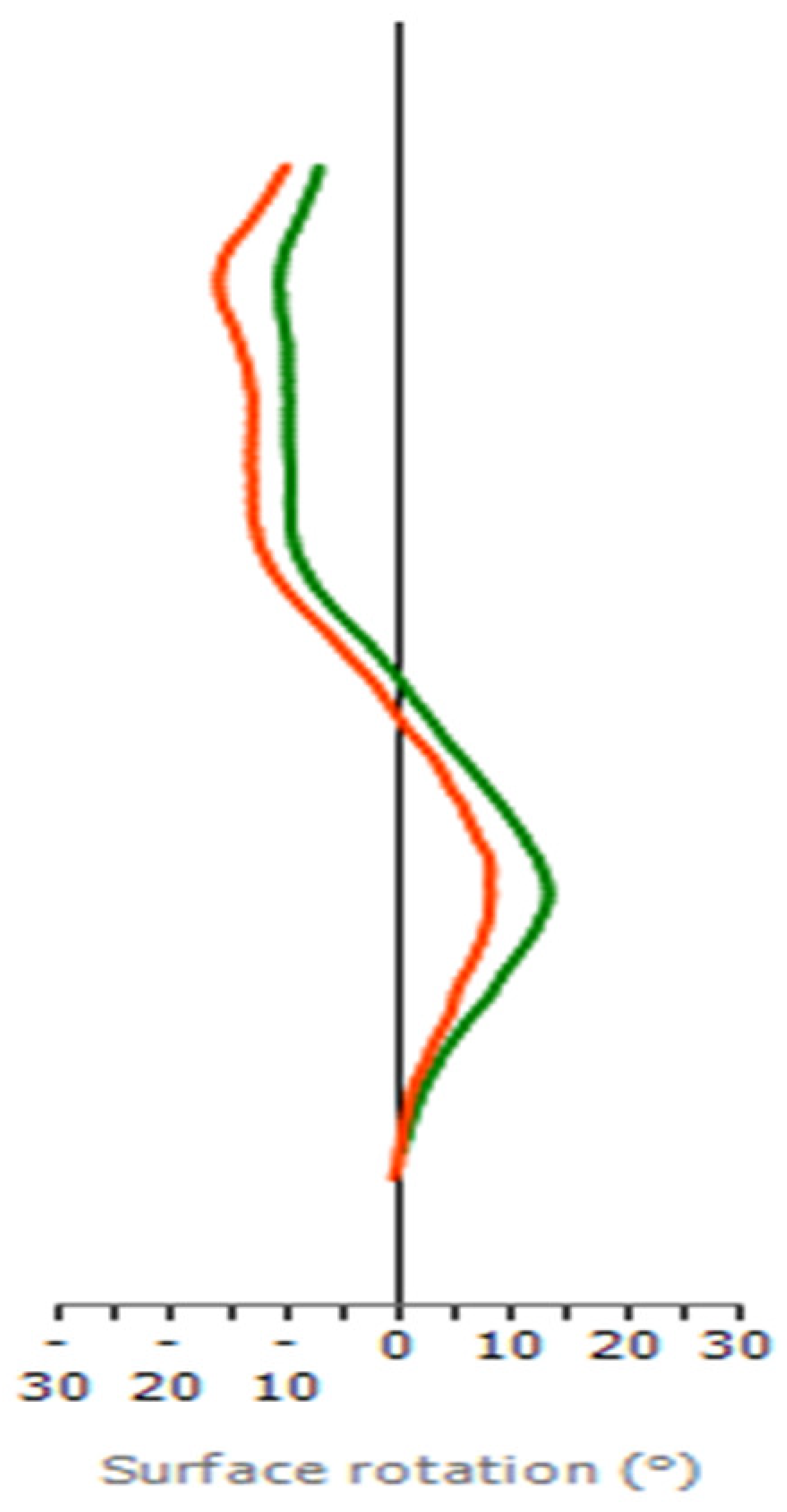
3.3. Case Report and Proof of Concept
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, J.; Henrikson, N.B.; Morrison, C.C.; Blasi, P.R.; Nguyen, M.; Lin, J.S. Screening for Adolescent Idiopathic Scoliosis: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, M.R.; Senyurt, H.; Krauspe, R. Epidemiology of adolescent idiopathic scoliosis. J. Child. Orthop. 2013, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Loughenbury, P.R.; Gentles, S.L.; Murphy, E.J.; Tomlinson, J.E.; Borse, V.H.; Dunsmuir, R.A.; Gummerson, N.W.; Millner, P.A.; Rao, A.S.; Rowbotham, E.; et al. Estimated cumulative X-ray exposure and additional cancer risk during the evaluation and treatment of scoliosis in children and young people requiring surgery. Spine Deform. 2021, 9, 949–954. [Google Scholar] [CrossRef]
- Luo, T.D.; Stans, A.A.; Schueler, B.A.; Larson, A.N. Cumulative Radiation Exposure with EOS Imaging Compared with Standard Spine Radiographs. Spine Deform. 2015, 3, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Simony, A.; Hansen, E.J.; Christensen, S.B.; Carreon, L.Y.; Andersen, M.O. Incidence of cancer in adolescent idiopathic scoliosis patients treated 25 years previously. Eur. Spine J. 2016, 25, 3366–3370. [Google Scholar] [CrossRef] [PubMed]
- De Mauroy, J.; Weiss, H.; Aulisa, A.; Aulisa, L.; Brox, J.; Durmala, J.; Fusco, C.; Grivas, T.; Hermus, J.; Kotwicki, T.; et al. 7th SOSORT consensus paper: Conservative treatment of idiopathic & Scheuermann’s kyphosis. Scoliosis 2010, 5, 9. [Google Scholar] [CrossRef]
- Bolzinger, M.; Bernardini, I.; Thevenin Lemoine, C.; Gallini, A.; Accadbled, F.; de Gauzy, J.S. Monitoring adolescent idiopathic scoliosis by measuring ribs prominence using surface topography device. Spine Deform. 2021, 9, 1349–1354. [Google Scholar] [CrossRef]
- Rehm, J.; Germann, T.; Akbar, M.; Pepke, W.; Kauczor, H.-U.; Weber, M.-A.; Spira, D. 3D-modeling of the spine using EOS imaging system: Inter-reader reproducibility and reliability. PLoS ONE 2017, 12, e0171258. [Google Scholar] [CrossRef]
- Melhem, E.; Assi, A.; El Rachkidi, R.; Ghanem, I. EOS(®) biplanar X-ray imaging: Concept, developments, benefits, and limitations. J. Child. Orthop. 2016, 10, 1–14. [Google Scholar] [CrossRef]
- Bagheri, A.; Liu, X.-C.; Tassone, C.; Thometz, J.; Tarima, S. Reliability of Three-Dimensional Spinal Modeling of Patients with Idiopathic Scoliosis Using EOS System. Spine Deform. 2018, 6, 207–212. [Google Scholar] [CrossRef]
- Knott, P.; Pappo, E.; Cameron, M.; Demauroy, J.; Rivard, C.; Kotwicki, T.; Zaina, F.; Wynne, J.; Stikeleather, L.; Bettany-Saltikov, J.; et al. SOSORT 2012 consensus paper: Reducing X-ray exposure in pediatric patients with scoliosis. Scoliosis 2014, 9, 4. [Google Scholar] [CrossRef]
- Lv, Z.; Lv, W.; Wang, L.; Ou, J. Development and validation of machine learning-based models for prediction of adolescent idiopathic scoliosis: A retrospective study. Medicine 2023, 102, e33441. [Google Scholar] [CrossRef]
- Frerich, J.M.; Hertzler, K.; Knott, P.; Mardjetko, S. Comparison of Radiographic and Surface Topography Measurements in Adolescents with Idiopathic Scoliosis. Open Orthop. J. 2012, 6, 261–265. [Google Scholar] [CrossRef]
- Komeili, A.; Westover, L.M.; Parent, E.C.; Moreau, M.; El-Rich, M.; Adeeb, S. Surface topography asymmetry maps categorizing external deformity in scoliosis. Spine J. 2014, 14, 973–983.e2. [Google Scholar] [CrossRef] [PubMed]
- Knott, P.; Sturm, P.; Lonner, B.; Cahill, P.; Betsch, M.; McCarthy, R.; Kelly, M.; Lenke, L.; Betz, R. Multicenter Comparison of 3D Spinal Measurements Using Surface Topography with Those From Conventional Radiography. Spine Deform. 2016, 4, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Tabard-Fougère, A.; Bonnefoy-Mazure, A.; Hanquinet, S.; Lascombes, P.; Armand, S.; Dayer, R. Validity and Reliability of Spine Rasterstereography in Patients with Adolescent Idiopathic Scoliosis. Spine 2017, 42, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Rayward, L.; Pearcy, M.; Izatt, M.; Green, D.; Labrom, R.; Askin, G.; Little, J.P. Predicting spinal column profile from surface topography via 3D non-contact surface scanning. PLoS ONE 2023, 18, e0282634. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, J. Relationship between Muscle Tone of the Erector Spinae and the Concave and Convex Sides of Spinal Curvature in Low-Grade Scoliosis among Children. Children 2021, 8, 1168. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.-J.; Moe, M.M.; Vaithinathan, R.; Wong, H.-K. Curve Progression in Idiopathic Scoliosis: Follow-up Study to Skeletal Maturity. Spine 2009, 34, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Nault, M.-L.; Beauséjour, M.; Roy-Beaudry, M.; Mac-Thiong, J.-M.; de Guise, J.; Labelle, H.; Parent, S. A Predictive Model of Progression for Adolescent Idiopathic Scoliosis Based on 3D Spine Parameters at First Visit. Spine 2020, 45, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Alfraihat, A.; Samdani, A.F.; Balasubramanian, S. Predicting curve progression for adolescent idiopathic scoliosis using random forest model. PLoS ONE 2022, 17, e0273002. [Google Scholar] [CrossRef]
- Yahara, Y.; Tamura, M.; Seki, S.; Kondo, Y.; Makino, H.; Watanabe, K.; Kamei, K.; Futakawa, H.; Kawaguchi, Y. A deep convolutional neural network to predict the curve progression of adolescent idiopathic scoliosis: A pilot study. BMC Musculoskelet. Disord. 2022, 23, 610. [Google Scholar] [CrossRef]
- Richards, B.S.; Bernstein, R.M.; D’amato, C.R.; Thompson, G.H. Standardization of criteria for adolescent idiopathic scoliosis brace studies: SRS Committee on Bracing and Nonoperative Management. Spine 2005, 30, 2068–2075. [Google Scholar] [CrossRef]
- Yang, Y. Can the strengths of AIC and BIC be shared? A conflict between model indentification and regression estimation. Biometrika 2005, 92, 937–950. [Google Scholar] [CrossRef]
- Smorgick, Y.; Nassar, M.; Tamir, E.; Tal, S.; Mirovsky, Y.; Anekstein, Y. Clinical and Radiographical Characteristics in Male and Female Adolescent Idiopathic Scoliosis Surgical Candidates. Isr. Med. Assoc. J. 2019, 21, 213–216. [Google Scholar] [PubMed]
- Lenz, M.; Oikonomidis, S.; Harland, A.; Fürnstahl, P.; Farshad, M.; Bredow, J.; Eysel, P.; Scheyerer, M.J. Scoliosis and Prognosis—A systematic review regarding patient-specific and radiological predictive factors for curve progression. Eur. Spine J. 2021, 30, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Larasati, A.; Deyong, C.; Slevitch, L. Comparing Neural Network and Ordinal Logistic Regression to Analyze Attitude Responses. Serv. Sci. 2011, 3, 304–312. [Google Scholar] [CrossRef]
- Applebaum, A.; Ference, R.; Cho, W. Evaluating the role of surface topography in the surveillance of scoliosis. Spine Deform. 2020, 8, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Groisser, B.N.; Hillstrom, H.J.; Thakur, A.; Morse, K.W.; Cunningham, M.; Hresko, M.T.; Kimmel, R.; Wolf, A.; Widmann, R.F. Reliability of automated topographic measurements for spine deformity. Spine Deform. 2022, 10, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Jandoo, T. WHO guidance for digital health: What it means for researchers. Digit. Health 2020, 6, 2055207619898984. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.S.; Aroeira, R.M.C.; Gressler, V.; Greco, M.; Pertence, A.E.M.; Lamounier, J.A. Accuracy of photogrammetry for detecting adolescent idiopathic scoliosis progression. Spine J. 2019, 19, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S.; Rajapaske, C.R.; Reddy, R.; Diebo, B.; Knott, P.; Jones, B.C.; Kumar, D.; Zhu, W.; Lou, E.; Shapira, N.; et al. Quantitative imaging of the spine in adolescent idiopathic scoliosis: Shifting the paradigm from diagnostic to comprehensive prognostic evaluation. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1273–1285. [Google Scholar] [CrossRef] [PubMed]

| Variable | Estimate | Standard Error | p-Value |
|---|---|---|---|
| Intercept | 26.243 | 9.997 | 0.012 |
| Age | −1.137 | 0.695 | 0.109 |
| Gender | −7.689 | 2.644 | 0.005 |
| T6 | −3.590 | 1.014 | 0.001 |
| T7 | 5.912 | 1.698 | 0.002 |
| T8 | −2.684 | 0.869 | 0.005 |
| Scoliotic angle | 0.729 | 0.093 | <0.001 |
| Predicting True Outcomes | Y1 | Y2 | Y3 |
|---|---|---|---|
| Progression | (0.034, 1.000) | (<0.001, 0.063) | (<0.001, 0.042) |
| Stable | (0.008, 0.502) | (0.228, 0.722) | (0.025, 0.664) |
| Improvement | (<0.001, 0.221) | (0.004, 0.709) | (0.083, 0.996) |
| True Group | Classification Based on the Model | Incorrectly Classified n (%) | ||
|---|---|---|---|---|
| Progression n (%) | Stable n (%) | Improvement n (%) | ||
| Progression | 6 (75%) | 2 (25%) | 0 (%) | 2 (25%) |
| Stable | 1 (5%) | 16 (85%) | 2 (10%) | 3 (15%) |
| Improving | 0 (0%) | 6 (55%) | 5 (45%) | 6 (55%) |
| ASR (°) | T8 | T9 | T10 | T11 | T12 | L1 | L2 | L3 | L4 |
|---|---|---|---|---|---|---|---|---|---|
| 1st visit | −13 | −10 | −5 | −1 | 4 | 7 | 8 | 5 | 2 |
| 2nd visit | −9 | −7 | −3 | 1 | 5 | 10 | 13 | 10 | 4 |
| Variable | Y1 | Y2 | Y3 |
|---|---|---|---|
| Probability | 0.925 | 0.073 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.; Liu, X.-C.; Yang, K.; Tassone, C.; Escott, B.; Thometz, J. 3D Back Contour Metrics in Predicting Idiopathic Scoliosis Progression: Retrospective Cohort Analysis, Case Series Report and Proof of Concept. Children 2024, 11, 159. https://doi.org/10.3390/children11020159
Patel M, Liu X-C, Yang K, Tassone C, Escott B, Thometz J. 3D Back Contour Metrics in Predicting Idiopathic Scoliosis Progression: Retrospective Cohort Analysis, Case Series Report and Proof of Concept. Children. 2024; 11(2):159. https://doi.org/10.3390/children11020159
Chicago/Turabian StylePatel, Milan, Xue-Cheng Liu, Kai Yang, Channing Tassone, Benjamin Escott, and John Thometz. 2024. "3D Back Contour Metrics in Predicting Idiopathic Scoliosis Progression: Retrospective Cohort Analysis, Case Series Report and Proof of Concept" Children 11, no. 2: 159. https://doi.org/10.3390/children11020159
APA StylePatel, M., Liu, X.-C., Yang, K., Tassone, C., Escott, B., & Thometz, J. (2024). 3D Back Contour Metrics in Predicting Idiopathic Scoliosis Progression: Retrospective Cohort Analysis, Case Series Report and Proof of Concept. Children, 11(2), 159. https://doi.org/10.3390/children11020159








