Helmet Continuous Positive Airway Pressure for Acute Bronchiolitis Respiratory Failure in a Pediatric Ward: Is It a Replicable Experience?
Abstract
1. Introduction
2. Materials and Methods
2.1. Respiratory Assistance Protocol
- (1)
- As a rescue therapy for patients initially treated with HFNC or nasal cannula oxygen therapy who had SpO2 < 92% with FiO2 of 0.6, in the absence of hypercapnic respiratory failure and/or a critical increase in work when breathing.
- (2)
- If one or more of the following were detected at the first evaluation of the patient:
- Signs of respiratory fatigue with moderate/severe dyspnea (respiratory rate > 50 breaths/min);
- Use of accessory respiratory muscles and paradoxical abdominal movement;
- Hypoxemia despite high FiO2 (P/F ≤ 250) ± mild hypercapnia (up to 50 mmHg in arterial blood gas).
2.2. H-CPAP Management
2.2.1. H-CPAP Equipment
- A transparent, latex-free polyvinyl chloride helmet;
- An anti-asphyxia valve positioned on the outside of the head hood, allowing for manual opening if the source of gas flow malfunctions, or automatic activation if the pressure inside the helmet drops to <3 cmH2O;
- An expiratory limb;
- An inspiratory limb;
- An audio silencer to reduce noise inside the helmet by approximately 10 Db;
- Two sealed accesses for probes and catheters of 3.5–7.0 mm;
- An airtight patient access porthole with screw closure;
- A PEEP valve.
2.2.2. H-CPAP Procedure and Monitoring
- Positioning the infant at a 45° angle using an oxygen nasal cannula or HFNC until the H-CPAP circuit was fully assembled;
- Connecting the FiO2 analyzer to the gas source;
- Clearing nasal secretions;
- Inserting a nasogastric tube;
- Setting the PEEP valve initially at 5 cmH2O (0.49 kPa), and gradually increasing that to a maximum of 7.5 cmH2O (0.68 kPa), while adjusting FiO2 to maintain SpO2 between 93 and 97%;
- Setting the flow rate according to the infant’s body size, typically from 30 L/min to 50 L/min.
2.3. Cost-Saving Analysis
- Cost A (PICU + PW) = {(days of H-CPAP treatment × Total daily cost of hospitalization in PICU) + [(total length of hospitalization − days of H-CPAP treatment) × total daily cost of hospitalization in PW]}.
- COST B (PW) = (total length of stay in PW) × (total daily cost of hospitalization in PW).
- Cost savings per individual patient = (COST A − COST B).
- Total cost saving = sum of the total cost savings per individual patient.
2.4. Data Analysis
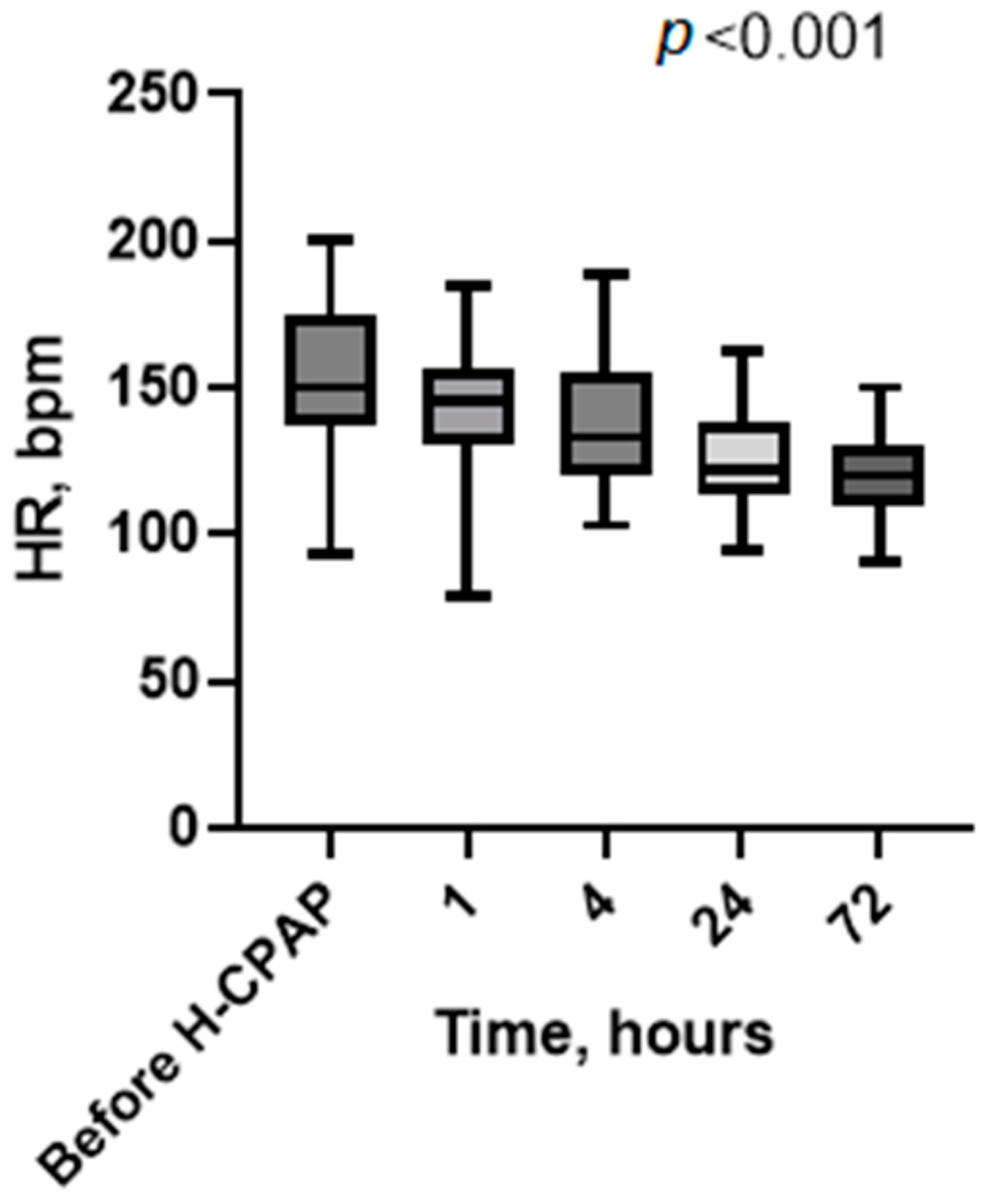
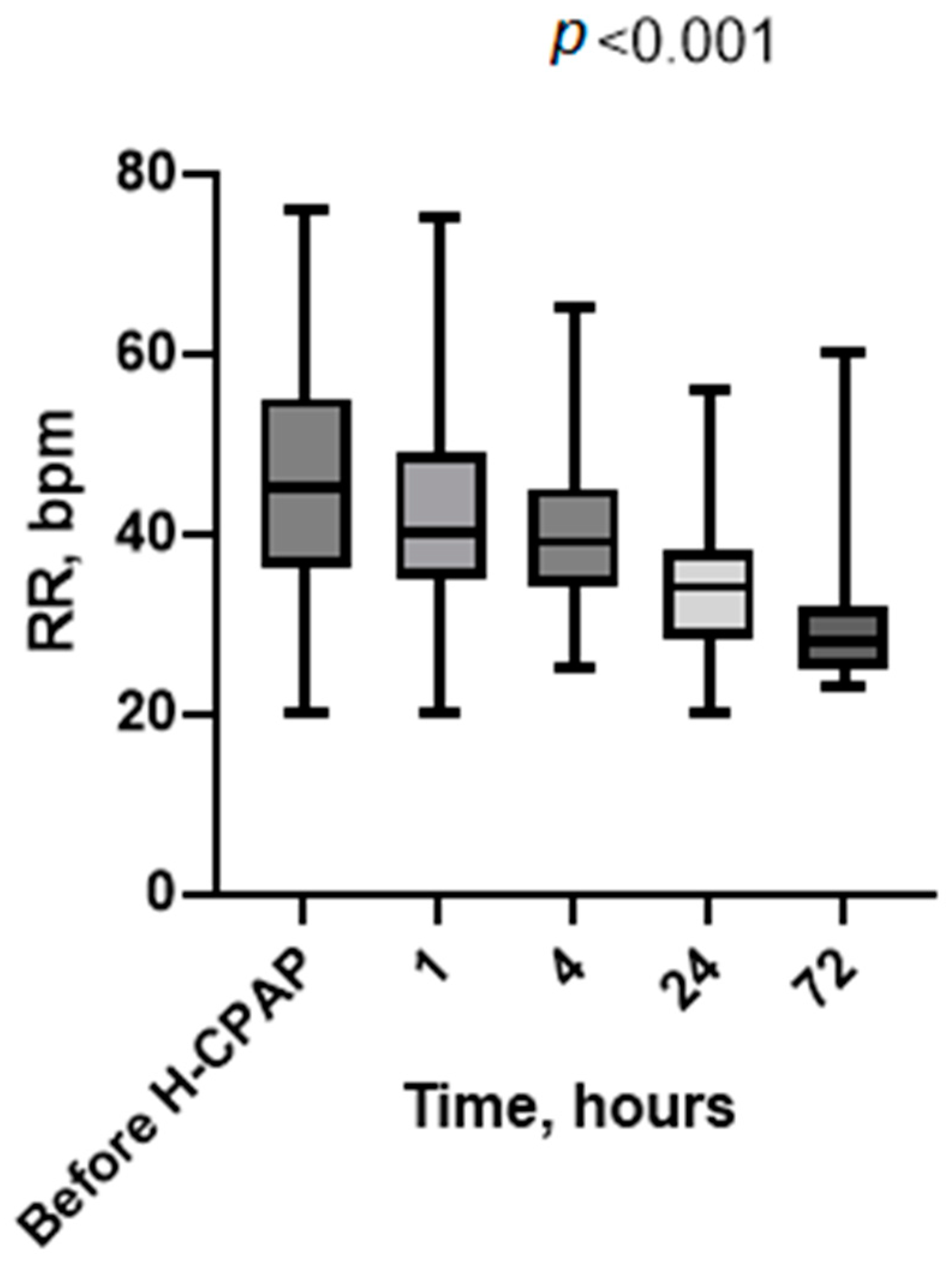
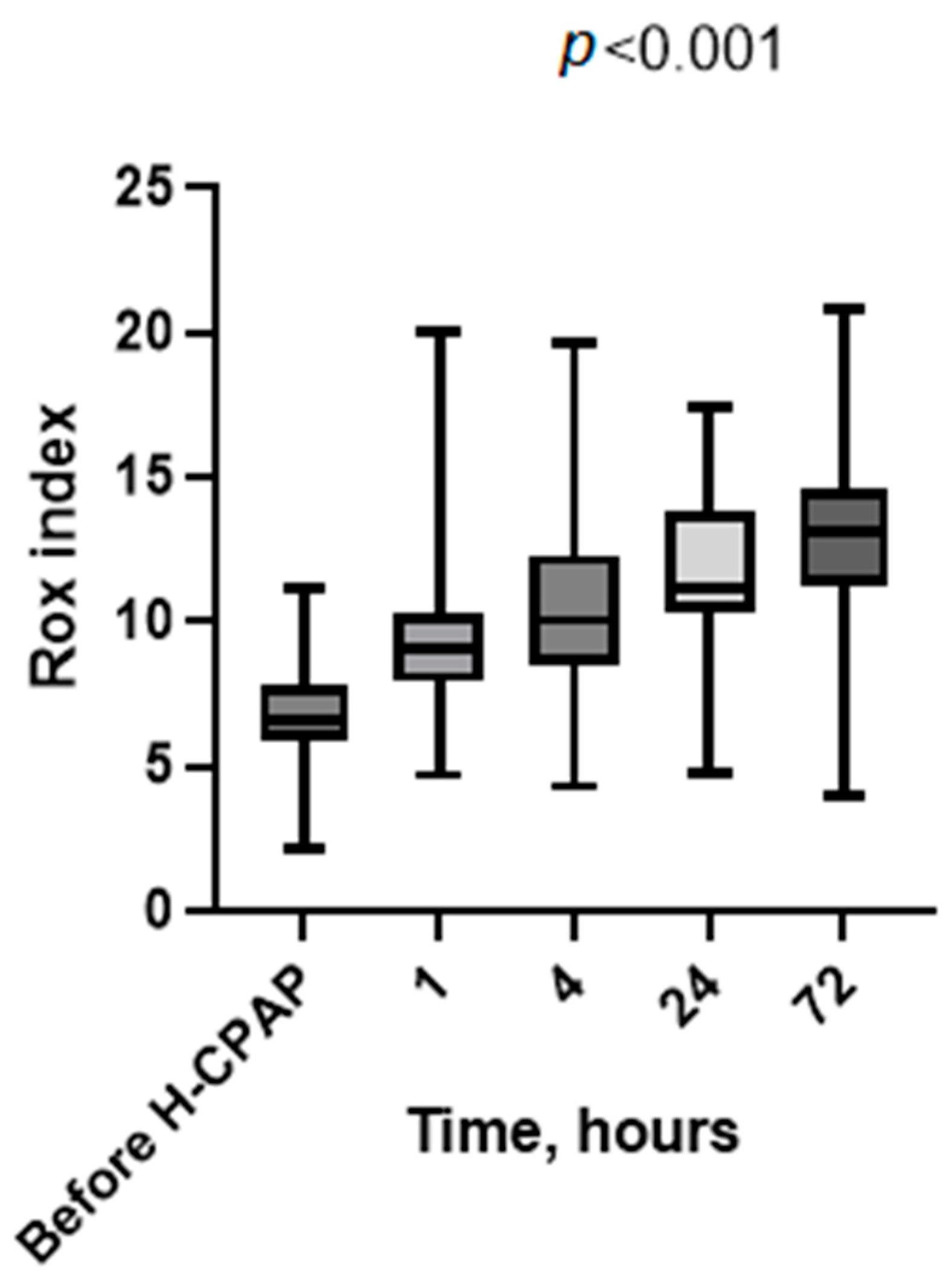
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zorc, J.J.; Hall, C.B. Bronchiolitis: Recent Evidence on Diagnosis and Management. Pediatrics 2010, 125, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, P.S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Caffrey Osvald, E.; Clarke, J.R. NICE clinical guideline: Bronchiolitis in children: Table 1. Arch. Dis. Child. -Educ. Pract. 2016, 101, 46–48. [Google Scholar] [CrossRef]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef] [PubMed]
- Lazner, M.R.; Basu, A.P.; Klonin, H. Non-invasive ventilation for severe bronchiolitis: Analysis and evidence. Pediatr. Pulmonol. 2012, 47, 909–916. [Google Scholar] [CrossRef]
- Coppadoro, A.; Zago, E.; Pavan, F.; Foti, G.; Bellani, G. The use of head helmets to deliver noninvasive ventilatory support: A comprehensive review of technical aspects and clinical findings. Crit. Care 2021, 25, 327. [Google Scholar] [CrossRef]
- Jat, K.R.; Dsouza, J.M.; Mathew, J.L. Continuous positive airway pressure (CPAP) for acute bronchiolitis in children. Cochrane Database Syst. Rev. 2022, 4, CD010473. [Google Scholar] [CrossRef]
- Essouri, S.; Laurent, M.; Chevret, L.; Durand, P.; Ecochard, E.; Gajdos, V.; Devictor, D.; Tissières, P. Improved clinical and economic outcomes in severe bronchiolitis with pre-emptive nCPAP ventilatory strategy. Intensive Care Med. 2014, 40, 84–91. [Google Scholar] [CrossRef]
- Piluso, M.; Scarpazza, P.; Oggionni, E.; Celeste, A.; Bencini, S.; Bernareggi, M.; Bonacina, C.; Cattaneo, R.; Melacini, C.; Raschi, S.; et al. Helmet Continuous Positive Airway Pressure in COVID-19 Related Acute Respiratory Distress Syndrome in Respiratory Intermediate Care Unit. Austin J. Infect. Dis. 2021, 8, 1061. [Google Scholar]
- Lucchini, A.; Giani, M.; Minotti, D.; Elli, S.; Bambi, S. Helmet cpap bundle: A narrative review of practical aspects and nursing interventions to improve patient’s comfort. Intensive Crit. Care Nurs. 2023, 74, 103335. [Google Scholar] [CrossRef]
- Aricò, M.O.; Wrona, D.; Lavezzo, G.; Valletta, E. Nasal CPAP in the Pediatric Ward to Reduce PICU Admissions for Severe Bronchiolitis? Pediatr. Rep. 2023, 15, 599–607. [Google Scholar] [CrossRef]
- Paredes González, E.; Bueno Campaña, M.; Salomón Moreno, B.; Rupérez Lucas, M.; de la Morena Martínez, R. Non-invasive ventilation in acute bronchiolitis on the ward. A viable option. An. Pediatr. Engl. Ed. 2019, 90, 119–121. [Google Scholar] [CrossRef]
- Agüera, M.; Melé-Casas, M.; Molina, M.M.; Pons-Odena, M.; de-Sevilla, M.F.; García-García, J.J.; Launes, C.; Monfort, L. Safety and effectiveness of bubble continuous positive airway pressure as respiratory support for bronchiolitis in a pediatric ward. Eur. J. Pediatr. 2022, 181, 4039–4047. [Google Scholar] [CrossRef] [PubMed]
- Bambino Gesù Pediatric Hospital. Bronchiolitis Protocol; Bambino Gesù Pediatric Hospital: Roma, Italy, 2024. [Google Scholar]
- Portelli, K.; Kandraju, H.; Ryu, M.; Shah, P.S. Efficacy and safety of dexmedetomidine for analgesia and sedation in neonates: A systematic review. J. Perinatol. 2024, 44, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Kannikeswaran, N.; Whittaker, P.; Sethuraman, U. Association between respiratory rate oxygenation index and need for positive pressure ventilation in children on high flow nasal cannula for bronchiolitis. Eur. J. Pediatr. 2022, 181, 3977–3983. [Google Scholar] [CrossRef]
- Siraj, S.; Stark, W.; McKinley, S.D.; Morrison, J.M.; Sochet, A.A. The bronchiolitis severity score: An assessment of face validity, construct validity, and interobserver reliability. Pediatr. Pulmonol. 2021, 56, 1739–1744. [Google Scholar] [CrossRef]
- Essouri, S.; Durand, P.; Chevret, L.; Haas, V.; Perot, C.; Clement, A.; Devictor, D.; Fauroux, B. Physiological effects of noninvasive positive ventilation during acute moderate hypercapnic respiratory insufficiency in children. Intensive Care Med. 2008, 34, 2248–2255. [Google Scholar] [CrossRef]
- Cambonie, G.; Milési, C.; Jaber, S.; Amsallem, F.; Barbotte, E.; Picaud, J.C.; Matecki, S. Nasal continuous positive airway pressure decreases respiratory muscles overload in young infants with severe acute viral bronchiolitis. Intensive Care Med. 2008, 34, 1865–1872. [Google Scholar] [CrossRef]
- Tang, G.; Lin, J.; Zhang, Y.; Shi, Q. The Effects and Safety of Continuous Positive Airway Pressure in Children with Bronchiolitis: A Systematic Review and Meta-Analysis. J. Trop. Pediatr. 2021, 67, fmaa128. [Google Scholar] [CrossRef]
- Mayordomo-Colunga, J.; Rey, C.; Medina, A.; Martínez-Camblor, P.; Vivanco-Allende, A.; Concha, A. Helmet Versus Nasal-Prong CPAP in Infants with Acute Bronchiolitis. Respir. Care 2018, 63, 455–463. [Google Scholar] [CrossRef]
- Wolfler, A.; Calderini, E.; Iannella, E.; Conti, G.; Biban, P.; Dolcini, A.; Pirozzi, N.; Racca, F.; Pettenazzo, A.; Salvo, I.; et al. Evolution of Noninvasive Mechanical Ventilation Use: A Cohort Study Among Italian PICUs*. Pediatr. Crit. Care Med. 2015, 16, 418–427. [Google Scholar] [CrossRef]
- Chidini, G.; Piastra, M.; Marchesi, T.; De Luca, D.; Napolitano, L.; Salvo, I.; Wolfler, A.; Pelosi, P.; Damasco, M.; Conti, G.; et al. Continuous Positive Airway Pressure with Helmet Versus Mask in Infants with Bronchiolitis: An RCT. Pediatrics 2015, 135, e868–e875. [Google Scholar] [CrossRef] [PubMed]
- Chiappero, C.; Misseri, G.; Mattei, A.; Ippolito, M.; Albera, C.; Pivetta, E.; Cortegiani, A.; Gregoretti, C. Effectiveness and safety of a new helmet CPAP configuration allowing tidal volume monitoring in patients with COVID-19. Pulmonology 2023, 29, S9–S17. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Mason, K.P. Dexmedetomidine: Review, update, and future considerations of pediatric perioperative and periprocedural applications and limitations. Br. J. Anaesth. 2015, 115, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Plambech, M.Z.; Afshari, A. Dexmedetomidine in the pediatric population: A review. Minerva Anestesiol. 2015, 81, 320–332. [Google Scholar] [PubMed]
- Øymar, K.; Bårdsen, K. Continuous positive airway pressure for bronchiolitis in a general pediatric ward; a feasibility study. BMC Pediatr. 2014, 14, 122. [Google Scholar] [CrossRef]
- Pelletier, A.J.; Mansbach, J.M.; Camargo, C.A. Direct Medical Costs of Bronchiolitis Hospitalizations in the United States. Pediatrics 2006, 118, 2418–2423. [Google Scholar] [CrossRef]
- Coppadoro, A.; Benini, A.; Fruscio, R.; Verga, L.; Mazzola, P.; Bellelli, G.; Carbone, M.; Mulinacci, G.; Soria, A.; Noè, B.; et al. Helmet CPAP to treat hypoxic pneumonia outside the ICU: An observational study during the COVID-19 outbreak. Crit. Care 2021, 25, 80. [Google Scholar] [CrossRef]
- De Luca, D.; Pezza, L.; Vivalda, L.; Di Nardo, M.; Lepainteur, M.; Baraldi, E.; Piastra, M.; Ricciardi, W.; Conti, G.; Gualano, M.R. Critical care of severe bronchiolitis during shortage of ICU resources. Eclinicalmedicine 2024, 69, 102450. [Google Scholar] [CrossRef]

| Variable | Result |
|---|---|
| Female (n) (%) | 11 (42) |
| Male (n) (%) | 15 (58) |
| Gestational age (wg) (median, range) | 39 (30–41) |
| Weight at hospitalization (g) (median, range) | 5100 (4200–5900) |
| Age at hospitalization (days) (median, range) | 72 (16–345) |
| Length of hospitalization (days) (median, range) | 5 (1–7) |
| Clinical score at t0 (n) (%) | |
| 6–10 | 26 (100) |
| >10 | 0 (0) |
| Viral status (n) | |
| RSV | 13/26 |
| RSV-rhinovirus | 2/26 |
| RSV-influenza | 1/26 |
| RSV-metapneumovirus | 2/26 |
| RSV-negative | 4/26 |
| Variable | Result |
|---|---|
| IMV after H-CPAP | 4 (15) |
| HFNC before H-CPAP (n) (%) | 18 (69) |
| Indications to start H-CPAP (n) (%) | |
| Severe respiratory distress | 22 (84) |
| Hypercapnia and severe respiratory distress | 2 (8) |
| Apnea | 2 (8) |
| Interface tolerance (n) (%) | 22 (85) |
| Duration of H-CPAP (hours) (median, range) | 72 (4–96) |
| Weaning HFNC after H-CPAP (n) (%) | 26 (100%) |
| Enteral feeding with nasogastric tube (n) (%) | 26 (100) |
| HCPAP adverse effects | |
| Gastric distension (n) | 0 |
| Pneumothorax (n) | 0 |
| Bradycardia due to pharmacological sedation (n) (%) | 2 (7) |
| Direct costs | EUR |
| In PW | 376 |
| In PICU | 2398 |
| Indirect costs | |
| In PW | 116 |
| In PICU | 546 |
| Total daily costs of hospitalization (direct and indirect combined) | |
| In PW | 492 |
| In PICU | 2944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musolino, A.M.; Persia, S.; Supino, M.C.; Stoppa, F.; Rotondi Aufiero, L.; Nacca, R.; Papini, L.; Pisani, M.; Cristaldi, S.; Vittucci, A.C.; et al. Helmet Continuous Positive Airway Pressure for Acute Bronchiolitis Respiratory Failure in a Pediatric Ward: Is It a Replicable Experience? Children 2024, 11, 1273. https://doi.org/10.3390/children11111273
Musolino AM, Persia S, Supino MC, Stoppa F, Rotondi Aufiero L, Nacca R, Papini L, Pisani M, Cristaldi S, Vittucci AC, et al. Helmet Continuous Positive Airway Pressure for Acute Bronchiolitis Respiratory Failure in a Pediatric Ward: Is It a Replicable Experience? Children. 2024; 11(11):1273. https://doi.org/10.3390/children11111273
Chicago/Turabian StyleMusolino, Anna Maria, Sabrina Persia, Maria Chiara Supino, Francesca Stoppa, Lelia Rotondi Aufiero, Raffaella Nacca, Laura Papini, Mara Pisani, Sebastian Cristaldi, Anna Chiara Vittucci, and et al. 2024. "Helmet Continuous Positive Airway Pressure for Acute Bronchiolitis Respiratory Failure in a Pediatric Ward: Is It a Replicable Experience?" Children 11, no. 11: 1273. https://doi.org/10.3390/children11111273
APA StyleMusolino, A. M., Persia, S., Supino, M. C., Stoppa, F., Rotondi Aufiero, L., Nacca, R., Papini, L., Pisani, M., Cristaldi, S., Vittucci, A. C., Antilici, L., Cecchetti, C., Raponi, M., Nadkarni, V., & Villani, A. (2024). Helmet Continuous Positive Airway Pressure for Acute Bronchiolitis Respiratory Failure in a Pediatric Ward: Is It a Replicable Experience? Children, 11(11), 1273. https://doi.org/10.3390/children11111273





