Development and Validation of a Diagnostic Algorithm for Down Syndrome Using Birth Certificate and International Classification of Diseases Codes
Abstract
1. Introduction
2. Materials and Methods
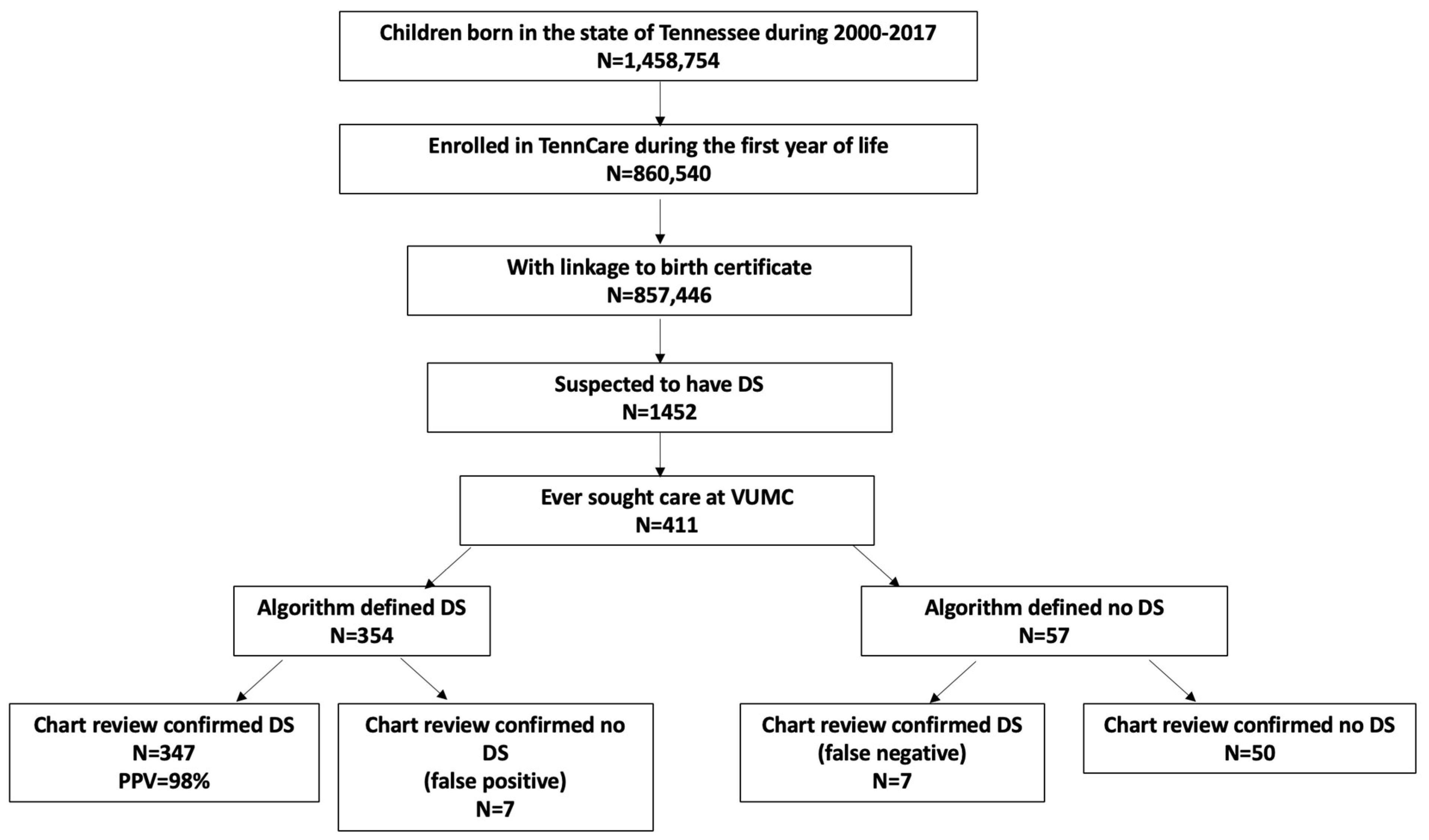
2.1. Study Design and Cohort
2.2. Algorithm Defining DS
- Having birth certificate indication for “karyotype-confirmed” DS;
- Having birth certificate indication for “karyotype-pending” DS or just DS if test type was not specified (i.e., prior to 2004) and having at least two healthcare encounters for DS during the first six years of life;
- Having at least three healthcare encounters for DS during the first six years of life, with the first and last encounter separated by at least 30 days.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stallings, E.B.; Isenburg, J.L.; Rutkowski, R.E.; Kirby, R.S.; Nembhard, W.N.; Sandidge, T.; Villavicencio, S.; Nguyen, H.H.; McMahon, D.M.; Nestoridi, E.; et al. National population-based estimates for major birth defects, 2016–2020. Birth Defects Res. 2024, 116, e2301. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat. Rev. Dis. Primers 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Trotter, T.; Santoro, S.L.; Christensen, C.; Grout, R.W.; Council On, G.; Burke, L.W.; Berry, S.A.; Geleske, T.A.; Holm, I.; et al. Health Supervision for Children and Adolescents with Down Syndrome. Pediatrics 2022, 149, e2022057010. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. Available online: https://www.nih.gov/include-project (accessed on 3 April 2024).
- Quan, H.; Parsons, G.A.; Ghali, W.A. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med. Care 2004, 42, 801–809. [Google Scholar] [CrossRef] [PubMed]
- van Walraven, C.; Austin, P. Administrative database research has unique characteristics that can risk biased results. J. Clin. Epidemiol. 2012, 65, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lash, T.L.; Mor, V.; Wieland, D.; Ferrucci, L.; Satariano, W.; Silliman, R.A. Methodology, design, and analytic techniques to address measurement of comorbid disease. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.S.; Chan, B.K.; Schembri, M.E.; Rainwater, J.A. Can administrative data be used to compare postoperative complication rates across hospitals? Med. Care 2002, 40, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.S.; Schembri, M.E.; Rainwater, J.A. Can administrative data be used to ascertain clinically significant postoperative complications? Am. J. Med. Qual. 2002, 17, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.M.; Cooke, C.R.; Davis, M.M. Fidelity of administrative data when researching Down syndrome. Med. Care 2014, 52, e52–e57. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.D.; Cai, T.T.; DasGupta, A.; Agresti, A.; Coull, B.A.; Casella, G.; Corcoran, C.; Mehta, C.; Ghosh, M.; Santner, T.J.; et al. Interval estimation for a binomial proportion—Comment—Rejoinder. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Northam, S.; Polancich, S.; Restrepo, E. Birth certificate methods in five hospitals. Public Health Nurs. 2003, 20, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Natoli, J.L.; Ackerman, D.L.; McDermott, S.; Edwards, J.G. Prenatal diagnosis of Down syndrome: A systematic review of termination rates (1995–2011). Prenat. Diagn. 2012, 32, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kucik, J.E.; Correa, A. Causes of death and case fatality rates among infants with down syndrome in metropolitan Atlanta. Birth Defects Res. Part A Clin. Mol. Teratol. 2007, 79, 775–780. [Google Scholar] [CrossRef] [PubMed]
| Qualified for Suspected DS | Confirmed DS | Total (n = 411) | |
|---|---|---|---|
| Yes (n = 354) | No (n = 57) | ||
| Having at least one ICD code specific for DS | 354 (100%) | 57 (100%) | 411 (100%) |
| DS coded on birth certificate | 101 (28.5%) | 1 (<1%) | 102 (24.8%) |
| Children with suspected DS (n = 411) | |
|---|---|
| Maternal characteristics | |
| Age at delivery, median (25th percentile, 75th percentile) | 31 (23, 38) |
| Education (n = 410) | |
| Some high school or less | 108 (26.3%) |
| High school graduate | 133 (32.4%) |
| At least some college education | 169 (41.2%) |
| Residence | |
| Urban | 134 (32.6%) |
| Suburban | 136 (33.1%) |
| Rural | 141 (34.3%) |
| Married | 241 (58.6%) |
| Smoking during pregnancy (n = 409) | 72 (17.6%) |
| Prenatal care started at first trimester (n = 381) | 264 (69.3%) |
| Parity (n = 405) | |
| Primiparous | 123 (30.4%) |
| 2 | 116 (28.6%) |
| 3+ | 166 (40.9%) |
| Delivery method | |
| Vaginal/assisted | 217 (52.8%) |
| Cesarean section | 194 (47.2%) |
| Infant characteristics | |
| Sex | |
| Male | 224 (54.5%) |
| Female | 187 (45.5%) |
| Race and Ethnicity | |
| Non-Hispanic White | 209 (50.9%) |
| Non-Hispanic Black | -- 1 |
| Hispanic | -- 1 |
| Other | -- 1 |
| Gestational age in weeks, median (25th percentile, 75th percentile) | 38 (36, 39) |
| Birth weight in grams, median (25th percentile, 75th percentile) | 2920 (2495, 3280) |
| Small for gestational age at 10th percentile (n = 410) | 59 (14.4%) |
| Singleton birth | 404 (98.3%) |
| One or more older siblings (n = 406) | 283 (69.7%) |
| Congenital heart disease | 343 (83.5%) |
| Birth year | |
| 2000–2004 | 74 (18.0%) |
| 2005–2009 | 118 (28.7%) |
| 2010–2017 | 219 (53.3%) |
| Criterion 1 (Karyotype-Confirmed DS) | Criterion 2 (Karyotype-Pending DS or Just DS and ≥2 ICD Diagnosis for DS) | Criterion 3 (≥3 ICD Diagnosis for DS) | Study Population—Children with Suspected DS (n = 411) | Children with Chart-Review-Confirmed DS (n = 354) | Children with Chart-Review-Confirmed No DS (n = 57) | PPV |
|---|---|---|---|---|---|---|
| X 3 | 1 | 1 | 0 | 100.0% | ||
| X 3 | X 3 | 34 | 34 | 0 | 100.0% | |
| X 3 | 3 | 3 | 0 | 100.0% | ||
| X 3 | X 3 | 63 | 63 | 0 | 100.0% | |
| X 3 | 253 | 246 | 7 1 | 97.2% | ||
| 57 | 7 2 | 50 | NA 4 |
| N (%) | |
|---|---|
| Type (n = 283 1) | |
| Nondisjunction | 266 (94.0%) |
| Translocation/Mosaic | 17 (6.0%) |
| Diagnosis/confirmation method 2 (n = 246 1) | |
| Karyotype | 227 (92.3%) |
| Fluorescence in situ hybridization | 20 (8.1%) |
| Timing of first diagnosis (n = 293 1) | |
| Prenatal | 83 (28.3%) |
| Postnatal | 210 (71.7%) |
| Infant age in days when first DS-specific ICD diagnosis appeared in claims data | 0 (0, 40) 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, L.; Bird, K.; Nian, H.; Maxwell-Horn, A.; Lee, R.; Ding, T.; Riddell, C.; Gebretsadik, T.; Snyder, B.; Hartert, T.; et al. Development and Validation of a Diagnostic Algorithm for Down Syndrome Using Birth Certificate and International Classification of Diseases Codes. Children 2024, 11, 1271. https://doi.org/10.3390/children11101271
Ammar L, Bird K, Nian H, Maxwell-Horn A, Lee R, Ding T, Riddell C, Gebretsadik T, Snyder B, Hartert T, et al. Development and Validation of a Diagnostic Algorithm for Down Syndrome Using Birth Certificate and International Classification of Diseases Codes. Children. 2024; 11(10):1271. https://doi.org/10.3390/children11101271
Chicago/Turabian StyleAmmar, Lin, Kristin Bird, Hui Nian, Angela Maxwell-Horn, Rees Lee, Tan Ding, Corinne Riddell, Tebeb Gebretsadik, Brittney Snyder, Tina Hartert, and et al. 2024. "Development and Validation of a Diagnostic Algorithm for Down Syndrome Using Birth Certificate and International Classification of Diseases Codes" Children 11, no. 10: 1271. https://doi.org/10.3390/children11101271
APA StyleAmmar, L., Bird, K., Nian, H., Maxwell-Horn, A., Lee, R., Ding, T., Riddell, C., Gebretsadik, T., Snyder, B., Hartert, T., & Wu, P. (2024). Development and Validation of a Diagnostic Algorithm for Down Syndrome Using Birth Certificate and International Classification of Diseases Codes. Children, 11(10), 1271. https://doi.org/10.3390/children11101271







