Abstract
Aim: to systematically review and meta-analyze the impact on morbidity and mortality of peritoneal drainage (PD) compared to laparotomy (LAP) in preterm neonates with surgical NEC (sNEC) or spontaneous intestinal perforation (SIP). Methods: Medical databases were searched until June 2022 for studies comparing PD and LAP as primary surgical treatment of preterm neonates with sNEC or SIP. The primary outcome was survival during hospitalization; predefined secondary outcomes included need for parenteral nutrition at 90 days, time to reach full enteral feeds, need for subsequent laparotomy, duration of hospitalization and complications. Results: Three RCTs (N = 493) and 49 observational studies (N = 19,447) were included. No differences were found in the primary outcome for RCTs, but pooled observational data showed that, compared to LAP, infants with sNEC/SIP who underwent PD had lower survival [48 studies; N = 19,416; RR 0.85; 95% CI 0.79–0.90; GRADE: low]. Observational studies also showed that the subgroup of infants with sNEC had increased survival in the LAP group (30 studies; N = 9370; RR = 0.82; 95% CI 0.72–0.91; GRADE: low). Conclusions: Compared to LAP, PD as primary surgical treatment for sNEC or SIP has similar survival rates when analyzing data from RCTs. PD was associated with lower survival rates in observational studies.
1. Introduction
Necrotizing enterocolitis (NEC) is a major contributor to mortality and morbidity in extremely preterm infants [1]. Despite significant improvements in NEC prevention strategies over the last decade [2], its incidence continues to increase worldwide as advances in perinatal care have led to increased survival of extremely preterm infants [3]. Globally, it has been reported that 7% of all very low birth weight (VLBW < 1500 g at birth) infants will suffer an episode of NEC over their neonatal intensive care unit (NICU) hospitalization [3].
Peritoneal drainage (PD), which involves insertion of a Penrose drain into the peritoneum at the bedside, was first reported as a treatment for necrotizing enterocolitis and spontaneous intestinal perforation (SIP) in 1977 by Ein et al. [4], in a case series of five patients who were unstable and would not tolerate laparotomy (LAP). PD aims to decompress the abdomen and remove peritoneal toxic effluents without requiring open surgery, and has established itself as an alternative to LAP, sometimes in hemodynamically unstable infants as a temporizing procedure, but also as a less aggressive and sometimes definitive first line treatment [5].
To date, given the overall high mortality [6] and poor neurodevelopmental outcomes [7,8] associated with surgical NEC (sNEC), the first-line surgical treatment approach for sNEC and SIP remains controversial. Previous meta-analysis [9,10] have analyzed data from two published randomized controlled trials (RCTs) [11,12] and numerous observational studies [5] that have compared LAP to PD as primary surgical treatment for NEC and/or SIP, analyzing differences in mortality and other outcomes. Over the last 10 years, however, new evidence, including the largest RCT so far [13] and multiple observational studies have been published, providing new data that might help us answer the question of which is the most appropriate surgical option for preterm neonates with sNEC or SIP.
The objective of this study was to conduct a systematic review and meta-analysis comparing the mortality and morbidities of LAP and PD when used as primary surgical intervention for sNEC or SIP in preterm neonates.
2. Methods
The design of this study followed the recommendations of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) statement [14] and MOOSE (Meta-analysis of Observational Studies in Epidemiology) statement [15]. The protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews database, prior to the start of the literature review (CRD42022302866).
2.1. Eligibility of Studies
RCTs and observational studies of preterm neonates who were diagnosed with sNEC or SIP during NICU admission were included. sNEC was defined as NEC requiring surgical intervention according to the different definitions of study authors. Studies were selected when providing data comparing LAP and PD as primary intervention for sNEC or SIP. Studies only including data from one intervention (PD or LAP) were excluded. Only studies with human participants were included in the review, with both full-published articles and conference abstracts evaluated and included if meeting inclusion criteria. Case reports, case series, reviews, letters to editor and commentaries were not included.
2.2. Intervention Group
PD was defined as placement of a Penrose drain in the lower abdomen at the bedside in the NICU under local anaesthesia and/or sedation. PD was considered primary intervention when performed as the initial surgical intervention following diagnosis of sNEC or SIP, irrespective of the need for later LAP.
2.3. Comparator
LAP was defined as surgical abdominal exploration with direct observation either in the operating room or at bedside in the NICU.
2.4. Outcomes
The primary outcome was survival during hospitalization, and predefined secondary outcomes included need for parenteral nutrition at 90 days, time to reach full enteral feeds, duration of hospitalization and complications including abdominal abscess, intestinal stricture and intestinal fistula.
2.5. Search Strategy
PubMed, Embase, CINAHL and Cochrane CENTRAL were searched from inception until June 2022. No language restrictions were applied. Searches were conducted by an information specialist, and authors of studies with missing data were contacted via email for additional data or clarification. Reference lists of included studies were also scanned for possible extra studies.
2.6. Study Selection
Two authors (GSG and BJ) screened the titles and abstracts independently for potential eligibility, and the same two authors read the subsequent full texts to decide on final inclusion.
2.7. Data Extraction
Authors GSG and BJ independently extracted the data with a standardized data collection form which was specifically designed for the study. Potential discrepancies during the data extraction process were resolved by discussion and consensus with the third author (AP).
2.8. Risk of Bias
Two authors (GSG and BJ) conducted risk of bias assessment independently for all the included studies. Cochrane risk of bias tool (ROB) 2.0 [16] was used for RCTs. GSG and BJ independently assessed ROB for the following domains: random number generation, allocation concealment, blinding of intervention and outcome assessors, completeness of follow up, selectivity of reporting and other potential biases. ROB was assigned as low, unclear and high risk based on the Cochrane Collaboration guidelines. The Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was used for observational studies [17]. Studies were considered low risk of bias only when risk was deemed low for all domains; moderate risk of bias when at least one domain had moderate risk; serious when at least one domain had serious risk; and critical when at least one domain had critical risk of bias.
2.9. Data Synthesis and Analysis
Meta-analysis of pooled data was performed with fixed effects models for RCTs and random effects models for observational studies to account for clinical heterogeneity of included studies, with inverse-variance weighing, following Cochrane Handbook guidelines [18]. Risk ratio (RR) for categorical variables and mean difference (MD) for continuous variables were used as effect measures, both with 95% confidence intervals (CI). Meta-regression was performed to account for heterogeneity and for the possible influence on results of mean gestational age, gestational age differences between study groups, and sample size. Data were analyzed using Review Manager version 5.4.1 and R 4.1.0.
The heterogeneity of studies was evaluated using the I2 statistic and was interpreted according to the Cochrane Handbook guidelines [18]: 0–40%: might not be important; 30–60%: may represent moderate heterogeneity; 50–90%: may represent substantial heterogeneity; 75–100%: considerable heterogeneity. Publication bias was evaluated by funnel plot.
2.10. Subgroup Analysis
Pre-defined subgroup analysis was performed for studies including only sNEC patients, for studies including only extremely low birth weight infants (ELBW defined as <1000 g at birth), and for studies published in the last 10 years (2012 to 2022).
2.11. Summary of Findings
The most significant information about the magnitude of effect of the intervention, the summary of data available and the certainty of evidence were presented in a “Summary of findings” table that followed the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) guidelines [19]. The certainty of evidence was classified into one of the four categories: high, moderate, low and very low. In case of discrepancies, discussions were held with other two reviewers before reaching consensus.
3. Results
The literature search retrieved 1901 potential records which were screened. After removal of 766 duplicates, 1135 records were screened and, finally, 143 full-text articles were assessed for eligibility for inclusion. Finally, 3 RCTs (N = 493 patients) [11,12,13] and 49 observational studies (N = 19,447 patients) [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] were included in the systematic review and meta-analysis. The flow diagram of the literature search and study selection is shown in Figure 1.

Figure 1.
Flow diagram of the literature search and study selection.
3.1. Characteristics of Included Studies
All three RCTs were multicenter and included infants with diagnosis of both SIP or sNEC. The RCT by Blakely et al. [13] reported outcomes for SIP and sNEC separately and the other two studies reporting overall results for the full cohort. Only preterm infants were included in these trials, with the trials by Blakely et al. [13] and Rees et al. [12] including infants <1000 g and Moss et al. [11] including infants <1500 g. Regarding observational studies, most of them (42 studies) were retrospective, with only 5 prospective and 2 ambispective studies. Eleven were multicenter studies, and thirty-eight were conducted in a single center. Thirty studies exclusively included sNEC patients, while eighteen included both NEC and SIP infants and only one study enrolled only SIP patients. The primary outcome was reported in all RCTs and all observational studies except one study by Murcia-Pascual et al. [61]. Supplementary Tables S1 and S2 summarize the characteristics of the included studies (observational studies and randomized controlled trials, respectively).
3.2. Risk of Bias Assessment
The risk of bias assessment using Cochrane ROB 2.0 tool showed low risk of bias in all domains for RCTs: bias arising from the randomization process, bias due to deviations from intended intervention, bias due to missing outcome data, bias in the measurement of the outcome, and bias in selection of the reported result (Figure 2). Observational studies analyzed with ROBINS-I were found to have moderate to serious risk of bias (Figure 3). Specifically, most observational studies had moderate to serious risk of bias in the domain ‘bias due to confounding’, due to lack of proper adjustment for confounding factors and likely different profile risk between infants receiving laparotomy and peritoneal drainage, having the latter group usually smaller, more immature and sicker infants receiving the intervention. Most of these observational studies also had moderate risk of bias in the domains ‘bias in classification of interventions’ and ‘bias in selection of the reported result’.
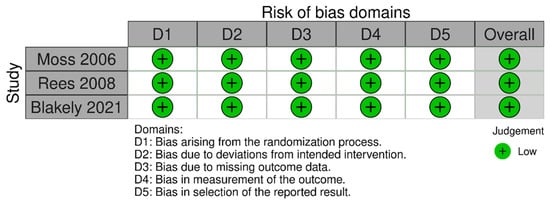
Figure 2.
Risk of bias assessment of randomized controlled trials with ROB 2.0 tool [11,12,13].
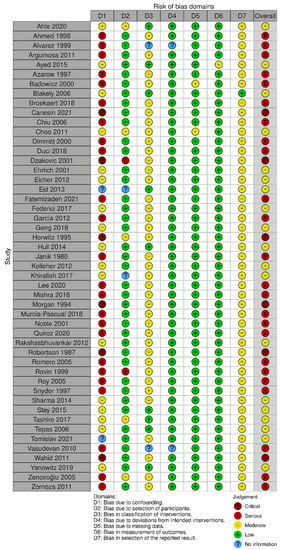
Figure 3.
Risk of bias diagram for observational studies via ROBINS-1 [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68].
3.3. Primary Outcome
Pooled analyses from 3 RCTs showed no differences in survival between preterm neonates randomized to LAP or PD for sNEC or SIP [3 RCTs; N = 493 patients; Relative risk (RR) 0.97; 95% confidence interval (CI) 0.86–1.09; GRADE: Moderate; Figure 4].
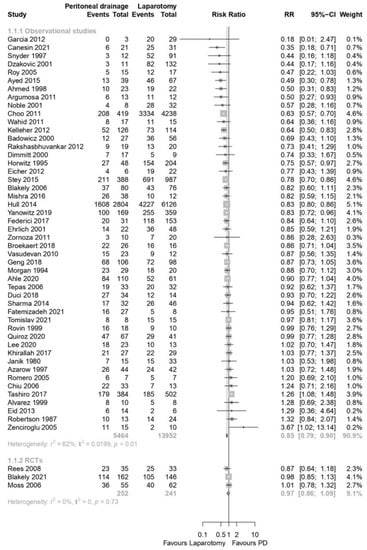
Figure 4.
Forest plot for primary outcome: survival during hospital stay [11,12,13,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68].
Observational data from 48 studies showed that, compared to LAP, preterm infants with sNEC or SIP who underwent PD had lower survival (48 studies; N = 19,416 patients; RR: 0.85; 95% CI 0.79–0.90; GRADE: low; Figure 4). There was no publication bias, as assessed with the creation of a funnel plot (Supplementary Figure S1).
3.4. Secondary Outcomes
No statistically significant differences were found between the two groups for the different pre-specified secondary outcomes in preterm neonates with sNEC or SIP, including need for TPN at 90 days, time to full feeds, duration of hospital stay and complications, including stricture, abdominal abscess and intestinal fistula (Table 1). These results were similar when including only evidence from RCTs and when including pooled data from observational studies.

Table 1.
Pre-specified secondary outcomes for included RCTs and observational studies.
3.5. Subgroup Analysis
When analyzing studies that only included sNEC infants, pooled observational data showed increased survival in the LAP group (30 studies; N = 9370; RR = 0.82; 95% CI 0.72–0.91; GRADE: low), with only one RCT by Blakely et al. [13] reporting sNEC outcomes separately. This trial did not find significant differences in the primary outcome evaluated in this systematic review, although an increase in survival without neurodevelopmental impairment was found when comparing the two groups using a Bayesian approach to meta-analysis. The study by Moss et al. [11] reported similar results for infants with and without pneumatosis intestinalis. Only one observational study, published by Ahle et al. [63] included exclusively SIP patients, without significant differences in survival between the two groups. These results were similar to the ones reported in the RCT by Blakely et al. [13], in which no significant differences were observed when analyzing the subgroup of infants with SIP.
For the subgroup of ELBW infants, there were no statistically significant differences for the primary outcome between the two groups when including pooled data from observational studies (16 studies; N = 2072; RR = 0.93; 95% CI 0.81–1.07; GRADE: low), or RCTs (3 studies; 466 patients; RR = 0.96; 95% CI 0.85–1.08; GRADE: Moderate, Supplemental Figure S2).
Analysis of evidence from observational studies published in the last 10 years (2012–2021) also showed higher survival rates for the LAP group compared to PD (23 studies, N = 13,271, RR = 0.87, 95% CI 0.81–0.94, GRADE: low), with only 1 RCT by Blakely et al. [13] published in the last 10 years and no differences in the primary outcome.
3.6. Meta-Regression
No statistically significant result was obtained when performing meta-regression for an association between survival in PD versus LAP with regards to sample size (coefficient −0.001, p-value 0.357), mean gestational age (coefficient −0.01, p value 0.59, Supplementary Figure S3) and gestational age differences between study groups (coefficient −0.03, p-value 0.12).
3.7. Summary of Findings and Certainty of Evidence
The certainty of evidence according to the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) guidelines was graded as low to moderate due to study design and risk of bias. Table 2 (primary outcome) and Supplementary Table S3 (secondary outcomes) summarize certainty of evidence.

Table 2.
Summary of findings for the primary outcome.
4. Discussion
In this systematic review and meta-analysis including three RCTs and 49 observational studies for a total of 19,940 preterm infants with sNEC or SIP, we found no differences in survival rates when analyzing high-quality data from RCTs at low risk of bias. When pooling data from observational studies, we found that compared to LAP preterm infants who received PD may have lower survival rates, but this result must be interpreted with caution, given the moderate to serious risk of bias found in the observational studies. The overall certainty of evidence was graded as low to moderate.
The controversy regarding the ideal first line surgical treatment between LAP and PD for sNEC and SIP in neonates has led to the conduct of three RCTs till date, all of them published in the last two decades. The most recent and largest trial to date is the one published by Blakely et al. [13], whose primary outcome was a composite outcome of death or neurodevelopmental impairment at 18–22 months corrected age, and which included mortality as a secondary outcome. This trial did not find differences for primary and pre-specified secondary outcomes, but in a pre-defined Bayesian sub-analysis of only sNEC patients, PD was found to be associated with an increase in risk of death or moderate-severe cerebral palsy compared to LAP. The previous two trials, published by Moss et al. [11] and Rees et al. [12], did not find statistically significant differences in mortality; however, their statistical methods were frequentist instead of Bayesian, their results were not reported independently for NEC and SIP patients, and both these trials had smaller sample sizes which might have impacted their statistical power to detect differences for key outcomes between the two approaches. The lack of statistical power and the clinical heterogeneity makes it challenging to extract clinically relevant conclusions from the results of the three trials. Although some of the studies and commentaries propose that a better response to the research question may be obtained with larger, adequately powered RCTs, the truth is that those are especially difficult to conduct for surgical interventions in sNEC or SIP in preterm neonatal populations [69], and it is not clear that a larger randomized study might be feasible at the moment. The commonly encountered barriers to designing such trials include difficulties with research ethic board’s approval, challenges with informed consent and low incidence of sNEC and SIP in preterm neonates.
For all these reasons, observational data are a key resource when interpreting evidence in surgical neonatal populations. Unlike RCTs, observational studies are usually able to provide enough statistical power even for small differences, but their design is less robust, as suggested by our analysis, with a significant risk of bias, especially in the confounding domain. Non-randomized studies, in our case and specifically in the PD group, tend to include lower birth weight and more unstable patients who may not be candidates to tolerate a LAP [31,43,66], putting these studies at high risk for residual confounding even after adjustment. With observational data, meta-analysis should always be interpreted with caution due to risk of bias and heterogeneity among included studies [70].
4.1. Previous Systematic Reviews and Important Differences from Our Study
The only previous meta-analysis to include a large number of observational studies is the one published by Van Heesewijk et al. [5], which compares the two surgical approaches and evaluated the mortality rates for two RCTs and 25 observational studies published between 1994 and 2016. Similarly to what we describe in this article, they observed higher mortality rates in the PD group on analysis of observational studies and did not find any differences in mortality on pooled analysis from RCTs. The latest meta-analysis comparing the two approaches for sNEC or SIP by Li et al. [71] is the only one published to date that has included the recent trial by Blakely et al. [13]. However, in this review the authors included only 10 observational studies and concluded that no statistically significant differences were found in mortality, which might be explained by a smaller sample size compared to our study, especially given that their confidence interval was of borderline significance, and that they did not include some studies that would have met inclusion criteria as per their description [72].
Our study contributes to this line of investigation by comprehensively including all observational studies published to our knowledge until date, with analysis of a total of 19,940 patients that provides better statistical power compared to previous systematic reviews, and which may explain the difference in the survival between the two surgical approaches for observational data. However, as previously stated, these differences may be more likely to be related to the baseline differences between the study groups, and even though underpowered, the trial data are likely to be more reliable.
As opposed to previous systematic reviews, we also included several secondary outcomes that were not assessed in previous meta-analyses. Similar to findings from previous individual RCTs, our pooled analysis did not show differences in the rates of parenteral nutrition at 90 days, the time to reach full feeds, the duration of hospital stays nor the rates of different complications, which included intestinal strictures, abdominal abscesses and intestinal fistulas. These results were similar for pooled data from RCTs and observational studies. There was significant heterogeneity for most of the outcomes that we meta-analyzed. We explored some possible causes for the heterogeneity of studies with meta-regression techniques, but we did not find that sample size, mean gestational age or gestational age differences between study groups had an impact on the differences between studies.
In addition, our study also analyzed the outcomes between the two surgical approaches for different subgroups, including publications in the contemporary era (2012–2022), ELBW infants and most importantly data for sNEC population alone. As previously stated, this last subgroup of infants is of particular interest, since although sometimes difficult to differentiate in presentation, sNEC and SIP are two clearly separate clinical entities with different pathophysiology and prognosis, and the recent trial by Blakely et al. [13] has suggested that the effect of the two interventions might be different for infants with sNEC but not necessarily for those with SIP. For this subgroup of infants, we found similar results to the overall analysis, with only the trial by Blakely et al. [13] reporting the individual sNEC results and pooled observational data showing increase in survival with LAP, with evidence being low quality due to observational study design and risk of bias. Only the trial by Blakely et al. [13] and the observational study by Ahle et al. [63] reported SIP outcomes independently, without significant differences in survival found in any of these studies but also without overall statistical power to draw meaningful conclusions from their analyses.
The main strengths of our systematic review and meta-analysis are the large sample size, including all RCTs and observational studies comparing the two surgical approaches published until date, comprehensive search of the literature, the inclusion of predefined secondary outcomes and subgroup analysis and the use of meta-regression techniques to explore possible causes of heterogeneity. However, our review also has limitations that must be acknowledged. Firstly, the number of included RCTs is limited to three, and two of them are relatively small, with only 117 [11] and 69 [12] patients, respectively. Secondly, all the observational studies had moderate to serious risk of bias, as shown by our risk of bias analysis via ROBINS-I, which limits the validity of the conclusions drawn from pooled data from these observational studies. Third, long-term data were not reported by most RCTs or observational studies, with only the RCT by Blakely et al. reporting long term outcomes [13]. Fourth, there is a subset of preterm neonates with sNEC or SIP that receive PD followed by LAP due to non-improvement and were not addressed independently in our study. Lastly, a significant number of studies were not able to differentiate between sNEC and SIP, which may present in a similar manner but have different short and long-term prognosis, and in other studies the diagnostic criteria for SIP and NEC were not always clear, making the results of these studies difficult to interpret. Individual data for the two diseases would be needed to draw firm conclusions on the differences in outcomes between the two surgical approaches.
4.2. Implications for Clinicians and Researchers
Adequately powered RCT would be the ideal design to confirm these findings, but as previously discussed, they are difficult to conduct due to rarity of sNEC and SIP in preterm neonates. If one were to design a definitive trial, to detect an absolute risk reduction of 10% in mortality (from 40% to 30%) with statistical power of 80% and α error of 0.05, a sample size of 350 ELBW infants per arm would be needed, which would be more than double the size of the biggest trial conducted so far and might be challenging from a logistical perspective even in the setting of a multicenter design. Given the difficulty in designing a trial like this, observational data may be useful; but future studies should include larger sample sizes and aim for balanced study groups, adjusting for possible confounders using multivariable models.
In conclusion, the results of this systematic review and meta-analysis suggest that, compared to LAP, PD as primary intervention for sNEC or SIP may have similar survival rates on analysis of data from clinical trials but may be associated with lower survival rates when pooling data from observational studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/children10071170/s1, Table S1. Characteristics of included observational studies; Table S2. Characteristics of included RCTs; Figure S1. Funnel plot evaluating publication bias; Figure S2. Forest plot for primary outcome, subgroup analysis of infants <1000 g; Figure S3. Bubble plot for gestational age metaregression; Table S3. Summary of findings, secondary outcomes.
Author Contributions
Conceptualization, G.S.-G. and B.J.; Data curation, G.S.-G. and B.J.; Formal analysis, G.S.-G. and B.J.; Investigation, G.S.-G.; Methodology, G.S.-G. and B.J.; Project administration, B.J.; Resources, A.P.; Software, G.S.-G.; Supervision, A.P. and B.J.; Writing—original draft, G.S.-G.; Writing—review and editing, G.S.-G., A.P. and B.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the study design.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sets and analysis are available upon request to the corresponding author.
Conflicts of Interest
The authors do not have any conflict of interest. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- Patel, R.M.; Kandefer, S.; Walsh, M.C.; Bell, E.F.; Carlo, W.A.; Laptook, A.R.; Sánchez, P.J.; Shankaran, S.; Van Meurs, K.P.; Ball, M.B.; et al. Causes and Timing of Death in Extremely Premature Infants from 2000 through 2011. N. Engl. J. Med. 2015, 372, 331–340. [Google Scholar] [CrossRef]
- Patel, A.L.; Panagos, P.G.; Silvestri, J.M. Reducing Incidence of Necrotizing Enterocolitis. Clin. Perinatol. 2017, 44, 683–700. [Google Scholar] [CrossRef]
- Alsaied, A.; Islam, N.; Thalib, L. Global incidence of Necrotizing Enterocolitis: A systematic review and Meta-analysis. BMC Pediatr. 2020, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Ein, S.H.; Marshall, D.G.; Girvan, D. Peritoneal drainage under local anesthesia for perforations from necrotizing enterocolitis. J. Pediatr. Surg. 1977, 12, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Van Heesewijk, A.E.; Rush, M.L.; Schmidt, B.; Kirpalani, H.; DeMauro, S.B. Agreement between study designs: A systematic review comparing observational studies and randomized trials of surgical treatments for necrotizing enterocolitis. J. Matern. Neonatal Med. 2018, 33, 1965–1973. [Google Scholar] [CrossRef]
- Fitzgibbons, S.C.; Ching, Y.; Yu, D.; Carpenter, J.; Kenny, M.; Weldon, C.; Lillehei, C.; Valim, C.; Horbar, J.D.; Jaksic, T. Mortality of necrotizing enterocolitis expressed by birth weight categories. J. Pediatr. Surg. 2009, 44, 1072–1076. [Google Scholar] [CrossRef]
- Rees, C.M.; Pierro, A.; Eaton, S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F193–F198. [Google Scholar] [CrossRef]
- Adams-Chapman, I. Necrotizing Enterocolitis and Neurodevelopmental Outcome. Clin. Perinatol. 2018, 45, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.C.; Basani, L.; Simmer, K.; Samnakay, N.; Deshpande, G. Peritoneal drainage versus laparotomy as initial surgical treatment for perforated necrotizing enterocolitis or spontaneous intestinal perforation in preterm low birth weight infants. Cochrane Database Syst. Rev. 2011, 6, CD006182. [Google Scholar] [CrossRef] [PubMed]
- Sola, J.E.; Tepas, J.J., III; Koniaris, L.G. Peritoneal drainage versus laparotomy for necrotizing enterocolitis and intestinal perforation: A meta-analysis. J. Surg. Res. 2010, 161, 95–100. [Google Scholar] [CrossRef]
- Moss, R.L.; Dimmitt, R.A.; Barnhart, D.C.; Sylvester, K.G.; Brown, R.L.; Powell, D.M.; Islam, S.; Langer, J.C.; Sato, T.T.; Brandt, M.L.; et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N. Engl. J. Med. 2006, 354, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.M.; Eaton, S.; Kiely, E.M.; Wade, A.M.; McHugh, K.; Pierro, A. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann. Surg. 2008, 248, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Blakely, M.L.; Tyson, J.E.; Lally, K.P.; Hintz, S.R.; Eggleston, B.; Stevenson, D.K.; Besner, G.E.; Das, A.; Ohls, R.K.; Truog, W.E.; et al. Initial Laparotomy Versus Peritoneal Drainage in Extremely Low Birthweight Infants with Surgical Necrotizing Enterocolitis or Isolated Intestinal Perforation: A Multicenter Randomized Clinical Trial. Ann. Surg. 2021, 274, e370–e380. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Brooke, B.S.; Schwartz, T.A.; Pawlik, T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021, 156, 787–788. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.; Chandler, J.; Welch, V.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Schuenemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Janik, J.S.; Ein, S.H. Peritoneal drainage under local anesthesia for necrotizing enterocolitis (NEC) perforation: A second look. J. Pediatr. Surg. 1980, 15, 565–568. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Azmy, A.F.; Young, D.G. Surgery for necrotizing enterocolitis. Br. J. Surg. 1987, 74, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Morgan, L.J.; Shochat, S.J.; Hartman, G.E. Peritoneal drainage as primary management of perforated NEC in the very low birth weight infant. J. Pediatr. Surg. 1994, 29, 310–315. [Google Scholar] [CrossRef]
- Horwitz, J.R.; Lally, K.P.; Cheu, H.W.; Vazquez, W.D.; Grosfeld, J.L.; Ziegler, M.M. Complications after surgical intervention for ne-crotizing enterocolitis: A multicenter review. J. Pediatr. Surg. 1995, 30, 994–998. [Google Scholar] [CrossRef]
- Azarow, K.S.; Ein, S.H.; Shandling, B.; Wesson, D.; Superina, R.; Filler, R.M. Laparotomy or drain for perforated necrotizing entero-colitis: Who gets what and why? Pediatr. Surg. Int. 1997, 12, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.L.; Gittes, G.K.; Murphy, J.; Sharp, R.J.; Ashcraft, K.W.; A Amoury, R. Survival after necrotizing enterocolitis in infants weighing less than 1000 g: 25 Years’ experience at a single institution. J. Pediatr. Surg. 1997, 32, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Ein, S.; Moore, A. The role of peritoneal drains in treatment of perforated necrotizing enterocolitis: Recommendations from recent experience. J. Pediatr. Surg. 1998, 33, 1468–1470. [Google Scholar] [CrossRef]
- Alvarez, E.M.; Cuellar, R.R.; Azcarate, A.B.; Pinchetti, S.A.; Bande, J.; Korman, R.; Kurlat, I.R. Peritoneal Drainage as Initial Treatment for Perforated Necrotizing Enter-ocolitis (NEC) in Prematures Infants with Birth Weight (BW) < 1250 g. Pediatr. Res. 1999, 45, 235. [Google Scholar]
- Rovin, J.D.; Rodgers, B.M.; Burns, R.; McGahren, E.D. The role of peritoneal drainage for intestinal perforation in infants with and without necrotizing enterocolitis. J. Pediatr. Surg. 1999, 34, 143–147. [Google Scholar] [CrossRef]
- Badowicz, B.; Latawiec-Mazurkiewicz, I. Necrotising Enterocolitis (NEC)—Methods of Treatment and Outcome: A Comparative Analysis of Scottish (Glasgow) and Polish (Western Pomerania) Cases. Eur. J. Pediatr. Surg. 2000, 10, 177–181. [Google Scholar] [CrossRef]
- Dimmitt, R.A.; Meier, A.H.; Skarsgard, E.D.; Halamek, L.P.; Smith, B.M.; Moss, R. Salvage laparotomy for failure of peritoneal drainage in necrotizing enterocolitis in infants with extremely low birth weight. J. Pediatr. Surg. 2000, 35, 856–859. [Google Scholar] [CrossRef]
- Dzakovic, A.; Notrica, D.M.; Smith, E.; Wesson, D.E.; Jaksic, T. Primary peritoneal drainage for increasing ventilatory requirements in critically ill neonates with necrotizing enterocolitis. J. Pediatr. Surg. 2001, 36, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.F.; Sato, T.T.; Short, B.L.; Hartman, G.E. Outcome of perforated necrotizing enterocolitis in the very low-birth weight ne-onate may be independent of the type of surgical treatment. Am. Surg. 2001, 67, 752–756. [Google Scholar] [CrossRef]
- Noble, H.S.; Driessnack, M. Bedside peritoneal drainage in very low birth weight infants. Am. J. Surg. 2001, 181, 416–419. [Google Scholar] [CrossRef]
- Romero, R.M.; García-Casillas, M.A.; Matute, J.A.; Barrientos, G.; Zamora, E.; Megías, A.; Cerdá, J.; Sánchez, R.; Franco, M.L.; Molina, E.; et al. Papel del drenaje peritoneal en la enterocolitis del prematuro crítico [Role of peritoneal drainage in very low birth weight with enterocolitis]. Cir. Pediatr. 2005, 18, 88–92. (In Spanish) [Google Scholar] [PubMed]
- Roy, A.; Tayeb, M.; Khogeer, S.S.; Al-Salem, A.H. Predictors of gangrenous necrotizing enterocolitis and extent of disease. Early laparotomy versus peritoneal drainage. Saudi Med. J. 2005, 26, 447–452. [Google Scholar]
- Zenciroğlu, A.; Çakmak, O.; Demirel, N.; Baş, A.Y.; Yılmaz, D.; Karaman, I.; Erdoğan, D. Outcome of Primary Peritoneal Drainage for Perforated Necrotizing Enterocolitis: Comparison between Laparotomy and Drainage. Eur. J. Pediatr. Surg. 2005, 15, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Blakely, M.L.; Tyson, J.E.; Lally, K.P.; McDonald, S.; Stoll, B.J.; Stevenson, D.K.; Poole, W.K.; Jobe, A.H.; Wright, L.L.; Higgins, R.D.; et al. Laparotomy versus peritoneal drainage for ne-crotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: Outcomes through 18 months adjusted age. Pediatrics 2006, 117, e680–e687. [Google Scholar] [CrossRef]
- Chiu, B.; Pillai, S.B.; Almond, P.S.; Madonna, M.B.; Reynolds, M.; Luck, S.R.; Arensman, R.M. To drain or not to drain: A single institution experience with neonatal intestinal perforation. J. Périnat. Med. 2006, 34, 338–341. [Google Scholar] [CrossRef]
- Tepas, J.J., III; Sharma, R.; Hudak, M.L.; Garrison, R.D.; Pieper, P. Coming full circle: An evidence-based definition of the timing and type of surgical management of very low-birth-weight (<1000 g) infants with signs of acute intestinal perforation. J. Pediatr. Surg. 2006, 41, 418–422. [Google Scholar]
- Vasudevan, V.; Zhuge, Y.; Neville, H.; Sola, J. Peritoneal Drain versus Laparotomy for Very Low Birth Weight Neonates with Bowel Perforation. J. Surg. Res. 2010, 158, 277. [Google Scholar] [CrossRef]
- Argumosa Salazar, Y.; Córdoba, M.S.F.; Piñiera, J.G.; Anselmi, E.H.; Cano, M.B.; Monzón, C.M.; Ruiz, A.R.; Gutiérrez, A.M. Enterocolitis necrotizante y perforaciones intestinales en prematuros de muy bajo peso. ¿Cuál es la mejor opción quirúrgica? [Necrotizing entercolitis and intestinal perforation in very low weight premature infants: Which is the best surgical option?]. Cir. Pediatr. 2011, 24, 142–145. (In Spanish) [Google Scholar] [PubMed]
- Choo, S.; Papandria, D.; Zhang, Y.; Camp, M.; Salazar, J.H.; Scholz, S.; Rhee, D.; Chang, D.; Abdullah, F. Outcomes analysis after percutaneous abdominal drainage and exploratory laparotomy for necrotizing enterocolitis in 4657 infants. Pediatr. Surg. Int. 2011, 27, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.N.; Al-Onazi, M.; Al Mohaidly, M.; Al Rawaf, A.; Al Otaibi, A.; Al Hudhaif, J.; Ather, F. Peritoneal drainage versus laparotomy as an initial treatment in complicated necrotizing enterocolitis: A single institution experience. Ann. Pediatr. Surg. 2011, 7, 97–100. [Google Scholar] [CrossRef]
- Zornoza, M.; Peláez, D.; Romero, R.; Corona, C.; Tardáguila, A.; Rojo, R.; Carrera, N.; Cañizo, A.; Molina, E.; García-Casillas, M.A.; et al. Papel del drenaje peritoneal en la enterocolitis necrotizante de prematuros críticos de bajo peso [Role of peritoneal drainage in necrotizing enterocolitis in critical infants with extremely low birth weight]. Cir. Pediatr. 2011, 24, 146–150. (In Spanish) [Google Scholar] [PubMed]
- Eicher, C.; Seitz, G.; Bevot, A.; Moll, M.; Goelz, R.; Arand, J.; Poets, C.; Fuchs, J. Surgical Management of Extremely Low Birth Weight Infants with Neonatal Bowel Perforation: A Single-Center Experience and a Review of the Literature. Neonatology 2012, 101, 285–292. [Google Scholar] [CrossRef]
- García, H.; Franco-Gutiérrez, M.; Gutiérrez-Hernández, J.I. Cirugía en enterocolitis necrotizante en niños, supervivencia y morbilidad. [Survival and morbidity of infants with necrotizing enterocolitis treated with surgery]. Rev. Medica Del. Inst. Mex. Del. Seguro Soc. 2012, 50, 427–436. (In Spanish) [Google Scholar]
- Kelleher, J.; Mallick, H.; Soltau, T.D.; Harmon, C.M.; Dimmitt, R.A. Mortality and intestinal failure in surgical necrotizing enterocolitis. J. Pediatr. Surg. 2013, 48, 568–572. [Google Scholar] [CrossRef]
- Rakshasbhuvankar, A.; Rao, S.; Minutillo, C.; Gollow, I.; Kolar, S. Peritoneal drainage versus laparotomy for perforated necrotising enterocolitis or spontaneous intestinal perforation: A retrospective cohort study. J. Paediatr. Child Health 2012, 48, 228–234. [Google Scholar] [CrossRef]
- Eid, A.; El-Sawaf, M.; Dawoud, H.; Rowisha, M. Neonatal necrotizing entero-colitis: A clinico-surgical study. Afr. J. Emerg. Med. 2013, 3, S13–S14. [Google Scholar] [CrossRef]
- Hull, M.A.; Fisher, J.G.; Gutierrez, I.M.; Jones, B.A.; Kang, K.H.; Kenny, M.; Zurakowski, D.; Modi, B.P.; Horbar, J.D.; Jaksic, T. Mortality and Management of Surgical Necrotizing Enterocolitis in Very Low Birth Weight Neonates: A Prospective Cohort Study. J. Am. Coll. Surg. 2013, 218, 1148–1155. [Google Scholar] [CrossRef]
- Sharma, R.; Tepas, J.J., III; Mollitt, D.L.; Pieper, P.; Wludyka, P. Surgical management of bowel perforations and outcome in very low-birth-weight infants (< or =1200 g). J. Pediatr. Surg. 2004, 39, 190–194. [Google Scholar]
- Ayed, M.; Moore, A.; Shah, P.; Synnes, A.; Sankaran, K.; Kalapesi, Z.; Lee, S. 54: Outcome of Infants with Necrotising Enterocolitis (NEC): The Impact of Laparotomy Versus Peritoneal Drainage on Neurodevelopment. Paediatr. Child Health 2015, 20, e52–e53. [Google Scholar] [CrossRef]
- Stey, A.; Barnert, E.S.; Tseng, C.-H.; Keeler, E.; Needleman, J.; Leng, M.; Kelley-Quon, L.I.; Shew, S.B. Outcomes and Costs of Surgical Treatments of Necrotizing Enterocolitis. Pediatrics 2015, 135, e1190–e1197. [Google Scholar] [CrossRef]
- Mishra, P.; Foley, D.; Purdie, G.; Pringle, K.C. Intestinal perforation in premature neonates: The need for subsequent laparotomy after placement of peritoneal drains. J. Paediatr. Child Health 2016, 52, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Federici, S.; DEBiagi, L.; Straziuso, S.; Leva, E.; Brisighelli, G.; Mattioli, G.; Pio, L.; Bagolan, P.; Totonelli, G.; Noccioli, B.; et al. Multicenter retrospective study on management and outcome of newborns affected by surgical necrotizing enterocolitis. Minerva Chir. 2017, 72, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Wagenaar, A.E.; Perez, E.A.; Sola, J.E. Peritoneal drainage is associated with higher survival rates for necrotizing enter-ocolitis in premature, extremely low birth weight infants. J. Surg. Res. 2017, 218, 132–138. [Google Scholar] [CrossRef]
- Khirallah, M.; Eid, A. Peritoneal drainage versus laparotomy in necrotizing enterocolitis: A continued asked question. Ann. Pediatr. Surg. 2017, 13, 81–84. [Google Scholar] [CrossRef]
- Broekaert, I.; Keller, T.; Schulten, D.; Hünseler, C.; Kribs, A.; Dübbers, M. Peritoneal drainage in pneumoperitoneum in extremely low birth weight infants. Eur. J. Pediatr. 2018, 177, 853–858. [Google Scholar] [CrossRef]
- Duci, M.; Fascetti-Leon, F.; Erculiani, M.; Priante, E.; Cavicchiolo, M.E.; Verlato, G.; Gamba, P. Neonatal independent predictors of severe NEC. Pediatr. Surg. Int. 2018, 34, 663–669. [Google Scholar] [CrossRef]
- Geng, Q.; Wang, Y.; Li, L.; Guo, C. Early postoperative outcomes of surgery for intestinal perforation in NEC based on intestinal location of disease. Medicine 2018, 97, e12234. [Google Scholar] [CrossRef]
- Pascual, F.J.M.; Pérez, J.I.G.; Uribe, A.S.; Cruz, V.V.; Cotán, L.D.; Rueda, F.V.; Esteban, R.M.P. Drenaje peritoneal como tratamiento definitivo en la enterocolitis ne-crotizante de prematuros de bajo peso. Cir. Pediatr. 2018, 31, 130–133. (In Spanish) [Google Scholar]
- Yanowitz, T.D.; Sullivan, K.M.; Piazza, A.J.; Brozanski, B.; Zaniletti, I.; Sharma, J.; DiGeronimo, R.; Nayak, S.P.; Wadhawan, R.; Reber, K.M.; et al. Does the initial surgery for necrotizing enterocolitis matter? Comparative outcomes for laparotomy vs. peritoneal drain as initial surgery for necrotizing enterocolitis in infants < 1000 g birth weight. J. Pediatr. Surg. 2019, 54, 712–717. [Google Scholar]
- Ahle, S.; Badru, F.; Damle, R.; Osei, H.; Munoz-Abraham, A.S.; Bajinting, A.; Barbian, M.E.; Bhatia, A.M.; Gingalewski, C.; Greenspon, J.; et al. Multicenter retrospective comparison of spontaneous intestinal perforation outcomes between primary peritoneal drain and primary laparotomy. J. Pediatr. Surg. 2020, 55, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Namgoong, J.-M.; Kim, S.C.; Kim, D.Y. Usefulness of peritoneal drainage in extremely low birth weight infants with intestinal perforation: A single-center experience. Ann. Surg. Treat. Res. 2020, 98, 153–157. [Google Scholar] [CrossRef]
- Quiroz, H.J.; Rao, K.; Brady, A.-C.; Hogan, A.R.; Thorson, C.M.; Perez, E.A.; Neville, H.L.; Sola, J.E. Protocol-Driven Surgical Care of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation. J. Surg. Res. 2020, 255, 396–404. [Google Scholar] [CrossRef]
- Canesin, W.; Volpe, F.; Gonçalves-Ferri, W.; Manso, P.; Aragon, D.; Sbragia, L. Primary peritoneal drainage in neonates with necrotizing enterocolitis associated with congenital heart disease: A single experience in a Brazilian tertiary center. Braz. J. Med. Biol. Res. 2021, 54, e10220. [Google Scholar] [CrossRef] [PubMed]
- Fatemizadeh, R.; Mandal, S.; Gollins, L.; Shah, S.; Premkumar, M.; Hair, A. Incidence of spontaneous intestinal perforations exceeds necrotizing enterocolitis in extremely low birth weight infants fed an exclusive human milk-based diet: A single center expe-rience. J. Pediatr. Surg. 2021, 56, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Tomislav, Ć.; Iva, V.; Petra, Ž.; Vesna, B.; Buljević, D.; Dorotea, N.; Boris, F.G.; Ivana, S.; Miram, P.; Stanko, Ć.; et al. Short-term outcomes for preterm infants with surgical necrotizing enterocolitis. Arch. Dis. Child. 2021, 106, A58. [Google Scholar]
- Rintala, R.J.; Pakarinen, M.P.; Koivusalo, A.I. Neonatal surgery: Towards evidence-based practice and management. Semin. Pediatr. Surg. 2014, 23, 303–308. [Google Scholar] [CrossRef]
- Metelli, S.; Chaimani, A. Challenges in meta-analyses with observational studies. Evid.-Based Ment. Health 2020, 23, 83–87. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Zhu, Z. Initial surgical treatment of necrotizing enterocolitis: A meta-analysis of peritoneal drainage versus lapa-rotomy. Eur. J. Pediatr. 2022, 181, 2593–2601. [Google Scholar] [CrossRef] [PubMed]
- Solis-Garcia, G.; Jasani, B. Laparotomy vs. peritoneal drainage: The need for better evidence: Letter to editor on the manuscript “Initial surgical treatment of necrotizing enterocolitis: A meta-analysis of peritoneal drainage versus laparotomy”. Eur. J. Pediatr. 2022, 181, 3559–3560. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).