Respiratory Syncytial Virus: A Systematic Review and Meta-Analysis of Tomographic Findings (2000–2022)
Abstract
1. Introduction
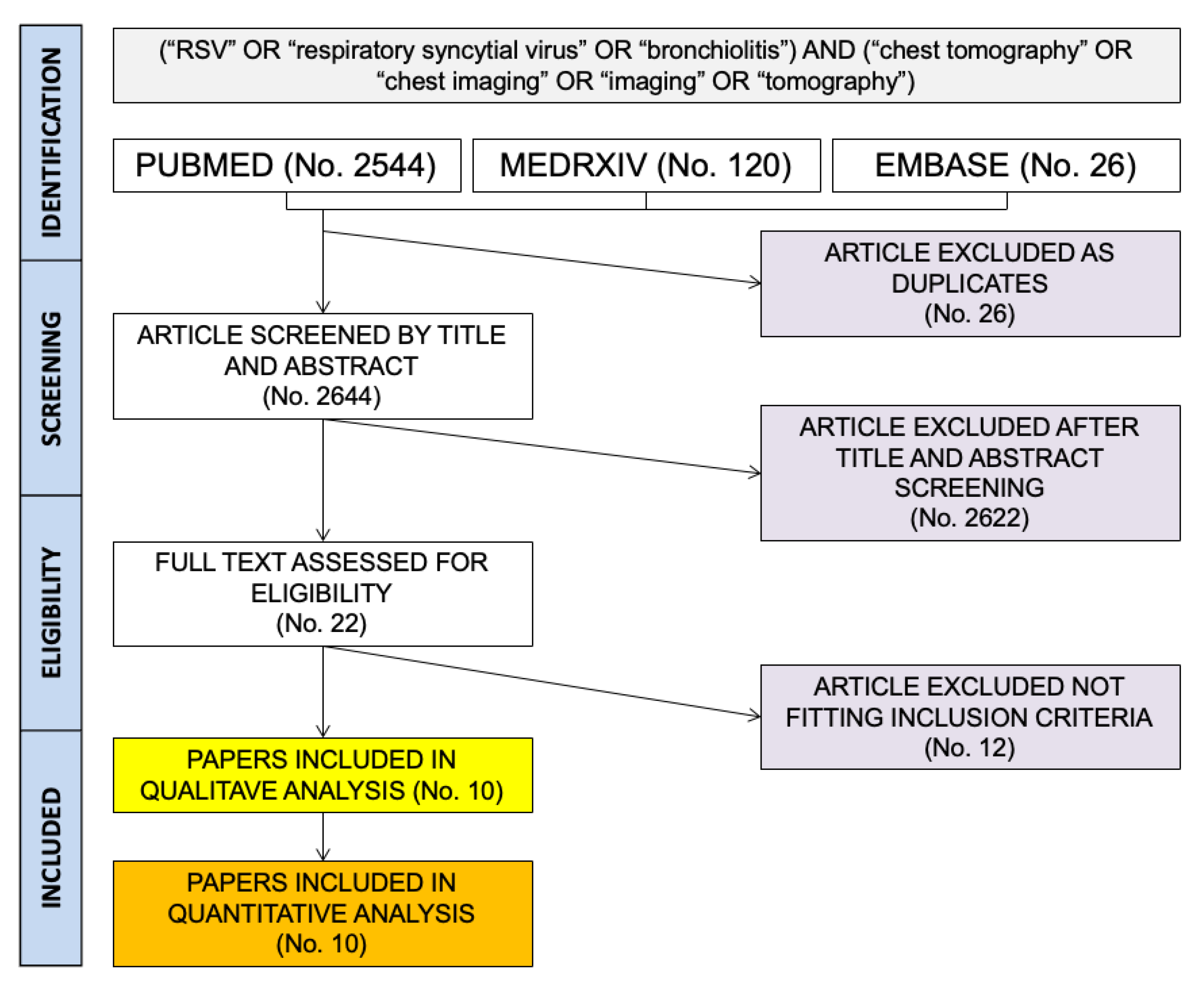
2. Materials and Methods
- Reporting crude number of assessed cases of respiratory infections;
- Reporting the number of RSV cases diagnosed;
- Reporting the number of CT scans actually performed;
- Any laboratory diagnosis of RSV infections (i.e., polymerase chain reaction [PCR], immunofluorescence, antigen testing, etc.).
- Settings of the study;
- Characteristics of the index cases, whether they were otherwise healthy children and adolescents (age < 18 years), adults (age ≥ 18 years), or individuals affected by any immune deficiency;
- Number of included respiratory infection cases;
- Number of total cases assessed;
- Number of RSV infection episodes;
- Number of other lower respiratory tract viral infections, particularly influenza, SARS-CoV-2, and all other viral infections.
- The following main findings [27,36,45,46]: laterality of the involvement (unilateral vs. bilateral); extensive vs. focal involvement; imaging pattern (i.e., ground glass opacities [GGOs]; nodular lesions; signs of Tree-in-Bud; signs of organizing pneumonia; signs of septal thickening; signs of crazy paving); signs of pleural effusions.
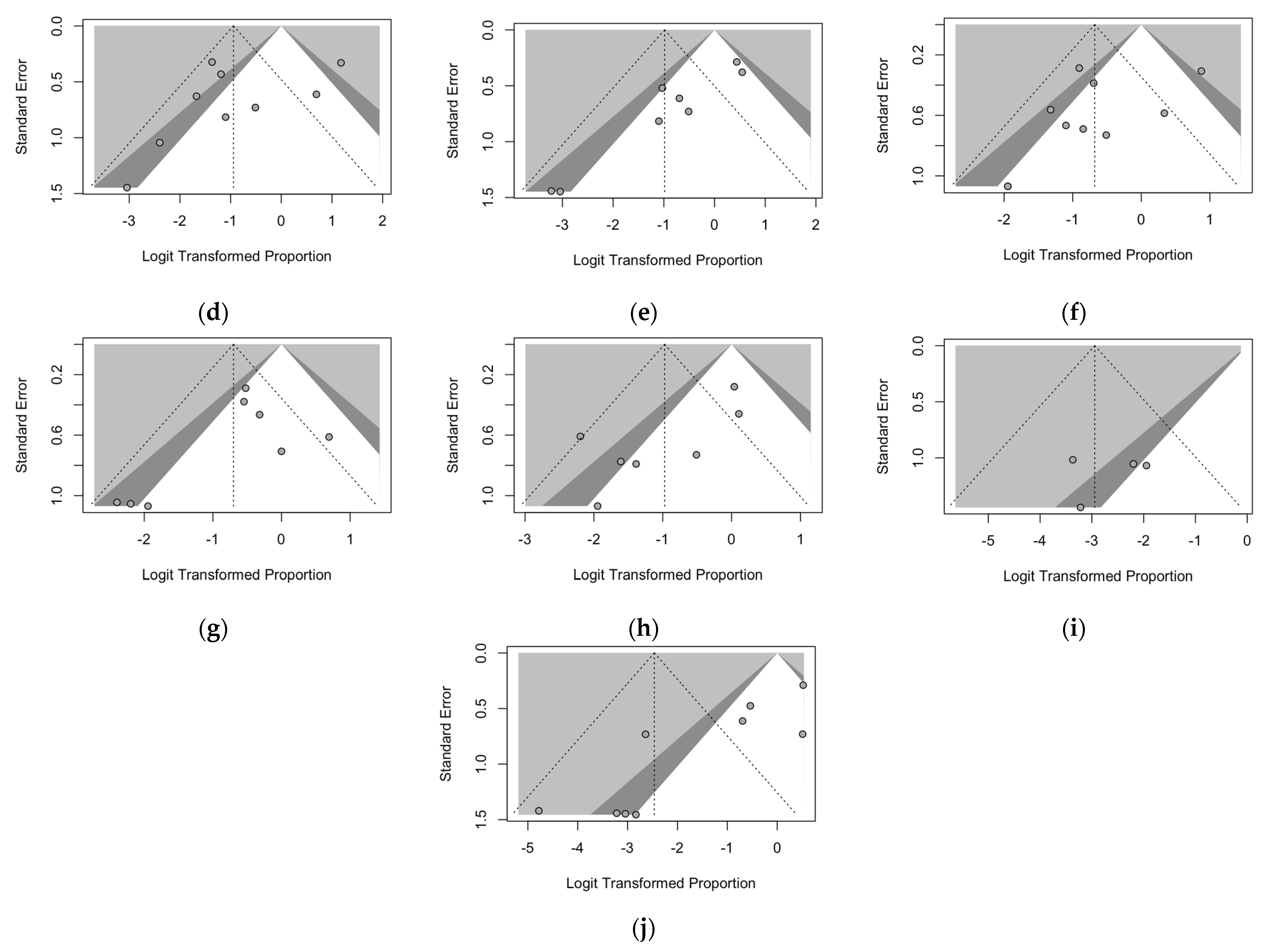
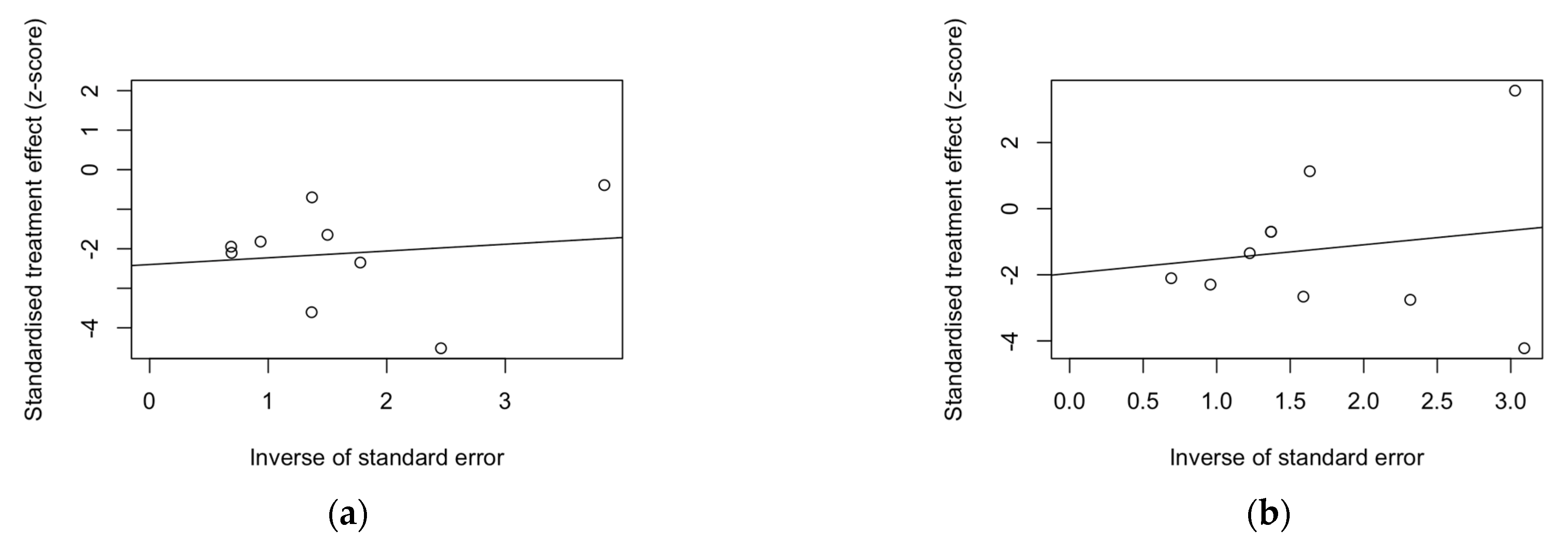
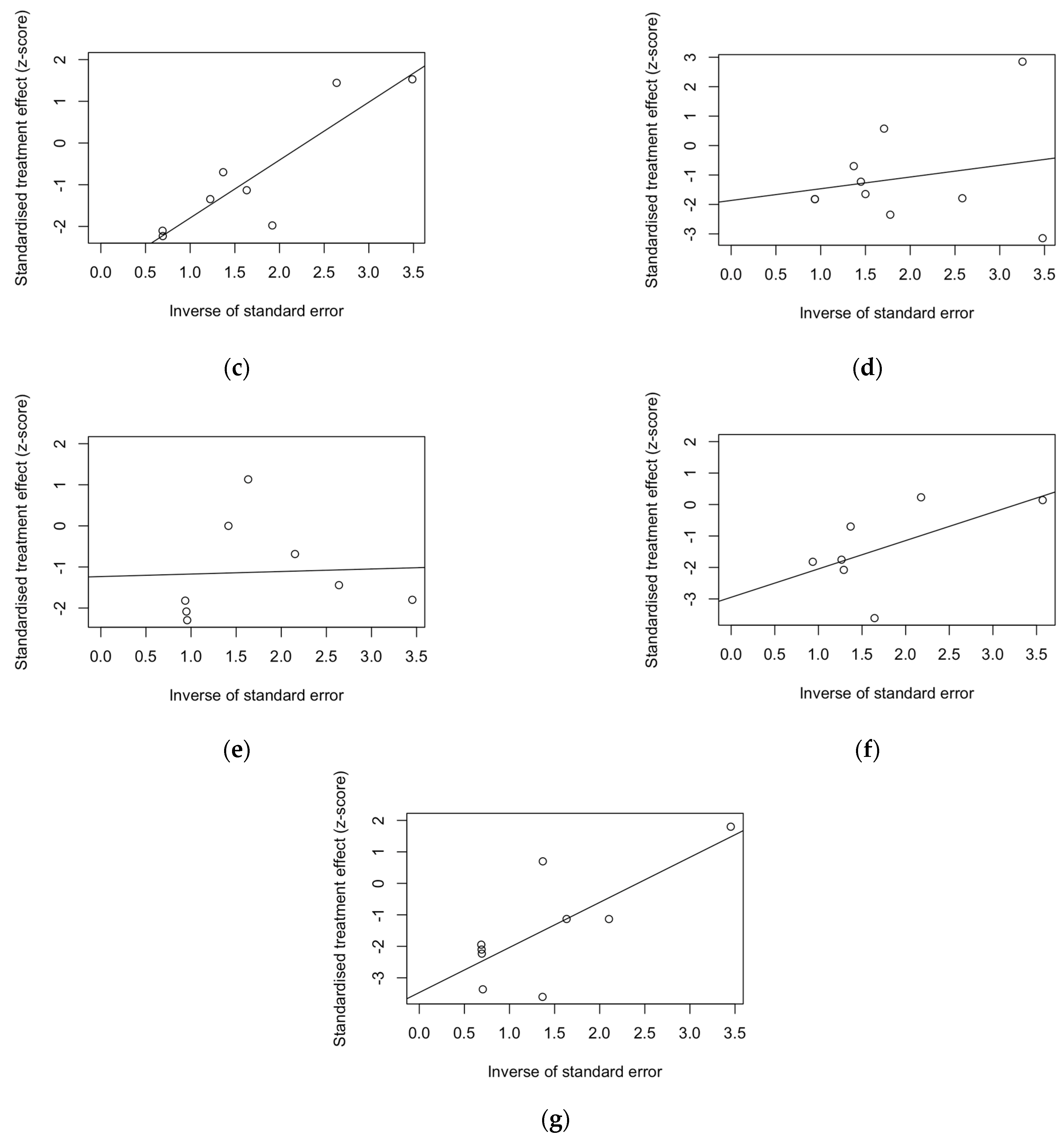
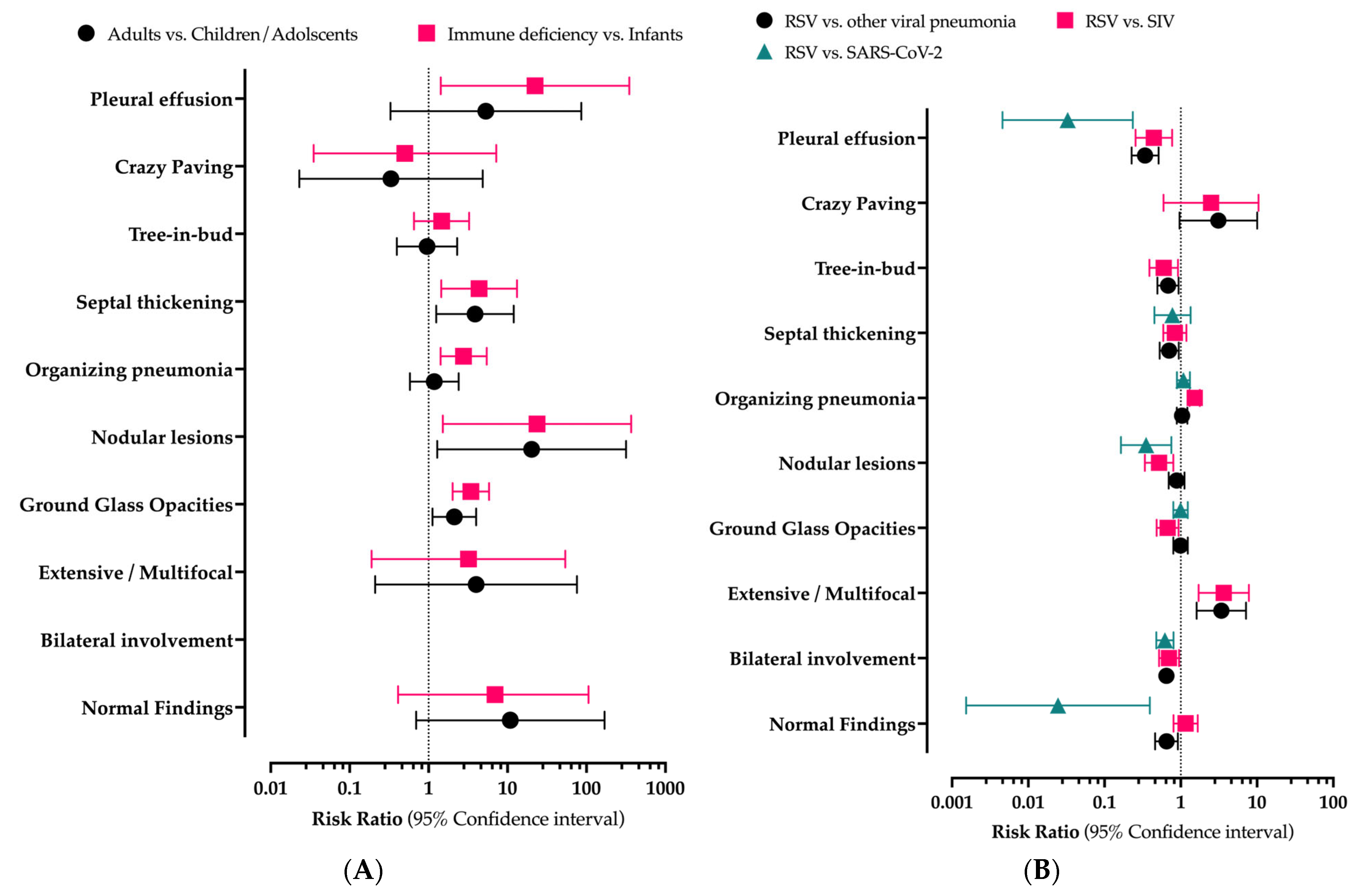
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Ariza-Heredia et al. [42] | ☹ | ? | ☺ | ☺ | ☹ | ☺ | ☺ |
| Escuissato et al. [41] | ☹ | ? | ☹ | ☺ | ☹ | ☺ | ☹ |
| Herbst et al. [52] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | ☺ |
| Jia et al. [53] | ☺ | ? | ☹ | ☹ | ☺ | ☺ | ☹ |
| Kim et al. [55] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | ☺ |
| Mayer et al. [56] | ☹ | ? | ☺ | ☺ | ☹ | ☺ | ☺ |
| Nabeya et al. [57] | ☹ | ? | ☺ | ☺ | ☺ | ☺ | ☺ |
| Ren et al. [54] | ☺ | ? | ☺ | ☹ | ☺ | ☺ | ☺ |
| Shen et al. [58] | ☺ | ? | ☺ | ☹ | ☺ | ☺ | ☺ |
| Shiley et al. [50] | ☺ | ? | ☺ | ☺ | ☺ | ☺ | ☺ |
| Item | COVID-19 (3 Studies) | Seasonal Influenza (7 Studies) | All Non-RSV Viral Infections (7 Studies) |
|---|---|---|---|
| Risk Ratio (95% Confidence Interval) | |||
| Normal findings | 0.025 (0.002; 0.394) | 1.157 (0.803; 1.666) | 0.650 (0.462; 0.916) |
| Bilateral involvement | 0.620 (0.479; 0.802) | 0.701 (0.518; 0.949) | 0.648 (0.535; 0.800) |
| Extensive/multifocal | n.a. | 3.667 (1.714; 7.842) | 3.410 (1.616; 7.195) |
| Ground glass opacities | 0.995 (0.801; 1.237) | 0.673 (0.483; 0.938) | 0.995 (0.801; 1.237) |
| Nodular lesions | 0.351 (0.164; 0.752) | 0.520 (0.339; 0.799) | 0.882 (0.695; 1.119) |
| Tree-in-bud | n.a. | 0.597 (0.388; 0.921) | 0.681 (0.495; 0.938) |
| Organizing pneumonia | 1.084 (0.891; 1.319) | 1.522 (1.306; 1.775) | 1.041 (0.887; 1.222) |
| Septal thickening | 0.778 (0.450; 1.346) | 0.835 (0.589; 1.184) | 0.705 (0.529; 0.941) |
| Crazy paving | n.a. | 2.500 (0.596; 10.490) | 3.111 (0.965; 10.033) |
| Pleural effusion | 0.033 (0.005; 0.235) | 0.443 (0.255; 0.769) | 0.341 (0.227; 0.511) |
| Item | COVID-19 | Seasonal Influenza | All Non-RSV Viral Infections | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 | Q | p | t2 | I2 | Q | p | t2 | I2 | Q | p | t2 | |
| Normal findings | - | - | - | - | 42.8 | 5.25 | 0.155 | 0.066 | 58.4 | 9.63 | 0.047 | 0.460 |
| Bilateral involvement | - | - | - | - | 0.0 | 0.12 | 0.729 | 0.001 | 0.0 | 0.21 | 0.647 | 0.001 |
| Ground glass opacities | 22.9 | 1.30 | 0.254 | 0.389 | 0.0 | 1.45 | 0.835 | 0.001 | 0.0 | 3.46 | 0.749 | 0.001 |
| Nodular lesions | - | - | - | - | 0.0 | 1.37 | 0.505 | 0.001 | 0.0 | 1.39 | 0.707 | 0.001 |
| Tree-in-bud | - | - | - | - | 16.9 | 3.61 | 0.307 | <0.001 | 50.8 | 8.13 | 0.087 | 0.305 |
| Organizing pneumonia | 62.7 | 2.68 | 0.102 | 0.404 | 0.0 | 2.83 | 0.726 | 0.001 | 0.0 | 1.51 | 0.959 | 0.001 |
| Septal thickening | - | - | - | - | 12.5 | 4.57 | 0.334 | <0.001 | 8.1 | 5.44 | 0.365 | <0.001 |
| Crazy paving | - | - | - | - | 66.7 | 3.00 | 0.083 | 2.145 | 0.0 | 0.73 | 0.696 | 0.001 |
| Pleural effusion | - | - | - | - | 48.7 | 1.95 | 0.163 | 0.228 | 0.0 | 2.57 | 0.463 | 0.085 |
| Finding | t | df | Bias (SE) | Intercept (SE) | p-Value |
|---|---|---|---|---|---|
| Normal findings | −2.64 | 7 | −2.399 (0.910) | 0.172 | 0.034 |
| Bilateral involvement | - | - | - | - | - |
| Extensive/multifocal | - | - | - | - | - |
| Ground glass opacities | −1.10 | 8 | −1.957 (1.784) | 0.434 (0.941) | 0.305 |
| Nodular lesions | −5.52 | 6 | −3.184 (0.577) | 1.388 (0.299) | 0.002 |
| Organizing pneumonia | −1.37 | 8 | −1.865 (1.358) | 0.399 (0.652) | 0.207 |
| Septal thickening | −1.19 | 6 | −1.234 (1.036) | 0.062 (0.527) | 0.279 |
| Tree in bud | −2.75 | 5 | −2.948 (1.071) | 0.902 (0.553) | 0.040 |
| Crazy paving | - | - | - | - | - |
| Pleural effusion | −4.32 | 7 | −3.467 (0.803) | 1.432 (0.485) | 0.004 |

References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021, 222, S577–S583. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Nair, H.; Theodoratou, E.; Rudan, I.; Nokes, D.J.; Ngama HND, M.; Munywoki, P.K.; Dherani, M.; Nair, H.; James Nokes, D.; Gessner, B.D.; et al. Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta-Analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Leader, S.; Kohlhase, K. Respiratory Syncytial Virus-Coded Pediatric Hospitalizations, 1997 to 1999. Pediatr. Infect. Dis. J. 2002, 21, 629–661. [Google Scholar] [CrossRef]
- Na’amnih, W.; Kassem, E.; Tannous, S.; Kagan, V.; Jbali, A.; Hanukayev, E.; Freimann, S.; Obolski, U.; Muhsen, K. Incidence and Risk Factors of Hospitalisations for Respiratory Syncytial Virus among Children Aged Less than Two Years. Epidemiol. Infect. 2022, 150, 1–30. [Google Scholar] [CrossRef]
- Jans, J.; Wicht, O.; Widjaja, I.; Ahout, I.M.L.; de Groot, R.; Guichelaar, T.; Luytjes, W.; de Jonge, M.I.; de Haan, C.A.M.; Ferwerda, G. Characteristics of RSV-Specific Maternal Antibodies in Plasma of Hospitalized, Acute RSV Patients under Three Months of Age. PLoS ONE 2017, 12, e0170877. [Google Scholar] [CrossRef] [PubMed]
- Chida-Nagai, A.; Sato, H.; Sato, I.; Shiraishi, M.; Sasaki, D.; Izumi, G.; Yamazawa, H.; Cho, K.; Manabe, A.; Takeda, A. Risk Factors for Hospitalisation Due to Respiratory Syncytial Virus Infection in Children Receiving Prophylactic Palivizumab. Eur. J. Pediatr. 2022, 181, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Abu Raya, B.; Baraldi, E.; Flanagan, K.; Martinon Torres, F.; Tsolia, M.; Zielen, S. RSV Prevention in All Infants: Which Is the Most Preferable Strategy? Front. Immunol. 2022, 13, 880368. [Google Scholar] [CrossRef]
- Baraldi, E.; Checcucci Lisi, G.; Costantino, C.; Heinrichs, J.H.; Manzoni, P.; Riccò, M.; Roberts, M.; Vassilouthis, N. RSV Disease in Infants and Young Children: Can We See a Brighter Future? Hum. Vaccin. Immunother. 2022, 16, 1232–1238. [Google Scholar] [CrossRef]
- Nowalk, M.P.; D’Agostino, H.; Dauer, K.; Stiegler, M.; Zimmerman, R.K.; Balasubramani, G.K. Estimating the Burden of Adult Hospitalized RSV Infection Including Special Populations. Vaccine 2022, 40, 4121–4127. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Ali, A.; Lopardo, G.; Scarpellini, B.; Stein, R.T.; Ribeiro, D. Systematic Review on Respiratory Syncytial Virus Epidemiology in Adults and the Elderly in Latin America. Int. J. Infect. Dis. 2020, 90, 170–180. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, L.; Zhang, Y.; Zhang, X.; Zheng, M.; Kyaw, M.H. Burden of Respiratory Syncytial Virus Infections in China: Systematic Review and Meta-Analysis. J. Glob. Health 2015, 5, 020417. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.; Hall, C.B.; Katkin, J.P.; Shi, N.; Masaquel, A.S.; McLaurin, K.K.; Mahadevia, P.J. Healthcare Costs within a Year of Respiratory Syncytial Virus among Medicaid Infants. Pediatr. Pulmonol. 2010, 45, 772–781. [Google Scholar] [CrossRef]
- McLaurin, K.K.; Farr, A.M.; Wade, S.W.; Diakun, D.R.; Stewart, D.L. Respiratory Syncytial Virus Hospitalization Outcomes and Costs of Full-Term and Preterm Infants. J. Perinatol. 2016, 36, 990–996. [Google Scholar] [CrossRef]
- Rietveld, E.; de Jonge, H.C.C.; Polder, J.J.; Vergouwe, Y.; Veeze, H.J.; Moll, H.A.; Steyerberg, E.W. Anticipated Costs of Hospitalization for Respiratory Syncytial Virus Infection in Young Children at Risk. Pediatr. Infect. Dis. J. 2004, 23, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, J.; Brannman, L.; Wade, S.W.; Gonzales, T.; Kong, A.M. Healthcare Resource Utilization and Costs in the 12 Months Following Hospitalization for Respiratory Syncytial Virus or Unspecified Bronchiolitis among Infants. J. Med. Econ. 2020, 23, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Li, X.; Dacosta-Urbieta, A.; Billard, M.N.; Wildenbeest, J.; Korsten, K.; Martinón-Torres, F.; Heikkinen, T.; Cunningham, S.; Snape, M.D.; et al. Economic Burden and Health-Related Quality-of-Life among Infants with Respiratory Syncytial Virus Infection: A Multi-Country Prospective Cohort Study in Europe. Vaccine 2023, 41, 2707–2715. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Vidal Valero, M. “A Good Day”: FDA Approves World’s First RSV Vaccine. Nature 2023, 617, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Giersing, B.K.; Karron, R.A.; Vekemans, J.; Kaslow, D.C.; Moorthy, V.S. Meeting Report: WHO Consultation on Respiratory Syncytial Virus (RSV) Vaccine Development, Geneva, 25–26 April 2016. In Proceedings of the Vaccine; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 37, pp. 7355–7362. [Google Scholar]
- Mazur, N.I.; Martinón-Torres, F.; Baraldi, E.; Fauroux, B.; Greenough, A.; Heikkinen, T.; Manzoni, P.; Mejias, A.; Nair, H.; Papadopoulos, N.G.; et al. Lower Respiratory Tract Infection Caused by Respiratory Syncytial Virus: Current Management and New Therapeutics. Lancet Respir. Med. 2015, 3, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Mosalli, R.; Alqarni, S.A.; Khayyat, W.W.; Alsaidi, S.T.; Almatrafi, A.S.; Bawakid, A.S.; Paes, B. Respiratory Syncytial Virus Nosocomial Outbreak in Neonatal Intensive Care: A Review of the Incidence, Management, and Outcomes. Am. J. Infect. Control. 2021; ahead of print. [Google Scholar] [CrossRef]
- Azzari, C.; Baraldi, E.; Bonanni, P.; Bozzola, E.; Coscia, A.; Lanari, M.; Manzoni, P.; Mazzone, T.; Sandri, F.; Checcucci Lisi, G.; et al. Epidemiology and Prevention of Respiratory Syncytial Virus Infections in Children in Italy. Ital. J. Pediatr. 2021, 47, 198. [Google Scholar] [CrossRef]
- Altmayer, S.; Zanon, M.; Pacini, G.S.; Watte, G.; Barros, M.C.; Mohammed, T.L.; Verma, N.; Marchiori, E.; Hochhegger, B. Comparison of the Computed Tomography Findings in COVID-19 and Other Viral Pneumonia in Immunocompetent Adults: A Systematic Review and Meta-Analysis. Eur. Radiol. 2020, 30, 6485–6496. [Google Scholar] [CrossRef] [PubMed]
- Barr, R.; Green, C.A.; Sande, C.J.; Drysdale, S.B. Respiratory Syncytial Virus: Diagnosis, Prevention and Management. Ther. Adv. Infect. Dis. 2019, 6, 204993611986579. [Google Scholar] [CrossRef]
- Martínez-Sanz, J.; Gilaberte Reyzábal, S.; Salillas, J.; Lago Gómez, M.R.; Rodríguez-Zurita, M.E.; Torralba, M. Respiratory Syncytial Virus Infection among Adults during Influenza Season: A Frequently Overlooked Diagnosis. J. Med. Virol. 2019, 91, 1679–1683. [Google Scholar] [CrossRef]
- Celik, K.; Olukman, O.; Demirol, H.; Terek, D.; Gulfidan, G.; Devrim, I.; Gulcu, P.; Arslanoglu, S.; Calkavur, S. Prevalence of Respiratory Pathogens during Two Consecutive Respiratory Syncytial Virus Seasons at a Tertiary Medical Care Center. Arch. Argent. Pediatr. 2019, 117, E356–E362. [Google Scholar] [CrossRef] [PubMed]
- Sawano, M.; Takeshita, K.; Ohno, H.; Oka, H. RT-PCR Diagnosis of COVID-19 from Exhaled Breath Condensate: A Clinical Study. J. Breath. Res. 2021, 15, 037103. [Google Scholar] [CrossRef]
- Zakharkina, T.; Koczulla, A.R.; Mardanova, O.; Hattesohl, A.; Bals, R. Detection of Microorganisms in Exhaled Breath Condensate during Acute Exacerbations of COPD. Respirology 2011, 16, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, P.; Vaschetto, R.; Carpagnano, G.E. Is It Feasible to Collect Exhaled Breath Condensate in COVID-19 Patients Undergoing Noninvasive Ventilatory Support? ERJ Open Res. 2021, 7, 00071–02021. [Google Scholar] [CrossRef]
- Riccò, M.; Zaniboni, A.; Satta, E.; Ranzieri, S.; Marchesi, F. Potential Use of Exhaled Breath Condensate for Diagnosis of SARS-CoV-2 Infections: A Systematic Review AndMeta-Analysis. Diagnostics 2022, 12, 2245. [Google Scholar] [CrossRef] [PubMed]
- Blairon, L.; Thomas, I.; Lê, P.Q.; Beukinga, I.; Tré-Hardy, M. Diagnosis of Respiratory Syncytial Virus and Influenza A and B with Cobas® Liat® from Nasopharyngeal Aspirations in Pediatrics. Diagn. Microbiol. Infect. Dis. 2021, 100, 115326. [Google Scholar] [CrossRef] [PubMed]
- Garrana, S.H.; Som, A.; Ndakwah, G.S.; Yeung, T.; Febbo, J.; Heeger, A.P.; Lang, M.; McDermott, S.; Mendoza, D.P.; Zhang, E.W.; et al. Comparison of Chest CT Findings of COVID-19, Influenza, and Organizing Pneumonia: A Multireader Study. Am. J. Roentgenol. 2021, 217, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Gori, L.; Amendolea, A.; Buonsenso, D.; Salvadori, S.; Supino, M.C.; Musolino, A.M.; Adamoli, P.; Coco, A.D.; Trobia, G.L.; Biagi, C.; et al. Prognostic Role of Lung Ultrasound in Children with Bronchiolitis: Multicentric Prospective Study. J. Clin. Med. 2022, 11, 4233. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.; Khera, D.; Toteja, N.; Sureka, B.; Choudhary, B.; Ganakumar, V.M.; Singh, K. Point-of-Care Thoracic Ultrasound in Children with Bronchiolitis. Indian. J. Pediatr. 2022, 89, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Nazif, J.M.; Taragin, B.H.; Azzarone, G.; Rinke, M.L.; Liewehr, S.; Choi, J.; Esteban-Cruciani, N. Clinical Factors Associated with Chest Imaging Findings in Hospitalized Infants with Bronchiolitis. Clin. Pediatr. 2017, 56, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Kloth, C.; Thaiss, W.M.; Beck, R.; Haap, M.; Fritz, J.; Beer, M.; Horger, M. Potential Role of CT-Textural Features for Differentiation between Viral Interstitial Pneumonias, Pneumocystis Jirovecii Pneumonia and Diffuse Alveolar Hemorrhage in Early Stages of Disease: A Proof of Principle. BMC Med. Imaging 2019, 19, 39. [Google Scholar] [CrossRef]
- Escuissato, D.L.; Gasparetto, E.L.; Marchiori, E.; de Melo Rocha, G.; Inoue, C.; Pasquini, R.; Müller, N.L. Pulmonary Infections After Bone Marrow Transplantation: High-Resolution CT Findings in 111 Patients. Am. J. Radiol. 2005, 185, 608–615. [Google Scholar] [CrossRef]
- Ariza-Heredia, E.J.; Fishman, J.E.; Cleary, T.; Smith, L.; Razonable, R.R.; Abbo, L. Clinical and Radiological Features of Respiratory Syncytial Virus in Solid Organ Transplant Recipients: A Single-Center Experience. Transpl. Infect. Dis. 2012, 14, 64–71. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Luijendijk, H.J. How to Create PICO Questions about Diagnostic Tests. BMJ Evid. Based Med. 2021, 26, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, L.; Ben, S. Meta-Analysis of Chest CT Features of Patients with COVID-19 Pneumonia. J. Med. Virol. 2021, 93, 241–249. [Google Scholar] [CrossRef]
- Wang, J.G.; Mo, Y.F.; Su, Y.H.; Wang, L.C.; Liu, G.B.; Li, M.; Qin, Q.Q. Computed Tomography Features of COVID-19 in Children A Systematic Review and Meta-Analysis. Medicine 2021, 100, e22571. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- R Development Core Team. R a Language and Environment for Statistical Computing: Reference Index; R Foundation for Statistical Computing: Vienna, Austria, 2010; ISBN 3900051070. [Google Scholar]
- Marinari, L.A.; Danny, M.A.; Simpson, S.A.; Schmitt, J.E.; Miller, W.T. Lower Respiratory Tract Infection with Human Metapneumovirus: Chest CT Imaging Features and Comparison with Other Viruses. Eur. J. Radiol. 2020, 128, 108988. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.T.; Mickus, T.J.; Barbosa, E.; Mullin, C.; van Deerlin, V.M.; Shiley, K.T. CT of Viral Lower Respiratory Tract Infections in Adults: Comparison among Viral Organisms and between Viral and Bacterial Infections. Am. J. Roentgenol. 2011, 197, 1088–1095. [Google Scholar] [CrossRef]
- Gasparetto, E.L.; Escuissato, D.L.; Marchiori, E.; Ono, S.; Frare e Silva, R.L.; Müller, N.L. High-Resolution CT Findings of Respiratory Syncytial Virus Pneumonia After Bone Marrow Transplantation. Am. J. Roetgen 2004, 182, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Herbst, T.; van Deerlin, V.M.; Miller, W.T. The CT Appearance of Lower Respiratory Infection Due to Parainfluenza Virus in Adults. Am. J. Roentgenol. 2013, 201, 550–554. [Google Scholar] [CrossRef]
- Jia, Z.; Yan, X.; Gao, L.; Ding, S.; Bai, Y.; Zheng, Y.; Cui, Y.; Wang, X.; Li, J.; Lu, G.; et al. Comparison of Clinical Characteristics Among COVID-19 and Non-COVID-19 Pediatric Pneumonias: A Multicenter Cross-Sectional Study. Front. Cell Infect. Microbiol. 2021, 11, 663884. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.L.; Wang, X.F.; Xu, J.; Li, J.; Meng, Q.; Xie, G.Q.; Huang, B.; Zhu, W.C.; Lin, J.; Tang, C.H.; et al. Comparison of Acute Pneumonia Caused by SARS-COV-2 and Other Respiratory Viruses in Children: A Retrospective Multi-Center Cohort Study during COVID-19 Outbreak. Mil. Med. Res. 2021, 8, 13. [Google Scholar] [CrossRef]
- Kim, M.C.; Kim, M.Y.; Lee, H.J.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. CT Findings in Viral Lower Respiratory Tract Infections Caused by Parainfluenza Virus, Influenza Virus and Respiratory Syncytial Virus. Medicine 2016, 95, e4003. [Google Scholar] [CrossRef]
- Mayer, J.L.; Lehners, N.; Egerer, G.; Kauczor, H.U.; Heuel, C.P. CT-Morphological Characterization of Respiratory Syncytial Virus (Rsv) Pneumonia in Immune-Compromised Adults. RoeFo-Fortschritte auf dem Gebiete der Roentgenstrahlen und der Bildgebenden Verfahren 2014, 186, 686–692. [Google Scholar] [CrossRef]
- Nabeya, D.; Kinjo, T.; Parrott, G.L.; Nakachi, S.; Yamashiro, T.; Ikemiyagi, N.; Arakaki, W.; Masuzaki, H.; Fujita, J. Chest Computed Tomography Abnormalities and Their Relationship to the Clinical Manifestation of Respiratory Syncytial Virus Infection in a Genetically Confirmed Outbreak. Intern. Med. 2020, 59, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sun, J.; Zhao, D.; Li, S.; Xiao, W.; Cai, X.; Yan, J.; Zhu, W.; Guo, Q.; Wen, X.; et al. Characteristics of Nosocomial Infections in Children Screened for SARS-CoV-2 Infection in China. Med. Sci. Monit. 2020, 26, e928835-1. [Google Scholar] [CrossRef]
- Shiley, K.T.; van Deerlin, V.M.; Miller, W.T. Chest CT Features of Community-Acquired Respiratory Viral Infections in Adult Inpatients with Lower Respiratory Tract Infections. J. Thorac. Imaging 2010, 25, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Knapper, F.; Ellis, J.; Bernatoniene, J.; Williams, P. The Burden of Respiratory Syncytial Virus Disease in Children and Adults Hospitalized in a Large Tertiary Hospital in the United Kingdom: A Retrospective Study. Pediatr. Infect. Dis. J. 2022, 41, E541–E543. [Google Scholar] [CrossRef]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.Y. Respiratory Syncytial Virus Disease Burden in Adults Aged 60 Years and Older in High-Income Countries: A Systematic Literature Review and Meta-Analysis. Influenza Other Respir. Viruses 2022, 17, e13031. [Google Scholar] [CrossRef]
- Hazir, T.; Nisar, Y.B.; Qazi, S.A.; Khan, S.F.; Raza, M.; Zameer, S.; Masood, S.A. Chest Radiography in Children Aged 2–59 Months Diagnosed with Non-Severe Pneumonia as Defined by World Health Organization: Descriptive Multicentre Study in Pakistan. Br. Med. J. 2006, 333, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Matar, L.D.; Mcadams, B.H.P.; Palmer, S.M.; Howell, D.N.; Henshaw, N.G.; Davis, R.D.; Tapson, V.F. Respiratory Viral Infections in Lung Transplant Recipients: Radiologic Findings with Clinical Correlation 1 Reprint Requests To. Radiology 1999, 213, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. The Pathology of Influenza Virus Infections. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Bösmüller, H.; Matter, M.; Fend, F.; Tzankov, A. The Pulmonary Pathology of COVID-19. Virchows Arch. 2021, 478, 137–150. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Rodrigues, J.C.L.; Hare, S.S.; Edey, A.; Devaraj, A.; Jacob, J.; Johnstone, A.; McStay, R.; Nair, A.; Robinson, G. An Update on COVID-19 for the Radiologist—A British Society of Thoracic Imaging Statement. Clin. Radiol. 2020, 75, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.T.; Panosian, J.S. Causes and Imaging Patterns of Tree-in-Bud Opacities. Chest 2013, 144, 1883–1892. [Google Scholar] [CrossRef]
- Shuai, W.; Chen, X.; Shan, Y.; Li, W.; Ma, W.; Lu, Q.; Li, D. Clinical Characteristics and CT Findings in 148 Non-COVID-19 Influenza-Like Illness Cases: A Retrospective Control Study. Front. Public. Health 2021, 9, 616963. [Google Scholar] [CrossRef]
- Staadegaard, L.; Caini, S.; Wangchuk, S.; Thapa, B.; de Almeida, W.A.F.; de Carvalho, F.C.; Njouom, R.; Fasce, R.A.; Bustos, P.; Kyncl, J.; et al. The Global Epidemiology of RSV in Community and Hospitalized Care: Findings from 15 Countries. Open Forum Infect. Dis. 2021, 8, ofab159. [Google Scholar] [CrossRef] [PubMed]
- Begley, K.M.; Leis, A.M.; Petrie, J.G.; Johnson, E.; McSpadden, E.; Lamerato, L.E.; Wei, M.; Martin, E.T. Title: Epidemiology of RSV-A and RSV-B in Adults and Children with Medically-Attended 1 Acute Respiratory Illness over Three Seasons. MedRxiv 2022. [Google Scholar] [CrossRef]
- Löwensteyn, Y.N.; Mazur, N.I.; Nair, H.; Willemsen, J.E.; van Thiel, G.; Bont, L.; Garba, M.A.; Giwa, F.J.; Rasooly, M.H.; Shirpoor, A.; et al. Describing Global Pediatric RSV Disease at Intensive Care Units in GAVI-Eligible Countries Using Molecular Point-of-Care Diagnostics: The RSV GOLD-III Study Protocol. BMC Infect. Dis. 2021, 21, 962. [Google Scholar] [CrossRef]
- Billard, M.; van de Ven, P.M.; Baraldi, B.; Kragten-Tabatabaie, L.; Bont, L.J.; Wildenbeest, J.G. International Changes in Respiratory Syncytial Virus (RSV) Epidemiology during the COVID-19 Pandemic: Association with School Closures. Influenza Other Respir. Viruses 2022, 16, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Özcan, H.N.; Özsürekci, Y.; Oğuz, B.; Oygar, P.D.; Gürlevik, S.L.; Karakaya, J.; Tekşam, Ö.; Ceyhan, M.; Haliloğlu, M. Chest Imaging for COVID-19: A Single-Center Comparative Results in Pediatric Patients. Turk. J. Pediatr. 2022, 64, 19–31. [Google Scholar] [CrossRef]
- Auvinen, R.; Syrjänen, R.; Ollgren, J.; Nohynek, H.; Skogberg, K. Clinical Characteristics and Population-Based Attack Rates of Respiratory Syncytial Virus versus Influenza Hospitalizations among Adults—An Observational Study. Influenza Other Respir. Viruses, 2021; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Debes, S.; Haug, J.B.; de Blasio, B.F.; Jonassen, C.M.; Dudman, S.G. Etiology of Viral Respiratory Tract Infections in Hospitalized Adults, and Evidence of the High Frequency of Prehospitalization Antibiotic Treatment in Norway. Health Sci. Rep. 2021, 4, e403. [Google Scholar] [CrossRef] [PubMed]
- Atamna, A.; Babich, T.; Froimovici, D.; Yahav, D.; Sorek, N.; Ben-Zvi, H.; Leibovici, L.; Bishara, J.; Avni, T. Morbidity and Mortality of Respiratory Syncytial Virus Infection in Hospitalized Adults: Comparison with Seasonal Influenza. Int. J. Infect. Dis. 2021, 103, 489–493. [Google Scholar] [CrossRef]
- Hurley, L.P.; Allison, M.A.; Kim, L.; O’Leary, S.T.; Crane, L.A.; Brtnikova, M.; Beaty, B.L.; Allen, K.E.; Poser, S.; Lindley, M.C.; et al. Primary Care Physicians’ Perspectives on Respiratory Syncytial Virus (RSV) Disease in Adults and a Potential RSV Vaccine for Adults. Vaccine 2019, 37, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Long, C.E.; Schnabel, K.C. Respiratory Syncytial Virus Infections in Previously Healthy Working Adults. Clin. Infect. Dis. 2001, 33, 792–796. [Google Scholar] [CrossRef]
- Allen, K.E.; Beekmann, S.E.; Polgreen, P.; Poser, S.; Pierre, J.S.; Santibañez, S.; Gerber, S.I.; Kim, L. Survey of Diagnostic Testing for Respiratory Syncytial Virus (RSV) in Adults: Infectious Disease Physician Practices and Implications for Burden Estimates. Diagn. Microbiol. Infect. Dis. 2018, 92, 206–209. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Zaniboni, A.; Ranzieri, S. Respiratory Syncytial Virus: Knowledge, Attitudes and Beliefs of General Practitioners from North-Eastern Italy (2021). Pediatr. Rep. 2022, 14, 147–165. [Google Scholar] [CrossRef]
- Bohmwald, K.; Espinoza, J.A.; Rey-Jurado, E.; Gómez, R.S.; González, P.A.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Human Respiratory Syncytial Virus: Infection and Pathology. Semin. Respir. Crit. Care Med. 2016, 37, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Bohmwald, K.; Soto, J.A.; Andrade-Parra, C.; Fernández-Fierro, A.; Espinoza, J.A.; Ríos, M.; Eugenin, E.A.; González, P.A.; Opazo, M.C.; Riedel, C.A.; et al. Lung Pathology Due to HRSV Infection Impairs Blood–Brain Barrier Permeability Enabling Astrocyte Infection and a Long-Lasting Inflammation in the CNS. Brain Behav. Immun. 2021, 91, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E.; Gonzales, R.A.; Olson, S.J.; Wright, P.F.; Graham, B.S. The Histopathology of Fatal Untreated Human Respiratory Syncytial Virus Infection. Mod. Pathol. 2007, 20, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Shilovskiy, I.P.; Yumashev, K.V.; Nikolsky, A.A.; Vishnyakova, L.I.; Khaitov, M.R. Molecular and Cellular Mechanisms of Respiratory Syncytial Viral Infection: Using Murine Models to Understand Human Pathology. Biochemistry 2021, 86, 290–306. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Imrey, P.B. Limitations of Meta-Analyses of Studies with High Heterogeneity. JAMA Netw. Open 2020, 3, e1919325. [Google Scholar] [CrossRef] [PubMed]
- Van Brusselen, D.; de Troeyer, K.; ter Haar, E.; vander Auwera, A.; Poschet, K.; van Nuijs, S.; Bael, A.; Stobbelaar, K.; Verhulst, S.; van Herendael, B.; et al. Bronchiolitis in COVID-19 Times: A Nearly Absent Disease? Eur. J. Pediatr. 2021, 180, 1969–1973. [Google Scholar] [CrossRef]
- Di Mattia, G.; Nenna, R.; Mancino, E.; Rizzo, V.; Pierangeli, A.; Villani, A.; Midulla, F. During the COVID-19 Pandemic Where Has Respiratory Syncytial Virus Gone? Pediatr. Pulmonol. 2021, 56, 3106–3109. [Google Scholar] [CrossRef]
- Ozeki, S.; Kawada, J.; Yamashita, D.; Yasufuku, C.; Akano, T.; Kato, M.; Suzuki, K.; Tano, C.; Matsumoto, K.; Mizutani, S.; et al. Impact of the COVID-19 Pandemic on the Clinical Features of Pediatric RSV Infection in Japan. Open Forum Infect Dis. 2022, 9, ofac562. [Google Scholar] [CrossRef]
- Stamm, P.; Sagoschen, I.; Weise, K.; Plachter, B.; Münzel, T.; Gori, T.; Vosseler, M. Influenza and RSV Incidence during COVID-19 Pandemic—An Observational Study from in-Hospital Point-of-Care Testing. Med. Microbiol. Immunol. 2021, 210, 277–282. [Google Scholar] [CrossRef]
- Rabbone, I.; Monzani, A.; Scaramuzza, A.E.; Cavalli, C. See-Sawing COVID-19 and RSV Bronchiolitis in Children under 2 Years of Age in Northern Italy. Acta Paediatr. Int. J. Paediatr. 2022, 11, 2174–2175. [Google Scholar] [CrossRef]
- Castagno, E.; Raffaldi, I.; del Monte, F.; Garazzino, S.; Bondone, C. New Epidemiological Trends of Respiratory Syncytial Virus Bronchiolitis during COVID-19 Pandemic. World J. Pediatr. 2022, 19, 502–504. [Google Scholar] [CrossRef]
- Ujiie, M.; Tsuzuki, S.; Nakamoto, T.; Iwamoto, N.; Ujiie, M. Resurgence of Respiratory Syncytial Virus Infections during COVID-19 Pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021, 27, 2969–2970. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Baldassarre, A.; Provenzano, S.; Corrado, S.; Cerviere, M.P.; Parisi, S.; Marchesi, F.; Bottazzoli, M. Infodemiology of RSV in Italy (2017–2022): An Alternative Option for the Surveillance of Incident Cases in Pediatric Age? Children 2022, 9, 1984. [Google Scholar] [CrossRef]
- Sansone, N.M.S.; Boschiero, M.N.; Marson, F.A.L. Epidemiologic Profile of Severe Acute Respiratory Infection in Brazil During the COVID-19 Pandemic: An Epidemiological Study. Front. Microbiol. 2022, 13, 911036. [Google Scholar] [CrossRef]
- Vihta, K.-D.; Pouwels, K.B.; Peto, T.E.A.; Pritchard, E.; House, T.; Studley, R.; Rourke, E.; Cook, D.; Diamond, I.; Crook, D.; et al. Omicron-Associated Changes in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Symptoms in the United Kingdom. Clin. Infect. Dis. 2023, 76, e133–e141. [Google Scholar] [CrossRef]
- Vihta, K.-D.; Pouwels, K.B.; Peto, T.E.A.; Pritchard, E.; Eyre, D.W.; House, T.; Gethings, O.; Studley, R.; Rourke, E.; Cook, D.; et al. Symptoms and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Positivity in the General Population in the United Kingdom. Clin. Infect. Dis. 2021, 75, e329–e337. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Choi, W.; You, S.H.; Park, S.; Kim, J.Y.; Nam, D.R.; Lee, J.W.; Jung, S.Y. Safety Profiles of MRNA COVID-19 Vaccines Using World Health Organization Global Scale Database (VigiBase): A Latent Class Analysis. Infect. Dis. Ther. 2022, 12, 443–458. [Google Scholar] [CrossRef]
- DeSanti, R.L.; Cowan, E.A.; Kory, P.D.; Lasarev, M.R.; Schmidt, J.; Al-Subu, A.M. Lung Ultrasound Artifact Findings in Pediatric Patients Admitted to the Intensive Care Unit for Acute Respiratory Failure. J. Ultrasound 2022, 25, 929–937. [Google Scholar] [CrossRef] [PubMed]
- La Regina, D.P.; Bloise, S.; Pepino, D.; Iovine, E.; Laudisa, M.; Cristiani, L.; Nicolai, A.; Nenna, R.; Mancino, E.; Di Mattia, G.; et al. Lung Ultrasound in Bronchiolitis. Pediatr. Pulmonol. 2021, 56, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Bobillo-Perez, S.; Sorribes, C.; Gebellí, P.; Lledó, N.; Castilla, M.; Ramon, M.; Rodriguez-Fanjul, J. Lung Ultrasound to Predict Pediatric Intensive Care Admission in Infants with Bronchiolitis (LUSBRO Study). Eur. J. Pediatr. 2021, 180, 2065–2072. [Google Scholar] [CrossRef]
- Supino, M.C.; Buonsenso, D.; Scateni, S.; Scialanga, B.; Mesturino, M.A.; Bock, C.; Chiaretti, A.; Giglioni, E.; Reale, A.; Musolino, A.M. Point-of-Care Lung Ultrasound in Infants with Bronchiolitis in the Pediatric Emergency Department: A Prospective Study. Eur. J. Pediatr. 2019, 178, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, S.; Duggan, N.M.; Cohen, A.R.; Shokoohi, H. Lung Ultrasound in Children with Respiratory Tract Infections: Viral, Bacterial or Covid-19? A Narrative Review. Open Access Emerg. Med. 2020, 12, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Amis, E.S.; Butler, P.F.; Applegate, K.E.; Birnbaum, S.B.; Brateman, L.F.; Hevezi, J.M.; Mettler, F.A.; Morin, R.L.; Pentecost, M.J.; Smith, G.G.; et al. American College of Radiology White Paper on Radiation Dose in Medicine. J. Am. Coll. Radiol. 2007, 4, 272–284. [Google Scholar] [CrossRef]
- Strauss, K.J.; Goske, M.J. Estimated Pediatric Radiation Dose during CT. Pediatr. Radiol. 2011, 41 (Suppl. S2), 472–482. [Google Scholar] [CrossRef]
- Brenner, D.J.; Elliston, C.D.; Hall, E.J.; Berdon, W.E. Estimated Risks of Radiation-Induced Fatal Cancer from Pediatric CT. Am. J. Roentgenol. 2001, 176, 289–296. [Google Scholar] [CrossRef]
- Andabaka, T.; Nickerson, J.W.; Rojas-Reyes, M.X.; Rueda, J.D.; Bacic Vrca, V.; Barsic, B. Monoclonal Antibody for Reducing the Risk of Respiratory Syncytial Virus Infection in Children. Cochrane Database Syst. Rev. 2013, 2013, CD006602. [Google Scholar] [CrossRef]
- Frogel, M.P.; Stewart, D.L.; Hoopes, M.; Fernandes, A.W.; Mahadevia, P.J. A Systematic Review of Compliance with Palivizumab Administration for RSV Immunoprophylaxis. J. Manag. Care Pharm. 2010, 16, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Olchanski, N.; Hansen, R.N.; Pope, E.; D’Cruz, B.; Fergie, J.; Goldstein, M.; Krilov, L.R.; McLaurin, K.K.; Nabrit-Stephens, B.; Oster, G.; et al. Palivizumab Prophylaxis for Respiratory Syncytial Virus: Examining the Evidence around Value. Open Forum Infect. Dis. 2018, 5, ofy031. [Google Scholar] [CrossRef] [PubMed]
- Mac, S.; Sumner, A.; Duchesne-Belanger, S. Cost-Effectiveness of Palivizumab for Respiratory Syncytial Virus: A Systematic Review. Pediatrics 2019, 143, 20184064. [Google Scholar] [CrossRef] [PubMed]
- Viguria, N.; Navascués, A.; Juanbeltz, R.; Echeverría, A.; Ezpeleta, C.; Castilla, J. Effectiveness of Palivizumab in Preventing Respiratory Syncytial Virus Infection in High-Risk Children. Hum. Vaccin. Immunother. 2021, 17, 1867–1872. [Google Scholar] [CrossRef]
- Sánchez Luna, M.; Manzoni, P.; Paes, B.; Baraldi, E.; Cossey, V.; Kugelman, A.; Chawla, R.; Dotta, A.; Rodríguez Fernández, R.; Resch, B.; et al. Expert Consensus on Palivizumab Use for Respiratory Syncytial Virus in Developed Countries. Paediatr. Respir. Rev. 2020, 33, 35–44. [Google Scholar] [CrossRef]
- Mitchell, I.; Li, A.; Bjornson, C.L.; Lanctot, K.L.; Paes, B.A. Respiratory Syncytial Virus Immunoprophylaxis with Palivizumab: 12-Year Observational Study of Usage and Outcomes in Canada. Am. J. Perinatol. 2021; ahead of print. [Google Scholar] [CrossRef]
- Zylbersztejn, A.; Almossawi, O.; Gudka, N.; Tompsett, D.; de Stavola, B.; Standing, J.F.; Smyth, R.; Hardelid, P. Access to Palivizumab among Children at High Risk of Respiratory Syncytial Virus Complications in English Hospitals. Br. J. Clin. Pharmacol. 2022, 88, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.D.L.; Ferreira, M.A.P.; da Silva Xavier, C.; de Souza, I.T.A.; Cruz, L.N.; Polanczyk, C.A. A Post-Incorporation Study on the Use of Palivizumab in the Brazilian Public Health System. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, e5. [Google Scholar] [CrossRef]
- Meissner, H.C.; Long, S.S.; Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised Indications for the Use of Palivizumab and Respiratory Syncytial Virus Immune Globulin Intravenous for the Prevention of Respiratory Syncytial Virus Infections. Pediatrics 2003, 112, 1447–1452. [Google Scholar] [CrossRef]
- Cutrera, R.; Wolfler, A.; Picone, S.; Rossi, G.A.; Gualberti, G.; Merolla, R.; del Vecchio, A.; Villani, A.; Midulla, F.; Dotta, A. Impact of the 2014 American Academy of Pediatrics Recommendation and of the Resulting Limited Financial Coverage by the Italian Medicines Agency for Palivizumab Prophylaxis on the RSV-Associated Hospitalizations in Preterm Infants during the 2016–2017 Epidemic Season: A Systematic Review of Seven Italian Reports. Ital. J. Pediatr. 2019, 45, 139. [Google Scholar] [PubMed]
- American Academy of Pediatrics. Committee on Infectious Diseases and Bronchiolitis Guidelines Committee Updated Guidance for Palivizumab Prophylaxis among Infants and Young Children at Increased Risk of Hospitalization for Respiratory Syncytial Virus Infection. Pediatrics 2014, 134, e620–e638. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.P.; Yuan, Y.; Takas, T.; Domachowske, J.B.; Madhi, S.A.; Manzoni, P.; Simões, E.A.F.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Engl. J. Med. 2020, 383, 415–425. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Voirin, N.; Virlogeux, V.; Demont, C.; Kieffer, A. Potential Impact of Nirsevimab on RSV Transmission and Medically Attended Lower Respiratory Tract Illness Caused by RSV: A Disease Transmission Model. Infect. Dis. Ther. 2021, 11, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Domachowske, J.B.; Anderson, E.J.; Goldstein, M. The Future of Respiratory Syncytial Virus Disease Prevention and Treatment. Infect. Dis. Ther. 2021, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Domachowske, J.; Madhi, S.A.; Simões, E.A.F.; Atanasova, V.; Cabañas, F.; Furuno, K.; Garcia-Garcia, M.L.; Grantina, I.; Nguyen, K.A.; Brooks, D.; et al. Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. N. Engl. J. Med. 2022, 386, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Obolski, U.; Kassem, E.; Na’amnih, W.; Tannous, S.; Kagan, V.; Muhsen, K. Unnecessary Antibiotic Treatment of Children Hospitalized with RSV-Bronchiolitis: Risk Factors and Prescription Patterns. J. Glob. Antimicrob. Resist. 2021, 27, 303–308. [Google Scholar] [CrossRef]
- Cunningham, C.K.; Karron, R.A.; Muresan, P.; Kelly, M.S.; McFarland, E.J.; Perlowski, C.; Libous, J.; Oliva, J.; Jean-Philippe, P.; Moye, J.; et al. Evaluation of Recombinant Live-Attenuated Respiratory Syncytial Virus (RSV) Vaccines RSV/ΔNS2/Δ1313/I1314L and RSV/276 in RSV-Seronegative Children. J. Infect. Dis. 2022, 226, 2069–2078. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]

| Item | Definition |
|---|---|
| Population of interest | Children and adults with diagnosis of respiratory infection |
| Investigated test result | Report of the following imaging features: bronchitis, alveolar involvement, tree-in-bud, bronchial wall thickening, ground glass opacities, nodules, consolidation, pleural effusions |
| Comparator test result | Imaging of features of respiratory syncytial virus (RSV) infections compared to other viral respiratory infections |
| Outcome | Association of imaging features with RSV infection |
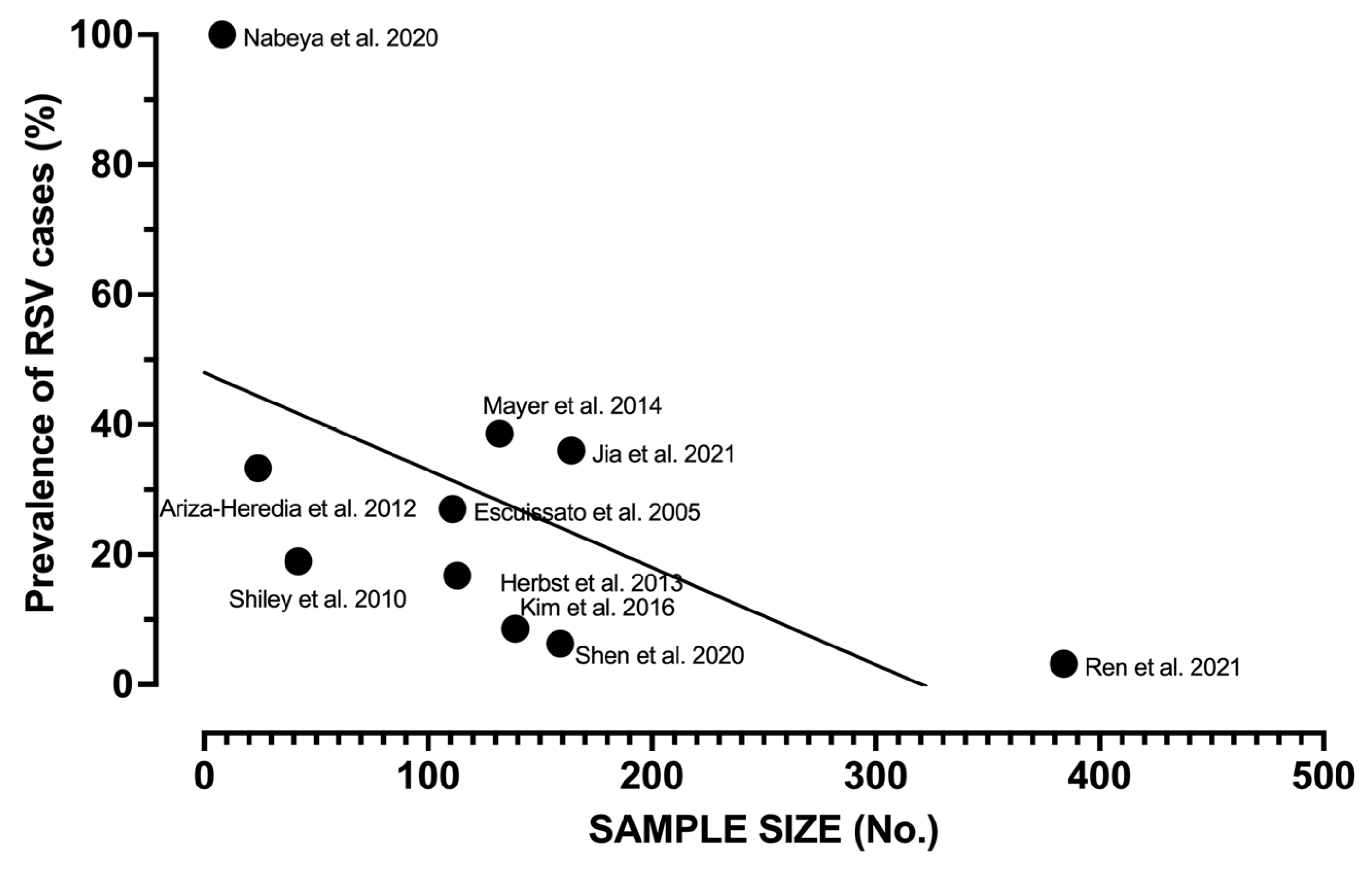
| Authors | Year | Timeframe | Country | Patients | Category | Diagnosis of RSV | Total Sample (No.) | RSV (No., %) | No Signs (No., %) |
|---|---|---|---|---|---|---|---|---|---|
| Ariza-Heredia et al. [42] | 2012 | 1 Januaty 1997 31 December 2009 | USA | All adult patients who underwent solid organ transplant and were positive for RSV at either antigen or RT-qPCR | Immunocompromised | RT-qPCR/antigen testing | 24 | 8, 33.3% | 1, 12.5% |
| Escuissato et al. [41] | 2005 | January 1993 December 2003 | Brazil | All patients (No. 111) with a proven pulmonary infection after bone marrow transplatation and HR-CT within 24 h from the admission | Immunocompromised | Immunofluorescence | 111 | 30, 27.0% | 6, 20.0% |
| Herbst et al. [52] | 2013 | 15 November 2005 15 July 2011 | USA | All patients from 2 university-based hospitals having received testing for respiratory pathogens and admitted for LRTI and undergone CT within 7 days from the positive diagnosis | Adults (age ≥ 18 years) | RT-qPCR | 113 | 19, 16.8% | 4, 21.1% |
| Jia et al. [53] | 2021 | 16 January 2020 25 February 2020 | China, mainland | All pediatric patients (No. 947) having been assessed in 30 hospitals from 4 provinces for pneumonia | Children/adolescents (age < 18 years) | Immunofluorescence | 164 | 59, 36.0% | 28, 47.5% |
| Kim et al. [55] | 2016 | January 1998 December 2014 | Republic of Korea | All adult patients who underwent BAL and were diagnosed with LRTI caused by PIV, influenza virus or RSV, within 14 days from diagnosis | Adults (age ≥ 16 years) | RT-qPCR/immunofluorescence | 139 | 12, 8.6% | 3, 25.0% |
| Mayer et al. [56] | 2014 | November 2011 July 2012 | Germany | All immunocompromized patients from the same clinic having received a CT scan within 24 h after the beginning of clinical symptoms | Immunocompromised | RT-qPCR/antigen testing/immunofluorescence | 132 | 51, 38.6% | 7, 13.7% |
| Nabeya et al. [57] | 2020 | 2014 | Japan | Eight patients who, during the outbreak of 2014, received CT scans and were genetically related in terms of pathogens | Adults (age ≥ 18 years) | RT-qPCR | 8 | 8, 100% | 3, 37.5% |
| Ren et al. [54] | 2021 | 15 December 2019 15 March 2020 | China, mainland | All consecutive children diagnosed with community-acquired pneumonia | Children/adolescents (age < 18 years) | RT-qPCR | 384 | 12, 3.2% | n.a. |
| Shen et al. [58] | 2020 | 23 January 2020 20 March 2020 | China, mainland | All consecutive children diagnosed with community-acquired pneumonia | Children/adolescents (age < 18 years) | RT-qPCR | 159 | 10, 6.3% | 0, 0.0% |
| Shiley et al. [50] | 2010 | 1 November 2005 31 July 2007 | USA | All adults patients with a viral diagnosis, PCR analysis and CT scans | Adults (age ≥ 18 years) | RT-qPCR | 42 | 8, 19.0% | 0, 0.0% |
| Item | No. of Studies (No./10) | Total | Children/Adolescents (Age < 18 Years) | Adults (Age ≥ 18 Years) | Immune Deficiency | Chi2 p-Value |
|---|---|---|---|---|---|---|
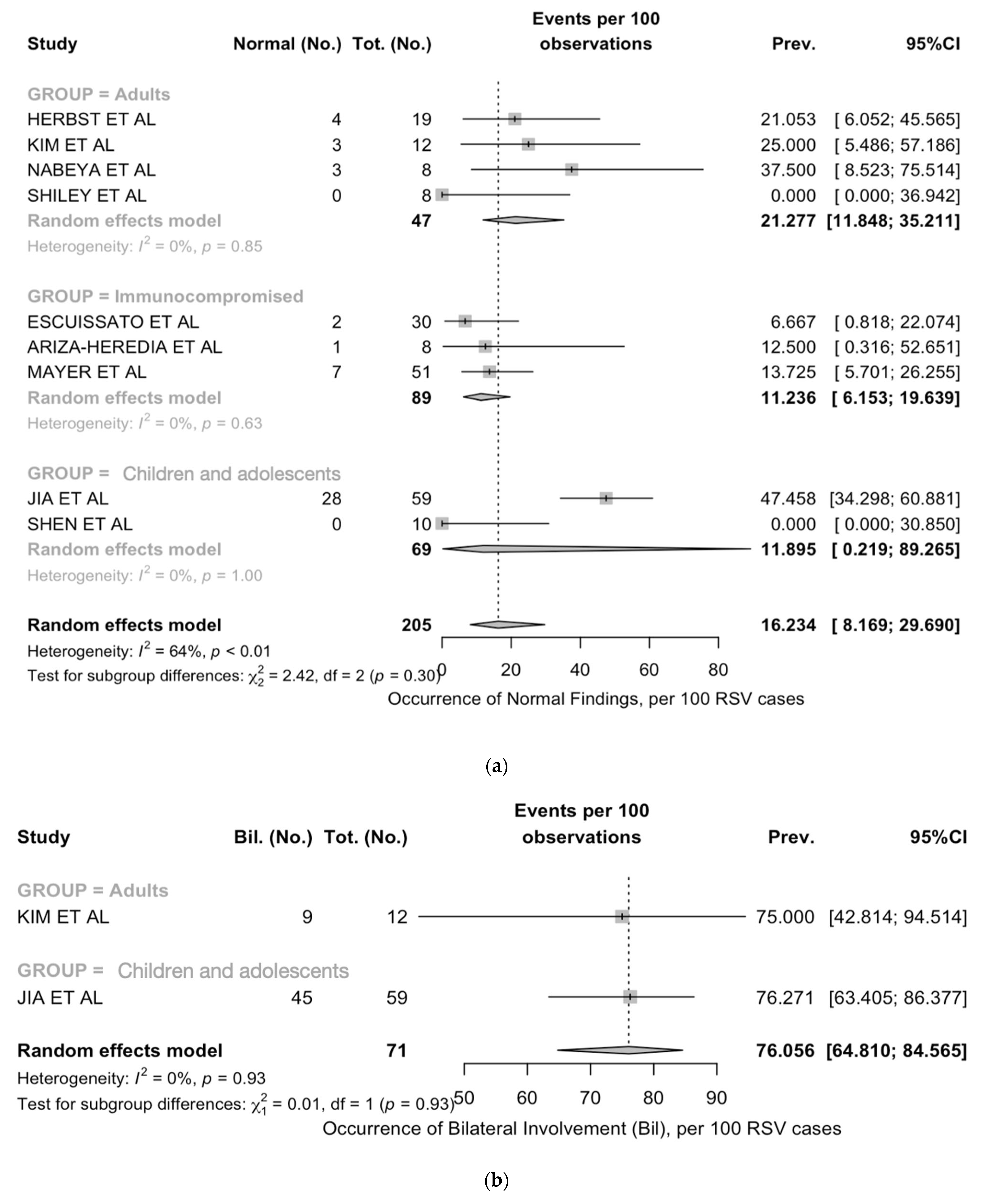
| Normal findings | 9 | 48/205, 23.4% | 0/18, 0.0% | 35/116, 30.2% | 13/71, 18.3% | 0.009 |
| Bilateral involvement | 2 | 54/71, 76.1% | n.a. | 54/71, 76.1% | n.a. | n.a. |
| Extensive/multifocal | 3 | 3/36, 16.7% | 0/8, 0.0% | 2/8, 25.0% | 4/20, 20.0% | 0.340 |
| Ground glass opacities | 10 | 78/217, 35.9% | 13/81, 16.0% | 16/47, 34.0% | 49/89, 55.1% | <0.001 |
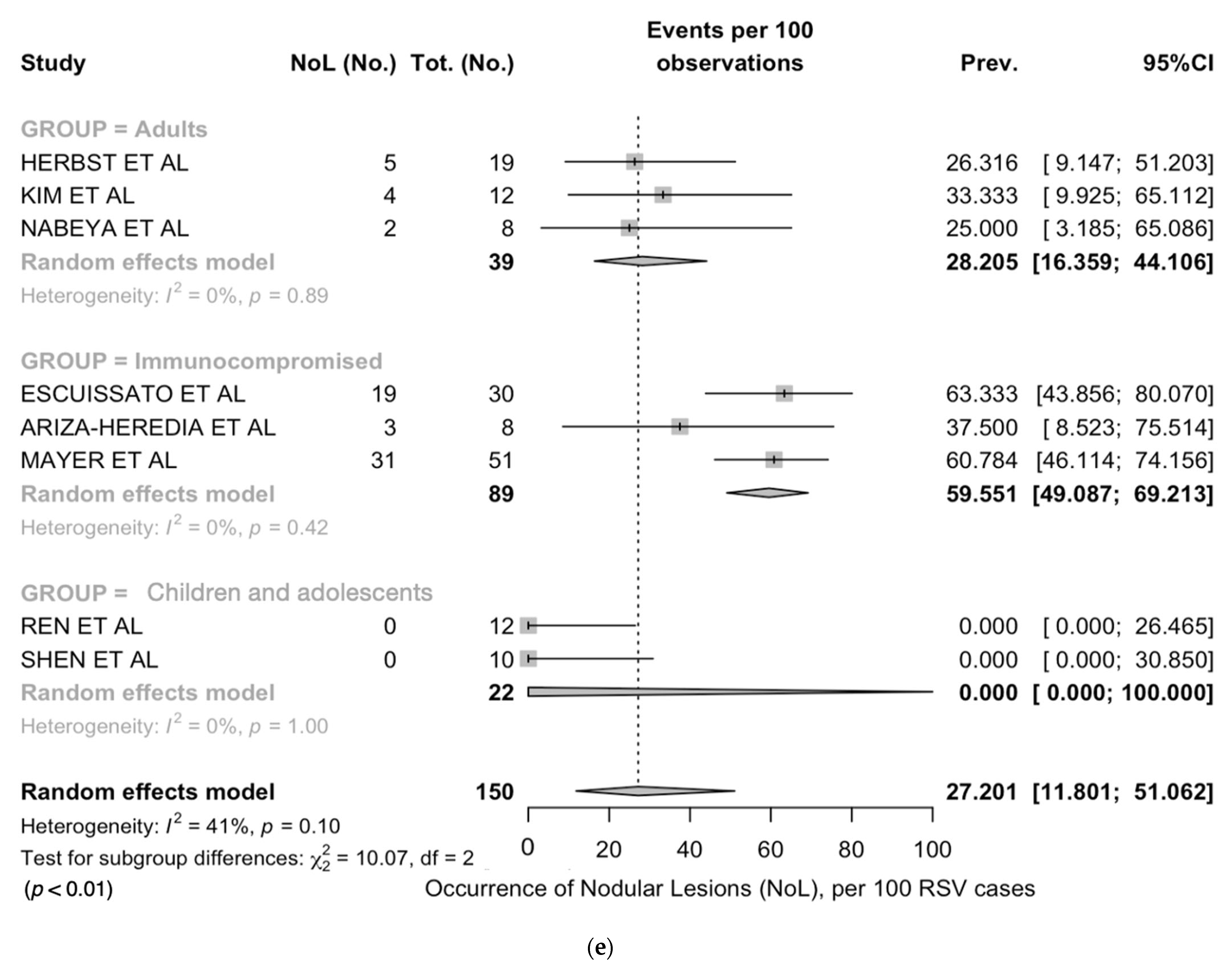
| Nodular lesions | 8 | 64/150, 42.7% | 0/22, 0.0% | 28/61, 45.9% | 36/67, 53.7% | <0.001 |
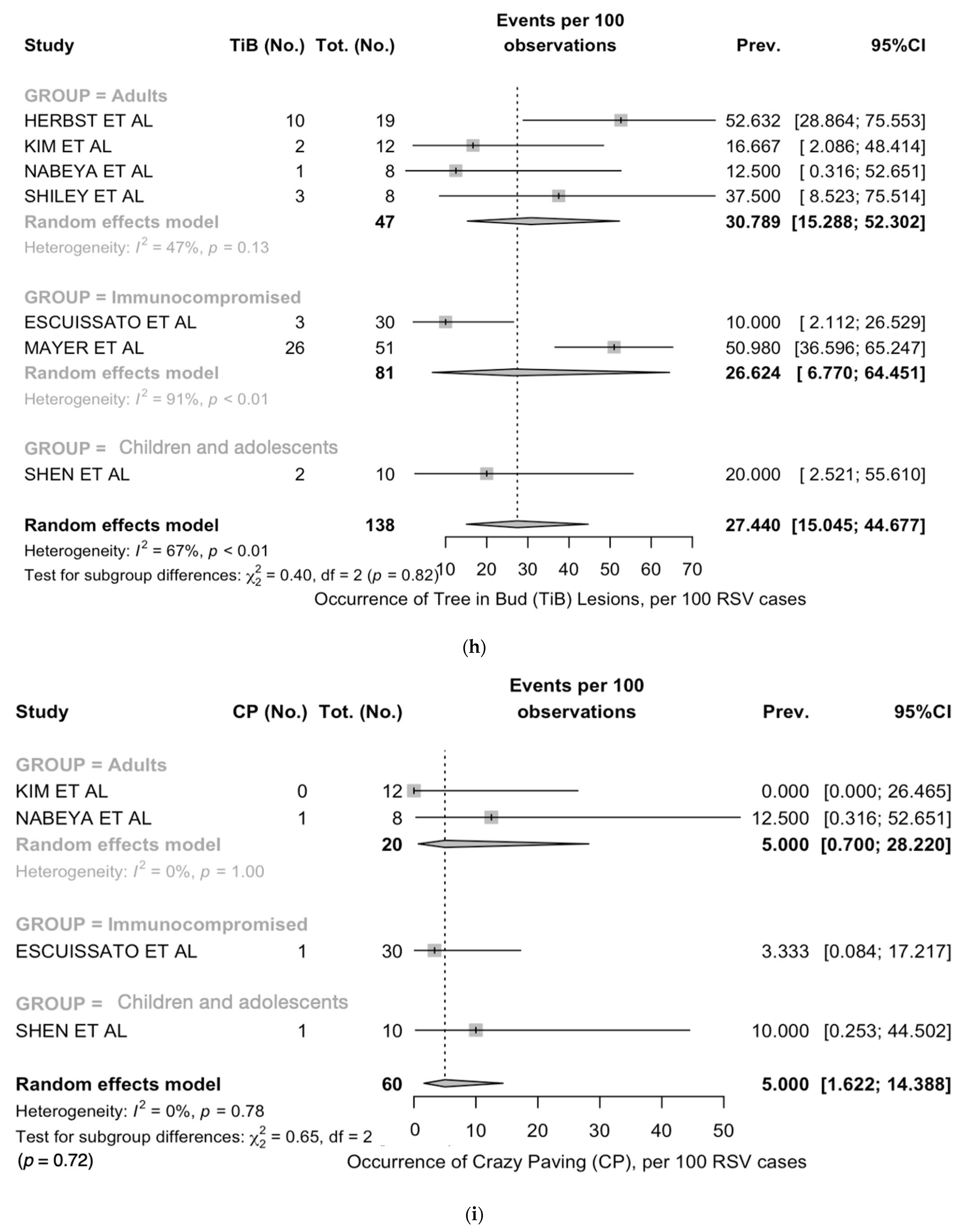
| Tree-in-bud | 7 | 47/138, 34.1% | 5/18, 27.8% | 13/49, 26.5% | 29/49, 40.9% | 0.002 |
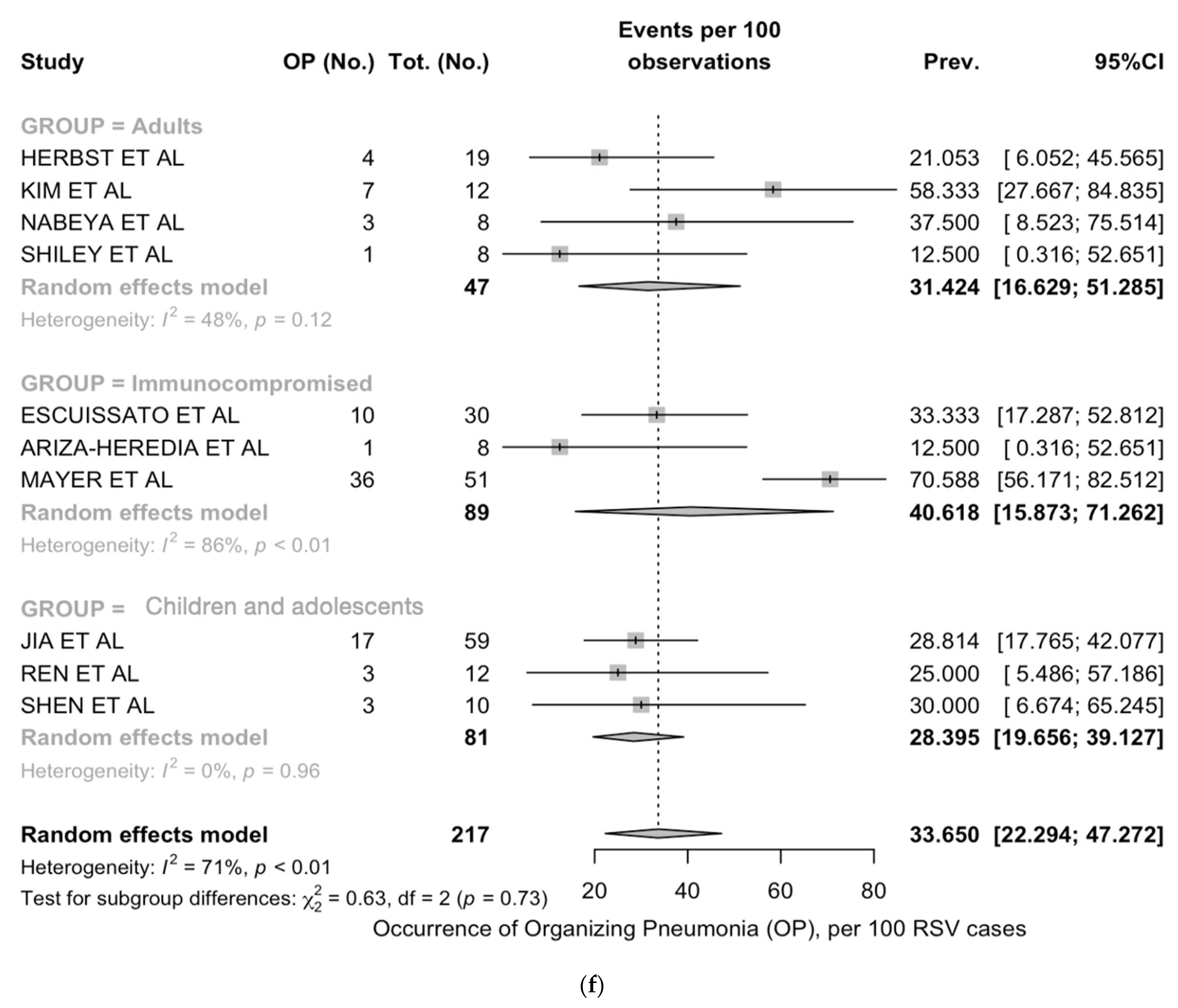
| Organizing pneumonia | 10 | 85/217, 39.2% | 7/30, 23.3% | 32/116, 27.6% | 46/71, 64.8% | <0.001 |
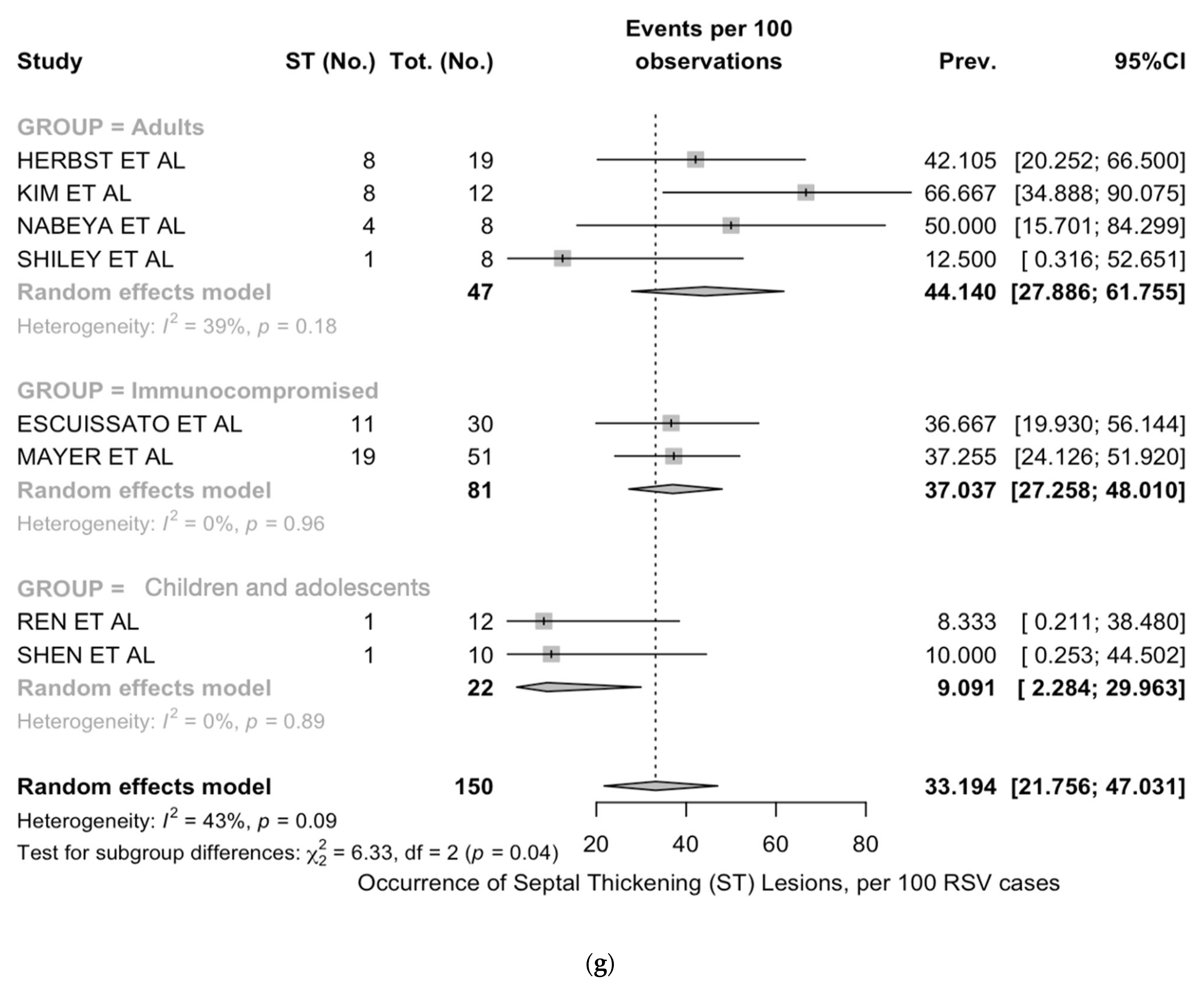
| Septal thickening | 7 | 53/150, 35.3% | 3/30, 10.0% | 19/49, 38.8% | 31/71, 43.7% | 0.004 |
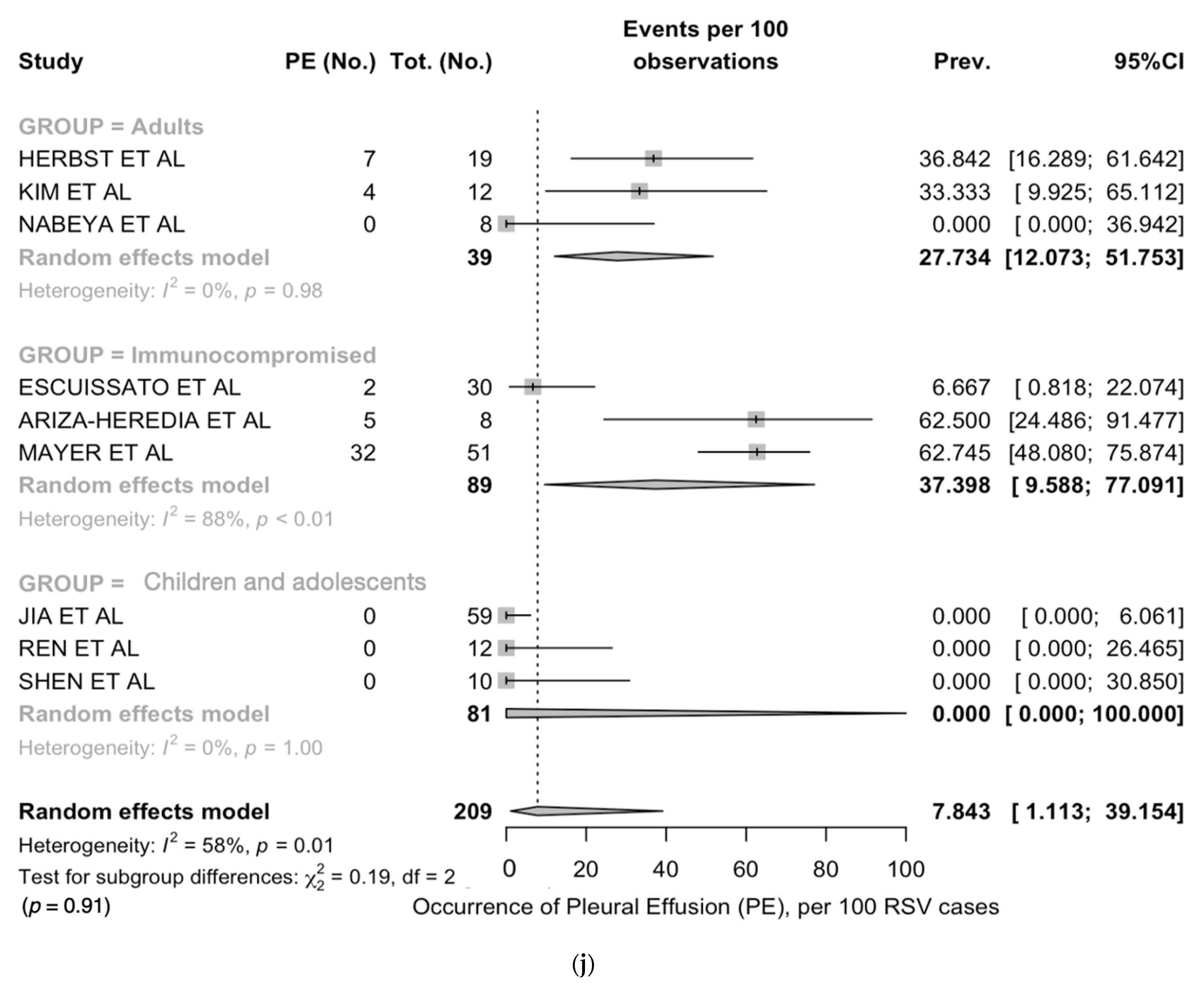
| Crazy paving | 4 | 3/60, 5.0% | 1/10, 10.0% | 1/30, 3.3% | 1/20, 5.0% | 0.704 |
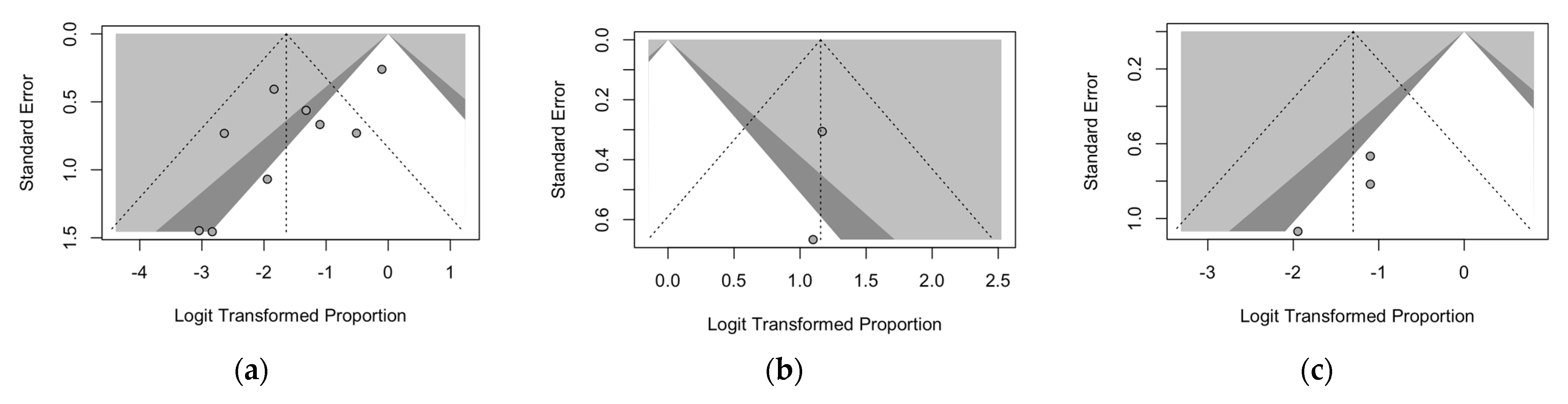
| Pleural effusion | 9 | 50/209, 23.9% | 0/22, 0.0% | 14/116, 12.1% | 36/71, 50.7% | <0.001 |
| Item | Prevalence (No. per 100 Studies) | I2 | Q | tau2 | p-Value |
|---|---|---|---|---|---|
| Normal findings | 16.2 (8.2; 29.7) | 64.0 | 22.20 | 0.745 | 0.005 |
| Bilateral involvement | 76.1 (64.8; 84.6) | 0.0 | 0.01 | 0.000 | 0.924 |
| Extensive/multifocal | 21.4 (10.0; 40.2) | 0.0 | 0.51 | 0.000 | 0.775 |
| Ground glass opacities | 28.0 (14.7; 46.8) | 80.7 | 46.66 | 1.241 | <0.001 |
| Nodular lesions | 27.2 (11.8, 51.1) | 41.1 | 11.88 | 1.473 | 0.1805 |
| Tree-in-bud | 27.4 (15.0; 44.7) | 67.3 | 18.34 | 0.606 | 0.005 |
| Organizing pneumonia | 33.7 (22.3; 47.3) | 70.8 | 30.85 | 0.481 | <0.001 |
| Septal thickening | 33.2 (21.8; 47.0) | 42.6 | 12.19 | 0.303 | 0.094 |
| Crazy paving | 5.0 (1.6; 14.4) | 0.0 | 1.08 | 0.000 | 0.783 |
| Pleural effusion | 7.8 (1.1; 39.2) | 58.2 | 19.12 | 6.063 | 0.014 |
| Children/Adolescents (Age < 18 Years) | Adults (Age ≥ 18 Years) | Immune Deficiency | ||||
|---|---|---|---|---|---|---|
| Prevalence (No. per 100 Studies) | I2 | Prevalence (No. per 100 Studies) | I2 | Prevalence (No. per 100 Studies) | I2 | |
| Normal findings | 11.9 (0.2; 89.3) | 0% | 21.3 (11.8; 35.2) | 85% | 11.2 (6.2; 19.6) | 0% |
| Bilateral involvement | 76.3 (63.4; 86.4) | - | 75.0 (42.8; 94.5) | - | ||
| Extensive/multifocal | - | - | 20.0 (7.7; 42.8) | 0% | 25.0 (10.0; 40.2) | - |
| Ground glass opacities | 16.0 (10.0; 25.7) | 0% | 34.1 (16.4; 57.8) | 61% | 46.8 (19.8; 75.8) | 90% |
| Nodular lesions | 0.0 (0.0; 100) | 0% | 28.2 (16.4; 44.1) | 0% | 59.5 (49.1; 69.2) | 0% |
| Tree-in-bud | 20.0 (2.5; 55.6) | - | 30.8 (15.3; 52.3) | 47% | 26.6 (6.8; 64.5) | 91% |
| Organizing pneumonia | 28.4 (19.7; 39.1) | 0% | 31.4 (16.6; 51.3) | 48% | 40.6 (15.9; 71.3) | 86% |
| Septal thickening | 9.1 (2.3; 30.0) | 0% | 44.1 (27.9; 61.8) | 39% | 37.0 (27.3; 48.0) | 0% |
| Crazy paving | 10.0 (0.3; 44.5) | - | 5.0 (0.7; 28.2) | 0% | 3.3 (0.1; 17.2) | - |
| Pleural effusion | 0.0 (0.0; 100) | 0% | 27.7 (12.1; 51.8) | 0% | 38.4 (9.6; 77.1) | 88% |
| Item | RSV (10 Studies) | COVID-19 (3 Studies) | Chi2 p-Value | Seasonal Influenza (7 Studies) | Chi2 p-Value | All Non-RSV Viral Infections (7 Studies) | Chi2 p-Value |
|---|---|---|---|---|---|---|---|
| Normal findings | 48/205, 23.4% | 0/87, 0.0% | <0.001 | 39/144, 27.1% | 0.513 | 58/381, 15.1% | 0.019 |
| Bilateral involvement | 54/71, 76.1% | 41/87, 47.1% | <0.001 | 24/45, 53.3% | 0.019 | 71/144, 49.3% | <0.001 |
| Extensive/multifocal | 6/28, 21.4% | n.a. | n.a. | 11/14, 78.6% | 0.001 | 19/26, 73.1% | <0.001 |
| Ground glass opacities | 78/217, 35.9% | 59/127, 46.5% | 0.051 | 37/153, 24.2% | 0.019 | 157/439, 35.8% | 0.967 |
| Nodular lesions | 64/150, 42.7% | 6/40, 15.0% | 0.002 | 23/101, 22.8% | 0.002 | 108/287, 37.6% | 0.358 |
| Tree in bud | 47/138, 34.1% | n.a. | n.a. | 23/113, 20.4% | 0.023 | 61/263, 23.2% | 0.027 |
| Organizing pneumonia | 85/150, 39.2% | 78/127, 61.4% | 0.498 | 132/153, 86.3% | <0.001 | 259/439, 59.0% | 0.686 |
| Septal thickening | 53/150, 35.3% | 11/40, 27.5% | 0.457 | 36/122, 29.5% | 0.374 | 80/321, 24.9% | 0.026 |
| Crazy paving | 3/60, 5.0% | n.a. | n.a. | 4/32, 12.5% | 0.379 | 21/135, 15.6% | 0.067 |
| Pleural effusion | 50/209, 23.9% | 1/127, 0.8% | <0.001 | 14/132, 10.6% | 0.003 | 33/405, 8.1% | <0.001 |
| Item | COVID-19 | Seasonal Influenza | All Non-RSV Viral Infections |
|---|---|---|---|
| Pooled Risk Ratio (95% Confidence Interval) | |||
| Normal findings | - | 0.785 (0.457; 1.347) | 1.737 (0.737; 4.095) |
| Bilateral involvement | - | 1.425 (1.047; 1.940) | 1.542 (1.248; 1.904) |
| Ground glass opacities | 0.391 (0.166; 0.922) | 0.730 (0.532; 1.003) | 0.636 (0.481; 0.841) |
| Nodular lesions | - | 0.937 (0.473; 1.859) | 0.740 (0.572; 0.959) |
| Tree-in-bud | - | 1.870 (1.125; 3.108) | 1.221 (0.601; 2.479) |
| Organizing pneumonia | 0.964 (0.332; 2.798) | 1.447 (0.907; 2.311) | 0.713 (0.549; 0.925) |
| Septal thickening | - | 1.024 (0.696; 1.508) | 1.433 (1.019; 2.015) |
| Crazy paving | - | 0.778 (0.021; 29.544) | 0.341 (0.096; 1.210) |
| Pleural effusion | 1.080 (0.047; 24.881) | 1.603 (0.625; 4.110) | 1.362 (0.717; 2.587) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Corrado, S.; Palmieri, S.; Marchesi, F. Respiratory Syncytial Virus: A Systematic Review and Meta-Analysis of Tomographic Findings (2000–2022). Children 2023, 10, 1169. https://doi.org/10.3390/children10071169
Riccò M, Corrado S, Palmieri S, Marchesi F. Respiratory Syncytial Virus: A Systematic Review and Meta-Analysis of Tomographic Findings (2000–2022). Children. 2023; 10(7):1169. https://doi.org/10.3390/children10071169
Chicago/Turabian StyleRiccò, Matteo, Silvia Corrado, Sara Palmieri, and Federico Marchesi. 2023. "Respiratory Syncytial Virus: A Systematic Review and Meta-Analysis of Tomographic Findings (2000–2022)" Children 10, no. 7: 1169. https://doi.org/10.3390/children10071169
APA StyleRiccò, M., Corrado, S., Palmieri, S., & Marchesi, F. (2023). Respiratory Syncytial Virus: A Systematic Review and Meta-Analysis of Tomographic Findings (2000–2022). Children, 10(7), 1169. https://doi.org/10.3390/children10071169







