Association between being Overweight in Young Childhood and during School Age and Puberty
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definition of Body Type
2.3. Confounders
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 March 2023).
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Al-Sulaiti, H.; Diboun, I.; Agha, M.V.; Mohamed, F.F.S.; Atkin, S.; Dömling, A.S.; Elrayess, M.A.; Mazloum, N.A. Metabolic Signature of Obesity-Associated Insulin Resistance and Type 2 Diabetes. J. Transl. Med. 2019, 17, 348. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and Cancer Risk: Emerging Biological Mechanisms and Perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, B.S.; Moss, A.; von Schnurbein, J.; Bickenbach, A.; Bollow, E.; Brandt, S.; Luetke-Brintrup, D.; Mühlig, Y.; Neef, M.; Ose, C.; et al. Do adolescents with extreme obesity differ according to previous treatment-seeking behavior? Youths with extreme obesity study (YES) cohort. Int. J. Obes. 2019, 43, 103–115. [Google Scholar] [CrossRef]
- Dhawan, D.; Sharma, S. Abdominal Obesity, Adipokines and Non-Communicable Diseases. J. Steroid. Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef]
- Chong, B.; Jayabaskaran, J.; Kong, G.; Chan, Y.H.; Chin, Y.H.; Goh, R.; Kannan, S.; Ng, C.H.; Loong, S.; Kueh, M.T.W.; et al. Trends and predictions of malnutrition and obesity in 204 countries and territories: An analysis of the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 57, 101850. [Google Scholar] [CrossRef]
- Tirthani, E.; Said, M.S.; Rehman, A. Genetics and Obesity; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wang, L.Y.; Chyen, D.; Lee, S.; Lowry, R. The Association Between Body Mass Index in Adolescence and Obesity in Adulthood. J. Adolesc. Health 2008, 42, 512–518. [Google Scholar] [CrossRef]
- Komiya, H.; Kurokawa, N. Changes in the physique of obese children in first grade elementary school students: Longitudinal study of six years. Jpn. J. Phys. Educ. Health Sport. Sci. 2018, 63, 495–504. [Google Scholar] [CrossRef][Green Version]
- Nakano, T.; Sei, M.; Ewis, A.A.; Munakata, H.; Onishi, C.; Nakahori, Y. Tracking Overweight and Obesity in Japanese Children; a Six Years Longitudinal Study. J. Med. Investig. 2010, 57, 114–123. [Google Scholar] [CrossRef][Green Version]
- Llewellyn, A.; Simmonds, M.; Owen, C.G.; Woolacott, N. Childhood Obesity as a Predictor of Morbidity in Adulthood: A Systematic Review and Meta-Analysis. Obes. Rev. 2016, 17, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Heslehurst, N.; Vieira, R.; Akhter, Z.; Bailey, H.; Slack, E.; Ngongalah, L.; Pemu, A.; Rankin, J. The Association between Maternal Body Mass Index and Child Obesity: A Systematic Review and Meta-Analysis. PLoS Med. 2019, 16, e1002817. [Google Scholar] [CrossRef] [PubMed]
- Correia-Costa, L.; Schaefer, F.; Afonso, A.C.; Correia, S.; Guimarães, J.T.; Guerra, A.; Barros, H.; Azevedo, A. Prenatal Alcohol Exposure Affects Renal Function in Overweight Schoolchildren: Birth Cohort Analysis. Pediatr. Nephrol. 2020, 35, 695–702. [Google Scholar] [CrossRef]
- Mizutani, T.; Suzuki, K.; Kondo, N.; Yamagata, Z. Association of Maternal Lifestyles Including Smoking during Pregnancy with Childhood Obesity. Obesity 2007, 15, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Education, Culture, Sports, Science and Technology. Available online: https://www.mext.go.jp/b_menu/toukei/chousa05/hoken/kekka/k_detail/1411711_00004.htm (accessed on 8 February 2023).
- Kuriyama, S.; Metoki, H.; Kikuya, M.; Obara, T.; Ishikuro, M.; Yamanaka, C.; Nagai, M.; Matsubara, H.; Kobayashi, T.; Sugawara, J.; et al. Cohort Profile: Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study (TMM BirThree Cohort Study): Rationale, Progress and Perspective. Int. J. Epidemiol. 2020, 49, 18–19m. [Google Scholar] [CrossRef] [PubMed]
- Ishikuro, M.; Obara, T.; Osanai, T.; Yamanaka, C.; Sato, Y.; Mizuno, S.; Miyashita, M.; Kikuya, M.; Sakurai, K.; Hozawa, A.; et al. Strategic Methods for Recruiting Grandparents: The Tohoku Medical Megabank Birth and Three-Generation Cohort Study. Tohoku J. Exp. Med. 2018, 246, 97–105. [Google Scholar] [CrossRef]
- Kuriyama, S.; Yaegashi, N.; Nagami, F.; Arai, T.; Kawaguchi, Y.; Osumi, N.; Sakaida, M.; Suzuki, Y.; Nakayama, K.; Hashizume, H.; et al. The Tohoku Medical Megabank Project: Design and Mission. J. Epidemiol. 2016, 26, 493–511. [Google Scholar] [CrossRef]
- Isojima, T.; Kato, N.; Ito, Y.; Kanzaki, S.; Murata, M. Growth Standard Charts for Japanese Children with Mean and Standard Deviation (SD) Values Based on the Year 2000 National Survey. Clin. Pediatr. Endocrinol. 2016, 25, 71–76. [Google Scholar] [CrossRef]
- Ogonowski, J.; Miazgowski, T. Intergenerational Transmission of Macrosomia in Women with Gestational Diabetes and Normal Glucose Tolerance. Eur. J. Obs. Gynecol. Reprod. Biol. 2015, 195, 113–116. [Google Scholar] [CrossRef]
- Kato, N.; Takimoto, H.; Sudo, N. The Cubic Functions for Spline Smoothed L, S and M Values for BMI Reference Data of Japanese Children. Clin. Pediatr. Endocrinol. 2011, 20, 47–49. [Google Scholar] [CrossRef]
- Ward, Z.J.; Long, M.W.; Resch, S.C.; Giles, C.M.; Cradock, A.L.; Gortmaker, S.L. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N. Engl. J. Med. 2017, 377, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Mulder, C.; Twisk, J.W.R.; Van Mechelen, W.; Chinapaw, M.J.M. Tracking of Childhood Overweight into Adulthood: A Systematic Review of the Literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfäffle, R.; Kiess, W.; Körner, A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 2018, 379, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch-Blüher, S.; Schwarz, P.; Klusmann, J.H. Childhood Obesity: Increased Risk for Cardiometabolic Disease and Cancer in Adulthood. Metabolism. 2019, 92, 147–152. [Google Scholar] [CrossRef]
- Sachdeva, P.; Ghosh, S.; Ghosh, S.; Han, S.; Banerjee, J.; Bhaskar, R.; Sinha, J.K. Childhood Obesity: A Potential Key Factor in the Development of Glioblastoma Multiforme. Life 2022, 12, 1673. [Google Scholar] [CrossRef]
- Evensen, E.; Emaus, N.; Kokkvoll, A.; Wilsgaard, T.; Furberg, A.S.; Skeie, G. The Relation between Birthweight, Childhood Body Mass Index, and Overweight and Obesity in Late Adolescence: A Longitudinal Cohort Study from Norway, the Tromsø Study, Fit Futures. BMJ Open 2017, 7, e015576. [Google Scholar] [CrossRef]
- Stettler, N.; Iotova, V. Early Growth Patterns and Long-Term Obesity Risk. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Briollais, L.; Rustand, D.; Allard, C.; Wu, Y.; Xu, J.; Rajan, S.G.; Hivert, M.F.; Doyon, M.; Bouchard, L.; McGowan, P.O.; et al. DNA Methylation Mediates the Association between Breastfeeding and Early-Life Growth Trajectories. Clin. Epigenetics 2021, 13, 231. [Google Scholar] [CrossRef]
- Mollon, J.; David, A.S.; Zammit, S.; Lewis, G.; Reichenberg, A. Course of Cognitive Development from Infancy to Early Adulthood in the Psychosis Spectrum. JAMA Psychiatry 2018, 75, 270–279. [Google Scholar] [CrossRef]
- Russell, S.J.; Hope, S.; Croker, H.; Packer, J.; Viner, R.M. Is It Possible to Model the Impact of Calorie-Reduction Interventions on Childhood Obesity at a Population Level and across the Range of Deprivation: Evidence from the Avon Longitudinal Study of Parents and Children (ALSPAC). PLoS ONE 2022, 17, e0263043. [Google Scholar] [CrossRef]
- Bruce, A.; Mojarrad, N.G.; Santorelli, G. Association of Anthropometric Measures across the Life-Course with Refractive Error and Ocular Biometry at Age 15 Years. BMC Ophthalmol. 2020, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Torres-Castillo, N.; Garaulet, M. Infancy and Childhood Obesity Grade Predicts Weight Loss in Adulthood: The Ontime Study. Nutrients 2021, 13, 2132. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sinha, J.; Raghunath, M. “Obesageing”: Linking Obesity & Ageing. Indian J. Med. Res. 2019, 149, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Roblin, L. Childhood Obesity: Food, Nutrient, and Eating-Habit Trends and Influences. Appl. Physiol. Nutr. Metab. 2007, 32, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Hills, A.P.; Andersen, L.B.; Byrne, N.M. Physical Activity and Obesity in Children. Br. J. Sport. Med. 2011, 45, 866–870. [Google Scholar] [CrossRef] [PubMed]
- De Giuseppe, R.; Di Napoli, I.; Porri, D.; Cena, H. Pediatric Obesity and Eating Disorders Symptoms: The Role of the Multidisciplinary Treatment. A Systematic Review. Front. Pediatr. 2019, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Dabas, A.; Seth, A. Prevention and Management of Childhood Obesity. Indian J. Pediatr. 2018, 85, 546–553. [Google Scholar] [CrossRef]
- Lifschitz, C. Early Life Factors Influencing the Risk of Obesity. Pediatr. Gastroenterol. Hepatol. Nutr. 2015, 18, 217–223. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Xiong, G.; Chao, T.; Jin, Q.; Liu, R.; Hao, L.; Wei, S.; Yang, N.; Yang, X. Introduction of Complementary Feeding before 4 Months of Age Increases the Risk of Childhood Overweight or Obesity: A Meta-Analysis of Prospective Cohort Studies. Nutr. Res. 2016, 36, 759–770. [Google Scholar] [CrossRef]
- Rayfield, S.; Plugge, E. Systematic Review and Meta-Analysis of the Association between Maternal Smoking in Pregnancy and Childhood Overweight and Obesity. J. Epidemiol. Community Health 2017, 71, 162–173. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Cuppari, C.; Salpietro, V.; Filippelli, M.; Trovato, A.; Gitto, E.; Salpietro, C.; Arrigo, T. Obesity and Breastfeeding: The Strength of Association. Women Birth. 2015, 28, 81–86. [Google Scholar] [CrossRef] [PubMed]

| Variables | n | Overall, n = 246 1 | Man, n = 119 1 | Woman, n = 127 1 | p-Value 2 |
|---|---|---|---|---|---|
| Length at birth (cm) | 227 | 49.54 (1.89) | 49.69 (1.90) | 49.39 (1.88) | 0.3 |
| Missing, n | 19 | 10 | 9 | ||
| Body weight at birth (g) | 227 | 3,111 (356) | 3,142 (336) | 3,081 (372) | 0.2 |
| Missing, n | 19 | 10 | 9 | ||
| Gestational age (weeks) | 227 | 39.56 (1.28) | 39.60 (1.06) | 39.51 (1.45) | 0.7 |
| Missing, n | 19 | 10 | 9 | ||
| Body types at birth | 227 | >0.9 | |||
| LGA, n (%) | 36 (15.9%) | 16 (14.7%) | 20 (16.9%) | ||
| AGA, n (%) | 183 (80.6%) | 89 (81.7%) | 94 (79.7%) | ||
| SGA, n (%) | 8 (3.5%) | 4 (3.7%) | 4 (3.4%) | ||
| Missing, n | 19 | 10 | 9 | ||
| Height at age 18–24 months (cm) | 191 | 81.08 (3.01) | 81.62 (2.81) | 80.56 (3.12) | 0.015 * |
| Missing, n | 55 | 26 | 29 | ||
| Body weight at age 18–24 months (kg) | 191 | 10.74 (1.20) | 11.04 (1.21) | 10.45 (1.11) | 0.002 * |
| Missing, n | 55 | 26 | 29 | ||
| BMI at age 18–24 months (kg/m2) | 191 | 16.31 (1.20) | 16.55 (1.29) | 16.07 (1.07) | 0.009 * |
| Missing, n | 55 | 26 | 29 | ||
| Body types at age 18–24 months | 191 | 0.6 | |||
| Normal weight, n (%) | 155 (81.2%) | 77 (82.8%) | 78 (79.6%) | ||
| Overweight, n (%) | 36 (18.8%) | 16 (17.2%) | 20 (20.4%) | ||
| Missing, n | 55 | 26 | 29 | ||
| Height at age 36–47 months (cm) | 211 | 96.8 (3.9) | 96.9 (3.7) | 96.7 (4.0) | >0.9 |
| Missing, n | 35 | 16 | 19 | ||
| Body weight at age 36–47 months (kg) | 211 | 14.93 (1.76) | 14.91 (1.56) | 14.94 (1.94) | >0.9 |
| Missing, n | 35 | 16 | 19 | ||
| BMI at age 36–47 months (kg/m2) | 211 | 15.90 (1.17) | 15.85 (0.99) | 15.94 (1.32) | 0.7 |
| Missing, n | 35 | 16 | 19 | ||
| Body types at age 36–47 months | 211 | >0.9 | |||
| Normal weight, n (%) | 160 (75.8%) | 78 (75.7%) | 82 (75.9%) | ||
| Overweight, n (%) | 51 (24.2%) | 25 (24.3%) | 26 (24.1%) | ||
| Missing, n | 35 | 16 | 19 | ||
| Height at age 6 years (cm) | 179 | 116.7 (5.2) | 117.0 (5.1) | 116.3 (5.3) | 0.6 |
| Missing, n | 67 | 28 | 39 | ||
| Body weight at age 6 years (kg) | 179 | 22.2 (4.2) | 22.1 (4.0) | 22.3 (4.4) | 0.6 |
| Missing, n | 67 | 28 | 39 | ||
| BMI at age 6 years (kg/m2) | 179 | 16.20 (2.05) | 16.01 (1.86) | 16.38 (2.22) | 0.4 |
| Missing, n | 67 | 28 | 39 | ||
| Body types at age 6 years | 179 | 0.089 | |||
| Normal weight | 149 (83.2%) | 80 (87.9%) | 69 (78.4%) | ||
| Overweight | 30 (16.8%) | 11 (12.1%) | 19 (21.6%) | ||
| Missing, n | 67 | 28 | 39 | ||
| Height at age 11 years (cm) | 174 | 146 (7) | 145 (8) | 148 (7) | 0.005 * |
| Missing, n | 72 | 30 | 42 | ||
| Body weight at age 11 years (kg) | 174 | 41 (9) | 40 (10) | 42 (8) | 0.026 * |
| Missing, n | 72 | 30 | 42 | ||
| BMI at age 11 years (kg/m2) | 174 | 18.9 (3.2) | 18.7 (3.3) | 19.1 (3.1) | 0.2 |
| Missing, n | 72 | 30 | 42 | ||
| Body types at age 11 years | 174 | 0.4 | |||
| Normal weight | 139 (79.9%) | 69 (77.5%) | 70 (82.4%) | ||
| Overweight | 35 (20.1%) | 20 (22.5%) | 15 (17.6%) | ||
| Missing, n | 72 | 30 | 42 | ||
| Height at age 14 years (cm) | 176 | 161 (7) | 166 (6) | 157 (6) | <0.001 * |
| Missing, n | 70 | 32 | 38 | ||
| Body weight at age 14 years (kg) | 176 | 54 (10) | 56 (12) | 52 (8) | 0.037 * |
| Missing, n | 70 | 32 | 38 | ||
| BMI at age 14 years (kg/m2) | 176 | 20.9 (3.5) | 20.3 (3.7) | 21.4 (3.2) | 0.001 * |
| Missing, n | 70 | 32 | 38 | ||
| Body types at age 14 years | 176 | 0.15 | |||
| Normal weight, n (%) | 151 (85.8%) | 78 (89.7%) | 73 (82.0%) | ||
| Overweight, n (%) | 25 (14.2%) | 9 (10.3%) | 16 (18.0%) | ||
| Missing, n | 70 | 32 | 38 |
| Variables | n | Overall, n = 246 1 | Man, n = 119 1 | Woman, n = 127 1 | p-Value 2 |
|---|---|---|---|---|---|
| Maternal age at the time of childbirth (years) | 227 | 25.0 (3.5) | 24.8 (3.4) | 25.1 (3.7) | 0.7 |
| Missing, n | 19 | 9 | 10 | ||
| Maternal parity | 229 | 0.5 | |||
| Multipara, n (%) | 45 (19.7%) | 20 (18.0%) | 25 (21.2%) | ||
| Primipara, n (%) | 184 (80.3%) | 91 (82.0%) | 93 (78.8%) | ||
| Missing, n | 17 | 8 | 9 | ||
| Maternal BMI when pregnancy was detected (kg/m2) | 178 | 20.70 (2.94) | 20.57 (2.97) | 20.83 (2.93) | 0.5 |
| Missing, n | 68 | 35 | 33 | ||
| Alcohol consumption when pregnancy was detected | 226 | 0.3 | |||
| Yes, n (%) | 17 (7.5%) | 9 (8.3%) | 8 (6.8%) | ||
| No, n (%) | 168 (74.3%) | 75 (69.4%) | 93 (78.8%) | ||
| Unknown, n (%) | 41 (18.1%) | 24 (22.2%) | 17 (14.4%) | ||
| Missing, n | 20 | 11 | 9 | ||
| Smoking when pregnancy was detected | 227 | 0.2 | |||
| Yes, n (%) | 18 (7.9%) | 11 (10.1%) | 7 (5.9%) | ||
| No, n (%) | 172 (75.8%) | 77 (70.6%) | 95 (80.5%) | ||
| Unknown | 37 (16.3%) | 21 (19.3%) | 16 (13.6%) | ||
| Missing, n | 19 | 10 | 9 |
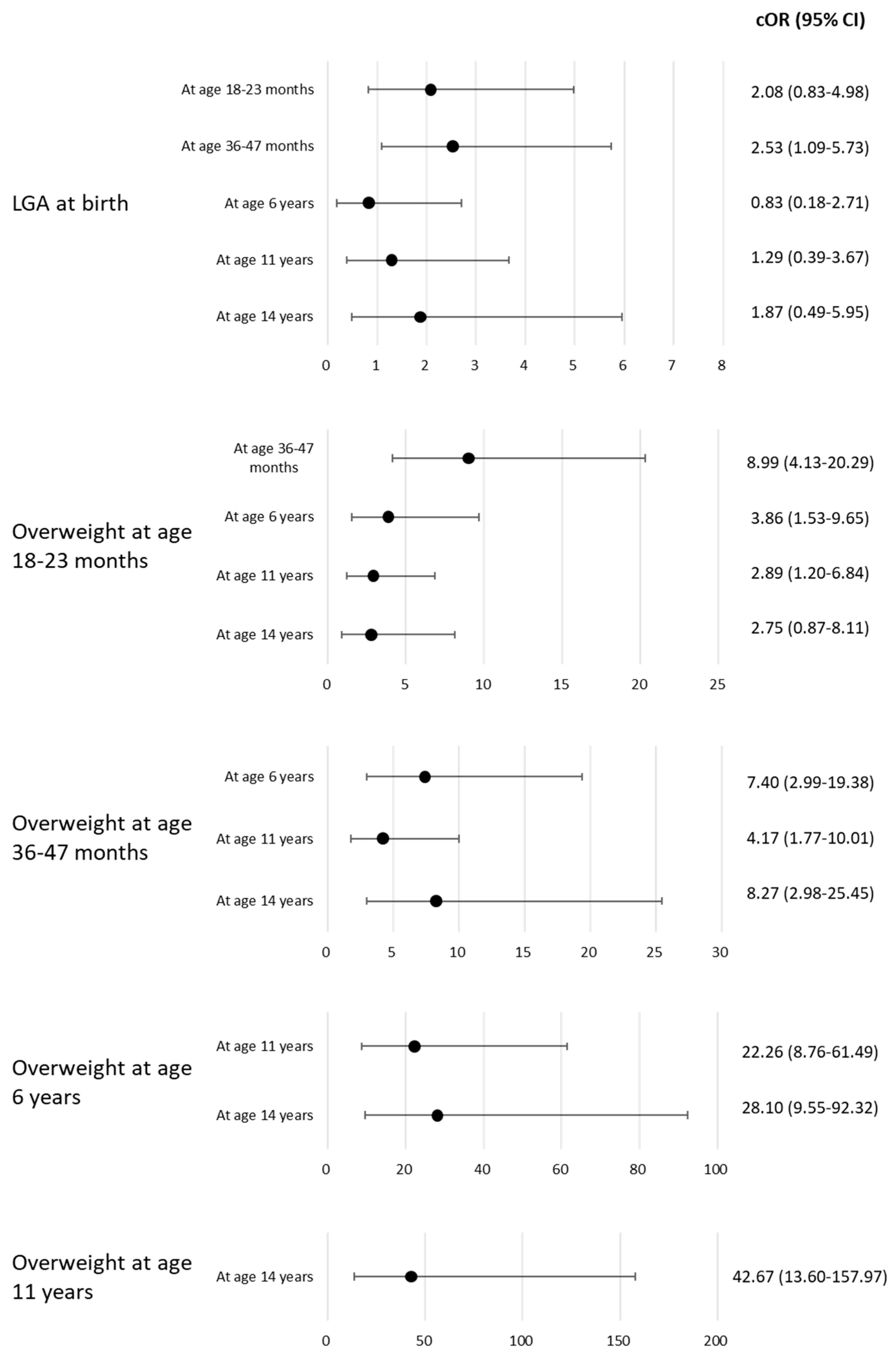
| Outcome | At Age 18–23 Months | At Age 36–47 Months | At Age 6 Years | At Age 11 Years | At Age 14 Years | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | n (%) | aOR * (95% CI) | n (%) | OR * (95% CI) | n (%) | OR * (95% CI) | n (%) | OR * (95% CI) | n (%) | OR *(95% CI) |
| At birth | 120 | 124 | 92 | 86 | 93 | |||||
| AGA | 97 | 1.00 | 101 | 1.00 | 78 | 1.00 | 72 | 1.00 | 77 | 1.00 |
| (80.8) | (Ref.) | (81.5) | (Ref.) | (84.8) | (Ref.) | (83.7) | (Ref.) | (82.8) | (Ref.) | |
| LGA | 19 | 1.26 | 18 | 2.59 | 11 | 0.00 | 11 | 0.45 | 12 | 5.38 |
| (15.8) | (0.32–4.22) | (14.5) | (0.80–7.98) | (12.0) | (N.A. −3.06 × 1047) | (12.8) | (0.02–3.35) | (82.8) | (0.17–115.71) | |
| SGA | 4 | 2.40 × 10−8 | 5 | 1.29 | 3 | 1.05 × 10−7 | 3 | 5.43 | 4 | 103.64 |
| (3.3) | (N.A. −1.13 × 10,148) | (4.0) | (0.06–9.76) | (3.3) | (N.A. −2.41 × 10,184) | (3.5) | (0.20–87.69) | (4.3) | (1.89–10,610.63) | |
| At age 18–23 months | 119 | 95 | 88 | 91 | ||||||
| Normal weight | 93 | 1.00 | 73 | 1.00 | 66 | 1.00 | 73 | 1.00 | ||
| (78.2) | (Ref.) | (76.8) | (Ref.) | (75.0) | (Ref.) | (80.2) | (Ref.) | |||
| Overweight | 26 | 13.42 | 22 | 6.94 | 22 | 5.22 | 18 | 1.61 | ||
| (21.8) | (4.46–45.42) | (23.2) | (1.64–33.46) | (25.0) | (1.25–24.79) | (19.8) | (0.14–14.96) | |||
| At age 36–47 months | 89 | 85 | 91 | |||||||
| Normal weight | 69 | 1.00 | 64 | 1.00 | 71 | 1.00 | ||||
| (77.5) | (Ref.) | (75.3) | (Ref.) | (78.0) | (Ref.) | |||||
| Overweight | 20 | 9.55 | 21 | 3.22 | 20 | 0.29 | ||||
| (22.5) | (1.85–61.41) | (24.7) | (0.70–15.14) | (22.0) | (0.01–4.47) | |||||
| At age 6 years | 91 | 80 | ||||||||
| Normal weight | 80 | 1.00 | 74 | 1.00 | ||||||
| (87.9) | (Ref.) | (92.5) | (Ref.) | |||||||
| Overweight | 11 | 1021.24 | 6 | 13.15 | ||||||
| At age 11 years | (12.1) | (48.96–81,487.89) | 79 | (0.97–261.80) | ||||||
| Normal weight | 69 | 1.00 | ||||||||
| (87.3) | (Ref.) | |||||||||
| Overweight | 10 | 60.46 | ||||||||
| (12.7) | (2.92–5001.37) | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinoda, G.; Nagaoka, Y.; Ueno, F.; Kurokawa, N.; Takahashi, I.; Onuma, T.; Noda, A.; Murakami, K.; Ishikuro, M.; Obara, T.; et al. Association between being Overweight in Young Childhood and during School Age and Puberty. Children 2023, 10, 909. https://doi.org/10.3390/children10050909
Shinoda G, Nagaoka Y, Ueno F, Kurokawa N, Takahashi I, Onuma T, Noda A, Murakami K, Ishikuro M, Obara T, et al. Association between being Overweight in Young Childhood and during School Age and Puberty. Children. 2023; 10(5):909. https://doi.org/10.3390/children10050909
Chicago/Turabian StyleShinoda, Genki, Yudai Nagaoka, Fumihiko Ueno, Naoyuki Kurokawa, Ippei Takahashi, Tomomi Onuma, Aoi Noda, Keiko Murakami, Mami Ishikuro, Taku Obara, and et al. 2023. "Association between being Overweight in Young Childhood and during School Age and Puberty" Children 10, no. 5: 909. https://doi.org/10.3390/children10050909
APA StyleShinoda, G., Nagaoka, Y., Ueno, F., Kurokawa, N., Takahashi, I., Onuma, T., Noda, A., Murakami, K., Ishikuro, M., Obara, T., Metoki, H., Sugawara, J., & Kuriyama, S. (2023). Association between being Overweight in Young Childhood and during School Age and Puberty. Children, 10(5), 909. https://doi.org/10.3390/children10050909






