Hybrid Palliation for Hypoplastic Borderline Left Ventricle: One More Chance to Biventricular Repair
Abstract
1. Introduction
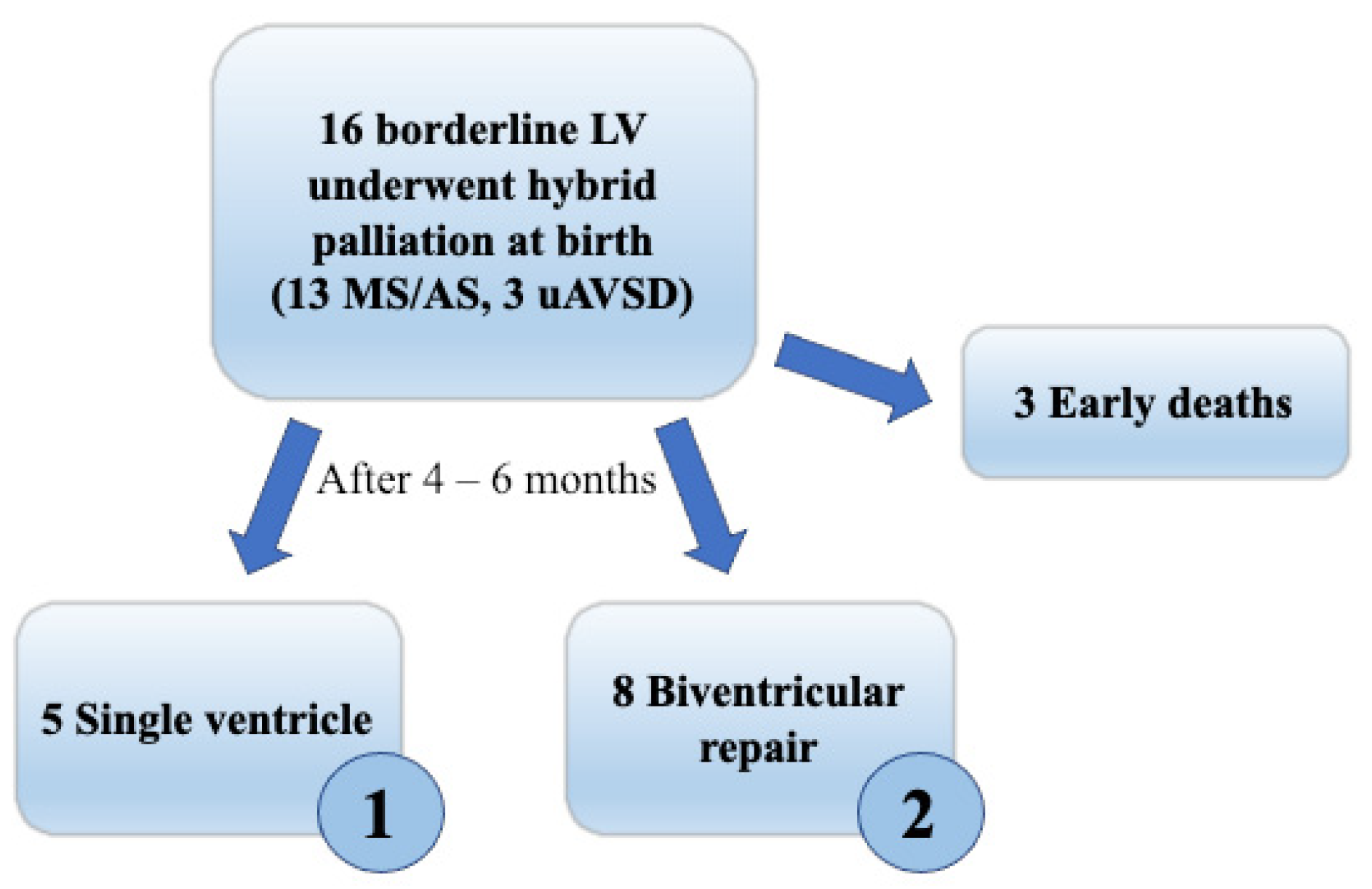
2. Materials and Methods
3. Results
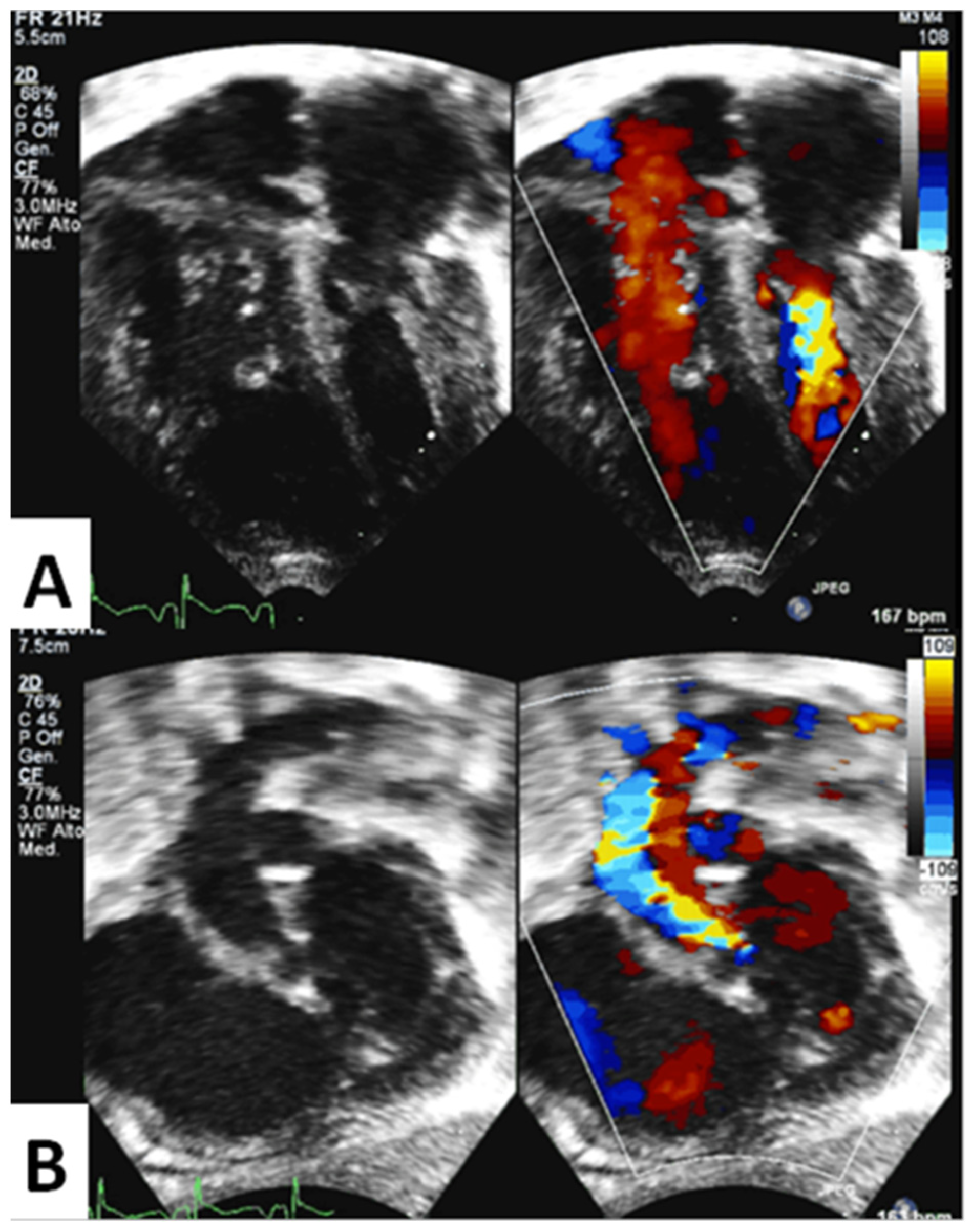
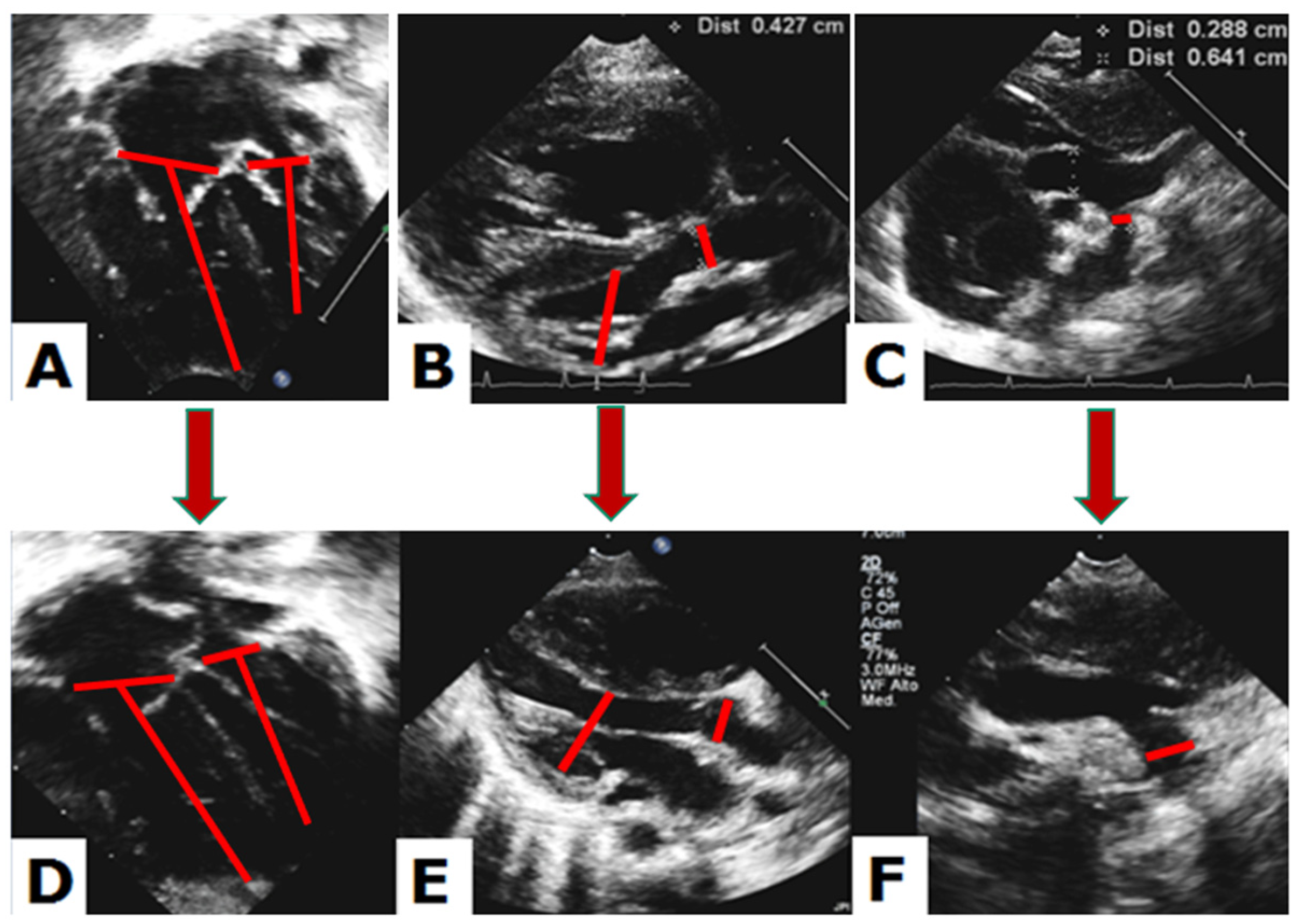
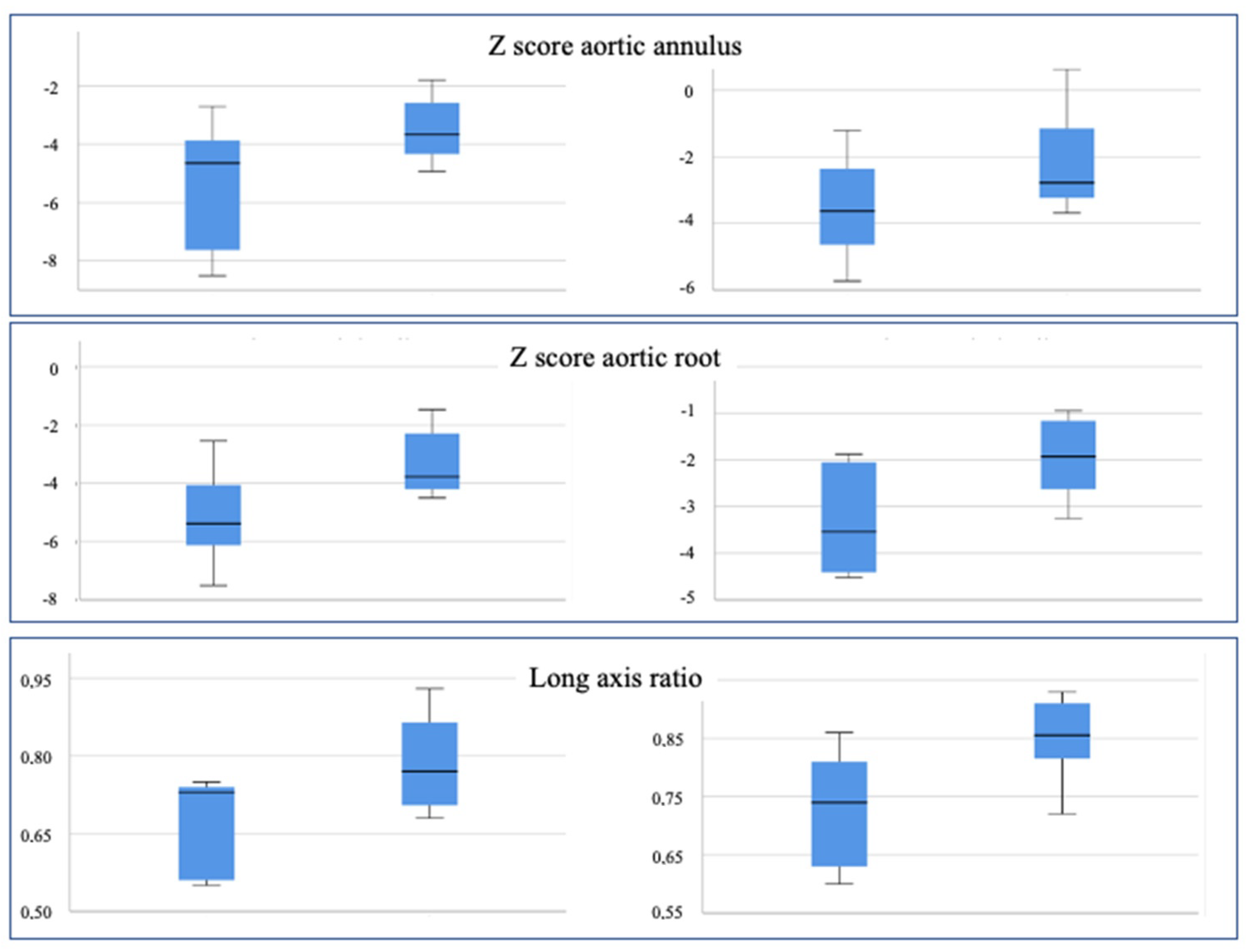
3.1. Quantitative Echocardiographic Evaluation
3.2. Qualitative Echocardiographic Evaluation
3.3. Application of Pre-Existing Risk Scores for Biventricular Repair
3.4. Follow-Up
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kearney, D.L. The pathological spectrum of left-ventricular hypoplasia. Semin. Cardiothorac. Vasc. Anesth. 2013, 17, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Hickey, E.J.; Caldarone, C.A.; McCrindle, B.W. Left ventricular hypoplasia: A spectrum of disease involving the left ventricular outflow tract, aortic valve, and aorta. J. Am. Coll. Cardiol. 2012, 59, S43–S54. [Google Scholar] [CrossRef]
- Tchervenkov, C.I.; Jacobs, J.P.; Weinberg, P.M.; Aiello, V.D.; Beland, M.J.; Colan, S.D.; Elliott, M.J.; Franklin, R.C.; Gaynor, J.W.; Krogmann, O.N.; et al. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol. Young 2006, 16, 339–368. [Google Scholar] [CrossRef]
- Feinstein, J.A.; Benson, D.W.; Dubin, A.M.; Cohen, M.S.; Maxey, D.M.; Mahle, W.T.; Pahl, E.; Villafane, J.; Bhatt, A.B.; Peng, L.F.; et al. Hypoplastic left heart syndrome: Current considerations and expectations. J. Am. Coll. Cardiol. 2012, 59, S1–S42. [Google Scholar] [CrossRef] [PubMed]
- Serraf, A.; Piot, J.D.; Bonnet, N.; Lacour-Gayet, F.; Touchot, A.; Bruniaux, J.; Belli, E.; Galletti, L.; Planche, C. Biventricular repair approach in ducto-dependent neonates with hypoplastic but morphologically normal left ventricle. J. Am. Coll. Cardiol. 1999, 33, 827–834. [Google Scholar] [CrossRef]
- Tchervenkov, C.I. Indications, criterions, and principles for biventricular repair. Cardiol. Young 2004, 14 (Suppl. S1), 97–100. [Google Scholar] [CrossRef]
- Ashburn, D.A.; McCrindle, B.W.; Tchervenkov, C.I.; Jacobs, M.L.; Lofland, G.K.; Bove, E.L.; Spray, T.L.; Williams, W.G.; Blackstone, E.H. Outcomes after the Norwood operation in neonates with critical aortic stenosis or aortic valve atresia. J. Thorac. Cardiovasc. Surg. 2003, 125, 1070–1082. [Google Scholar] [CrossRef]
- Baba, K.; Kotani, Y.; Chetan, D.; Chaturvedi, R.R.; Lee, K.J.; Benson, L.N.; Grosse-Wortmann, L.; Van Arsdell, G.S.; Caldarone, C.A.; Honjo, O. Hybrid versus Norwood strategies for single-ventricle palliation. Circulation 2012, 126, S123–S131. [Google Scholar] [CrossRef]
- Akinturk, H.; Michel-Behnke, I.; Valeske, K.; Mueller, M.; Thul, J.; Bauer, J.; Hagel, K.J.; Schranz, D. Hybrid transcatheter-surgical palliation: Basis for univentricular or biventricular repair: The Giessen experience. Pediatr. Cardiol. 2007, 28, 79–87. [Google Scholar] [CrossRef]
- Galantowicz, M. In favor of the Hybrid Stage 1 as the initial palliation for hypoplastic left heart syndrome. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2013, 16, 62–64. [Google Scholar] [CrossRef]
- Galantowicz, M.; Cheatham, J.P. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr. Cardiol. 2005, 26, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Galantowicz, M.; Cheatham, J.P.; Phillips, A.; Cua, C.L.; Hoffman, T.M.; Hill, S.L.; Rodeman, R. Hybrid approach for hypoplastic left heart syndrome: Intermediate results after the learning curve. Ann. Thorac. Surg. 2008, 85, 2063–2070. [Google Scholar] [CrossRef]
- Gibbs, J.L.; Wren, C.; Watterson, K.G.; Hunter, S.; Hamilton, J.R. Stenting of the arterial duct combined with banding of the pulmonary arteries and atrial septectomy or septostomy: A new approach to palliation for the hypoplastic left heart syndrome. Br. Heart J. 1993, 69, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.; Colan, S.D.; Frommelt, P.C.; Ensing, G.J.; Kendall, K.; Younoszai, A.K.; Lai, W.W.; Geva, T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J. Am. Soc. Echocardiogr. 2010, 23, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Colan, S.D.; McElhinney, D.B.; Crawford, E.C.; Keane, J.F.; Lock, J.E. Validation and re-evaluation of a discriminant model predicting anatomic suitability for biventricular repair in neonates with aortic stenosis. J. Am. Coll. Cardiol. 2006, 47, 1858–1865. [Google Scholar] [CrossRef]
- van Son, J.A.; Phoon, C.K.; Silverman, N.H.; Haas, G.S. Predicting feasibility of biventricular repair of right-dominant unbalanced atrioventricular canal. Ann. Thorac. Surg. 1997, 63, 1657–1663. [Google Scholar] [CrossRef]
- Rhodes, L.A.; Colan, S.D.; Perry, S.B.; Jonas, R.A.; Sanders, S.P. Predictors of survival in neonates with critical aortic stenosis. Circulation 1991, 84, 2325–2335. [Google Scholar] [CrossRef]
- Pesarin, F.; Salmaso, L. Permutation Tests for Complex Data: Theory, Applications and Software; Wiley: Hoboken, NJ, USA, 2010; pp. 1–11. [Google Scholar]
- Banka, P.; Schaetzle, B.; Komarlu, R.; Emani, S.; Geva, T.; Powell, A.J. Cardiovascular magnetic resonance parameters associated with early transplant-free survival in children with small left hearts following conversion from a univentricular to biventricular circulation. J. Cardiovasc. Magn. Reson. 2014, 16, 73. [Google Scholar] [CrossRef]
- Emani, S.M. Biventricular Repair in Patients with Borderline Left Heart-The “Growing” Experience. World J. Pediatr. Congenit. Heart Surg. 2019, 10, 18–19. [Google Scholar] [CrossRef]
- Schranz, D. Hybrid approach in hypoplastic left heart syndrome. Heart 2014, 100, 750–751. [Google Scholar] [CrossRef]
- Schwartz, M.L.; Gauvreau, K.; Geva, T. Predictors of outcome of biventricular repair in infants with multiple left heart obstructive lesions. Circulation 2001, 104, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Corno, A.F. Borderline left ventricle. Eur. J. Cardiothorac. Surg. 2005, 27, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mart, C.R.; Eckhauser, A.W. Development of an echocardiographic scoring system to predict biventricular repair in neonatal hypoplastic left heart complex. Pediatr. Cardiol. 2014, 35, 1456–1466. [Google Scholar] [CrossRef]
- Plymale, J.M.; Frommelt, P.C.; Nugent, M.; Simpson, P.; Tweddell, J.S.; Shillingford, A.J. The Infant with Aortic Arch Hypoplasia and Small Left Heart Structures: Echocardiographic Indices of Mitral and Aortic Hypoplasia Predicting Successful Biventricular Repair. Pediatr. Cardiol. 2017, 38, 1296–1304. [Google Scholar] [CrossRef]
- Grosse-Wortmann, L.; Yun, T.J.; Al-Radi, O.; Kim, S.; Nii, M.; Lee, K.J.; Redington, A.; Yoo, S.J.; van Arsdell, G. Borderline hypoplasia of the left ventricle in neonates: Insights for decision-making from functional assessment with magnetic resonance imaging. J. Thorac. Cardiovasc. Surg. 2008, 136, 1429–1436. [Google Scholar] [CrossRef]
- Cohen, M.S.; Jegatheeswaran, A.; Baffa, J.M.; Gremmels, D.B.; Overman, D.M.; Caldarone, C.A.; McCrindle, B.W.; Mertens, L. Echocardiographic features defining right dominant unbalanced atrioventricular septal defect: A multi-institutional Congenital Heart Surgeons’ Society study. Circ. Cardiovasc. Imaging 2013, 6, 508–513. [Google Scholar] [CrossRef]
- Jegatheeswaran, A.; Pizarro, C.; Caldarone, C.A.; Cohen, M.S.; Baffa, J.M.; Gremmels, D.B.; Mertens, L.; Morell, V.O.; Williams, W.G.; Blackstone, E.H.; et al. Echocardiographic definition and surgical decision-making in unbalanced atrioventricular septal defect: A Congenital Heart Surgeons’ Society multiinstitutional study. Circulation 2010, 122, S209–S215. [Google Scholar] [CrossRef]
- Overman, D.M.; Baffa, J.M.; Cohen, M.S.; Mertens, L.; Gremmels, D.B.; Jegatheeswaran, A.; McCrindle, B.W.; Blackstone, E.H.; Morell, V.O.; Caldarone, C.; et al. Unbalanced atrioventricular septal defect: Definition and decision making. World J. Pediatr. Congenit. Heart Surg. 2010, 1, 91–96. [Google Scholar] [CrossRef]
- Szwast, A.L.; Marino, B.S.; Rychik, J.; Gaynor, J.W.; Spray, T.L.; Cohen, M.S. Usefulness of left ventricular inflow index to predict successful biventricular repair in right-dominant unbalanced atrioventricular canal. Am. J. Cardiol. 2011, 107, 103–109. [Google Scholar] [CrossRef]
- Ballard, G.; Tibby, S.; Miller, O.; Krasemann, T.; Rosenthal, E.; Anderson, D.; Austin, C.; Qureshi, S.; Simpson, J. Growth of left heart structures following the hybrid procedure for borderline hypoplastic left heart. Eur. J. Echocardiogr. 2010, 11, 870–874. [Google Scholar] [CrossRef]
- Cavigelli-Brunner, A.; Bauersfeld, U.; Pretre, R.; Kretschmar, O.; Oxenius, A.; Valsangiacomo Buechel, E.R. Outcome of biventricular repair in infants with multiple left heart obstructive lesions. Pediatr. Cardiol. 2012, 33, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Kaplinski, M.; Cohen, M.S. Characterising adequacy or inadequacy of the borderline left ventricle: What tools can we use? Cardiol. Young 2015, 25, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Tuo, G.; Khambadkone, S.; Tann, O.; Kostolny, M.; Derrick, G.; Tsang, V.; Sullivan, I.; Marek, J. Obstructive left heart disease in neonates with a “borderline” left ventricle: Diagnostic challenges to choosing the best outcome. Pediatr. Cardiol. 2013, 34, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
| Group 1 | Group 2 | ||
|---|---|---|---|
| Gender | 1 M–4 F | 4 M–4 F | |
| Weight at birth | 3.03 ± 0.54 Kg | 3.25 ± 0.39 Kg | p 0.42 |
| Body surface area (BSA) at birth | 0.19 ± 0.01 | 0.20 ± 0.01 | p 0.61 |
| Weight at 5 months | 5.58 ± 1.1 Kg | 4.94 ± 0.64 Kg | p 0.20 p 0.11 OR 1.1 |
| BSA at 5 months | 0.31 ± 0.04 | 0.28 ± 0.03 | |
| Age at first intervention | 2.3 days | 2.3 days | |
| Mitral/aortic stenosis | 4 | 7 | |
| Unbalanced AV canal | 1 | 1 | |
| Endocardial fibroelastosis (EFE) | 3/5 | 5/8 | |
| Bicuspid aortic valve | 2/5 | 5/8 | |
| Single papillary muscle | 0/5 | 4/8 | OR 4.5 |
| Left superior vena cava | 0/5 | 5/8 | |
| Restrictive atrial septal defect | 2/5 | 5/8 | |
| Death during long-term follow up | 2/5 | 1/8 |
| Parameter | Group 1 at Birth | Group 2 at Birth | p-Value | Group 1 after 5 Months | Group 2 after 5 Months | p-Value |
|---|---|---|---|---|---|---|
| Aortic Annulus (z score) | −5.47 | −3.49 | 0.071 | −3.52 | −2.17 | 0.169 |
| Aortic Root (z score) | −5.13 | −3.31 | 0.053 | −3.28 | −1.65 | 0.06 |
| Aortic Root (mm/m2) | 2.79 | 3.33 | 0.071 | 2.61 | 3.31 | 0.041 |
| Long axis ratio | 0.67 | 0.79 | 0.054 | 0.73 | 0.85 | 0.032 |
| Mitral Annulus (z score) | −4.21 | −4.13 | 0.880 | −3.81 | −2.57 | 0.132 |
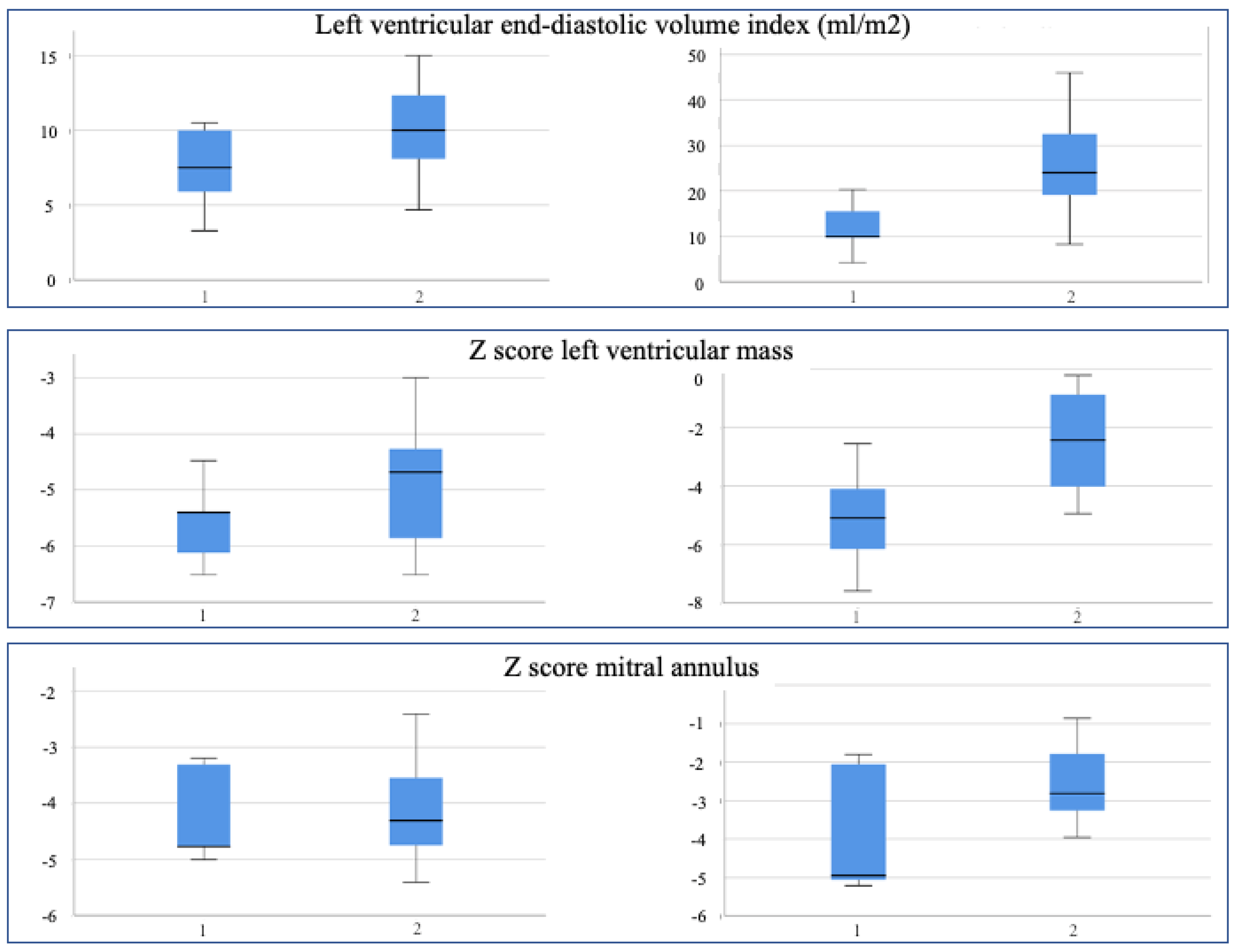
| LV Mass (z score) | −5.58 | −4.89 | 0.271 | −5.10 | −2.48 | 0.032 |
| LV Mass (g/m2) | 20.20 | 24.00 | 0.260 | 29.00 | 45.38 | 0.068 |
| Indexed EDV (ml/m2) | 7.44 | 10.08 | 0.168 | 11.94 | 25.69 | 0.034 |
| Indexed ESV (ml/m2) | 4.00 | 3.96 | 0.970 | 5.20 | 11.78 | 0.023 |
| Transverse Arch (z score) | −4.79 | −5.44 | 0.473 | −4.17 | −4.08 | 0.944 |
| Distal Arch (z score) | −4.20 | −4.70 | 0.646 | −2.55 | −3.86 | 0.403 |
| Aortic Isthmus (z score) | −4.11 | −5.41 | 0.273 | −3.08 | −3.80 | 0.570 |
| Parameter | Group 1 at Birth | Group 1 after 4 Months | p-Value | Group 2 at Birth | Group 2 after 4 Months | p-Value |
|---|---|---|---|---|---|---|
| Aortic Annulus (z score) | −5.47 | −3.52 | 0.043 | −3.49 | −2.17 | 0.036 |
| Aortic Root (z score) | −5.13 | −3.28 | 0.042 | −3.31 | −1.65 | 0.017 |
| Long axis ratio | 0.67 | 0.73 | 0.042 | 0.79 | 0.85 | 0.207 |
| Mitral Annulus (z score) | −4.21 | −3.81 | 0.502 | −4.13 | −2.57 | 0.012 |
| LV Mass (z score) | −5.58 | −5.10 | 0.345 | −4.89 | −2.48 | 0.012 |
| LV Mass (g/m2) | 20,20 | 29.00 | 0.078 | 24,00 | 45.38 | 0.012 |
| Indexed EDV (ml/m2) | 7.44 | 11.94 | 0.043 | 10,08 | 25.69 | 0.012 |
| Indexed ESV (ml/m2) | 4.00 | 5.20 | 0.08 | 3.96 | 11.78 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oreto, L.; Mandraffino, G.; Calaciura, R.E.; Poli, D.; Gitto, P.; Saitta, M.B.; Bellanti, E.; Carerj, S.; Zito, C.; Iorio, F.S.; et al. Hybrid Palliation for Hypoplastic Borderline Left Ventricle: One More Chance to Biventricular Repair. Children 2023, 10, 859. https://doi.org/10.3390/children10050859
Oreto L, Mandraffino G, Calaciura RE, Poli D, Gitto P, Saitta MB, Bellanti E, Carerj S, Zito C, Iorio FS, et al. Hybrid Palliation for Hypoplastic Borderline Left Ventricle: One More Chance to Biventricular Repair. Children. 2023; 10(5):859. https://doi.org/10.3390/children10050859
Chicago/Turabian StyleOreto, Lilia, Giuseppe Mandraffino, Rita Emanuela Calaciura, Daniela Poli, Placido Gitto, Michele Benedetto Saitta, Ermanno Bellanti, Scipione Carerj, Concetta Zito, Fiore Salvatore Iorio, and et al. 2023. "Hybrid Palliation for Hypoplastic Borderline Left Ventricle: One More Chance to Biventricular Repair" Children 10, no. 5: 859. https://doi.org/10.3390/children10050859
APA StyleOreto, L., Mandraffino, G., Calaciura, R. E., Poli, D., Gitto, P., Saitta, M. B., Bellanti, E., Carerj, S., Zito, C., Iorio, F. S., Guccione, P., & Agati, S. (2023). Hybrid Palliation for Hypoplastic Borderline Left Ventricle: One More Chance to Biventricular Repair. Children, 10(5), 859. https://doi.org/10.3390/children10050859








