Perinatal Characteristics and the Sensitization to Cow Milk, Egg Whites and Wheat in Children up to 3 Years of Age
Abstract
1. Introduction
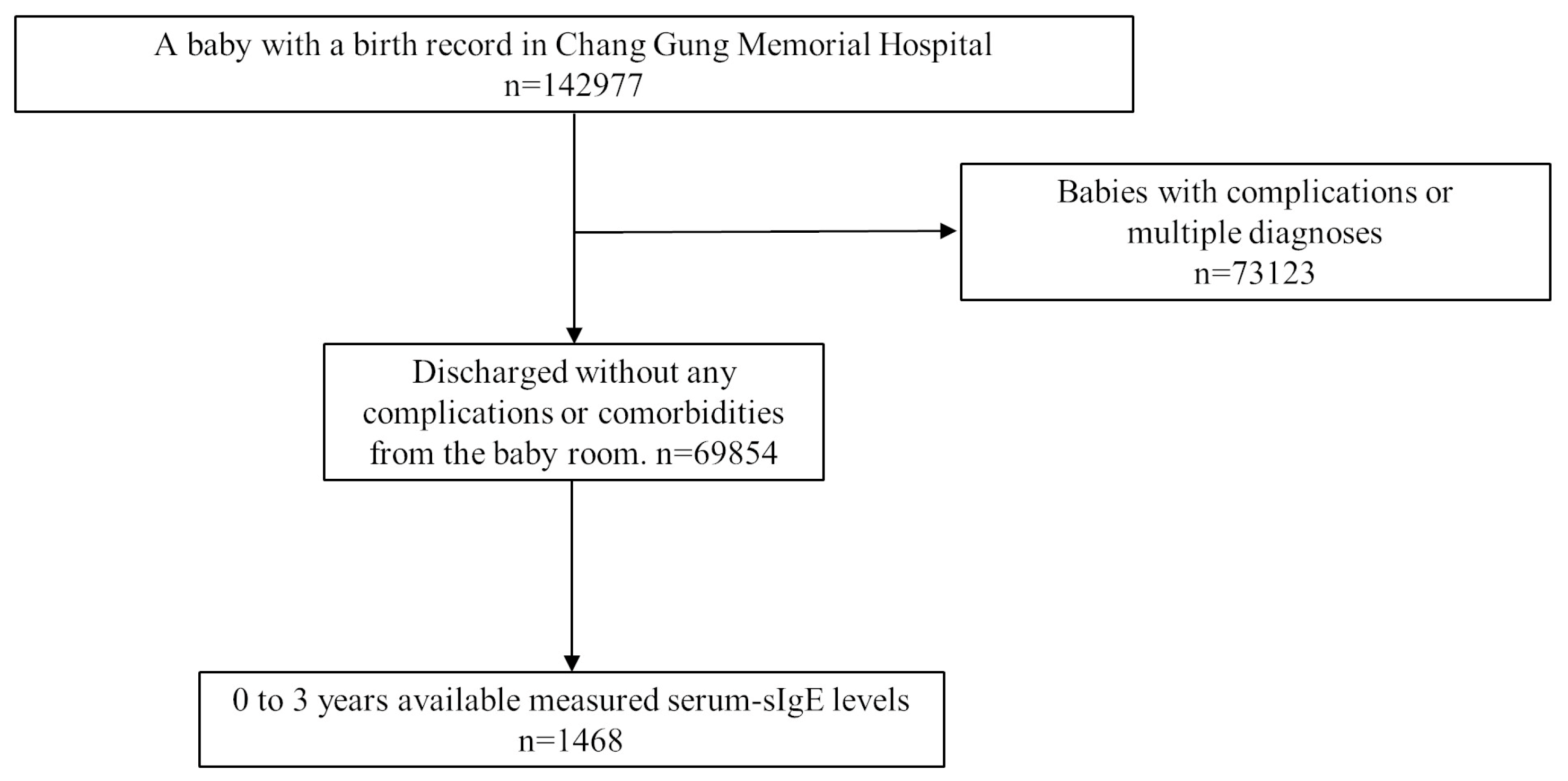
2. Methods
3. Results
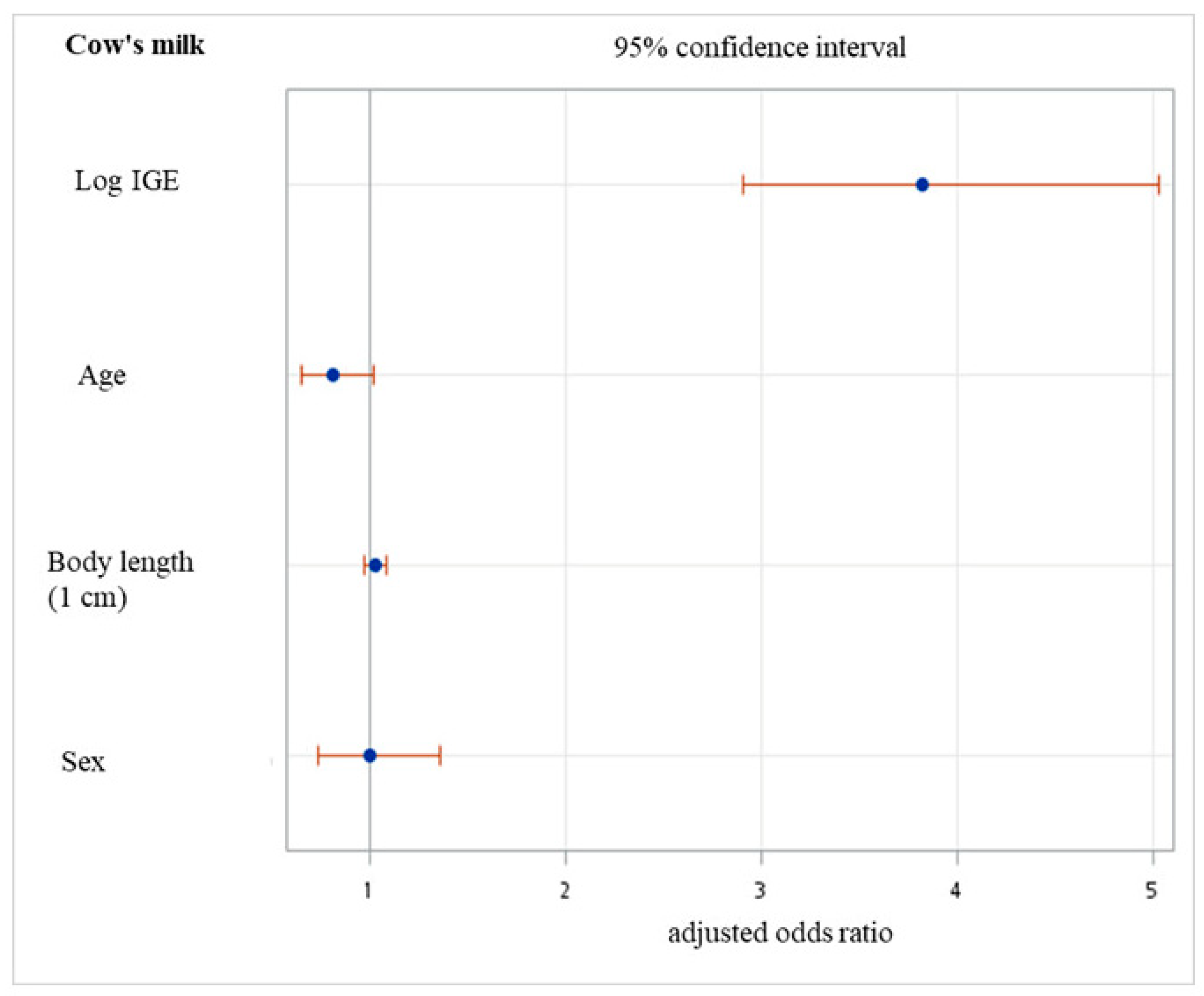
3.1. Is There a Role of Perinatal Characteristics for sIgE of CM?
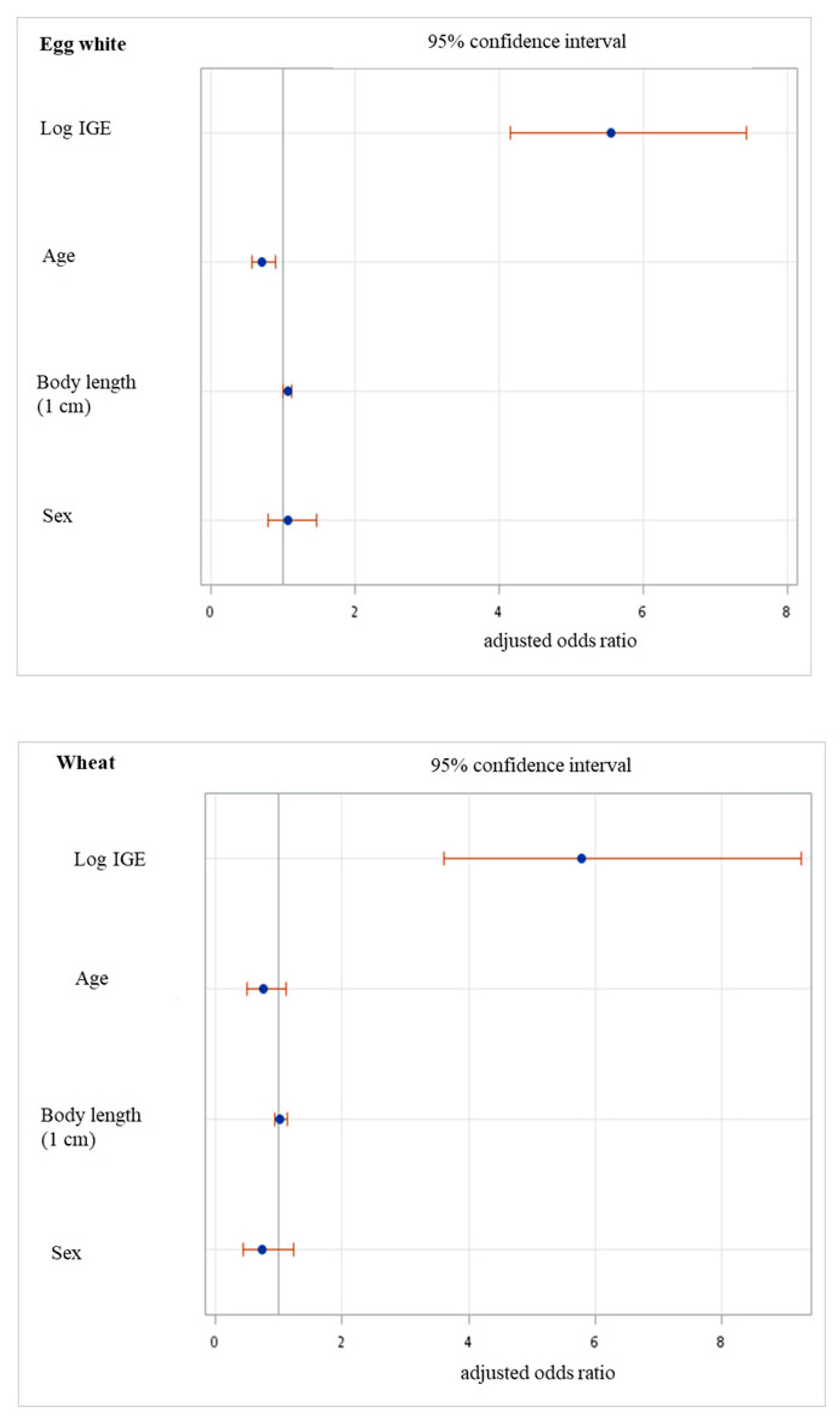
3.2. Is There an Effect of Perinatal Characteristics on sIgE of Egg White and Wheat?
3.3. Perinatal Characteristics and the Odds of Sensitization
3.4. Association between Allergic Diseases and Sensitization to Allergens
3.5. Effect of LEAP Trial on Evaluated Allergens
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alduraywish, S.A.; Lodge, C.J.; Campbell, B.; Allen, K.J.; Erbas, B.; Lowe, A.J.; Dharmage, S.C. The march from early life food sensitization to allergic disease: A systematic review and meta-analyses of birth cohort studies. Allergy 2016, 71, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Pereira, B.; Voigt, K.; Grundy, J.; Clayton, C.B.; Higgins, B.; Arshad, S.H.; Dean, T. Prevalence and cumulative incidence of food hypersensitivity in the first 3 years of life. Allergy 2008, 63, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Ching-Wei, L.; Yi-Fen, T.; Yu-Tsun, S.; Hong-Ren, Y.; Hsing-Jung, L.; Chih-Hsing, H.; Li-Fan, L.; Hui-Ju, T.; Jiu-Yao, W. Prenatal and perinatal risk factors of food allergy in Taiwanese young children. World Allergy Organ. J. 2022, 15, 100663. [Google Scholar] [CrossRef] [PubMed]
- Marenholz, I.; Grosche, S.; Kalb, B.; Rüschendorf, F.; Blümchen, K.; Schlags, R.; Harandi, N.; Price, M.; Hansen, G.; Seidenberg, J.; et al. Genome-wide association study identifies the SERPINB gene cluster as a susceptibility locus for food allergy. Nat. Commun. 2017, 8, 1056. [Google Scholar] [CrossRef]
- Huang, P.Y.; Huang, Y.H.; Guo, M.M.; Chang, L.S.; Kuo, H.C. Kawasaki Disease and Allergic Diseases. Front. Pediatr. 2020, 8, 614386. [Google Scholar] [CrossRef]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children--EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef]
- Laha, A.; Ghosh, A.; Moitra, S.; Saha, I.; Kumar Saha, G.; Bhattacharya, S.; Podder, S. Association of the STAT6 rs3024974 (C/T) Polymorphism with IgE-Mediated Food Sensitization among West Bengal Population in India. Int. Arch. Allergy Immunol. 2020, 181, 200–210. [Google Scholar] [CrossRef]
- Urashima, M.; Mezawa, H.; Okuyama, M.; Urashima, T.; Hirano, D.; Gocho, N.; Tachimoto, H. Primary Prevention of Cow’s Milk Sensitization and Food Allergy by Avoiding Supplementation with Cow’s Milk Formula at Birth: A Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 1137–1145. [Google Scholar] [CrossRef]
- Tachimoto, H.; Imanari, E.; Mezawa, H.; Okuyama, M.; Urashima, T.; Hirano, D.; Gocho, N.; Urashima, M. Effect of Avoiding Cow’s Milk Formula at Birth on Prevention of Asthma or Recurrent Wheeze Among Young Children: Extended Follow-up from the ABC Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2018534. [Google Scholar] [CrossRef]
- Sakihara, T.; Otsuji, K.; Arakaki, Y.; Hamada, K.; Sugiura, S.; Ito, K. Randomized trial of early infant formula introduction to prevent cow’s milk allergy. J. Allergy Clin. Immunol. 2021, 147, 224–232.e8. [Google Scholar] [CrossRef]
- De Boer, R.; Cartledge, N.; Lazenby, S.; Tobias, A.; Chan, S.; Fox, A.T.; Santos, A.F. Specific IgE as the best predictor of the outcome of challenges to baked milk and baked egg. J. Allergy Clin. Immunol. Pract. 2020, 8, 1459–1461.e5. [Google Scholar] [CrossRef]
- Sinai, T.; Goldberg, M.R.; Nachshon, L.; Amitzur-Levy, R.; Yichie, T.; Katz, Y.; Monsonego-Ornan, E.; Elizur, A. Reduced Final Height and Inadequate Nutritional Intake in Cow’s Milk-Allergic Young Adults. J. Allergy Clin. Immunol. Pract. 2019, 7, 509–515. [Google Scholar] [CrossRef]
- Benhamou, A.H.; Caubet, J.C.; Eigenmann, P.A.; Nowak-Wegrzyn, A.; Marcos, C.P.; Reche, M.; Urisu, A. State of the art and new horizons in the diagnosis and management of egg allergy. Allergy 2010, 65, 283–289. [Google Scholar] [CrossRef]
- Takahashi, K.; Yanagida, N.; Itonaga, T.; Nishino, M.; Nagakura, K.I.; Ogura, K.; Sato, S.; Ebisawa, M. Phenotyping of immediate-type food allergies based on 10 years of research: A latent class analysis. Pediatr. Allergy Immunol. 2022, 33, e13873. [Google Scholar] [CrossRef]
- Sato, S.; Ogura, K.; Takahashi, K.; Sato, Y.; Yanagida, N.; Ebisawa, M. Usefulness of antigen-specific IgE probability curves derived from the 3gAllergy assay in diagnosing egg, cow’s milk, and wheat allergies. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2017, 66, 296–301. [Google Scholar] [CrossRef]
- Laerum, B.N.; Svanes, C.; Wentzel-Larsen, T.; Gulsvik, A.; Iversen, M.; Gislason, T.; Jögi, R.; Norrman, E.; Janson, C.; Omenaas, E. The association between birth size and atopy in young North-European adults. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2005, 35, 1022–1027. [Google Scholar] [CrossRef]
- Chang, L.S.; Chen, Y.J.; Huang, P.Y.; Chen, K.D.; Lo, M.H.; Huang, Y.H.; Guo, M.M.; Kuo, H.C. Significantly Lower Immunoglobulin M Levels 6 Months After Disease Onset in Patients with Kawasaki Disease With Coronary Artery Lesions. J. Am. Heart Assoc. 2021, 10, e020505. [Google Scholar] [CrossRef]
- Han, M.; Shin, S.; Park, H.; Park, K.U.; Park, M.H.; Song, E.Y. Comparison of three multiple allergen simultaneous tests: RIDA allergy screen, MAST optigen, and polycheck allergy. BioMed Res. Int. 2013, 2013, 340513. [Google Scholar] [CrossRef]
- Woon, F.C.; Chin, Y.S.; Ismail, I.H.; Abdul Latiff, A.H.; Batterham, M.; Chan, Y.M.; On Behalf of The Micos Research, G. Maternal Vitamin D Levels during Late Pregnancy and Risk of Allergic Diseases and Sensitization during the First Year of Life-A Birth Cohort Study. Nutrients 2020, 12, 2418. [Google Scholar] [CrossRef]
- Wu, C.Y.; Huang, H.Y.; Pan, W.C.; Liao, S.L.; Hua, M.C.; Tsai, M.H.; Lai, S.H.; Yeh, K.W.; Chen, L.C.; Huang, J.L.; et al. Allergic diseases attributable to atopy in a population sample of Asian children. Sci. Rep. 2021, 11, 16052. [Google Scholar] [CrossRef]
- Kuo, H.C.; Chu, C.H.; Su, Y.J.; Lee, C.H. Atopic dermatitis in Taiwanese children: The laboratory values that correlate best to the SCORAD index are total IgE and positive Cheddar cheese IgE. Medicine 2020, 99, e21255. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chiang, W.L.; Chiang, T.L. Does cesarean delivery increase the occurrence of neurodevelopmental disorders in childhood? Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2022, 158, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.T.; Ke, H.H.; Shen, S.H.; Yeh, C.C.; Wang, P.H.; Ho, C.M.; Horng, H.C. A theoretical analysis of prophylactic common iliac arterial occlusion for potential massive bleeding during cesarean delivery: Decision-making considerations—A 2-year retrospective study. Taiwan. J. Obstet. Gynecol. 2022, 61, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef]
- Olivier, J.; Johnson, W.D.; Marshall, G.D. The logarithmic transformation and the geometric mean in reporting experimental IgE results: What are they and when and why to use them? Ann. Allergy Asthma Immunol. 2008, 100, 333–337. [Google Scholar] [CrossRef]
- Kim, J.D.; Kim, S.Y.; Kwak, E.J.; Sol, I.S.; Kim, M.J.; Kim, Y.H.; Kim, K.W.; Sohn, M.H. Reduction Rate of Specific IgE Level as a Predictor of Persistent Egg Allergy in Children. Allergy Asthma Immunol. Res. 2019, 11, 498–507. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Yang, H.K.; Won, H.J.; Kim, K.; Kim, J.; Ahn, K. The Natural Course of Immediate-Type Cow’s Milk and Egg Allergies in Children. Int. Arch. Allergy Immunol. 2020, 181, 103–110. [Google Scholar] [CrossRef]
- Chang, L.-S.; Li, J.-H.; Wang, P.-M.; Huang, C.-F.; Kuo, H.-C. Association between Serum Total and Specific Immunoglobulin E Levels and Body Height: A Cross-Sectional Study of Children and Adolescents. Children 2022, 9, 661. [Google Scholar] [CrossRef]
- Sung, W.H.; Yeh, K.W.; Huang, J.L.; Su, K.W.; Chen, K.F.; Wu, C.C.; Tsai, M.H.; Hua, M.C.; Liao, S.L.; Lai, S.H.; et al. Longitudinal changes in body mass index Z-scores during infancy and risk of childhood allergies. J. Microbiol. Immunol. Infect. 2022, 55, 956–964. [Google Scholar] [CrossRef]
- Anderson, E.L.; Fraser, A.; Martin, R.M.; Kramer, M.S.; Oken, E.; Patel, R.; Tilling, K. Associations of postnatal growth with asthma and atopy: The PROBIT Study. Pediatr. Allergy Immunol. 2013, 24, 122–130. [Google Scholar] [CrossRef]
- Greenhawt, M.J.; Fleischer, D.M.; Atkins, D.; Chan, E.S. The Complexities of Early Peanut Introduction for the Practicing Allergist. J. Allergy Clin. Immunol. Pract. 2016, 4, 221–225. [Google Scholar] [CrossRef]
- Lo, R.M.; Purington, N.; McGhee, S.A.; Mathur, M.B.; Shaw, G.M.; Schroeder, A.R. Infant Allergy Testing and Food Allergy Diagnoses Before and After Guidelines for Early Peanut Introduction. J. Allergy Clin. Immunol. Pract. 2021, 9, 302–310.e9. [Google Scholar] [CrossRef]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa’ad, A.; Baker, J.R., Jr.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F.; et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J. Allergy Clin. Immunol. 2017, 139, 29–44. [Google Scholar] [CrossRef]
- Aldakheel, F.M. Allergic Diseases: A Comprehensive Review on Risk Factors, Immunological Mechanisms, Link with COVID-19, Potential Treatments, and Role of Allergen Bioinformatics. Int. J. Environ. Res. Public Health 2021, 18, 12105. [Google Scholar] [CrossRef]
- Celi, G.; Brusca, I.; Scala, E.; Villalta, D.; Pastorello, E.; Farioli, L.; Cortellini, G.; Deleonardi, G.; Galati, P.; Losappio, L.; et al. House dust mite allergy in Italy-Diagnostic and clinical relevance of Der p 23 (and of minor allergens): A real-life, multicenter study. Allergy 2019, 74, 1787–1789. [Google Scholar] [CrossRef]
- Xu-De, Z.; Bei-Bei, G.; Xi-Juan, W.; Hai-Bo, L.; Li-Li, Z.; Feng-Xia, L. Serum IgE Predicts Difference of Population and Allergens in Allergic Diseases: Data from Weifang City, China. Mediat. Inflamm. 2021, 2021, 6627087. [Google Scholar] [CrossRef]
- Lee, A.; Mathilda Chiu, Y.H.; Rosa, M.J.; Jara, C.; Wright, R.O.; Coull, B.A.; Wright, R.J. Prenatal and postnatal stress and asthma in children: Temporal- and sex-specific associations. J. Allergy Clin. Immunol. 2016, 138, 740–747.e3. [Google Scholar] [CrossRef]
- Celi, G.; Brusca, I.; Scala, E.; Villalta, D.; Pastorello, E.; Farioli, L.; Cortellini, G.; Deleonardi, G.; Galati, P.; Losappio, L.; et al. House dust mite allergy and shrimp allergy: A complex interaction. Eur. Ann. Allergy Clin. Immunol. 2020, 52, 205–209. [Google Scholar] [CrossRef]
- Mitselou, N.; Hallberg, J.; Stephansson, O.; Almqvist, C.; Melén, E.; Ludvigsson, J.F. Cesarean delivery, preterm birth, and risk of food allergy: Nationwide Swedish cohort study of more than 1 million children. J. Allergy Clin. Immunol. 2018, 142, 1510–1514.e2. [Google Scholar] [CrossRef]
- Papathoma, E.; Triga, M.; Fouzas, S.; Dimitriou, G. Cesarean section delivery and development of food allergy and atopic dermatitis in early childhood. Pediatr. Allergy Immunol. 2016, 27, 419–424. [Google Scholar] [CrossRef]
- Eggesbø, M.; Botten, G.; Stigum, H.; Samuelsen, S.O.; Brunekreef, B.; Magnus, P. Cesarean delivery and cow milk allergy/intolerance. Allergy 2005, 60, 1172–1173. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Kim, K.W.; Choi, B.S.; Jee, H.M.; Sohn, M.H.; Kim, K.E. Relationship between mode of delivery in childbirth and prevalence of allergic diseases in Korean children. Allergy Asthma Immunol. Res. 2010, 2, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, M.F.; Banerji, A.; Rudders, S.A.; Clark, S.; Mullins, R.J.; Camargo, C.A., Jr. Season of birth and food allergy in children. Ann. Allergy Asthma Immunol. 2010, 104, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, E.; Han, Y.; Ahn, K.; Lee, S.I. The incidence and risk factors of immediate type food allergy during the first year of life in Korean infants: A birth cohort study. Pediatr. Allergy Immunol. 2011, 22, 715–719. [Google Scholar] [CrossRef]
- Zimmerman, B.; Forsyth, S.; Gold, M. Highly atopic children: Formation of IgE antibody to food protein, especially peanut. J. Allergy Clin. Immunol. 1989, 83, 764–770. [Google Scholar] [CrossRef]
- Kotaniemi-Syrjänen, A.; Reijonen, T.M.; Romppanen, J.; Korhonen, K.; Savolainen, K.; Korppi, M. Allergen-specific immunoglobulin E antibodies in wheezing infants: The risk for asthma in later childhood. Pediatrics 2003, 111, e255–e261. [Google Scholar] [CrossRef]
- Amin, P.; Levin, L.; Epstein, T.; Ryan, P.; LeMasters, G.; Khurana Hershey, G.; Reponen, T.; Villareal, M.; Lockey, J.; Bernstein, D.I. Optimum predictors of childhood asthma: Persistent wheeze or the Asthma Predictive Index? J. Allergy Clin. Immunol. Pract. 2014, 2, 709–715. [Google Scholar] [CrossRef]
- Moustaki, M.; Loukou, I.; Tsabouri, S.; Douros, K. The Role of Sensitization to Allergen in Asthma Prediction and Prevention. Front. Pediatr. 2017, 5, 166. [Google Scholar] [CrossRef]
- Turner, S. Perinatal programming of childhood asthma: Early fetal size, growth trajectory during infancy, and childhood asthma outcomes. Clin. Dev. Immunol. 2012, 2012, 962923. [Google Scholar] [CrossRef]
- Calvani, M.; Anania, C.; Cuomo, B.; D’Auria, E.; Decimo, F.; Indirli, G.C.; Marseglia, G.; Mastrorilli, V.; Sartorio, M.U.A.; Santoro, A.; et al. Non-IgE- or Mixed IgE/Non-IgE-Mediated Gastrointestinal Food Allergies in the First Years of Life: Old and New Tools for Diagnosis. Nutrients 2021, 13, 226. [Google Scholar] [CrossRef]
- Wang, L.C.; Chiang, B.L.; Huang, Y.M.; Shen, P.T.; Huang, H.Y.; Lin, B.F. Lower vitamin D levels in the breast milk is associated with atopic dermatitis in early infancy. Pediatr. Allergy Immunol. 2020, 31, 258–264. [Google Scholar] [CrossRef]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed. J. 2017, 40, 263–269. [Google Scholar] [CrossRef]
- Chang, L.-S.; Kuo, H.-C.; Suen, J.J.-B.; Yang, P.-H.; Hou, C.-P.; Sun, H.-R.; Lee, Z.-M.; Huang, Y.-H. Multimedia Mixed Reality Interactive Shared Decision-Making Game in Children with Moderate to Severe Atopic Dermatitis, a Pilot Study. Children 2023, 10, 574. [Google Scholar] [CrossRef]
- Chang, L.-S.; Chang, H.-Y.; Yang, Y.-H.; Lee, Z.-M.; Guo, M.M.-H.; Huang, Y.-H.; Kuo, H.-C. Allergen Tests of Fruit Sensitization Involving Children with Allergic Diseases. Children 2022, 9, 470. [Google Scholar] [CrossRef]
| M ± SD or n (%) | Negative sIgE to Milk | Positive sIgE to Milk | p |
|---|---|---|---|
| All (n) | 793 (72.1%) | 307 (27.9%) | |
| Age at sIgE tests | 2.0 ± 0.7 | 2.0 ± 0.6 | 0.286 |
| Sex of child | 0.012 * | ||
| Male | 443 | 197 | |
| Female | 350 | 110 | |
| M ± SD or n (%) | Negative sIgE to egg | Positive sIgE to egg | p |
| All (n) | 745 (67.9%) | 353 (32.1%) | |
| Age at sIgE tests | 2.0 ± 0.7 | 2.0 ± 0.7 | 0.207 |
| Sex of child | 0.001 * | ||
| Male | 407 | 232 | |
| Female | 338 | 121 | |
| M ± SD or n (%) | Negative sIgE to wheat | Positive sIgE to wheat | p |
| All (n) | 1000 (91.8%) | 89 (8.2%) | |
| Age at sIgE tests | 2.0 ± 0.7 | 2.1 ± 0.7 | 0.241 |
| Sex of child | 0.442 | ||
| Male | 576 | 55 | |
| Female | 424 | 34 |
| M ± SD or n (%) | Negative sIgE to Milk | Positive sIgE to Milk | p |
|---|---|---|---|
| Gestational age (weeks) | 38.5 ± 1.6 | 38.5 ± 1.3 | 0.613 |
| Birth weight (g) | 3013.9 ± 547.2 | 3076.3 ± 514.9 | 0.085 |
| Birth length (cm) | 48.9 ± 3.0 | 49.3 ± 2.8 | 0.063 |
| Birth head circumference (cm) | 33.4 ± 1.8 | 33.6 ± 1.7 | 0.139 |
| Birth chest circumference (cm) | 32.0 ± 2.3 | 32.2 ± 2.1 | 0.061 |
| Maternal age at birth (years) | 0.748 | ||
| <40 | 623 | 231 | |
| ≧40 | 70 | 28 | |
| Number of fetuses: | 0.764 | ||
| Single | 765 | 295 | |
| Multiple | 28 | 12 | |
| Parity | 0.443 | ||
| First | 777 | 300 | |
| Second or third | 16 | 7 | |
| Meconium stain | 0.687 | ||
| Negative | 717 | 280 | |
| Positive | 76 | 27 | |
| Meconium passage | 0.690 | ||
| Negative | 493 | 189 | |
| Positive | 299 | 117 | |
| Season of birth | 0.211 | ||
| Spring | 162 | 79 | |
| Summer | 205 | 79 | |
| Fall | 221 | 72 | |
| Winter | 205 | 77 | |
| Serum log total IgE (U/mL) | 1.8 ± 0.6 | 2.3 ± 0.5 | <0.001 * |
| Delivery method | 0.217 | ||
| Vaginal delivery | 602 | 222 | |
| Cesarean delivery | 191 | 85 |
| M ± SD or n (%) | Negative sIgE to Egg | Positive sIgE to Egg | p |
|---|---|---|---|
| Gestational age (weeks) | 38.5 ± 1.6 | 38.5 ± 1.3 | 0.629 |
| Birth weight (g) | 2998.2 ± 562.0 | 3100.9 ± 480.8 | 0.002 * |
| Birth length (cm) | 48.8 ± 3.1 | 49.5 ± 2.6 | <0.001 * |
| Birth head circumference (cm) | 33.4 ± 1.9 | 33.6 ± 1.6 | 0.066 |
| Birth chest circumference (cm) | 32.0 ± 2.4 | 32.2 ± 2.0 | 0.096 |
| Maternal age at birth (years) | 0.477 | ||
| <40 | 578 | 274 | |
| ≧40 | 63 | 35 | |
| Number of fetuses: | 0.324 | ||
| Single | 715 | 343 | |
| Multiple | 30 | 10 | |
| Parity | 0.129 | ||
| First | 727 | 348 | |
| Second or third | 18 | 5 | |
| Meconium stain | 0.788 | ||
| Negative | 677 | 319 | |
| Positive | 68 | 34 | |
| Meconium passage | 0.689 | ||
| Negative | 468 | 214 | |
| Positive | 276 | 138 | |
| Season of birth | 0.670 | ||
| Spring | 155 | 85 | |
| Summer | 194 | 90 | |
| Fall | 202 | 90 | |
| Winter | 194 | 88 | |
| Serum log total IgE (U/mL) | 1.7 ± 0.6 | 2.3 ± 0.5 | <0.001 * |
| Delivery method: | 0.796 | ||
| Vaginal delivery | 556 | 266 | |
| Cesarean delivery | 189 | 87 |
| M ± SD or n (%) | Negative sIgE to Wheat | Positive sIgE to Wheat | p |
|---|---|---|---|
| Gestational age (weeks) | 38.5 ± 1.5 | 38.7 ± 1.1 | 0.197 |
| Birth weight (g) | 3016.9 ± 548.3 | 3154.1 ± 413.9 | 0.004 * |
| Birth length (cm) | 48.9 ± 3.0 | 49.5 ± 2.3 | 0.025 * |
| Birth head circumference (cm) | 33.4 ± 1.8 | 33.7 ± 1.4 | 0.071 |
| Birth chest circumference (cm) | 32.0 ± 2.3 | 32.5 ± 1.8 | 0.015 * |
| Maternal age at birth (years) | 0.523 | ||
| <40 | 775 | 71 | |
| ≧40 | 87 | 10 | |
| Number of fetuses | 0.182 | ||
| Single | 961 | 88 | |
| Multiple | 39 | 1 | |
| Parity | 0.352 | ||
| First | 977 | 89 | |
| Second or third | 23 | 0 | |
| Meconium stain | 0.632 | ||
| Negative | 906 | 82 | |
| Positive | 94 | 7 | |
| Meconium passage | 0.909 | ||
| Negative | 622 | 56 | |
| Positive | 376 | 33 | |
| Season of birth | 0.516 | ||
| Spring | 226 | 14 | |
| Summer | 256 | 24 | |
| Fall | 265 | 26 | |
| Winter | 253 | 25 | |
| Serum log total IgE (U/mL) | 1.9 ± 0.6 | 2.5 ± 0.5 | <0.001 * |
| Delivery method: | 0.920 | ||
| Vaginal delivery | 748 | 67 | |
| Cesarean delivery | 252 | 22 |
| n (%) | Negative sIgE to Milk | Positive sIgE to Milk | p a | Negative sIgE to Egg | Positive sIgE to Egg | p a | Negative sIgE to Wheat | Positive sIgE to Wheat | p a |
|---|---|---|---|---|---|---|---|---|---|
| All (n) | 793 (72.1%) | 307 (27.9%) | 745 (67.9%) | 353 (32.1%) | 1000 (91.8%) | 89 (8.2%) | |||
| Asthma | 0.006 * | 0.154 | 1.000 | ||||||
| Negative | 492 | 143 | 447 | 186 | 579 | 47 | |||
| Positive | 301 | 164 | 298 | 167 | 421 | 42 | |||
| Dermatitis | 0.014 * | 1.000 | 1.000 | ||||||
| Negative | 493 | 222 | 490 | 224 | 647 | 55 | |||
| Positive | 300 | 85 | 255 | 129 | 353 | 34 | |||
| Rhinitis | 1.000 | 0.287 | 1.000 | ||||||
| Negative | 365 | 136 | 355 | 145 | 462 | 38 | |||
| Positive | 428 | 171 | 390 | 208 | 538 | 51 | |||
| Asthma and dermatitis | 1.000 | 0.212 | 1.000 | ||||||
| Negative | 730 | 275 | 690 | 313 | 915 | 78 | |||
| Positive | 63 | 32 | 55 | 40 | 85 | 11 | |||
| Asthma and rhinitis | 0.014 * | 0.273 | 1.000 | ||||||
| Negative | 594 | 202 | 553 | 241 | 725 | 62 | |||
| Positive | 199 | 105 | 192 | 112 | 275 | 27 | |||
| Dermatitis and rhinitis | 1.000 | 0.126 | 1.000 | ||||||
| Negative | 660 | 263 | 639 | 283 | 840 | 72 | |||
| Positive | 133 | 44 | 106 | 70 | 160 | 17 | |||
| Asthma, dermatitis, and rhinitis | 1.000 | 0.210 | 1.000 | ||||||
| Negative | 757 | 288 | 715 | 328 | 951 | 83 | |||
| Positive | 36 | 19 | 30 | 25 | 49 | 6 |
| 2015 and before 2015 (n = 171) | Adjusted Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Log total IgE | 1.003 | 1.001–1.004 | 0.003 * |
| Age | 1.165 | 0.659–2.06 | 0.598 |
| Birth body length | 1.08 | 0.955–1.222 | 0.222 |
| Sex | 1.415 | 0.665–3.012 | 0.368 |
| 2016 and after 2016 (n = 816) | |||
| Log total IgE | 1.001 | 1.000–1.001 | <0.0001 * |
| Age | 0.971 | 0.775–1.216 | 0.797 |
| Birth body length | 1.026 | 0.968–1.087 | 0.385 |
| Sex | 1.163 | 0.844–1.601 | 0.357 |
| 2015 and before 2015 (n = 170) | Adjusted Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Log total IgE | 1.001 | 1.000–1.002 | 0.039 * |
| Age | 1.178 | 0.678–2.046 | 0.561 |
| Birth body length | 1.073 | 0.954–1.208 | 0.241 |
| Sex | 1.331 | 0.639–2.774 | 0.445 |
| 2016 and after 2016 (n = 816) | |||
| Log total IgE | 1.002 | 1.002–1.003 | <0.0001 * |
| Age | 0.851 | 0.681–1.063 | 0.156 |
| Birth body length | 1.083 | 1.018–1.152 | 0.012 * |
| Sex | 1.23 | 0.893–1.693 | 0.205 |
| 2015 and before 2015 (n = 167) | Adjusted Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Log total IgE | 1.001 | 0.999–1.002 | 0.306 |
| Age | 0.852 | 0.336–2.157 | 0.735 |
| Birth body length | 1.135 | 0.904–1.425 | 0.274 |
| Sex | 0.963 | 0.282–3.286 | 0.952 |
| 2016 and after 2016 (n = 809) | |||
| Log total IgE | 1.001 | 1.001–1.001 | <0.0001 * |
| Age | 1.033 | 0.696–1.532 | 0.872 |
| Birth body length | 1.029 | 0.931–1.137 | 0.580 |
| Sex | 0.955 | 0.556–1.642 | 0.869 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-Y.; Lee, Z.-M.; Chang, L.-S.; Feng, W.-L.; Yang, Y.-H.; Ou-Yang, M.-C. Perinatal Characteristics and the Sensitization to Cow Milk, Egg Whites and Wheat in Children up to 3 Years of Age. Children 2023, 10, 860. https://doi.org/10.3390/children10050860
Chang H-Y, Lee Z-M, Chang L-S, Feng W-L, Yang Y-H, Ou-Yang M-C. Perinatal Characteristics and the Sensitization to Cow Milk, Egg Whites and Wheat in Children up to 3 Years of Age. Children. 2023; 10(5):860. https://doi.org/10.3390/children10050860
Chicago/Turabian StyleChang, Hsin-Yu, Zon-Min Lee, Ling-Sai Chang, Wei-Ling Feng, Yao-Hsu Yang, and Mei-Chen Ou-Yang. 2023. "Perinatal Characteristics and the Sensitization to Cow Milk, Egg Whites and Wheat in Children up to 3 Years of Age" Children 10, no. 5: 860. https://doi.org/10.3390/children10050860
APA StyleChang, H.-Y., Lee, Z.-M., Chang, L.-S., Feng, W.-L., Yang, Y.-H., & Ou-Yang, M.-C. (2023). Perinatal Characteristics and the Sensitization to Cow Milk, Egg Whites and Wheat in Children up to 3 Years of Age. Children, 10(5), 860. https://doi.org/10.3390/children10050860







