EEG Alpha Band Responses Reveal Amplification Benefits in Infants with Hearing Loss
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. EEG Procedure
2.3. Stimuli
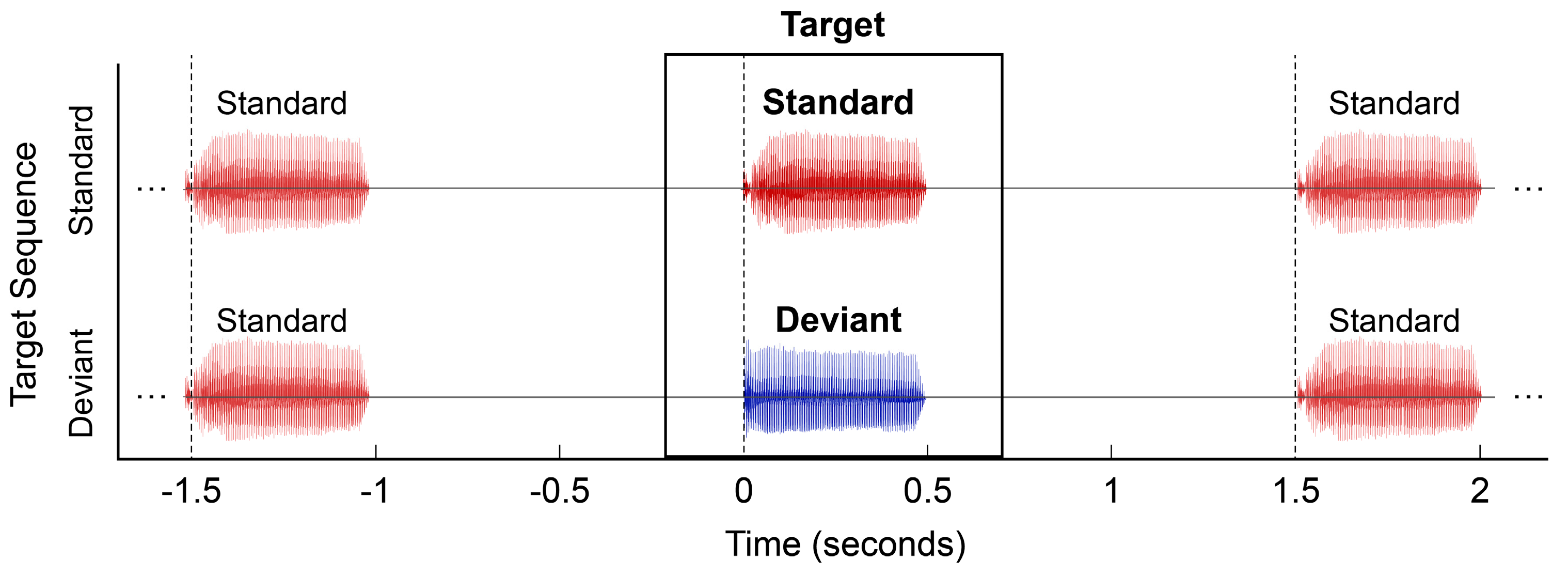
2.4. MMR Paradigm and Analysis Procedures
2.5. EEG Signal Processing
2.5.1. Band-Pass Filtering and Artifact Rejection
2.5.2. Latent EEG Extraction
2.5.3. ERPs and MMR
2.5.4. Time-Frequency Coherence
2.5.5. Alpha Band Separation
2.6. Statistical Analyses and Hypothesis Testing
2.6.1. Aided vs. Unaided Responses
2.6.2. Standard vs. Deviant Responses
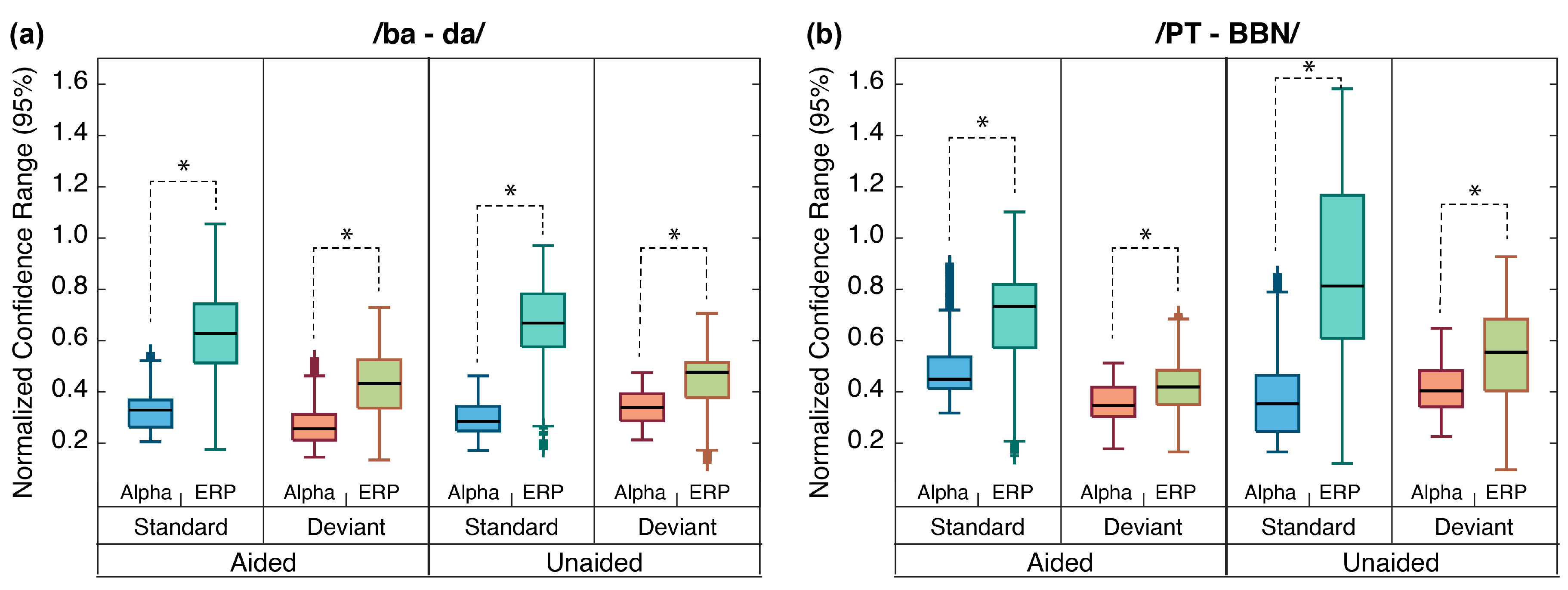
2.6.3. Alpha Responses vs. ERPs
2.6.4. Aided Alpha vs. Aided SII
2.6.5. Permutation t-Tests
3. Results
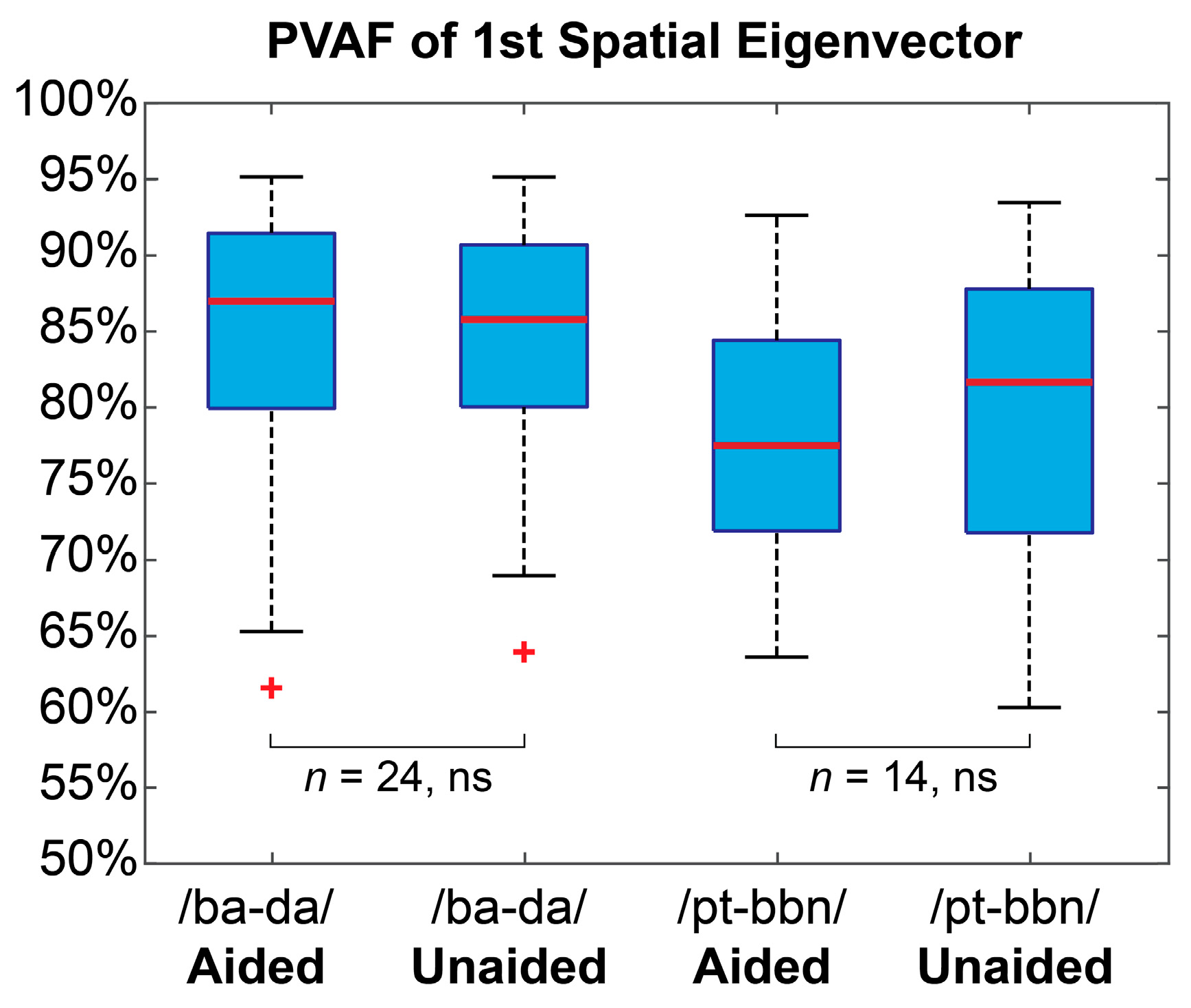
3.1. Latent EEG Representation
3.2. ERPs and MMR
3.3. Time-Frequency Representation
3.4. Cofactor Effects
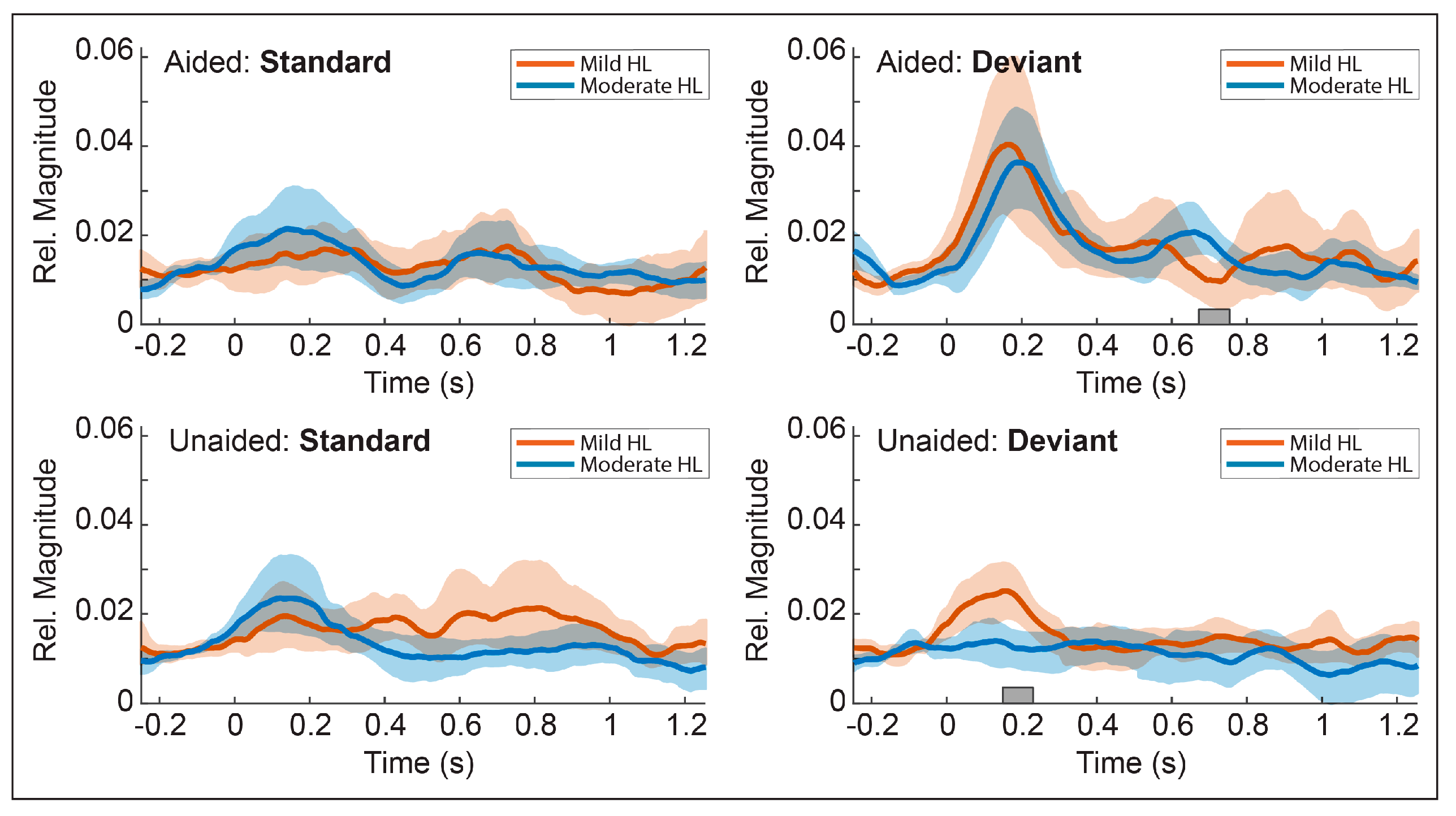
3.5. Temporal Envelopes of the Alpha Response
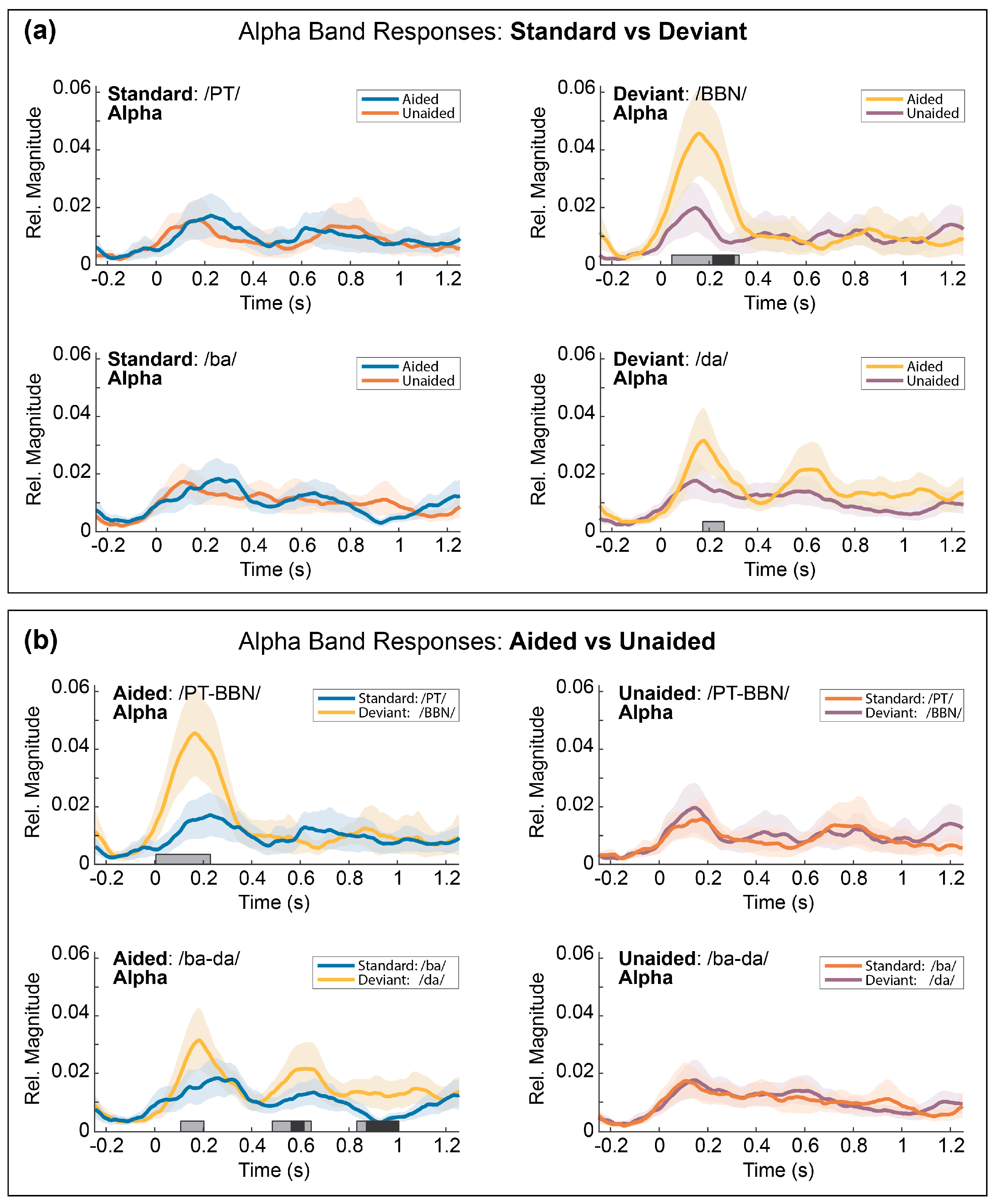
3.5.1. Comparison 1: Aided vs. Unaided Responses
3.5.2. Comparison 2: Standard vs. Deviant Responses
3.6. Alpha Band Mismatch vs. Aided SII
3.7. Alpha Band Mismatch vs. Aided SII
3.8. Post-Hoc Comparisons
Post-Hoc Results
4. Discussion
4.1. Alpha Coherence Reveals Aided Benefit
4.2. Auditory Processing and the Alpha Band
4.3. Alpha Coherence vs. ERPs
4.4. Alpha Coherence vs. Aided SII
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holte, L.; Walker, E.; Oleson, J.; Spratford, M.; Moeller, M.P.; Roush, P.; Tomblin, J.B.; Ou, H.; Lin, G.G.; Scott, J.G.; et al. NIH Public Access. Am. J. Audiol. 2012, 21, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Awad, R.; Oropeza, J.; Uhler, K.M. Meeting the joint committee on infant hearing standards in a large metropolitan children’s hospital: Barriers and next steps. Am. J. Audiol. 2019, 28, 251–259. [Google Scholar] [CrossRef]
- American Academy of Audiology. Clinical Practice Guidelines—Pediatric Amplification 2013. Available online: https://audiology-web.s3.amazonaws.com/migrated/PediatricAmplificationGuidelines.pdf_539975b3e7e9f1.74471798.pdf (accessed on 6 February 2023).
- Valente, M.; Abrams, H.; Benson, D.; Chisolm, T.; Citron, D.; Hampton, D.; Loavenbruck, A.; Ricketts, T.; Solodar, H.; Sweetow, R. Guidelines for the Audiologic Management of Adult Hearing Impairment; The American Academy of Audiology: Reston, VA, USA, 2006. [Google Scholar]
- Kushnerenko, E.; Ceponiene, R.; Balan, P.; Fellman, V.; Huotilaine, M.; Näätäne, R. Maturation of the auditory event-related potentials during the first year of life. Neuroreport 2002, 13, 47–51. [Google Scholar] [CrossRef]
- Bishop, D.V.M.; Hardiman, M.; Uwer, R.; Von Suchodoletz, W. Maturation of the long-latency auditory ERP: Step function changes at start and end of adolescence: REPORT. Dev. Sci. 2007, 10, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Cheour, M.; Leppanen, P.H.; Kraus, N. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clin. Neurophysiol. 2000, 111, 4–16. [Google Scholar] [CrossRef]
- Sharma Dorman, M.F.; Spahr, A.J. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implantation. Ear Hear. 2002, 23, 532–539. [Google Scholar] [CrossRef]
- Halliday, L.F.; Tuomainen, O.; Rosen, S. Language Development and Impairment in Children With Mild to Moderate Sensorineural Hearing Loss. J. Speech Lang. Hear. Res. 2017, 60, 1551–1567. [Google Scholar] [CrossRef] [PubMed]
- Koravand, A.; Jutras, B.; Lassonde, M. Auditory event related potentials in children with peripheral hearing loss. Clin. Neurophysiol. 2013, 124, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Calcus, A.; Tuomainen, O.; Campos, A.; Rosen, S.; Halliday, L.F. Functional brain alterations following mild-to-moderate sensorineural hearing loss in children. eLife 2019, 8, e46965. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin. Neurophysiol. 2007, 118, 2544–2590. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Lehtokoski, A.; Lennes, M.; Cheour, M.; Huotilainen, M.; Iivonen, A.; Vainio, M.; Alku, P.; Ilmoniemi, R.J.; Luuk, A.; et al. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature 1997, 385, 432–434. [Google Scholar] [CrossRef]
- Tervaniemi, M.; Medvedev, S.V.; Alho, K.; Pakhomov, S.V.; Roudas, M.S.; Van Zuijen, T.L.; Näätänen, R. Lateralized automatic auditory processing of phonetic versus musical information: A PET study. Hum. Brain Mapp. 2000, 10, 74–79. [Google Scholar] [CrossRef]
- Näätänen, R.; Alho, K. Mismatch negativity–a unique measure of sensory processing in audition. Int. J. Neurosci. 1995, 80, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Virtala, P.; Putkinen, V.; Kailaheimo-Lönnqvist, L.; Thiede, A.; Partanen, E.; Kujala, T. Infancy and early childhood maturation of neural auditory change detection and its associations to familial dyslexia risk. Clin. Neurophysiol. 2022, 137, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Cheour, M.; Alho, K.; Čeponiené, R.; Reinikainen, K.; Sainio, K.; Pohjavuori, M.; Aaltonen, O.; Näätänen, R.; Ceponiene, R.; Reinikainen, K.; et al. Maturation of mismatch negativity in infants. Int. J. Psychophysiol. 1998, 29, 217–226. [Google Scholar] [CrossRef]
- Cheour, A.K.; Sainio, K. The mismatch negativity to speech sounds at the age of three months. Dev. Neurophysiol. 1997, 13, 167–174. [Google Scholar] [CrossRef]
- He, C.; Hotson, L.; Trainor, L.J. Development of infant mismatch responses to auditory pattern changes between 2 and 4 months old. Eur. J. Neurosci. 2009, 29, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.; Micco, A.G.; Koch, D.B.; McGee, T.; Carrell, T.; Sharma, A.; Wiet, R.J.; Weingarten, C.Z. The mismatch negativity cortical evoked potential elicited by speech in cochlear-implant users. Hear. Res. 1993, 65, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, P.H.T.; Hämäläinen, J.A.; Guttorm, T.K.; Eklund, K.M.; Salminen, H.; Tanskanen, A.; Torppa, M.; Puolakanaho, A.; Richardson, U.; Pennala, R.; et al. Infant brain responses associated with reading-related skills before school and at school age. Neurophysiol. Clin. Neurophysiol. 2012, 42, 35–41. [Google Scholar] [CrossRef]
- Sharma, A.; Martin, K.; Roland, P.; Bauer, P.; Sweeney, M.H.; Gilley, P.; Dorman, M. P1 latency as a biomarker for central auditory development in children with hearing impairment. J. Am. Acad. Audiol. 2005, 16, 564–573. [Google Scholar] [CrossRef]
- Gilley, P.M.; Sharma, A.; Dorman, M.; Martin, K. Abnormalities in central auditory maturation in children with language-based learning problems. Clin. Neurophysiol. 2006, 117, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Gilley, P.M.; Sharma, A.; Dorman, M.; Martin, K. Developmental changes in refractoriness of the cortical auditory evoked potential. Clin. Neurophysiol. 2005, 116, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Gilley, P.M.; Sharma, A.; Dorman, M.F. Cortical Reorganization in children with cochlear implants. Brain Res. 2008, 1239, 56–65. [Google Scholar] [CrossRef]
- Ponton, C.W.; Eggermont, J.J. Of kittens and kids: Altered cortical maturation following profound deafness and cochlear implant use. Audiol. Neurootol. 2001, 6, 363–380. [Google Scholar] [CrossRef]
- Eisenberg, L.S.; Johnson, K.C.; Martinez, A.S. Clinical Assessment of Speech Perception for Infants and Toddlers. Audiol. Online 2005. [Google Scholar]
- Uhler, K.M.; Hunter, S.K.; Gilley, P.M. Mismatched Response (MMR) predicts behavioral speech discrimination outcomes in infants with hearing loss and normal hearing. Infancy 2021, 26, 327–348. [Google Scholar]
- Gilley, P.M.; Uhler, K.; Watson, K.; Yoshinaga-Itano, C. Spectral-temporal EEG dynamics of speech discrimination processing in infants during sleep. BMC Neurosci. 2017, 18, 34. [Google Scholar] [CrossRef]
- Uhler, K.M.; Hunter, S.K.; Tierney, E.; Gilley, P.M. The relationship between mismatch response and the acoustic change complex in normal hearing infants. Clin. Neurophysiol. 2018, 129, 1148–1160. [Google Scholar] [CrossRef]
- Paul, B.T.; Chen, J.; Le, T.; Lin, V.; Dimitrijevic, A. Cortical alpha oscillations in cochlear implant users reflect subjective listening effort during speech-in-noise perception. PLoS ONE 2021, 16, e0254162. [Google Scholar] [CrossRef]
- Dimitrijevic, A.; Smith, M.L.; Kadis, D.S.; Moore, D.R. Cortical alpha oscillations predict speech intelligibility. Front. Hum. Neurosci. 2017, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- The Colorado Infant Hearing Advisory Committee. Part III of CIHAC Guidelines: Recommended Guidelines for Pediatric Audiology Diagnosis. Colorado Department of Public Health & Environment. 2022. Available online: https://cdphe.colorado.gov/center-for-health-and-environmental-data/registries-and-vital-statistics/newborn-hearing-screening-provider-resources (accessed on 8 March 2023).
- Leppänen, P.H.T.; Tóth, D.; Honbolygó, F.; Lohvansuu, K.; Hämäläinen, J.A.; NeurodysWP7 Group; Demonet, J.-F.; Schulte-Körne, G.; Csépe, V. Reproducibility of Brain Responses: High for Speech Perception, Low for Reading Difficulties. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Ponton, C.W.; Don, M.; Eggermont, J.J.; Kwong, B. Integrated mismatch negativity (MMN(i)): A noise-free representation of evoked responses allowing single-point distribution-free statistical tests. Electroencephalogr. Clin. Neurophysiol.- Evoked Potentials 1997, 104, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kayser, J.; Tenke, C.E. Consensus on PCA for ERP data, and sensibility of unrestricted solutions. Clin. Neurophysiol. 2006, 117, 703–707. [Google Scholar] [CrossRef]
- Kayser, J.; Tenke, C.E. Optimizing PCA methodology for ERP component identification and measurement: Theoretical rationale and empirical evaluation. Clin. Neurophysiol. 2003, 114, 2307–2325. [Google Scholar] [CrossRef]
- Dien, J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. J. Neurosci. Methods 2010, 187, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.M.; Dien, J.; Donchin, E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology 2001, 38, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Nash-Kille, A.; Gilley, P.; Sharma, A. Cortical organization and variability in unilateral auditory neuropathy spectrum disorder: A case study. Hear. Balance Commun. 2014, 12, 41–51. [Google Scholar] [CrossRef]
- McCreery, R.; Walker, E.; Spratford, M.; Bentler, R.; Holte, L.; Roush, P.; Oleson, J.; Van Buren, J.; Moeller, M.P. Longitudinal Predictors of Aided Speech Audibility in Infants and Children. Ear Hear. 2015, 36, 24S–37S. [Google Scholar] [CrossRef]
- Rempala, G.; Yang, Y. On Permutation Procedures for Strong Control in Multiple Testing with Gene Expression Data. Stat. Interface 2014, 6, 1–17. [Google Scholar] [CrossRef]
- Westfall, P.H.; Young, S. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment; John Wiley and Sons: New York, NY, USA, 1993. [Google Scholar]
- Nicholson, K.J.; Sherman, M.; Divi, S.N.; Bowles, D.R.; Vaccaro, A.R. The Role of Family-wise Error Rate in Determining Statistical Significance. Clin. Spine Surg. 2022, 35. [Google Scholar] [CrossRef] [PubMed]
- Budd, T.W.; Barry, R.J.; Gordon, E.; Rennie, C.; Michie, P.T. Decrement of the N1 auditory event-related potential with stimulus repetition: Habituation vs. refractoriness. Int. J. Psychophysiol. 1998, 31, 51–68. [Google Scholar] [CrossRef]
- Fujioka, T.; Ross, B. Auditory processing indexed by stimulus-induced alpha desynchronization in children. Int. J. Psychophysiol. 2008, 68, 130–140. [Google Scholar] [CrossRef]
- Del Giudice, R.; Lechinger, J.; Wislowska, M.; Heib, D.P.J.; Hoedlmoser, K.; Schabus, M. Oscillatory brain responses to own names uttered by unfamiliar and familiar voices. Brain Res. 2014, 1591, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Dyball, A.; Xu Rattanasone, N.; Ibrahim, R.; Sharma, M. Alpha synchronisation of acoustic responses in active listening is indicative of native language listening experience. Int. J. Audiol. 2021, 61, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Tomblin, J.B.; Oleson, J.; Ambrose, S.; Walker, E.; Mccreery, R.; Moeller, M. Aided Hearing Moderates the Academic Outcomes of Children With Mild to Severe Hearing Loss. Ear Hear. 2020, 41, 1. [Google Scholar] [CrossRef]
- Moeller, M.P.; Tomblin, J.B. An Introduction to the Outcomes of Children with Hearing Loss Study. Ear Hear. 2015, 36, 4–13. [Google Scholar] [CrossRef]
- Wiseman, K.B.; McCreery, R.W.; Walker, E.A. Hearing Thresholds, Speech Recognition, and Audibility as Indicators for Modifying Intervention in Children With Hearing Aids. Ear Hear. 2023; Publish Ahead of Print. [Google Scholar]
- Bagatto, M.P.; Moodie, S.T.; Malandrino, A.C.; Richert, F.M.; Clench, D.A.; Scollie, S.D. The University of Western Ontario Pediatric Audiological Monitoring Protocol (UWO PedAMP). Trends Amplif. 2011, 15, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Sininger, Y.S.; Grimes, A.; Christensen, E. Auditory development in early amplified children: Factors influencing auditory-based communication outcomes in children with hearing loss. Ear Hear. 2010, 31, 166–185. [Google Scholar] [CrossRef]
- Grigg-Damberger, M.M. The visual scoring of sleep in infants 0 to 2 months of age. J. Clin. Sleep Med. 2016, 12, 429–445. [Google Scholar] [CrossRef]



| Total N = 38 (20 Female) | Mean (s.d.) | Range |
|---|---|---|
| Age at test | 3.84 (1.16) months | 1.75–6.32 months |
| Age at hearing aid fit | 2.74 (0.99) months | 1.12–6.17 months |
| Duration of hearing aid use | 1.10 (0.76) months | 0.04–2.93 months |
| Degree of hearing loss (PTA range) | Count | |
| Mild (15–39 dB HL) | n = 21 | |
| Moderate (40–59 dB HL) | n = 12 | |
| Moderately-severe (≥60 dB HL) | n = 5 | |
| ERP Contrast | Count | |
| /PT-BBN/ | n = 14 | |
| /ba-da/ | n = 24 |
| Contrast | Comparison | Aided Magnitude (CI 95%) | Unaided Magnitude (CI 95%) | Effect | Latency (ms) |
|---|---|---|---|---|---|
| /PT-BBN/ | Aided vs. Unaided Deviant | 0.046 (0.031,0.060) | 0.016 (0.011,0.028) | p < 0.05 | A: 164 ms, U: 156 ms |
| /PT-BBN/ | Aided vs. Unaided Standard | 0.017 (0.009,0.025) | 0.015 (0.008,0.023) | ns | |
| /ba-da/ | Aided vs. Unaided Deviant | 0.031 (0.018,0.041) | 0.018 (0.010,0.025) | p < 0.01 | A: 188 ms, U: 156 ms |
| /ba-da/ | Aided vs. Unaided Standard | 0.018 (0.013,0.025) | 0.017 (0.012,0.023) | ns | |
| Condition | Comparison | Deviant magnitude (CI 95%) | Standard magnitude (CI 95%) | Effect | Latency (ms) |
| Aided | Deviant/BBN/vs. Standard/PT/ | 0.046 (0.031,0.060) | 0.017 (0.009,0.025) | p < 0.05 | 152 ms |
| Unaided | Deviant/BBN/vs. Standard/PT/ | 0.016 (0.011,0.028) | 0.015 (0.008,0.023) | ns | |
| Aided | Deviant/da/vs. Standard/ba/ | 0.031 (0.018,0.041) | 0.015 (0.009,0.021) | p < 0.05 | 180 ms |
| Unaided | Deviant/da/vs. Standard/ba/ | 0.018 (0.010,0.025) | 0.017 (0.010,0.023) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhler, K.; Tollin, D.J.; Gilley, P.M. EEG Alpha Band Responses Reveal Amplification Benefits in Infants with Hearing Loss. Children 2023, 10, 600. https://doi.org/10.3390/children10030600
Uhler K, Tollin DJ, Gilley PM. EEG Alpha Band Responses Reveal Amplification Benefits in Infants with Hearing Loss. Children. 2023; 10(3):600. https://doi.org/10.3390/children10030600
Chicago/Turabian StyleUhler, Kristin, Daniel J. Tollin, and Phillip M. Gilley. 2023. "EEG Alpha Band Responses Reveal Amplification Benefits in Infants with Hearing Loss" Children 10, no. 3: 600. https://doi.org/10.3390/children10030600
APA StyleUhler, K., Tollin, D. J., & Gilley, P. M. (2023). EEG Alpha Band Responses Reveal Amplification Benefits in Infants with Hearing Loss. Children, 10(3), 600. https://doi.org/10.3390/children10030600






