Effects of Sports, Exercise Training, and Physical Activity in Children with Congenital Heart Disease—A Review of the Published Evidence
Abstract
1. Introduction
1.1. Background
1.2. Pathophysiologic Mechanisms of Sports and Training in CHD
1.3. Objectives
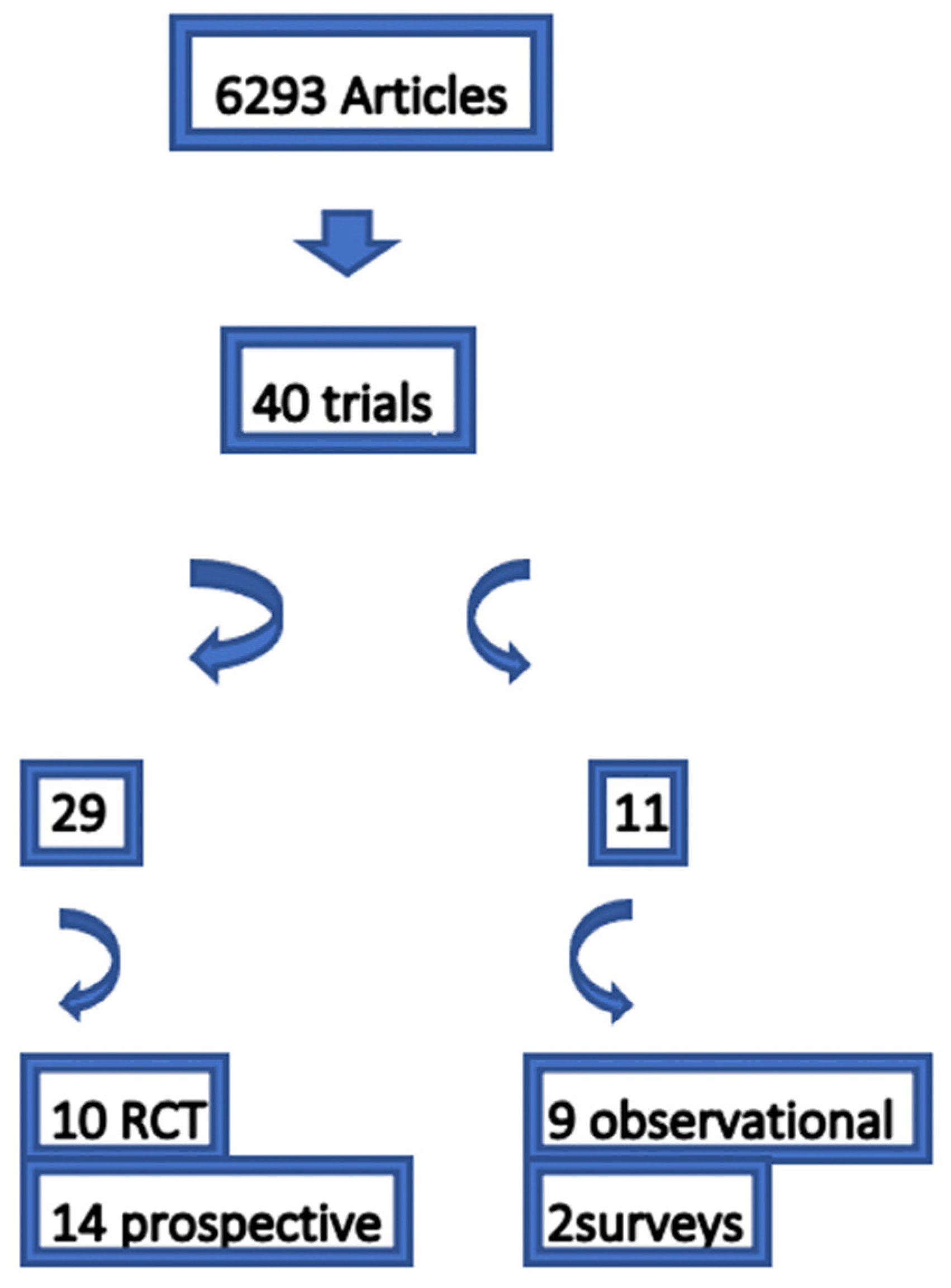
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Level of Evidence
3. Results
3.1. Motoric Skills
3.2. BMI
3.3. Cardiorespiratory Fitness
3.4. Physical Activity
3.5. Quality of Life and Other Questionnaires
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASD | atrial septum defect |
| ASO | arterial switch operation |
| VSD | ventricular septal defect |
| BMI | body mass index |
| CHD | congenital heart disease |
| CPET | cardiopulmonary exercise testing |
| CPVT | catecholaminergic polymorphic ventricular tachycardia |
| CRF | cardiorespiratory fitness |
| HR | heart rate |
| HRQoL | health-related quality of life |
| 6MWT | 6-minute walking test |
| MVPA | moderate to vigorous physical activity |
| PA | physical activity |
| QoL | quality of life |
| SCPC | superior cavopulmonary connection |
| TGA | transposition of the great arteries |
| TOF | tetralogy of Fallot |
| VO2max | max. oxygen consumption/uptake |
References
- Böhm, B.; Oberhoffer, R. Vascular health determinants in children. Cardiovasc. Diagn. Ther. 2019, 9 (Suppl. 2), S269–S280. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.S.; Warburton, D.E.; Janssen, I.; Paterson, D.H.; Latimer, A.E.; Rhodes, R.E.; Kho, M.E.; Hicks, A.; Leblanc, A.G.; Zehr, L.; et al. New Canadian physical activity guidelines. Appl. Physiol. Nutr. Metab. 2011, 36, 47–58. [Google Scholar] [CrossRef]
- Longmuir, P.E.; Brothers, J.A.; de Ferranti, S.D.; Hayman, L.L.; Van Hare, G.F.; Matherne, G.P.; Davis, C.K.; Joy, E.A.; McCrindle, B.W.; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Promotion of physical activity for children and adults with congenital heart disease: A scientific statement from the American Heart Association. Circulation 2013, 127, 2147–2159. [Google Scholar] [CrossRef]
- Physical Activity and Health: The Benefits of Physical Activity. Centers for Disease Control and Prevention Web Site. Available online: http://www.cdc.gov/physicalac-tivity/everyone/health/index.html (accessed on 9 November 2008).
- CDC. Guidelines for school and community programs to promote lifelong physical activity among young people. Morb. Mortal. Wkly. Rep. 1997, 46, 1–36. [Google Scholar]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. 5), S213–S256. [Google Scholar] [CrossRef]
- Siaplaouras, J.; Niessner, C.; Helm, P.C.; Jahn, A.; Flemming, M.; Urschitz, M.S.; Sticker, E.; Abdul-Khaliq, H.; Bauer, U.M.; Apitz, C. Physical Activity Among Children with Congenital Heart Defects in Germany: A Nationwide Survey. Front. Pediatr. 2020, 8, 170. [Google Scholar] [CrossRef]
- Graf, C.; Beneke, R.; Bloch, W.; Bucksch, J.; Dordel, S.; Eiser, S.; Ferrari, N.; Koch, B.; Krug, S.; Lawrenz, W.; et al. Recommendations for promoting physical activity for children and adolescents in Germany. A consensus statement. Obes. Facts 2014, 7, 178–190. [Google Scholar] [CrossRef]
- Ong, L.; Nolan, R.P.; Irvine, J.; Kovacs, A.H. Parental overprotection and heart-focused anxiety in adults with congenital heart disease. Int. J. Behav. Med. 2011, 18, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Longmuir, P.E.; McCrindle, B.W. Physical activity restrictions for children after the Fontan operation: Disagreement between parent, cardiologist, and medical record reports. Am. Heart J. 2009, 157, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Wadey, C.; Pieles, G.; Stuart, G.; Taylor, R.S.; Long, L. Physical activity interventions for people with congenital heart disease. Cochrane Database Syst. Rev. 2020, 10, CD013400. [Google Scholar] [CrossRef]
- West, S.L.; Banks, L.; Schneiderman, J.E.; Caterini, J.E.; Stephens, S.; White, G.; Dogra, S.; Wells, G.D. Physical activity for children with chronic disease; a narrative review and practical applications. BMC Pediatr. 2019, 19, 12. [Google Scholar] [CrossRef]
- Duppen, N.; Takken, T.; Hopman, M.T.; ten Harkel, A.D.; Dulfer, K.; Utens, E.M.; Helbing, W.A. Systematic review of the effects of physical exercise training programmes in children and young adults with congenital heart disease. Int. J. Cardiol. 2013, 168, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Millage, J.; Wong, D.; Yap, L.-A.; Bodiam, L.; Allison, A.; McCrindle, B.W.; Longmuir, P.E. Perceptions of Healthy Lifestyles among Children with Complex Heart Disease and Their Caregivers. CJC Open 2021, 3, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Barbour-Tuck, E.; Boyes, N.G.; Tomczak, C.R.; Lahti, D.S.; Baril, C.L.; Pockett, C.; Runalls, S.; Kakadekar, A.; Pharis, S.; Bradley, T.J.; et al. A cardiovascular disease risk factor in children with congenital heart disease: Unmasking elevated waist circumference—A CHAMPS* study *CHAMPS: Children’s Healthy-Heart Activity Monitoring Program in Saskatchewan. BMC Cardiovasc. Disord. 2020, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Neidenbach, R.; Achenbach, S.; Andonian, C.; Bauer, U.M.M.; Ewert, P.; Freilinger, S.; Gundlach, U.; Kaemmerer, A.S.; Nagdyman, N.; Nebel, K.; et al. Systematic assessment of health care perception in adults with congenital heart disease in Germany. Cardiovasc. Diagn. Ther. 2021, 11, 481–491. [Google Scholar] [CrossRef]
- Diller, G.P.; Giardini, A.; Dimopoulos, K.; Gargiulo, G.; Müller, J.; Derrick, G.; Giannakoulas, G.; Khambadkone, S.; Lammers, A.E.; Picchio, F.M.; et al. Predictors of morbidity and mortality in contemporary Fontan patients: Results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur. Heart J. 2010, 31, 3073–3083. [Google Scholar] [CrossRef]
- Zaqout, M.; Vandekerckhove, K.; Michels, N.; Bove, T.; François, K.; De Wolf, D. Physical Fitness and Metabolic Syndrome in Children with Repaired Congenital Heart Disease Compared with Healthy Children. J. Pediatr. 2017, 191, 125–132. [Google Scholar] [CrossRef]
- Acosta-Dighero, R.; Torres-Castro, R.; Rodríguez-Núñez, I.; Rosales-Fuentes, J.; Vilaró, J.; Fregonezi, G.; Lopetegui, B. Physical activity assessments in children with congenital heart disease: A systematic review. Acta Paediatr. 2020, 109, 2479–2490. [Google Scholar] [CrossRef]
- Tikkanen, A.U.; Oyaga, A.R.; Riaño, O.A.; Álvaro, E.M.; Rhodes, J. Paediatric cardiac rehabilitation in congenital heart disease: A systematic review. Cardiol. Young 2012, 22, 241–250. [Google Scholar] [CrossRef]
- Tran, D.; Maiorana, A.; Ayer, J.; Lubans, D.R.; Davis, G.M.; Celermajer, D.S.; d’Udekem, Y.; Cordina, R. Recommendations for exercise in adolescents and adults with congenital heart disease. Prog. Cardiovasc. Dis. 2020, 63, 350–366. [Google Scholar] [CrossRef]
- Wadey, C.A.; Pieles, G.; Stuart, G.; Taylor, R.; Long, L.; Williams, C.A. Cochrane corner: Physical activity interventions for people with congenital heart disease. Heart 2021, 107, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Caterini, J.E.; Campisi, E.S.; Cifra, B. Physical Activity Promotion in Pediatric Congenital Heart Disease: Are We Running Late? Can. J. Cardiol. 2020, 36, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Stuart, G.; Forsythe, L. Exercise prescription in young children with congenital heart disease: Time for a change in culture. Open Heart 2021, 8, e001669. [Google Scholar] [CrossRef]
- Brudy, L.; Meyer, M.; Oberhoffer, R.; Ewert, P.; Müller, J. Move more—Be happier? physical activity and health-related quality of life in children with congenital heart disease. Am. Heart J. 2021, 241, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, N.; Curran, T.; O’Neill, J.A.; Alexander, M.E.; Rhodes, J. Establishing a Comprehensive Pediatric Cardiac Fitness and Rehabilitation Program for Congenital Heart Disease. Pediatr. Cardiol. 2020, 41, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Takken, T.; Giardini, A.; Reybrouck, T.; Gewillig, M.; Hövels-Gürich, H.H.; Longmuir, P.E.; McCrindle, B.W.; Paridon, S.M.; Hager, A. Recommendations for physical activity, recreation sport, and exercise training in paediatric patients with congenital heart disease: A report from the Exercise, Basic & Translational Research Section of the European Association of Cardiovascular Prevention and Rehabilitation, the European Congenital Heart and Lung Exercise Group, and the Association for European Paediatric Cardiology. Eur. J. Prev. Cardiol. 2012, 19, 1034–1065. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96, Erratum in Eur. Heart J. 2021, 42, 548–549. [Google Scholar] [CrossRef]
- Budts, W.; Pieles, G.E.; Roos-Hesselink, J.W.; Sanz de la Garza, M.; D’Ascenzi, F.; Giannakoulas, G.; Müller, J.; Oberhoffer, R.; Ehringer-Schetitska, D.; Herceg-Cavrak, V.; et al. Recommendations for participation in competitive sport in adolescent and adult athletes with Congenital Heart Disease (CHD): Position statement of the Sports Cardiology & Exercise Section of the European Association of Preventive Cardiology (EAPC), the European Society of Cardiology (ESC) Working Group on Adult Congenital Heart Disease and the Sports Cardiology, Physical Activity and Prevention Working Group of the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2020, 41, 4191–4199. [Google Scholar] [CrossRef]
- Rhodes, J.; Curran, T.J.; Camil, L.; Rabideau, N.; Fulton, D.R.; Gauthier, N.S.; Gauvreau, K.; Jenkins, K.J. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics 2005, 116, 1339–1345. [Google Scholar] [CrossRef]
- Faulkner, G.E.; Buliung, R.N.; Flora, P.K.; Fusco, C. Active school transport, physical activity levels and body weight of children and youth: A systematic review. Prev. Med. 2009, 48, 3–8. [Google Scholar] [CrossRef]
- Ridgers, N.D.; Stratton, G.; Fairclough, S.J. Physical Activity Levels of Children during School Playtime. Sports Med. 2006, 36, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Stratton, G. Promoting children’s physical activity in primary school: An intervention study using playground markings. Ergonomics 2000, 43, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Stratton, G.; Leonard, J. The metabolism of the elementary school playground: The effects of an intervention study on children’s energy expenditure. Ped. Exerc. Sci. 2002, 14, 170. [Google Scholar]
- Stratton, G.; Mullan, E. The effect of multicolor playground markings on children’s physical activity level during recess. Prev. Med. 2005, 41, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, P.W.; Beveridge, S.K.; Watson, D.L. Increasing children’s school time physical activity using structured fitness breaks. Ped. Exerc. Sci. 2003, 15, 156–169. [Google Scholar] [CrossRef]
- Connolly, P.; McKenzie, T.L. Effects of a games intervention on the physical activity levels of children at recess. Res. Q. Exerc. Sport 1995, 66, A-60. [Google Scholar]
- Lee, S.M.; Burgeson, C.R.; Fulton, J.E.; Spain, C.G. Physical education and physical activity: Results from the school health policies and programs study 2006. J. Sch. Health 2007, 77, 435–463. [Google Scholar] [CrossRef] [PubMed]
- Pate, R.R.; Ward, D.S.; Saunders, R.P.; Felton, G.; Dishman, R.K.; Dowda, M. Promotion of physical activity among high-school girls: A randomized controlled trial. Am. J. Public Health 2005, 95, 1582–1587. [Google Scholar] [CrossRef]
- Pate, R.R.; Trost, S.G.; Mullis, R.; Sallis, J.F.; Wechsler, H.; Brown, D.R. Community interventions to promote proper nutrition and physical activity in youth. Prev. Med. 2000, 31, S138–S149. [Google Scholar] [CrossRef]
- Jago, R.; Baranowski, T. Non-curricular approaches for increasing physical activity in youth: A review. Prev. Med. 2004, 39, 157–163. [Google Scholar] [CrossRef]
- Baranowski, T.; Baranowski, J.C.; Cullen, K.W.; Thompson, D.I.; Nicklas, T.; Zakeri, I.E.; Rochon, J. The Fun, Food, and Fitness Project (FFFP): The Baylor GEMS pilot study. Ethn. Dis. 2003, 13, S30–S39. [Google Scholar] [PubMed]
- Pate, R.R.; Saunders, R.P.; Ward, D.S.; Felton, G.; Trost, S.G.; Dowda, M. Evaluation of a community-based intervention to promote physical activity in youth: Lessons from Active Winners. Am. J. Health Promot. 2003, 17, 171–182. [Google Scholar] [CrossRef] [PubMed]
- King, A.C. Community intervention for promotion of physical activity and fitness. Exerc. Sport Sci. Rev. 1991, 19, 211–259. [Google Scholar] [CrossRef] [PubMed]
- Resnicow, K.; Jackson, A.; Blissett, D.; Wang, T.; McCarty, F.; Rahotep, S.; Periasamy, S. Results of the healthy body healthy spirit trial. Health Psychol. 2005, 24, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, S.L.; Rhodes, R.E. Parental correlates of physical activity in children and early adolescents. Sports Med. 2006, 36, 79–97. [Google Scholar] [CrossRef]
- Ferreira, I.; van der Horst, K.; Wendel-Vos, W.; Kremers, S.; van Lenthe, F.J.; Brug, J. Environmental correlates of physical activity in youth—A review and update. Obes. Rev. 2007, 8, 129–154. [Google Scholar] [CrossRef]
- Böhm, B.; Karwiese, S.D.; Böhm, H.; Oberhoffer, R. Effects of Mobile Health Including Wearable Activity Trackers to Increase Physical Activity Outcomes among Healthy Children and Adolescents: Systematic Review. JMIR Mhealth Uhealth 2019, 7, e8298. [Google Scholar] [CrossRef]
- Bailey, R.C.; Olson, J.; Pepper, S.L.; Porszasz, J.; Barstow, T.J.; Cooper, D.M. The level and tempo of children’s physical activities: An observational study. Med. Sci. Sports Exerc. 1995, 27, 1033–1041. [Google Scholar] [CrossRef]
- Beets, M.W.; Beighle, A.; Erwin, H.E.; Huberty, J.L. After-school program impact on physical activity and fitness: A meta-analysis. Am. J. Prev. Med. 2009, 36, 527–537. [Google Scholar] [CrossRef]
- Schöffl, I.; Ehrlich, B.; Rottermann, K.; Weigelt, A.; Dittrich, S.; Schöffl, V. Jumping into a Healthier Future: Trampolining for Increasing Physical Activity in Children. Sports Med. Open 2021, 7, 53. [Google Scholar] [CrossRef]
- Luepker, R.V.; Perry, C.L.; McKinlay, S.M.; Nader, P.R.; Parcel, G.S.; Stone, E.J.; Webber, L.S.; Elder, J.P.; Feldman, H.A.; Johnson, C.C.; et al. Outcomes of a field trial to improve children’s dietary patterns and physical activity. The Child and Adolescent Trial for Cardiovascular Health. CATCH Collaborative Group. JAMA 1996, 275, 768–776. [Google Scholar] [CrossRef]
- Longmuir, P.E.; Tremblay, M.S.; Goode, R.C. Postoperative exercise training develops normal levels of physical activity in a group of children following cardiac surgery. Pediatr. Cardiol. 1990, 11, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, S.; Morrison, M.L.; McKeown, P.P.; Tennyson, C.; Sands, A.J.; McCrossan, B.; Grant, B.; Craig, B.G.; Casey, F.A. Exercise prescription improves exercise tolerance in young children with CHD: A randomised clinical trial. Open Heart 2021, 8, e001599. [Google Scholar] [CrossRef]
- Ferrer-Sargues, F.; Peiró-Molina, E.; Cebrià i Iranzo, M.C.; Moreno, J.C.; Cano-Sánchez, A.; Vázquez-Arce, M.; Albert, B.I.; Salvador-Coloma, P. Effects of Cardiopulmonary Rehabilitation on the Muscle Function of Children with Congenital Heart Disease: A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 5870. [Google Scholar] [CrossRef]
- Ferrer-Sargues, F.J.; Peiró-Molina, E.; Salvador-Coloma, P.; Carrasco Moreno, J.I.; Cano-Sánchez, A.; Vázquez-Arce, M.I.; Insa Albert, B.; Sepulveda Sanchis, P.; Cebrià IIranzo, M.À. Cardiopulmonary Rehabilitation Improves Respiratory Muscle Function and Functional Capacity in Children with Congenital Heart Disease. A Prospective Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 4328. [Google Scholar] [CrossRef] [PubMed]
- Kroll, K.H.; Kovach, J.R.; Ginde, S.; Jacobsen, R.M.; Danduran, M.; Foster, A.; Brosig, C.L. Impact of a paediatric cardiac rehabilitation programme upon patient quality of life. Cardiol. Young 2021, 31, 804–811. [Google Scholar] [CrossRef]
- Meyer, M.; Brudy, L.; Fuertes-Moure, A.; Hager, A.; Oberhoffer-Fritz, R.; Ewert, P.; Müller, J. E-Health Exercise Intervention for Pediatric Patients with Congenital Heart Disease: A Randomized Controlled Trial. J. Pediatr. 2021, 233, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Blais, A.Z.; Lougheed, J.; Yaraskavitch, J.; Adamo, K.B.; Longmuir, P.E. “I really like playing games together”: Understanding what influences children with congenital heart disease to participate in physical activity. Child Care Health Dev. 2020, 46, 457–467. [Google Scholar] [CrossRef]
- Lopez, J.R.; Voss, C.; Kuan, M.T.Y.; Hemphill, N.M.; Sandor, G.G.S.; Harris, K.C. Physical Activity Is Associated with Better Vascular Function in Children and Adolescents with Congenital Heart Disease. Can. J. Cardiol. 2020, 36, 1474–1481. [Google Scholar] [CrossRef]
- Sutherland, N.; Jones, B.; Westcamp Aguero, S.; Melchiori, T.; du Plessis, K.; Konstantinov, I.E.; Cheung, M.M.H.; d’Udekem, Y. Home- and hospital-based exercise training programme after Fontan surgery. Cardiol. Young 2018, 28, 1299–1305. [Google Scholar] [CrossRef]
- Altamirano-Diaz, L.; Rombeek, M.; De Jesus, S.; Welisch, E.; Prapavessis, H.; Dempsey, A.A.; Fraser, D.; Miller, M.R.; Norozi, K. Remote Lifestyle Counseling Influences Cardiovascular Health Outcomes in Youth with Overweight or Obesity and Congenital Heart Disease. Front. Pediatr. 2017, 5, 269. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, E.R.; Lundell, B.; Söderström, L.; Sjöberg, G. Can endurance training improve physical capacity and quality of life in young Fontan patients? Cardiol. Young 2018, 28, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, R.M.; Ginde, S.; Mussatto, K.; Neubauer, J.; Earing, M.; Danduran, M. Can a Home-based Cardiac Physical Activity Program Improve the Physical Function Quality of Life in Children with Fontan Circulation? Congenit. Heart Dis. 2016, 11, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Klausen, S.H.; Andersen, L.L.; Søndergaard, L.; Jakobsen, J.C.; Zoffmann, V.; Dideriksen, K.; Kruse, A.; Mikkelsen, U.R.; Wetterslev, J. Effects of eHealth physical activity encouragement in adolescents with complex congenital heart disease: The PReVaiL randomized clinical trial. Int. J. Cardiol. 2016, 221, 1100–1106. [Google Scholar] [CrossRef]
- Duppen, N.; Etnel, J.R.; Spaans, L.; Takken, T.; van den Berg-Emons, R.J.; Boersma, E.; Schokking, M.; Dulfer, K.; Utens, E.M.; Helbing, W.; et al. Does exercise training improve cardiopulmonary fitness and daily physical activity in children and young adults with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. Am. Heart J. 2015, 170, 606–614. [Google Scholar] [CrossRef]
- Dulfer, K.; Duppen, N.; Blom, N.A.; van Dijk, A.P.; Helbing, W.A.; Verhulst, F.C.; Utens, E.M. Effect of exercise training on sports enjoyment and leisure-time spending in adolescents with complex congenital heart disease: The moderating effect of health behavior and disease knowledge. Congenit. Heart Dis. 2014, 9, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Dulfer, K.; Duppen, N.; Blom, N.A.; Van Domburg, R.T.; Helbing, W.A.; Verhulst, F.C.; Utens, E.M.W.J. Effects of exercise training on behavioral and emotional problems in adolescents with tetralogy of Fallot or a Fontan circulation: A randomized controlled trial. Int. J. Cardiol. 2014, 172, e425–e427. [Google Scholar] [CrossRef] [PubMed]
- Longmuir, P.E.; Tyrrell, P.N.; Corey, M.; Faulkner, G.; Russell, J.L.; McCrindle, B.W. Home-based rehabilitation enhances daily physical activity and motor skill in children who have undergone the Fontan procedure. Pediatr. Cardiol. 2013, 34, 1130–1151. [Google Scholar] [CrossRef]
- Morrison, M.L.; Sands, A.J.; McCusker, C.G.; McKeown, P.P.; McMahon, M.; Gordon, J.; Grant, B.; Craig, B.G.; Casey, F.A. Exercise training improves activity in adolescents with congenital heart disease. BMJ Heart 2013, 99, 1122–1128. [Google Scholar] [CrossRef]
- Moalla, W.; Elloumi, M.; Chamari, K.; Dupont, G.; Maingourd, Y.; Tabka, Z.; Ahmaidi, S. Training effects on peripheral muscle oxygenation and performance in children with congenital heart diseases. Appl. Physiol. Nutr. Metab. 2012, 37, 621–630. [Google Scholar] [CrossRef]
- Moalla, W.; Dupont, G.; Costes, F.; Gauthier, R.; Maingourd, Y.; Ahmaidi, S. Performance and muscle oxygenation during isometric exercise and recovery in children with congenital heart diseases. Int. J. Sports Med. 2006, 27, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Pringsheim, M.; Engelhardt, A.; Meixner, J.; Halle, M.; Oberhoffer, R.; Hess, J.; Hager, A. Motor training of sixty minutes once per week improves motor ability in children with congenital heart disease and retarded motor development: A pilot study. Cardiol. Young 2013, 23, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Stieber, N.A.; Gilmour, S.; Morra, A.; Rainbow, J.; Robitaille, S.; Van Arsdell, G.; McCrindle, B.W.; Gibson, B.E.; Longmuir, P.E. Feasibility of improving the motor development of toddlers with congenital heart defects using a home-based intervention. Pediatr. Cardiol. 2012, 33, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Beek, E.A.; Binkhorst, M.; de Hoog, M.; de Groot, P.; van Dijk, A.; Schokking, M.; Hopman, M. Exercise performance and activity level in children with transposition of the great arteries treated by the arterial switch operation. Am. J. Cardiol. 2010, 105, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Brassard, P.; Poirier, P.; Martin, J.; Noël, M.; Nadreau, E.; Houde, C.; Cloutier, A.; Perron, J.; Jobin, J. Impact of exercise training on muscle function and ergoreflex in Fontan patients: A pilot study. Int. J. Cardiol. 2006, 107, 85–94. [Google Scholar] [CrossRef]
- Moons, P.; Barrea, C.; Suys, B.; Ovaert, C.; Boshoff, D.; Eyskens, B.; Vandenrijn, C.; Sluysmans, T. Improved perceived health status persists three months after a special sports camp for children with congenital heart disease. Eur. J. Pediatr. 2006, 165, 767–772. [Google Scholar] [CrossRef]
- Opocher, F.; Varnier, M.; Sanders, S.P.; Tosoni, A.; Zaccaria, M.; Stellin, G.; Milanesi, O. Effects of aerobic exercise training in children after the Fontan operation. Am. J. Cardiol. 2005, 95, 150–152. [Google Scholar] [CrossRef]
- Fredriksen, P.M.; Kahrs, N.; Blaasvaer, S.; Sigurdsen, E.; Gundersen, O.; Roeksund, O.; Norgaand, G.; Vik, J.T.; Soerbye, O.; Ingjer, E.; et al. Effect of physical training in children and adolescents with congenital heart disease. Cardiol. Young 2000, 10, 107–114. [Google Scholar] [CrossRef]
- Dean, P.N.; Gillespie, C.W.; Greene, E.A.; Pearson, G.D.; Robb, A.S.; Berul, C.I.; Kaltman, J.R. Sports participation and quality of life in adolescents and young adults with congenital heart disease. Congenit. Heart Dis. 2015, 10, 169–179. [Google Scholar] [CrossRef]
- Majnemer, A.; Rohlicek, C.; Dahan-Oliel, N.; Sahakian, S.; Mazer, B.; Maltais, D.B.; Schmitz, N. Participation in leisure activities in adolescents with congenital heart defects. Dev. Med. Child Neurol. 2020, 62, 946–953. [Google Scholar] [CrossRef]
- Chen, C.W.; Su, W.J.; Wang, J.K.; Yang, H.L.; Chiang, Y.T.; Moons, P. Physical self-concept and its link to cardiopulmonary exercise tolerance among adolescents with mild congenital heart disease. Eur. J. Cardiovasc. Nurs. 2015, 14, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, D.; Slinde, F.; Hulthén, L.; Sunnegårdh, J. Physical activity, sports participation and aerobic fitness in children who have undergone surgery for congenital heart defects. Acta Paediatr. 2009, 98, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, M.L.; McBride, M.G.; Paridon, S.; Goldmuntz, E. Association of Habitual Activity and Body Mass Index in Survivors of Congenital Heart Surgery: A Study of Children and Adolescents with Tetralogy of Fallot, Transposition of the Great Arteries, and Fontan Palliation. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Longmuir, P.E.; Corey, M.; McCrindle, B.W. Interactions with Home and Health Environments Discourage Physical Activity: Reports from Children with Complex Congenital Heart Disease and Their Parents. Int. J. Environ. Res. Public Health 2021, 18, 4903. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.D.; Green, A.; Henry, K. Physical activity and obesity in children with congenital cardiac disease. Cardiol. Young 2011, 21, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jae, S.Y.; Choo, J.; Yoon, J.K.; Kim, S.H.; Königstein, K.; Schmidt-Trucksäss, A.; Franklin, B.A. Mediating effects of exercise capacity on the association between physical activity and health-related quality of life among adolescents with complex congenital heart disease. Am. J. Hum. Biol. 2019, 31, e23297. [Google Scholar] [CrossRef]
- O’Byrne, M.L.; Desai, S.; Lane, M.; McBride, M.; Paridon, S.; Goldmuntz, E. Relationship between Habitual Exercise and Performance on Cardiopulmonary Exercise Testing Differs between Children with Single and Biventricular Circulations. Pediatr. Cardiol. 2017, 38, 472–483. [Google Scholar] [CrossRef]
- Knowles, R.L.; Day, T.; Wade, A.; Bull, C.; Wren, C.; Dezateux, C.; UK Collaborative Study of Congenital Heart Defects (UKCSCHD). Patient-reported quality of life outcomes for children with serious congenital heart defects. Arch. Dis. Child. 2014, 99, 413–419. [Google Scholar] [CrossRef]
- Ray, T.D.; Henry, K. Self-efficacy and physical activity in children with congenital heart disease: Is there a relationship? J. Spec. Pediatr. Nurs. 2011, 16, 105–112. [Google Scholar] [CrossRef]
- Amedro, P.; Gavotto, A.; Guillaumont, S.; Bertet, H.; Vincenti, M.; De La Villeon, G.; Bredy, C.; Acar, P.; Ovaert, C.; Picot, M.C.; et al. Cardiopulmonary fitness in children with congenital heart diseases versus healthy children. Heart 2018, 104, 1026–1036. [Google Scholar] [CrossRef]
- Andonian, C.; Langer, F.; Beckmann, J.; Bischoff, G.; Ewert, P.; Freilinger, S.; Kaemmerer, H.; Oberhoffer, R.; Pieper, L.; Neidenbach, R.C. Overweight and obesity: An emerging problem in patients with congenital heart disease. Cardiovasc. Diagn. Ther. 2019, 9 (Suppl. 2), S360–S368. [Google Scholar] [CrossRef] [PubMed]
- Willinger, L.; Brudy, L.; Meyer, M.; Oberhoffer-Fritz, R.; Ewert, P.; Müller, J. Overweight and Obesity in Patients with Congenital Heart Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 9931. [Google Scholar] [CrossRef] [PubMed]
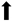 : improvement;
: improvement;  : reduction; FEV1: Forced expiratory volume.
: reduction; FEV1: Forced expiratory volume.
| Study | Study Design | Diagnosis | Number of Participants | Age | Intervention | Control | Outcome | Results: Parameters with Significant Improvement | LOE |
|---|---|---|---|---|---|---|---|---|---|
| Callaghan S et al. 2021 [54] | RCT | CHD, 100 males | 163 | 5–10 | One-day education session individual written exercise plan over the 4-month intervention period. | Usual care | CPET activity monitoring using an accelerometer. | Significant improvement in peak exercise capacity in the intervention group. Trend towards increased daily activity levels. | 1 |
| Ferrer-Sargues FJ et al. 2021 [55] | Prospective interventional Study 11/2017–01/2020 | CHD with reduced aerobic capacity | 15 | 12.4–15.7 | IMPROVE project (Initiative for Monitored Pediatric cardiac Rehabilitation Oriented by cardiopulmonary Exercise testing). IMPROVE was designed following the American College of Sports Medicine (ACSM). Cardiopulmonary 3-month rehabilitation program (CPRP), 2x/week for a total of 24 sessions. Sessions 70 min, endurance and strength-resistance training. Intensity was defined by the subject’s CPET, initially aiming for a heart rate (HR) near the first ventilatory threshold (VT1) HR and displacing this target frequency progressively towards the secondary ventilatory threshold (VT2) HR or a maximal HR of 75% of their peak HR in cases where the VT2 was not available. (a) Warm-up phase (5 min): diaphragmatic breathing, articular mobility exercises, and a light walk. (b) Endurance-training phase (20 min): treadmill, and a static bicycle, two min of warm-up, two min of cooldown. Ten to fifteen repetitions of each exercise, with a 20 s rest. Respiratory training (20 min): inspiratory muscle trainer threshold. | No | 6MWT handgrip strength muscle strength arms and limbs. | Statistically significant improvement was observed in the subjects’ handgrip strength, biceps brachii, and quadriceps femoris strength, as well as triceps surae fatigue process with maintenance of the results six months after the intervention. | 2 |
| Ferrer-Sargues FJ et al. 2021 [56] | Prospective interventional Study 11/2017–01/2020 | CHD with reduced aerobic capacity | 15 | 12–16 | IMPROVE project (Initiative for Monitored Pediatric cardiac Rehabilitation Oriented by cardiopulmonary Exercise testing). IMPROVE designed following the American College of Sports Medicine (ACSM). Cardiopulmonary 3-month rehabilitation program (CPRP), 2x/week for a total of 24 sessions. Sessions 70 min, endurance and strength-resistance training. Intensity was defined by the subject’s CPET, initially aiming for a heart rate (HR) near the first ventilatory threshold (VT1) HR and displacing this target frequency progressively towards the secondary ventilatory threshold (VT2) HR or a maximal HR of 75% of their peak HR in cases where the VT2 was not available. (a) Warm-up phase (5 min): diaphragmatic breathing, articular mobility exercises, and a light walk. (b) Endurance-training phase (20 min): treadmill, and a static bicycle, two min of warm-up, two min of cooldown. Ten to fifteen repetitions of each exercise, with a 20 s rest. Respiratory training (20 min): inspiratory muscle trainer threshold. | No | Inspiratory muscle function. Spirometry/maximum static inspiratory and expiratory pressures. 6MWT handgrip strength, muscle strength, arms and limbs. | Increase in peripheral muscle function after a three-month, 24-session CPRP in children with CHD. Improvement persisted 6 months after the completion. Improvement of inspiratory muscles. Improved 6MWT. Results suggest CPRP improves respiratory muscle function and functional capacity, with lasting results. | 2 |
| Kroll KH et al. 2021 [57] | Prospective interventional study | CHD (25 completed, 20 dropped out, 24 in progress) 25 (11 mental health diagnoses/ADHS) | 25 | 7–24 | “Steppin’ It Up” home-based year-long cardiac rehabilitation program. Four in-person visits occurring every 3 to 6 months. Garmin VivoFit2© Activity Monitor. | Exercise capacity was assessed by Progressive Aerobic Cardiovascular Endurance Run, (20-meter shuttle test run), patient functioning (social, emotional, school, psychosocial), patient general and cardiac-related quality of life, patient self-concept, and patient behavioral/emotional problems: HR-QoL/PQoL Piers’ Harris Children’s Self-Concept Scale Child Behavior Checklist | Progressive Aerobic Cardiovascular Endurance Run, significant increase from 5 to 10 median shuttles completed. PQoL in general not significantly improved, but cardiac-related quality of life improved Conversely, parents reported significant improvement in patients’ emotional functioning, social functioning, school functioning, psychosocial functioning, and total quality of life score. Overall self-concept, physical appearance and attributes, and freedom from anxiety improved | 2 | |
| Meyer et al. 2021 [58] | RCT | CHD | 70 35:35 | 10–18 | 3x/week 20 min web-based program over 24 weeks. | No training | Health-related physical fitness (HRPF) and health-related quality of life (HRQoL). | No improvement found. | 1 |
| Blais AZ et al. 2020 [59] | Prospective interventional study | CHD | 11 | 7–10 | For 10 consecutive weeks, participants attended a once-weekly multi-sport program (Sportball ©). Each lesson focused on a different ball sport (i.e., basketball, volleyball, and soccer). | No | Questionnaire self-report; focus group sessions 40 min. | Themes: (a) motivation, (b) self-efficacy, (c) peer influences, and (d) family influences. Enjoyment of physical activity is a primary source of motivation, intrinsic motivation. | 2 |
| Lopez et al. 2020 [60] | Prospective interventional study | CHD | 104 | 9–16 | ActiGraph accelerometer worn over the right hip during waking hours for 7 days. | Aortic pulse wave velocity (cm/s) was measured using standard two-dimensional echocardiography and Doppler ultrasound. | Higher levels of moderate-to-vigorous physical activity were associated with lower aortic pulse wave velocity. | 2 | |
| Sutherland N et al. 2018[61] | Prospective, randomized trial 01/2010–06/2014 | Fontan circulation (Home 11, Hospital 6) | 17 | 12–19 15 ± 3 | Home-based 2x1 h training/week (1 phone call + 1 visit week 4/5) Training: 5–10 min warm-up 20–30 min aerobic (target HR: 65–85% of HR at peak oxygen consumption); 20–30 min lower limb bodyweight resistance; 5–10 min cooldown. | Hospital-based: Training: 5–10 min warm-up 20–30 min aerobic (target-HR: 65–85% of HR at peak oxygen consumption) 20–30 min lower limb bodyweight resistance 5–10 min cooldown | CPET 6MWT QoL Reassessment after 8 weeks. | Oxygen consumption at anaerobic threshold increased from 19.3 ± 3.8 to 21.6 ± 6.0 mL/kg/minute. Peak oxygen pulse increased from 8.8 ± 2.5 to 9.5 ± 2.7 mL/beat. Total quality of life scale improved from 68 to 74%. Psychosocial health improved from 67 to 74%. 6MWT (521 ± 101 versus 569 ± 81 m). | 1 |
| Altamirano-Diaz L et al. 2017[62] | Prospective 05/2012–10/2015 | CHD and with overweight or obesity 19 operated/non-operated | 34 | 7–17 | Bi-weekly nutrition and fitness counseling delivered via smartphone over 12 months; 30 min for each session: 15–20 min for counseling and 10–15 min for charting. | Anthropometry, body composition cardiorespiratory exercise capacity, and cardio-metabolic risk factors assessed at baseline, 6 months, and 12 months. | Statistically significant decreases in waist circumference (WC), body mass, waist-to-height ratio z-score were observed at 6 and 12 months in the operated group. Significant linear increase in lean body mass was observed in both groups. No significant difference between blood tests. VO2max at baseline, 6 months, or 12 months | 2 | |
| Hedlund et al. 2017 [63] | Prospective Interventional | Fontan | 30 | 14.2 ±3.2 | 12-week endurance training subject’s organized physical exercise during an average school week. Duration in minutes was stated, and average perceived intensity was estimated using the Borg scale →individualized endurance training. The contract was to include 2 × 45 min of extra endurance training every week for 12 consecutive weeks, with maintained baseline activities such as physical education in school and other sports. The endurance training programs included sports such as running, jogging, skiing, cycling, riding, swimming, dancing, football, and so on. The purpose of the training program was to increase endurance training at a submaximal level with the aim to increase load gradually during the training program. | 25 healthy | CPET 6MWT tests were repeated after a 12-week endurance training program and after 1 year. Questionnaire QoL Pediatric Quality of Life Inventory Version 4.0 questionnaires for children and parents. | CRF increased significantly in the control group but not in the patients. | 2 |
| Jacobsen RM et al. 2016 [64] | Pilot study | Fontan (8 male) | 14 | 8–12 | Twelve-week moderate/high intensity home-based cardiac physical activity program. Home exercise routine. Three formalized in-person exercise sessions at 0, 6, and 12 weeks. A 45-minute home exercise routine of dynamic and static exercises. Fitbit Flex activity monitor | No | CPET HRQOL (child and parents) PedsQoL Shuttle Test Run to measure exercise capacity Fitbit Flex activity monitor. | Parents: overall HRQOL, physical function, school function, and psychosocial function. No measurable improvement in the patient-reported HRQOL. Objective exercise capacity significantly improved. VO2max from baseline to the 12-week session, improving from a mean of 41.8 to 42.3 mL/kg/min. | (2) |
| Klausen et al. 2016 [65] | RCT | CHD | 158 | 10–13 | Paediatric Rehabilitation for Vanguard in Lifeskills (PReVaiL) All patients received 45 min of group health education and 15 min of individual counseling with their parents. Intervention: 52-week Internet, mobile application, and SMS-based program delivering individually tailored text messages to encourage physical activity. The program encompassed three main approaches: health education, tailored interactive text encouragements, and a personal exercise planning tool. The patients allocated to the eHealth intervention were sent health information and a new encouragement every week. Patients recorded exercise duration and type in a mobile application that translated intensity into virtual points, a system designed to provide motivation. Adherence to the eHealth program was assessed by patient registration of physical activities via the eHealth application for at least two consecutive weeks during the trial. | Without training | CPET | Of 81 patients in the intervention group, just 46 (57%) patients used the eHealth application for at least two consecutive weeks and completed both exercise tests. Only eight (10%) patients were active users during the last week of the intervention. Just 57 (70%) of the patients in the intervention group adhered to the intervention using the eHealth application for at least two consecutive weeks. PA and VO2max did not change significantly. Few patients used the app. | 1 |
| Duppen et al. 2015 [66] | RCT | TOF Fontan | 56 37 control 93 | 10–25 | Twelve-week standardized aerobic exercise training program. Training program consisted of 3 1-hour exercise sessions per week The exercise sessions consisted of 40 min aerobic dynamic cardiovascular training, 10 min warming up, and 10 min cooling down. HR-monitor (aim to train submaximal level–baseline HR +60/70%) | Care as usual | CRF (CPET 2 weeks before and 2 weeks after training pro gram Stroke volume (cardiac MRI) PA (accelerometer) | VO2max increased in the exercise group by 5.0% (1.7 ± 4.2 mL/kg per minute) but not in the control group (0.9 ± 5.2 mL/kg per minute). Workload increased significantly in the exercise group compared with the control group (6.9 ± 11.8 vs. 0.8 ± 13.9 W). →in TOF, CRF increased (VO2max, O2pulse). in Fontan, it did not. PA level did not significantly raise after training. | 1 |
| Dulfer K et al. 2014 [67,68] | RCT | TOF/Fontan | 93 | 10–25 | Twelve-week period 3x/week 1 h standardized exercise training. Training: 10 min warm-up, 40 min aerobic dynamic cardiovascular training, 10 min cooldown. | Care as usual | Web-based questionnaires and interviews by phone At baseline and after 12 weeks. Sports enjoyment and leisure-time spending. 2. Web-based age-appropriate HRQoL questionnaires 3. Psychological follow-up: phone interview, web-based questionnaire 0 + 3 months follow-up, focus on behavioral and emotional problems. | Exercise training decreased passive, but not active, leisure-time spending. It did not influence sports enjoyment. 2. children, aged 10–15 years in the exercise group improved significantly in self-reported cognitive functioning and parent-reported social functioning. Youngsters aged 16–25 years did not change their HRQoL. Fewer negative emotions than healthy peers. Less bodily pain, less role limitation due to physical limitations, and less role limitation due to emotional problems In contrast, they reported decreased general health. Patients in the control group had higher sustained scores on bodily pain. 3. No effects on behavioral and emotional problems. | 1 |
| Longmuir PE et al. 2013 [69] | RCT | Fontan (36 male) | 61 | 5.9–11.7 | Twelve-month, parent-delivered home training programs to enhance physical activity, motor skills, fitness, and activity. Static and dynamic exercise, play-based physical activity. Monthly, specific written/illustrated instructions for four 1-week sessions 1.5–2.0 h/week | 12-month, parent-delivered home education programs. Educational activities (e.g., games, stories, Web sites). Families received healthy lifestyle information, such as healthy eating, injury prevention, and activity benefits Accelerometry | MVPA baseline, 6, 12, 24 months gross motor skills, fitness, and activity attitudes | Gross motor skill was significantly greater at the end of the 2-year study period for both intervention groups combined. MVPA at 2 years was significantly greater. | 1 |
| Morrison et al. 2013 [70] | RCT | CHD | 143 | 12–20 | The activity day was conducted as a motivational interview-style group exploring the nature of motivation towards exercise using small groups and visualization techniques. The concept of motivation was introduced. Participants completed a motivational rating sheet assessing their own feelings towards the importance of exercise, confidence, and readiness with regard to increasing their activity presession and postsession. Each participant was seen individually, and suggestions were discussed for ways to increase their activity over the next 6 months in a manner suitable for their diagnosis. They were also given a written exercise training plan to implement at home. Each participant was contacted once a month to check on progress with their exercise plan and discuss any problems. | No exercise | MVPA accelerator CPET endurance reassessment after the 6-month intervention period | Increased MVPA in intervention group. This study is unique in that it employed psychological methods to maximize the impact of its interventions. Consideration for issues, such as maintenance of activity, psychological well-being, and promotion of good lifestyle choices. | 1 |
| Moalla et al. 2012 [71] | RCT | CHD | 18 | 12–15 | Individualized 12-week aerobic cycling training group (TG) | No intervention | Effect of training on peripheral muscular performance and oxygenation. Maximal voluntary contraction (MVC) and endurance at 50% MVC (time to exhaustion, Tlim) of the knee extensors were measured before and after training. During the 50% MVC exercise and recovery, near-infrared spectroscopy (NIRS) was used to assess the fall in muscle oxygenation, i.e., deoxygenation (DmO2 ) of the vastus lateralis, the mean rate of decrease in muscle oxygenation, the half time of recovery (T1/2R), and the recovery speed to maximal oxygenation (RS). | After training, significant improvements were observed in TG for MVC (101.6 ± 14.0 vs. 120.2 ± 19.4 N·m) and Tlim (66.2 ± 22.6 vs. 86.0 ± 23.0 s). Increased oxygenation (0.20 ± 0.13 vs. 0.15 ± 0.07 a.u.) and faster mean rate of decrease in muscle oxygenation were also shown after training in TG (1.22 ± 0.45 vs. 1.71 ± 0.78%·s−1). Moreover, a shorter recovery time was observed in TG after training for T1/2R (27.2 ± 6.1 vs. 20.8 ± 4.2 s) and RS (63.1 ± 18.4 vs. 50.3 ± 11.4 s). A significant relationship between the change in DmO2 and both MVC (r = 0.95) and Tlim (r = 0.90) in TG was observed | 1 |
| Moalla et al. 2012 [72] | Prospective interventional Study | CHD | 25 | 13.5 ± 1.8 | Three-day multi-sports camp | Healthy controls | The perceived health status was measured using the Child Health Questionnaire–Child Form, CHQ-CF87, completed by the child at the start of the camp (T1), at the end of the camp (T2), and 3 months after the camp concluded (T3). Habitual physical activities were assessed by means of a modified version of the Baecke questionnaire, which was completed by one of the parents at T1 and T3. | Improvement during camp attendance. No change in habitual activity afterwards. | 2 |
| Müller et al. 2012 [73] | Pilot study | CHD 04/2007–07/2011 | 14 | 4–6 | Three-month low-dose motor training program of 60 min once per week. | Motor developmental test MOT 4–6 months before and after 3 months. | Delayed motor development significantly increased motoric skills | (2) | |
| Stieber NA et al. 2011 [74] | Prospective interventional study | toddler CHD (post-op ASO or SCPC) (5 female) | 20 | 12–26 months | Ten weeks (5 two-week sessions), play-based, parent-delivered rehabilitation program on gross, fine, and visual motor functions. The total daily time requested was 20 min: 10 min for each of the two motor development goals identified for that 2-week period. Parents were contacted biweekly. | No | Peabody developmental motor scale-version 2 (PDMS-2) before and after the 10 week + video SCPC scored lower at baseline. | No significant differences between the baseline and follow-up age-adjusted scores in either group. Rehabilitation program enables post-SCPC children to increase their rate of development to an age-appropriate rate. | 3 |
| Beek et al. 2010 [75] | Observational study | TGA switch | 17 | 12.1 ± 2 | Pedometer and diary. | 20 | Seven-day period using a pedometer and diary questionnaire was used to assess physical activity participation and overprotection | No significant differences in physical activity pattern or overprotection. | 3 |
| Brassard P et al. 2006 [76] | Pilot study | Fontan | 7 | 16 ± 5 | Eight-week aerobic and resistance training program at the Pavillon de Prévention des Maladies Cardiaques de l’Hôpital Laval (n = 2) or at home (n = 3). Aerobic training was individually prescribed to allow the subjects to work progressively at 50–80% of their VO2 peak throughout the 8 weeks. | Control group of Fontan patients (n = 4) performed their normal activities without exercise training. Healthy (7) | CPET ergometer skeletal muscle function evaluation ergoreflex contribution | Fontan exercising group: no significant change in absolute or relative VO2 peak and in skeletal muscle strength. Neuromuscular function was positively influenced by exercise training. Lower ergoreflex contribution to absolute values of systolic blood pressure resulted from exercise training. An 87% reduction in the ergoreflex contribution to absolute values of diastolic blood pressure ( p = 0.2) and a 68% reduction in relative values of diastolic blood pressure ( p = 0.36) in the Fontan exercising group was observed. | 2 |
| Moalla 2006 [72] | Prospective interventional Study | CHD | 9 | 13.5 ± 1.8 | Maximal volunteered contraction (MVC) and endurance at 50 % of MVC (time to exhaustion, Tlim) of the knee extensor were measured. | 14 healthy | Near-infrared spectroscopy (NIRS) was used to evaluate StO2 and BV in vastus lateralis. The drop in muscle oxygen saturation (DmO2), half-time of recovery (T1⁄2R), and recovery speed to maximal oxygen saturation (Rs) were analyzed. | Patients with CHD showed lower MVC and Tlim than control children. StO2 and BV values in both groups were similar at rest and decreased at the onset of contraction. DmO2 was larger in patients, which reflected pronounced deoxygenation. During recovery, the patients exhibited a longer T1⁄2R and RS than control children. We concluded that reduced strength and endurance in patients with CHD were associated with an impairment of StO2 and BV and a slower reoxygenation during recovery. Mismatch between oxygen delivery and oxygen consumption during exercise. Patients with CHD showed reduced skeletal muscle strength and endurance of the knee extensors in comparison with the control children. Parameters of NIRS recovered significantly faster in control subjects than patients with CHD. | |
| Moons et al. 2006 [77] | RCT | CHD (+14 healthy) | 17 | 12–16 | 9CHD Training: 12 weeks, 3 sessions/week, 1 h. (1) 10 min warm-up; (2) 45 min interval training alternating 10 min active periods at an HR within ± 5 bpm to the VT (individualized intensity) and passive 5 min periods of pedaling against an unloaded charge; (3) 5 min recovery. Cycle ergometer, pulse monitor. | 14 healthy 8 CHD | CPET 6MWT | No significant difference for FEV1/FVC, TLC, and FVC between the two groups. After training period, T-CHD group significantly increased their WD. After 12 weeks of training, the ratios of power output at VT/peak, HR at VT(HRVT)/HRmax, VO2 at VT(VO2VT)/VO2max, and VE at VT (VEVT)/VE max increased significantly in T-CHD. | 1 |
| Rhodes et al. 2006 [30] | Prospective interventional study | CHD | 15 | 8–17 | 1 h session 2x/week for 12 weeks . | 18 CHD without training | Restudied 6.9 ± 1.6 months after completion of the cardiac rehabilitation program (1 year after the precardiac rehabilitation study). | The cardiac rehabilitation patients’ exercise function did not change significantly over the 6.9-month period. Predicted peak oxygen consumption and peak work rate remained significantly superior to baseline, precardiac rehabilitation values. These changes were also associated with improvements in self-esteem, behavior, and emotional state. In contrast, among the control subjects, small but statistically insignificant declines in peak oxygen consumption and peak work rate were observed on the final exercise test compared with values obtained at baseline, 1 year earlier. | 2 |
| Opocher et al. 2005 [78] | Prospective interventional Study | Fontan | 10 | 7–12 | The training program lasted for 8 months. Ten lessons were held at a local gymnasium in Padova, Italy, twice a week for the first 3 weeks and once a month for the next 4 months of the training program. The remaining part of the program was held at each child’s home twice a week for 30 to 45 min under the supervision of parents. Each child was given a wristband heart rate monitor and instructed to keep their heart rate in the prescribed aerobic range. The exercise level during training was designed to range from 50% to 70% of maximal oxygen consumption. Parents were requested to write the frequency and duration of each exercise session in a log. Moreover, we contacted the families monthly by phone to inquire about the children’s compliance with the training program and to encourage continued participation. | CPET | Max. oxygen pulse significantly increased training results in an improvement in an aerobic capacity. Important increase in maximal oxygen consumption as well as a decrease in the heart rate curve and an increase in the oxygen pulse curve during submaximal exercise. Reduced heart rates and improved cardiovascular efficiency during usual daily activities. | 2 | |
| Rhodes et al. 2005 [30] | Pilot study | CHD (mostly Fontan) | 19 | 8–17 | Rehabilitation sessions were conducted for 1 hour twice a week for 12 weeks at the clinic. 5 to 10 minutes of stretching exercise. 45 minutes of aerobic and light weight/resistance exercises. Activities included aerobic dance, step aerobics, calisthenics (sit-ups, crunches, jumping jacks, push-ups, etc.), kickboxing, and jumping rope. When the weather permitted, outdoor games such as capture the flag and relay races were conducted. Resistance exercises were performed with 3 and 5 lb barbells, light elastic bands, and cords. Games, rubber balls, music, and simple, age-appropriate prizes (e.g., baseball cards) were incorporated into the activities to promote enthusiasm and motivation. Attempts were also made to vary activities and to accommodate the moment-to-moment desires of the patients to optimize participation and interest. The last 5 to 10 min of each session were devoted to cooling down and stretching. Patients were also encouraged to exercise at home on at least two additional occasions per week. Heart rate was checked (manually) at the start of each session and on two or three additional occasions during each session. The patients were encouraged to exercise at an intensity sufficient to raise their heart rates to levels equal to that associated with the ventilatory anaerobic threshold. | CPET | VO2max increased. Oxygen pulse increased . | 2 | |
| Fredriksen et al. 2000 [79] | Prospective interventional | CHD | 129 | 10–16 | Rehabilitation facility- or home-based training for 5 months, 2x/week. The subjects were introduced to swimming, football, volleyball, and also more general activities which had the purpose of facilitating strength, balance, coordination, flexibility, and stamina. Outdoor activities included downhill skiing, cross-country skiing, and hiking. The activities had one common feature; the intensity of the exercise should induce 65—80% of peak heart rate for at least half of the time spent in physical activity, using the peak heart rate assessed at the exercise test prior to training. | 38 healthy | CPET +PA before 1–2 w after the end of intervention. Survey (psychosocial) (Youth self-report; child behavior checklist) Polar heart rate counter Activity monitor | Controls gained weight. VO2max(l/min) increased in exercise groups significantly but did not reach values of control group. PA also significantly increased. Control group had a higher VO2max at the beginning compared to the intervention group, decreased in follow-up. Decrease in withdrawal and somatic complaints. No change in anxiety/depression. | 2 |
| Longmuir PE et al. 1990 [53] | Prospective interventional study | CHD | 40 | ? | Three-month home exercise program (directly postoperative, included endurance activities and jogging, a special program these groups used before). | Healthy children | Questionnaire of PA child and parents Exercise and scoring identical to an earlier study (1985). Reassessment after 6 months. | PA improved in those of the healthy pairs and persisted in the 5-year follow-up. | 2 |
| Author | Study Design | Diagnosis | Number of Participants | Age | Intervention | Control | Outcome | Results | LOE |
|---|---|---|---|---|---|---|---|---|---|
| Dean PN et al. 2015 [80] | prospective observational study | CHD | 177 | 13–30 | PedsQoL Questionnaire regarding PA/sedentary | Adolescents: 18.6% reported physical activity of at least 60 min 7 days per week, and 39.5% reported physical activity 5 or more days per week, compared with 28.7% and 49.5%, respectively, of the nationally representative sample. Majority CHD recreational or competitive sports participation. CHD commonly participate in competitive sports. Participation in competitive sports and physical activity associated with improved QoL and exercise capacity and decreased BMI. | 2 | ||
| Majnemer A et al. 2019 [81] | Prospective observational study | CHD 39 male | 80 | 15 years 8 months (1 year 8 months; range 11 years 5 months–19 years 11 months) | Children’s Assessment of Participation and Enjoyment (CAPE) outcome measure of leisure participation. | Participants exhibited impaired motor (43.5%), behavioral (23.7%), and cognitive (29.9%) development. The most intense participation was in social (mean [SD] 3.3 [0.99]) and recreational (2.9 [0.80]) activity types on the CAPE. Male sex (p < 0.05) and younger age were associated with greater physical activity (<15 years: 1.87; ≥15 years: 1.31, p < 0.05). Greater engagement in social activities was related to better cognition (r = 0.28, p < 0.05), higher motor function (r = 0.30–0.36, p < 0.01), and fewer behavioral difficulties (r = 0.32 to 0.47, p < 0.01). Cognitive ability (r = 0.27, p < 0.05), dexterity and aiming/catching (r = 0.27–0.33, p < 0.05), and behavior problems (r = 0.38–0.49, p = 0.001) were correlated with physical activity participation. Persistence in tasks, an aspect of motivation, correlated with physical (r = 0.45, p < 0.001) and social activity involvement (r = 0.28, p < 0.05). | 2 | ||
| Chen CW et al. 2015 [82] | Observational study | CHD | 413 | 12–20 | Physical self-description questionnaires and three-minute step tests. | The male participants had significantly greater scores in overall physical self-concept, competence in sports, physical appearance, body fat, physical activity, endurance, and strength. | |||
| Arvidsson D et al. 2009 [83] | Observational study | 45 34 | 9–11 14–16 | PA during seven consecutive days was assessed using the Acti- Regò activity monitor (PreMed AS, Ytre Enebakk, Norway), CPET. | PA levels similar to controls. most did not achieve the recommended 60 min of daily MVPA. Statistically significant associations between PA level, time spent on MVPA and aerobic fitness were found in girl patients, and between time spent on MVPA and aerobic fitness in younger control boys. | 2 | |||
| O´Byrne ML et al. 2018 [84] | Retrospective cross-sectional trial | TOF TGA Fallot | 253 | 8–18 13.1 | Association between body mass index and duration of habitual exercise (measured by questionnaire). | Increased habitual activity associated with lower body mass index. | 3 | ||
| Longmuir PE et al. 2021 [85] | Retrospective | CHD 26 male | 45 | 6–12.4 | Separate parent (n = 3) and child focus groups (n = 3), 4–6 participants per group, facilitated the expression of personal opinions. Questionnaire/interviews regarding PA. Focus groups were 60–90 min. Interviews were 30 (children) to 60 min (parents). Audio recordings. | Children with CCHD and their parents reported interactions. Social environment at home, social health environment, and social and physical environments related to peer interactions at school/daycare . | 3 | ||
| Ray TA et al. 2011 [86] | observational study | CHD 51 males | 84 | 10–14 | Self-reported measures and clinical data. Previous day physical activity recall clinical data (BMI, etc.). | Low rates of physical activity and a higher obesity rate. Physical activity and body mass index were not significantly correlated. | 3 | ||
| Kim HJ et al. 2018 [87] | Prospective observational study 11/2015–02/2016 | CHD 61 Male | 111 | 13–18 | CPET global physical activity questionnaire (GPAQ) HRQOL/PedsQoL. | Boys more likely to participate in total physical activity (p = 0.06). Boys higher exercise capacity (p < 0.01). Self-reported and parent proxy-reported HRQOL were positively associated (p = 0.003; ß = 0.12; ß = 0.63, p < 0.001) with physical activity (ß = 0.16, p = 0.049) and exercise capacity.ß = 0.66, p < 0.001). Parent HRQOL differed. Improving exercise capacity to potentially enhance HRQOL in adolescents with complex CHD. | 2 | ||
| O´Byrne ML et al. 2017 [88] | single-center cross-sectional observational study with prospective and retrospective data 03/2012–12/2013 | TGA Fontan Repaired biventricular | 175 | 8–17.5 | CPET Habitual exercise questionnaire. | VO2max lower in Fontan than normal cardiac anatomy (p < 0.0001) or TGA (p < 0.0001). Habitual exercise was not associated with VO2max in Fontan as compared to biventricular circulation. | 3 | ||
| Knowles RL et al. 2014 [89] | Survey Cross-sectional study | CHD | 477 | 10–14 | 464 control classmates | Participation in sports activities is positively associated with increased emotional well-being. Child self-report measures of QoL. | 2 | ||
| Ray TD et al. 2010 [90] | Survey Cross sectional study | CHD 51 male | 84 | 10–14 | Self-efficacy instrument (SES; Dishman et al., 2002), the five physical activity items from the YRBS (Centers for Disease Control and Prevention [CDC], 1991. | Self-efficacy scores were moderately correlated with physical activity participation (r = 0.47; p < 0.001). | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dold, S.K.; Haas, N.A.; Apitz, C. Effects of Sports, Exercise Training, and Physical Activity in Children with Congenital Heart Disease—A Review of the Published Evidence. Children 2023, 10, 296. https://doi.org/10.3390/children10020296
Dold SK, Haas NA, Apitz C. Effects of Sports, Exercise Training, and Physical Activity in Children with Congenital Heart Disease—A Review of the Published Evidence. Children. 2023; 10(2):296. https://doi.org/10.3390/children10020296
Chicago/Turabian StyleDold, Simone K., Nikolaus A. Haas, and Christian Apitz. 2023. "Effects of Sports, Exercise Training, and Physical Activity in Children with Congenital Heart Disease—A Review of the Published Evidence" Children 10, no. 2: 296. https://doi.org/10.3390/children10020296
APA StyleDold, S. K., Haas, N. A., & Apitz, C. (2023). Effects of Sports, Exercise Training, and Physical Activity in Children with Congenital Heart Disease—A Review of the Published Evidence. Children, 10(2), 296. https://doi.org/10.3390/children10020296






