Cardiopulmonary Ultrasound Patterns of Transient Acute Respiratory Distress of the Newborn: A Retrospective Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Equipment, Study Design
2.1.1. Lung Ultrasound (LUS) Data
- –
- –
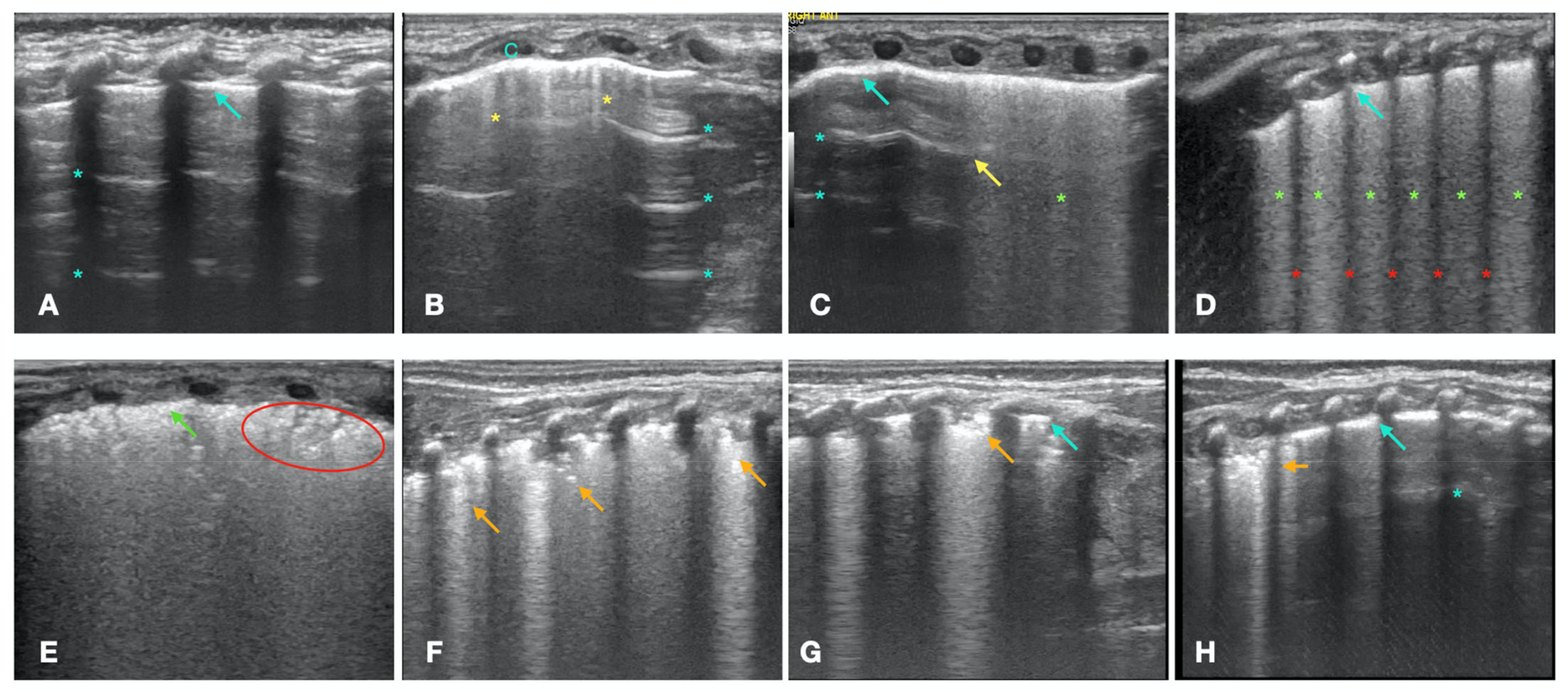
- A- Pattern with normal lung sliding. The A-pattern is characterized by the presence of A-lines and less than 3 isolated B-lines (Figure 1A). This pattern describes the normal lung. The presence of lung sliding, which is the normal movement of the visceral pleura against the motionless parietal pleura, proves the absence of pneumothorax.
- Double lung point. The double lung point represents a sharp sonographic demarcation between the upper and lower lung fields, with less compact B-lines in the former than in the latter, suggesting a gravity-dependent pattern (Figure 1C) [17]. The presence of the double lung point suggests increased fluid in the interstitial space, due to a decreased clearance of pulmonary fluid during labor and delivery (wet lung) [20].
- B2-pattern. The B2-pattern consists in the confluence of B-lines that occupy the entire intercostal space between two ribs, suggesting a further increase in the interstitial fluid with a gravity-dependent pattern. Pleural line is normal (Figure 1D) [16]. According to the literature, this was still interpreted as a sign of wet lung [21,22].
- White lung with irregular pleural line. The white lung is characterized by compact B-lines that cause the acoustic shadow of the ribs to disappear within the entire scanning zone, anteriorly and posteriorly without spared areas, with thickened and irregular pleural line (Figure 1E). This pattern is usually accompanied by the ground-glass opacity (GOS) sign, characterized by mild, regularly distributed lung consolidations with no obvious air bronchogram (Figure 1E), or by the snowflake (SFS) sign [21,22], characterized by regularly distributed lung consolidations with air bronchogram that resembles a snow pattern [23,24]. This pattern is typical of the respiratory distress syndrome (RDS), which is caused by a dysfunction or lack of lung surfactant [21,22].
- Irregular atelectasis. This pattern is characterized by the presence of lung consolidations with irregular margins, along with a few spared areas [25,26]. The presence of atelectasis is characterized on LUS by tissue-like images with anechogenic borders with or without air bronchogram [27]. The presence of the atelectasis is irregularly distributed in the lung, may be more evident on one side, and does not follow a gravity-dependent pattern (Figure 1F-H) [24]. We defined this pattern as pulmonary consolidation.
2.1.2. Echocardiographic Data
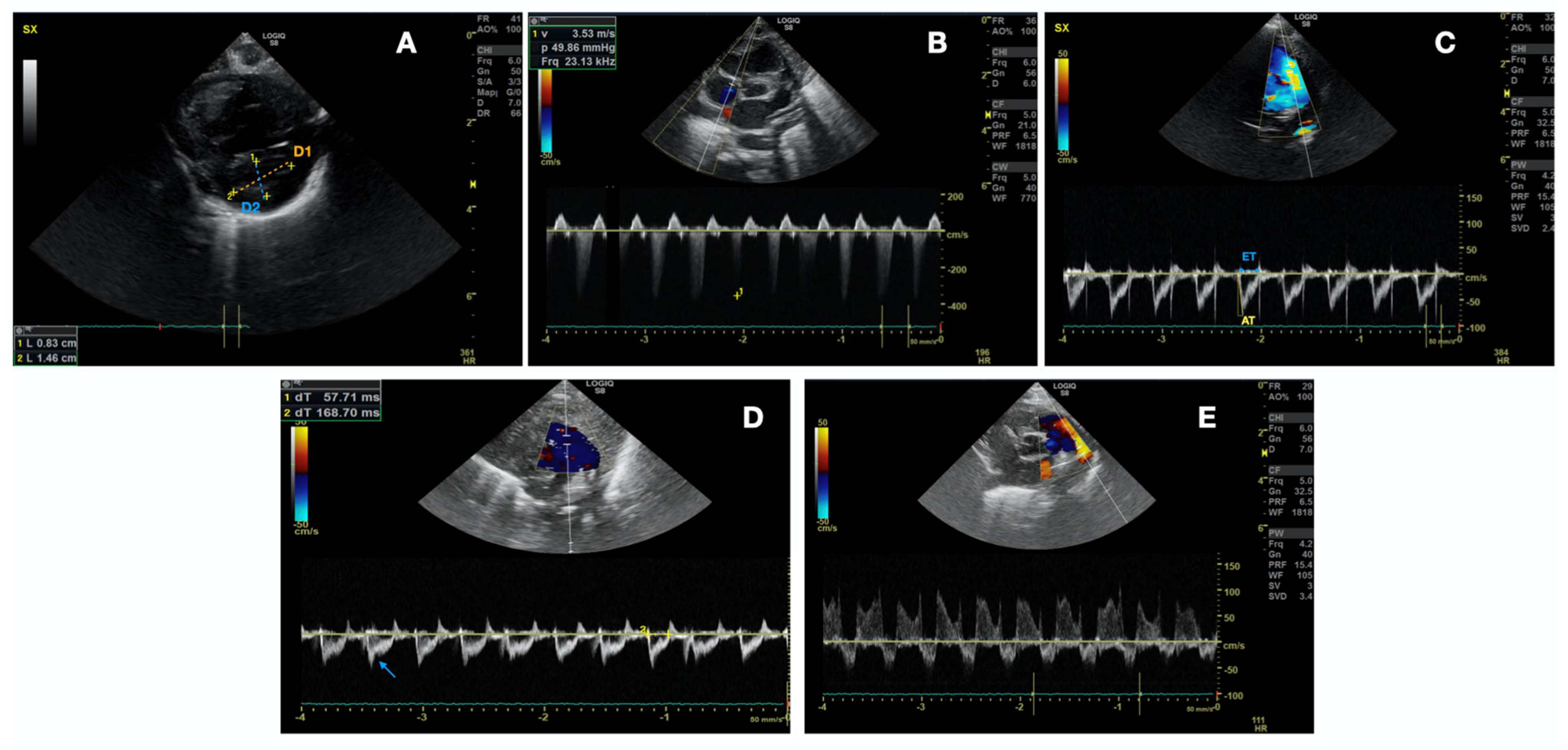
- Left ventricle telesystolic eccentricity index (EI) > 1.15 (Figure 2A). EI was obtained from the parasternal short axis at the mid-papillary muscle level. The formula (EI = D2/D1) was used, where D1 is the ventricular diameter perpendicular to the interventricular septum bisecting D2, the diameter parallel to the interventricular septum [28]. As the right-to-left ventricular pressure ratio increases, septal curvature typically flattens and may even reverse curvature, providing higher EI.
- Pulmonary artery pressure-systolic (PAPs) systemic or supra-systemic (Figure 2B). PAPs estimation was based on Doppler measurement of tricuspid regurgitation and pulmonary regurgitation jet on parasternal short axis view. The velocity at end-diastole (at the QRS complex on the ECG) is converted into a pressure gradient through the modified Bernoulli equation: (RVSP = 4 × v2), where v is the maximum velocity of the tricuspid valve regurgitation jet measured using continuous wave (CW) Doppler. Right atrial pressure was ignored.
- Pulmonary artery acceleration time to right ventricular ejection time ratio (PAAT/RVET) < 0.3 (Figure 2C) +/− pulmonary notch (Figure 2D). PAAT/RVET was taken on the parasternal short axis view or parasternal long axis [29]. Pulmonary artery acceleration time (PAAT) is defined as the interval between the onset of systolic pulmonary arterial flow and peak flow velocity. Right ventricle ejection time (RVET) is the interval between the onset of right ventricle ejection to the point of systolic pulmonary arterial flow cessation. PAAT may be shortened in the case of increased PVR for several reasons: enhanced early pulmonary ejection, increased pulmonary vascular resistance, and loss of lung compliance leading to a rapid increase and reduction of flow velocity. PAAT, in fact, represents pulmonary flow acceleration, which increases as the vascular resistance is augmented, based on Newton’s law of motion. Therefore, the time to peak velocity in the pulmonary artery decreases and PAAT shortens, while the ejection time stays the same, causing a decreased PAAT/RVET ratio.
2.1.3. Placental Histology
2.2. Statistical Analysis
3. Results
3.1. LUS and Echo Findings
3.2. Acute Neonatal Distress (AND) Phenotypes
- Undefined phenotype (AND-u) (n = 2, 8.3%): normal LUS with TnECHO findings indicative of normal PVR;
- Vascular phenotype (AND-v) (n = 4, 16.6%): normal LUS with TnECHO findings indicative of increased PVR;
- Wet lung phenotype (AND-w) (n = 4, 16.6%): LUS signs of increased lung fluid with TnECHO findings indicative of normal PVR;
- Vascular-wet lung phenotype (AND-vw) (n = 4, 16.6%): LUS signs of increased lung fluid with TnECHO findings indicative of increased PVR;
- Vascualar-RDS (n = 2, 8.3%): LUS signs of RDS with TnECHO findings indicative of increased PVR.
- Consolidation Phenotype (AND-c) (n = 6, 25%): irregular lung consolidation at LUS with TnECHO findings indicative of normal PVR;
- Vascular-consolidation Phenotype (AND-vc) (n = 2, 8.3%): irregular lung consolidation at LUS with TnECHO findings indicative of increased PVR;
3.3. AND Phenotypes, Perinatal Features, and Neonatal Outcomes
3.4. CPUS Diagnosis versus Clinical Discharge Diagnosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alhassen, Z.; Vali, P.; Guglani, L.; Lakshminrusimha, S.; Ryan, R.M. Recent Advances in Pathophysiology and Management of Transient Tachypnea of Newborn. J. Perinatol. 2021, 41, 6–16. [Google Scholar] [CrossRef]
- Gizzi, C.; Klifa, R.; Pattumelli, M.G.; Massenzi, L.; Taveira, M.; Shankar-Aguilera, S.; De Luca, D. Continuous Positive Airway Pressure and the Burden of Care for Transient Tachypnea of the Neonate: Retrospective Cohort Study. Am. J. Perinatol. 2015, 32, 939–943. [Google Scholar] [CrossRef]
- Gyamfi-Bannerman, C.; Zupancic, J.A.F.; Sandoval, G.; Grobman, W.A.; Blackwell, S.C.; Tita, A.T.N.; Reddy, U.M.; Jain, L.; Saade, G.R.; Rouse, D.J.; et al. Cost-effectiveness of Antenatal Corticosteroid Therapy vs No Therapy in Women at Risk of Late Preterm Delivery: A Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 462–468. [Google Scholar] [CrossRef] [PubMed]
- McGillick, E.V.; Te Pas, A.B.; van den Akker, T.; Keus, J.M.H.; Thio, M.; Hooper, S.B. Evaluating Clinical Outcomes and Physiological Perspectives in Studies Investigating Respiratory Support for Babies Born at Term With or at Risk of Transient Tachypnea: A Narrative Review. Front. Pediatr. 2022, 10, 878536. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.; Giles, B.L.; Dell, S.D. Full-Term Neonatal Respiratory Distress and Chronic Lung Disease. Pediatr. Ann. 2019, 48, e175–e181. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, R.L.; Egan, E.A.; Gessner, I.H.; Eitzman, D.V. Persistence of fetal cardiopulmonary circulation: One manifestation of transient tachypnea of the newborn. Pediatrics 1976, 58, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Brenner, D.J. Cancer risks from diagnostic radiology: The impact of new epidemiological data. Br. J. Radiol. 2012, 85, e1316–e1317. [Google Scholar] [CrossRef]
- Corsini, I.; Parri, N.; Gozzini, E.; Coviello, C.; Leonardi, V.; Poggi, C.; Giacalone, M.; Bianconi, T.; Tofani, L.; Raimondi, F.; et al. Lung Ultrasound for the Differential Diagnosis of Respiratory Distress in Neonates. Neonatology 2019, 115, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.D. NICE guidance on diabetes in pregnancy: Management of diabetes and its complications from preconception to the postnatal period. NICE clinical guideline 63. London, March 2008. Diabet. Med. 2008, 25, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S14–S31. [Google Scholar] [CrossRef] [PubMed]
- ACOG Writing Group. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef]
- Peng, C.C.; Chang, J.H.; Lin, H.Y.; Cheng, P.J.; Su, B.H. Intrauterine inflammation, infection, or both (Triple I): A new concept for chorioamnionitis. Pediatr. Neonatol. 2018, 59, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ott, W.J. Sonographic diagnosis of fetal growth restriction. Clin. Obstet. Gynecol. 2006, 49, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Macones, G.A.; Hankins, G.D.; Spong, C.Y.; Hauth, J.; Moore, T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: Update on definitions, interpretation, and research guidelines. Obstet. Gynecol. 2008, 112, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Smargiassi, A.; Demi, L.; Inchingolo, R. Artifactual Lung Ultrasonography: It Is a Matter of Traps, Order, and Disorder. Appl. Sci. 2020, 10, 1570. [Google Scholar] [CrossRef]
- Liu, J.; Copetti, R.; Sorantin, E.; Lovrenski, J.; Rodriguez-Fanjul, J.; Kurepa, D.; Feng, X.; Cattaross, L.; Zhang, H.; Hwang, M.; et al. Protocol and Guidelines for Point-of-Care Lung Ultrasound in Diagnosing Neonatal Pulmonary Diseases Based on International Expert Consensus. J. Vis. Exp. 2019, 145, e58990. [Google Scholar] [CrossRef]
- Sharma, D.; Farahbakhsh, N. Role of chest ultrasound in neonatal lung disease: A review of current evidences. J. Matern. Fetal Neonatal Med. 2019, 32, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Pierro, M.; Chioma, R.; Ciarmoli, E.; Villani, P.; Storti, E.; Copetti, R. Lung ultrasound guided pulmonary recruitment during mechanical ventilation in neonates: A case series. J. Neonatal Perinat. Med. 2022, 15, 357–365. [Google Scholar] [CrossRef]
- Buonsenso, D.; Soldati, G.; Curatola, A.; Morello, R.; De Rose, C.; Vacca, M.E.; Lazzareschi, I.; Musolino, A.M.; Valentini, P. Lung Ultrasound Pattern in Healthy Infants During the First 6 Months of Life. J. Ultrasound Med. 2020, 39, 2379–2388. [Google Scholar] [CrossRef] [PubMed]
- Copetti, R.; Cattarossi, L. The ‘double lung point’: An ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology 2007, 91, 203–209. [Google Scholar] [CrossRef]
- Srinivasan, S.; Aggarwal, N.; Makhaik, S.; Jhobta, A.; Kapila, S.; Bhoil, R. Role of lung ultrasound in diagnosing and differentiating transient tachypnea of the newborn and respiratory distress syndrome in preterm neonates. J. Ultrason. 2022, 22, e1–e5. [Google Scholar] [CrossRef]
- Guo, B.B.; Pang, L.; Yang, B.; Zhang, C.; Chen, X.Y.; OuYang, J.B.; Wu, C.J. Lung Ultrasound for the Diagnosis and Management of Neonatal Respiratory Distress Syndrome: A Minireview. Front. Pediatr. 2022, 10, 864911. [Google Scholar] [CrossRef] [PubMed]
- Vergine, M.; Copetti, R.; Brusa, G.; Cattarossi, L. Lung ultrasound accuracy in respiratory distress syndrome and transient tachypnea of the newborn. Neonatology 2014, 106, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Cattarossi, L. Lung Ultrasound (LUS) and neonatal respiratory distress. Ital. J. Pediatr. 2015, 41, A13. [Google Scholar] [CrossRef]
- Ruoss, J.L.; Bazacliu, C.; Cacho, N.; De Luca, D. Lung Ultrasound in the Neonatal Intensive Care Unit: Does It Impact Clinical Care? Children 2021, 8, 1098. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, S.W.; Liu, F.; Li, Q.P.; Kong, X.Y.; Feng, Z.C. The diagnosis of neonatal pulmonary atelectasis using lung ultrasonography. Chest 2015, 147, 1013–1019. [Google Scholar] [CrossRef]
- Chioma, R.; Amabili, L.; Ciarmoli, E.; Copetti, R.; Villani, P.G.; Natile, M.; Vento, G.; Storti, E.; Pierro, M. Lung UltraSound Targeted Recruitment (LUSTR): A Novel Protocol to Optimize Open Lung Ventilation in Critically Ill Neonates. Children 2022, 9, 1035. [Google Scholar] [CrossRef]
- More, K.; Soni, R.; Gupta, S. The role of bedside functional echocardiography in the assessment and management of pulmonary hypertension. Semin. Fetal Neonatal Med. 2022, 27, 101366. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Patel, M.D.; Groh, G.; Choudhry, S.; Murphy, J.; Holland, M.R.; Hamvas, A.; Grady, M.R.; Singh, G.K. Pulmonary Artery Acceleration Time Provides a Reliable Estimate of Invasive Pulmonary Hemodynamics in Children. J. Am. Soc. Echocardiogr. 2016, 29, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Breinig, S.; Dicky, O.; Ehlinger, V.; Dulac, Y.; Marcoux, M.O.; Arnaud, C. Echocardiographic Parameters Predictive of Poor Outcome in Persistent Pulmonary Hypertension of the Newborn (PPHN): Preliminary Results. Pediatr. Cardiol. 2021, 42, 1848–1853. [Google Scholar] [CrossRef]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, H.M.A.; Aladawy, M.A.; Mansor, T.M.; Abo El Magd, H.A. Comparative Study between Chest X-Ray and Lung Ultrasound in Neonatal Respiratory Distress. Ann. Neonatol. J. 2021, 3, 125–143. [Google Scholar] [CrossRef]
- Chen, S.W.; Fu, W.; Liu, J.; Wang, Y. Routine application of lung ultrasonography in the neonatal intensive care unit. Medicine 2017, 96, e5826. [Google Scholar] [CrossRef] [PubMed]
- Maymon, E.; Chaim, W.; Furman, B.; Ghezzi, F.; Shoham Vardi, I.; Mazor, M. Meconium stained amniotic fluid in very low risk pregnancies at term gestation. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998, 80, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Mundhra, R.; Agarwal, M. Fetal outcome in meconium stained deliveries. J. Clin. Diagn. Res. 2013, 7, 2874–2876. [Google Scholar] [CrossRef] [PubMed]
- Nangia, S.; Thukral, A.; Chawla, D. Tracheal suction at birth in non-vigorous neonates born through meconium-stained amniotic fluid. Cochrane Database Syst. Rev. 2021, 6, Cd012671. [Google Scholar] [CrossRef] [PubMed]
- Swarnam, K.; Soraisham, A.S.; Sivanandan, S. Advances in the management of meconium aspiration syndrome. Int. J. Pediatr. 2012, 2012, 359571. [Google Scholar] [CrossRef]
- Piastra, M.; Yousef, N.; Brat, R.; Manzoni, P.; Mokhtari, M.; De Luca, D. Lung ultrasound findings in meconium aspiration syndrome. Early Hum. Dev. 2014, 90 (Suppl. 2), S41–S43. [Google Scholar] [CrossRef]
- Chiruvolu, A.; Miklis, K.K.; Chen, E.; Petrey, B.; Desai, S. Delivery Room Management of Meconium-Stained Newborns and Respiratory Support. Pediatrics 2018, 142, e20181485. [Google Scholar] [CrossRef] [PubMed]
- Gordon, E.; South, M.; McDougall, P.N.; Dargaville, P.A. Blood aspiration syndrome as a cause of respiratory distress in the newborn infant. J. Pediatr. 2003, 142, 200–202. [Google Scholar] [CrossRef]
- Siefkes, H.M.; Lakshminrusimha, S. Management of systemic hypotension in term infants with persistent pulmonary hypertension of the newborn: An illustrated review. Arch. Dis. Child Fetal Neonatal Ed. 2021, 106, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Pierro, M.; Villamor-Martinez, E.; van Westering-Kroon, E.; Alvarez-Fuente, M.; Abman, S.H.; Villamor, E. Association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: A systematic review, meta-analysis and meta-regression. Thorax 2021, 77, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Chioma, R.; Storti, E.; De Luca, G.; Fantinato, M.; Pierro, M. Targeted management of evolving and established chronic lung disease of prematurity assisted by cardiopulmonary ultrasound: A case report of four patients. Front. Pediatr. 2022, 10, 2396. [Google Scholar] [CrossRef]
- Armangil, D.; Yurdakök, M.; Korkmaz, A.; Yiğit, S.; Tekinalp, G. Inhaled beta-2 agonist salbutamol for the treatment of transient tachypnea of the newborn. J. Pediatr. 2011, 159, 398–403.e1. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Yoo, J.H.; Jung, J.A.; Byun, S.Y. The effects of inhaled albuterol in transient tachypnea of the newborn. Allergy Asthma Immunol. Res. 2014, 6, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Basiri, B.; Sadeghi, N.; Sabzehei, M.K.; Ashari, F.E. Effects of Inhaled Salbutamol on Transient Tachypnea of the Newborn. Respir. Care 2022, 67, 433–439. [Google Scholar] [CrossRef]
- Kassab, M.; Khriesat, W.M.; Anabrees, J. Diuretics for transient tachypnoea of the newborn. Cochrane Database Syst. Rev. 2015, 2015, Cd003064. [Google Scholar] [CrossRef] [PubMed]
- Kao, B.; Stewart de Ramirez, S.A.; Belfort, M.B.; Hansen, A. Inhaled epinephrine for the treatment of transient tachypnea of the newborn. J. Perinatol. 2008, 28, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Bruschettini, M.; Hassan, K.O.; Romantsik, O.; Banzi, R.; Calevo, M.G.; Moresco, L. Interventions for the management of transient tachypnoea of the newborn—An overview of systematic reviews. Cochrane Database Syst. Rev. 2022, 2, Cd013563. [Google Scholar] [CrossRef] [PubMed]
- Schaubel, D.; Johansen, H.; Dutta, M.; Desmeules, M.; Becker, A.; Mao, Y. Neonatal characteristics as risk factors for preschool asthma. J. Asthma 1996, 33, 255–264. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Picone, C.; Markowitz, W.; El Khwad, M.; Shen, W.H.; Tafari, N. Association of transient tachypnea of the newborn and childhood asthma. Pediatr. Pulmonol. 2006, 41, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Brat, R.; Yousef, N.; Klifa, R.; Reynaud, S.; Shankar Aguilera, S.; De Luca, D. Lung Ultrasonography Score to Evaluate Oxygenation and Surfactant Need in Neonates Treated with Continuous Positive Airway Pressure. JAMA Pediatr. 2015, 169, e151797. [Google Scholar] [CrossRef] [PubMed]
- Corsini, I.; Ficial, B.; Ciarcià, M.; Capasso, L.; Migliaro, F.; Rodriguez-Fanjul, J.; Clemente, M.; Raimondi, F.; Dani, C. Lung ultrasound scores in neonatal clinical practice: A narrative review of the literature. Pediatr. Pulmonol. 2022, 57, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Chioma, R.; Amabili, L.; Ciarmoli, E.; Copetti, R.; Villani, P.; Stella, M.; Storti, E.; Pierro, M. The importance of lung recruitability: A novel ultrasound pattern to guide lung recruitment in neonates. J. Neonatal Perinatal Med. 2022, 15, 767–776. [Google Scholar] [CrossRef] [PubMed]
| LUS Pattern | LUS Diagnostic Score | LUS Diagnosis 1 |
|---|---|---|
| A pattern | 0 | Normal Lung |
| B1 pattern | 1 | |
| Double lung point | 2 | Wet lung |
| B2 pattern | 3 | |
| White lung + pleural line abnormalities | 4 | RDS |
| +/− Ground-glass opacity sign or | ||
| Snowflake sign | ||
| Irregular atelectasis | 5 | Pulmonary consolidation |
| Characteristics | n = 24 |
|---|---|
| Maternal data | |
| Maternal diabetes, n (%) | 2 (8.3%) |
| Maternal hypertensive disorders, n | 0 |
| Triple-I, n (%) | 8 (33.3%) |
| IUGR, n (%) | 1 (4.1%) |
| Fetal tachicardia, n (%) | 6 (25%) |
| Maternal tachicardia, n (%) | 1 (4.1 %) |
| Maternal fever, n (%) | 3 (12.5%) |
| Intrapartum antibiotics, n (%) | 8 (33.3%) |
| Chorioamnionitis, n (%) | 9 (37.5%) |
| Placenta malperfusion, n (%) | 12 (50%) |
| Perinatal infant data | |
| Gestational age (weeks), mean (SD) | 39.2 (2.1) |
| Birth weight (grams), mean (SD) | 3330.2 (566.6) |
| Need for any kind of resuscitation at birth, n (%) | 11 (45.8%) |
| Need for endotracheal intubation at birth, n (%) | 0 |
| Apgar at 1 min, median (IQR] | 9 (5–9) |
| Apgar at 5 min, median (IQR) | 9 (8–10) |
| Stained amniotic fluid, n (%) | 10 (41.6%) |
| Meconium stained amniotic fluid, n (%) | 9 (37.5%) |
| Blood-stained amniotic fluid, n (%) | 1 (4.1 %) |
| Infant data related to NICU stay | |
| Maximum CRP (mg/dL), mean (SD) | 3.8 (5.1) |
| Positive blood culture, n (%) | 1 (4.1 %) |
| Antibiotic therapy, n (%) | 10 (41.6%) |
| Antibiotics duration (days), mean (SD) | 2 (3.4) |
| Length of stay (days), mean (SD) | 8.1 (3.7) |
| Duration of CPAP (hours), mean (SD) | 28.5 (57) |
| Duration of HFNC (hours), mean (SD) | 21.3 (51.2) |
| Overall duration of NRS (hours), mean (SD) | 49 (46.7) |
| Duration of NRS/low-flow oxygen (hours), mean (SD) | 62 (69) |
| Maximum FiO2, mean (SD) | 0.36 (0.11) |
| Surfactant administration via INSURE, n (%) | 4 (18.2) |
| Hours of life at CPUS, mean (SD) | 13.9 (7.7) |
| FiO2 at CPUS, mean (SD) | 0.35 (0.09) |
| Discharge diagnosis | |
| Transient tachypnea of the newborn, n (%) | 22 (91.6%) |
| Respiratory distress syndrome, n (%) | 1 (4.1%) |
| Meconium aspiration syndrome, n (%) | 1 (4.1%) |
| Lung Diagnosis | Raised PVR | AND Phenotypes |
|---|---|---|
| Normal Lung (n = 6, 25%) | No | Undefined (n = 2, 8.3%) |
| Yes | Vascular (n = 4, 16.6%) | |
| Wet lung (n = 8, 33.3%) | No | Wet lung (n = 4, 16.6%) |
| Yes | Vascular wet lung (n = 4, 16.6%) | |
| RDS (n = 2, 8.3%) | Yes | Vascular-RDS (n = 2, 8.3%) |
| Pulmonary consolidation (n = 8, 33.3%) | No | Consolidation (n = 6, 25%) |
| Yes | Vascular-consolidation (n = 2, 8.3%) |
| Undefined (AND-u) | Vascular (AND-v) | Wet Lung (AND-w) | Vascular-Wet Lung (AND-vw) | Vascular-RDS | Consolidation (AND-c) | Vascular-Consolidation (AND-vc) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| (n = 2) | (n = 4) | (n = 4) | (n = 4) | (n = 2) | (n = 6) | (n = 2) | ||
| Gestational age (weeks), median (IQR) | 40.4 (39.7–41.1) | 41 (35.8–41.9) | 37.5 (36–40.3) | 36.8 (35.8–40-4) | 37.9 (35.7–40.1) | 40.2 (38.6–41) | 40.3 (39.41.7) | 0.45 |
| Birth weight (grams), median (IQR) | 3695 (3520–3870) | 3392 (2772–3695) | 3587 (3031–4287) | 2812 (2715–3205) | 3175 (2100–4250) | 3405 (3078–3537) | 3515 (3175–3855) | 0.72 |
| Hour of life at echocardiography (h), median (IQR) | 17.5 (11–24) | 18.5 (9.75–21.25) | 10.5 (5.5–20) | 9 (5.25–18) | 24 (24–24) | 14 (5.25–22-5) | 6 (2–10) | 0.28 |
| Ejection fraction (%), median (IQR) | 64.5 (60–69) | 64 (60–65) | 64 (60.75–65) | 63 (58.5–66) | 61.5 (58–64) | 65 (58.75–72) | 65 (65–65) | 0.95 |
| PAAT/RVET, median (IQR) | 0.45 (0.44–0.46) | 0.31 (0.22–0.46) | 0.41 (0.32–0.52) | 0.23 (0.17–0.40) | 0.37 (0.34–0.40) | 0.45 (0.38–0.48) | 0.20 (0.20–0.20) | 0.1 |
| Mid-systolic notch of the flow across the pulmonary artery, n (%) | 0 | 2 (50%) | 1 (25%) | 3 (75%) | 0 | 0 | 2 (100%) | 0.03 |
| Tricuspid valve insufficiency, n (%) | 0 | 3 (75%) | 2 (50%) | 2 (50%) | 0 | 1 (16.6%) | 2 (100%) | 0.2 |
| Trans-ductal flow pattern | 0.22 | |||||||
| R-to-L > 30% n (%) | 0 | 2 (50%) | 1 (25%) | 1 (25%) | 2 (100%) | 4 (66.7%) | 2 (100%) | |
| Growing, n (%) | 0 | 2 (50%) | 1 (25%) | 3 (75%) | 0 | 0 | 0 | |
| Pulsatile, n (%) | 0 | 0 | 1 (25%) | 0 | 0 | 0 | 0 | |
| Restrictive, n (%) | 1 (50%) | 0 | 0 | 0 | 0 | 1 (16.7%) | 0 | |
| No-PDA, n (%) | 1 (50%) | 0 | 1 (25%) | 0 | 0 | 1 (16.7%) | 0 | |
| Trans-ductal peak systolic velocity (m/s), median [IQR] | 2 (2–2) † | 0.9 (0.9–0.9) ¶ | 1 (0.8–1.2) | 1 (0.99–1.37) | 1.05 (1–1.1) | 1.8 (1.65–2.35) *,¶ | 0.8 (0.7–0.9) *,† | 0.049 |
| Tele-systolic eccentricity index, median (IQR) | 0.97 (0.95–1) †,*,¶ | 1.63 (1.51–1.73) †,‡ | 1.1 (0.92–1.65) § | 1.45 (1.33–1.87) | 1.65 (1.5–1.7) § | 1.18 (0.97-1.4) ‡ | 1.62 (1.55–1.7) * | 0.045 |
| Hour of life at lung ultrasound (h), median (IQR) | 17.5 (11–24) | 18.5 (9.75–21.25) | 14.5 (5.75–21.75) | 9 (5.2–18) | 23.5 (23,24) | 15 (5.25–25.75) | 13 (2–24) | 0.69 |
| Pleural line | 0.08 | |||||||
| Normal, n (%) | 1 (50%) | 3 (75%) | 4 (100%) | 3 (75%) | 0 | 2 (33.3%) | 0 | |
| Thickened, n (%) | 1 (50%) | 1 (25%) | 0 | 1 (25%) | 2 (100%) | 2 (33.3%) | 0 | |
| Irregular, n (%) | 0 | 0 | 0 | 0 | 0 | 2 (33.3%) | 2 (100%) | |
| Focal subpleuric micriatelectasis, n (%) | 0 | 0 † | 0 * | 1 (25%) | 0 | 6 (100%) *,† | 2 (100%) | <0.001 |
| Anterior right AND score, median (IQR) | 0.5 (0–1) | 0 (0–0) | 0.5 (0–1.75) | 1.5 (0.25–4.25) | 3.5 (3–4) | 1 (0–3) | 0.5 (0–1) | 0.14 |
| Anterior left AND score, median (IQR) | 0.5 (0–1) | 0 (0–0) | 0.5 (0–2.5) | 2 (0.25–4.5) | 3.5 (3–4) | 1 (0–3) | 0.5 (0–1) | 0.15 |
| Posterior right AND score, median (IQR) | 1.5 (0–3) | 0 (0–0) | 3 (3–3) | 1.5 (0.25–2.75) | 4.5 (4–5) | 4 (2.25–5) | 2.5 (0–5) | 0.06 |
| Posterior left AND score, median (IQR) | 1.5 (0–3) | 0 (0–0) *,†,⁑ | 3 (3–3) ¶ | 2 (0.5–2.75) ‡ | 4.5 (4–5) † | 5 (3–5) * | 5 (5–5) ‡,⁑ | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierro, M.; Chioma, R.; Benincasa, C.; Gagliardi, G.; Amabili, L.; Lelli, F.; De Luca, G.; Storti, E. Cardiopulmonary Ultrasound Patterns of Transient Acute Respiratory Distress of the Newborn: A Retrospective Pilot Study. Children 2023, 10, 289. https://doi.org/10.3390/children10020289
Pierro M, Chioma R, Benincasa C, Gagliardi G, Amabili L, Lelli F, De Luca G, Storti E. Cardiopulmonary Ultrasound Patterns of Transient Acute Respiratory Distress of the Newborn: A Retrospective Pilot Study. Children. 2023; 10(2):289. https://doi.org/10.3390/children10020289
Chicago/Turabian StylePierro, Maria, Roberto Chioma, Consuelo Benincasa, Giacomo Gagliardi, Lorenzo Amabili, Francesca Lelli, Giovanni De Luca, and Enrico Storti. 2023. "Cardiopulmonary Ultrasound Patterns of Transient Acute Respiratory Distress of the Newborn: A Retrospective Pilot Study" Children 10, no. 2: 289. https://doi.org/10.3390/children10020289
APA StylePierro, M., Chioma, R., Benincasa, C., Gagliardi, G., Amabili, L., Lelli, F., De Luca, G., & Storti, E. (2023). Cardiopulmonary Ultrasound Patterns of Transient Acute Respiratory Distress of the Newborn: A Retrospective Pilot Study. Children, 10(2), 289. https://doi.org/10.3390/children10020289






