Cardiac Magnetic Resonance Strain in Beta Thalassemia Major Correlates with Cardiac Iron Overload
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
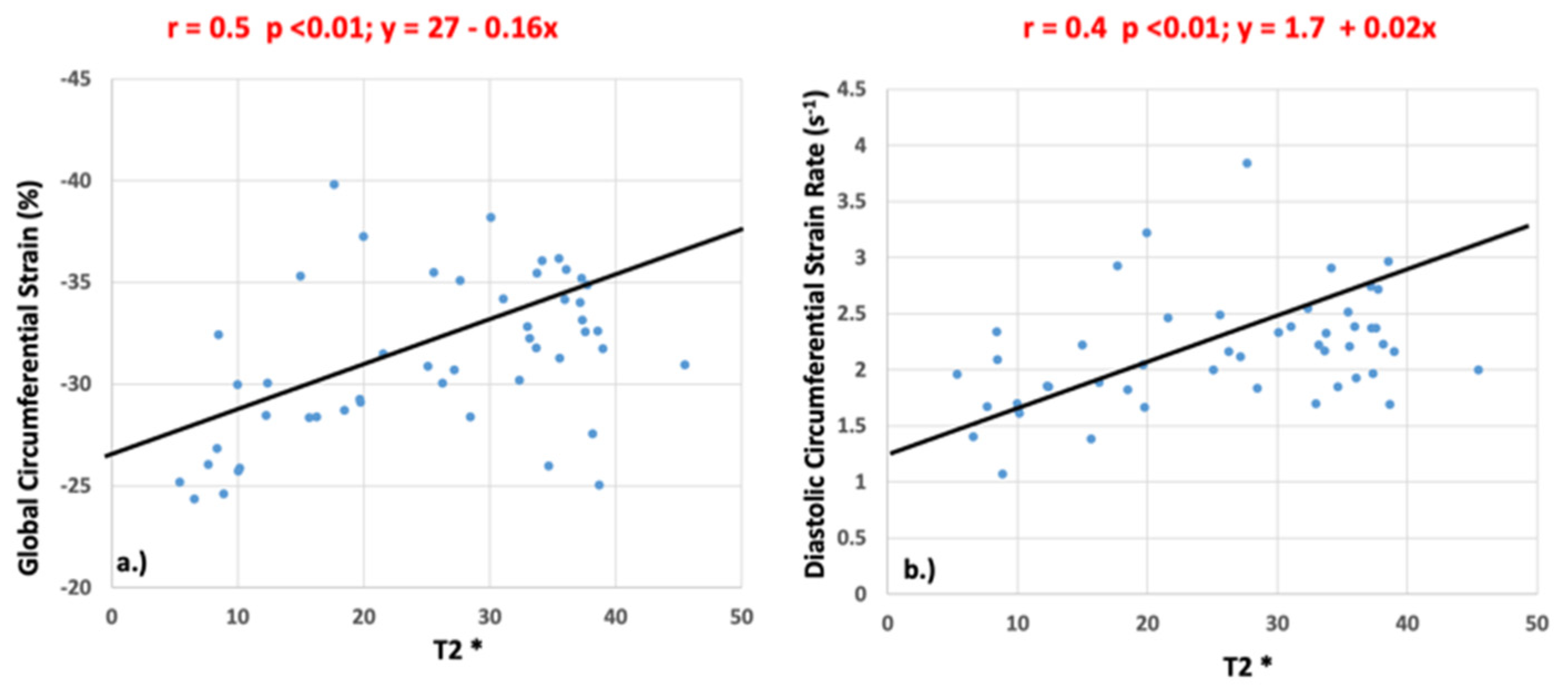
3.2. Myocardial Functional Analysis
3.3. Hematological Data
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kremastinos, D.T.; Farmakis, D.; Aessopos, A.; Hahalis, G.; Hamodraka, E.; Tsiapras, D.; Keren, A. Beta-thalassemia cardiomyopathy: History, present considerations, and future perspectives. Circ. Heart Fail. 2010, 3, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Cohen, A.; Eleftheriou, A.; Piga, A.; Porter, J.; Taher, A. Guidelines for the Clinical Management of Thalassaemia; Thalassaemia International Federation: Strovolos, Cyprus, 2008. [Google Scholar]
- Zurlo, M.; De Stefano, P.; Borgna-Pignatti, C.; Di Palma, A.; Melevendi, C.; Piga, A.; Di Gregorio, F.; Burattini, M.; Terzoli, S. Survival and causes of death in thalassaemia major. Lancet 1989, 2, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Khan, M.; Darlison, M.; Westwood, M.A.; Ingram, D.; Pennell, D.J. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Holden, S.; Davis, B.; Prescott, E.; Charrier, C.; Bunce, N.; Firmin, D.; Wonke, B.; Porter, J.; Walker, J.M.; et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur. Heart J. 2001, 22, 2171–2179. [Google Scholar] [CrossRef]
- Kirk, P.; Roughton, M.; Porter, J.; Walker, J.; Tanner, M.; Patel, J.; Wu, D.; Taylor, J.; Westwood, M.; Anderson, L.; et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation 2009, 120, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef]
- Anderson, L.J. Assessment of iron overload with T2* magnetic resonance imaging. Prog. Cardiovasc. Dis. 2011, 54, 287–294. [Google Scholar] [CrossRef] [PubMed]
- D’Hooge, J.; Heimdal, A.; Jamal, F.; Kukulski, T.; Bijnens, B.; Rademakers, F.; Hatle, L.; Suetens, P.; Sutherland, G. Regional strain and strain rate measurements by cardiac ultrasound: Principles, implementation and limitations. Eur. J. Echocardiogr. 2000, 1, 154–170. [Google Scholar] [CrossRef]
- Scatteia, A.; Baritussio, A.; Bucciarelli-Ducci, C. Strain imaging using cardiac magnetic resonance. Heart Fail. Rev. 2017, 22, 465–476. [Google Scholar] [CrossRef]
- Storey, P.; Thompson, A.A.; Carqueville, C.L.; Wood, J.C.; de Freitas, R.A.; Rigsby, C.K. R2* imaging of transfusional iron burden at 3T and comparison with 1.5T. J. Magn. Reson. Imaging 2007, 25, 540–547. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Di Odoardo, L.A.F.; Giuditta, M.; Cassinerio, E.; Roghi, A.; Pedrotti, P.; Vicenzi, M.; Sciumbata, V.M.; Cappellini, M.D.; Pierini, A. Myocardial deformation in iron overload cardiomyopathy: Speckle tracking imaging in a beta-thalassemia major population. Intern. Emerg. Med. 2017, 12, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Ari, M.E.; Ekici, F.; Çetin, I.I.; Tavil, E.B.; Yaralı, N.; Işık, P.; Hazırolan, T.; Tunç, B. Assessment of left ventricular functions and myocardial iron load with tissue Doppler and speckle tracking echocardiography and T2* MRI in patients with β-thalassemia major. Echocardiography 2017, 34, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, N.; Mohtasham, M.A.; Khaleel, A.J.; Parnianfard, N.; Kasani, K.; Golshan, R. Comparison of global strain values of myocardium in beta-thalassemia major patients with iron load using specific feature tracking in cardiac magnetic resonance imaging. Int. J. Cardiovasc. Imaging 2020, 36, 1343–1349. [Google Scholar] [CrossRef]
- Ojha, V.; Ganga, K.P.; Seth, T.; Roy, A.; Naik, N.; Jagia, P.; Gulati, G.S.; Kumar, S.; Sharma, S. Role of CMR feature-tracking derived left ventricular strain in predicting myocardial iron overload and assessing myocardial contractile dysfunction in patients with thalassemia major. Eur. Radiol. 2021, 31, 6184–6192. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; La Sala, L. Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis. Int. J. Mol. Sci. 2022, 23, 10944. [Google Scholar] [CrossRef]
- Claus, P.; Omar, A.M.S.; Pedrizzetti, G.; Sengupta, P.P.; Nagel, E. Tissue Tracking Technology for Assessing Cardiac Mechanics: Principles, Normal Values, and Clinical Applications. JACC Cardiovasc. Imaging 2015, 8, 1444–1460. [Google Scholar] [CrossRef]
- Seldrum, S.; Pierard, S.; Moniotte, S.; Vermeylen, C.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.-L.; Gerber, B.L. Iron overload in polytransfused patients without heart failure is associated with subclinical alterations of systolic left ventricular function using cardiovascular magnetic resonance tagging. J. Cardiovasc. Magn. Reson. 2011, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Moody, W.E.; Umar, F.; Edwards, N.C.; Taylor, T.J.; Stegemann, B.; Townend, J.; Hor, K.N.; Steeds, R.; Mazur, W.; et al. Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: Normal values. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.; Schuster, A.; Jogiya, R.; Kutty, S.; Beerbaum, P.; Nagel, E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J. Cardiovasc. Magn. Reson. 2012, 14, 43. [Google Scholar] [CrossRef]
- Schuster, A.; Morton, G.; Hussain, S.T.; Jogiya, R.; Kutty, S.; Asrress, K.N.; Makowski, M.R.; Bigalke, B.; Perera, D.; Beerbaum, P.; et al. The intra-observer reproducibility of cardiovascular magnetic resonance myocardial feature tracking strain assessment is independent of field strength. Eur. J. Radiol. 2013, 82, 296–301. [Google Scholar] [CrossRef]
- Mancuso, L.; Vitrano, A.; Mancuso, A.; Sacco, M.; Ledda, A.; Maggio, A. Left Ventricular Diastolic Dysfunction in β-Thalassemia Major with Heart Failure. Hemoglobin 2018, 42, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Efthimiadis, G.K.; Hassapopoulou, H.P.; Tsikaderis, D.D.; Karvounis, H.I.; Giannakoulas, G.A.; Parharidis, G.E.; Louridas, G.E. Survival in thalassaemia major patients. Circ. J. 2006, 70, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.A.; Wonke, B.; Maceira, A.M.; Prescott, E.; Walker, J.M.; Porter, J.; Pennell, D.J. Left ventricular diastolic function compared with T2* cardiovascular magnetic resonance for early detection of myocardial iron overload in thalassemia major. J. Magn. Reson. Imaging 2005, 22, 229–233. [Google Scholar] [CrossRef]
- Moussavi, F.; Ghasabeh, M.A.; Roodpeyma, S.; Alavi, S.; Shakiba, M.; Gheiratmand, R.; Omidghaemi, M. Optimal method for early detection of cardiac disorders in thalassemia major patients: Magnetic resonance imaging or echocardiography? Blood Res. 2014, 49, 182–186. [Google Scholar] [CrossRef]
- Backhaus, S.J.; Metschies, G.; Billing, M.; Schmidt-Rimpler, J.; Kowallick, J.T.; Gertz, R.J.; Lapinskas, T.; Pieske-Kraigher, E.; Pieske, B.; Lotz, J.; et al. Defining the optimal temporal and spatial resolution for cardiovascular magnetic resonance imaging feature tracking. J. Cardiovasc. Magn. Reson. 2021, 23, 60. [Google Scholar] [CrossRef]
- Kumfu, S.; Fucharoen, S.; Chattipakorn, S.C.; Chattipakorn, N. Cardiac complications in beta-thalassemia: From mice to men. Exp. Biol. Med. 2017, 242, 1126–1135. [Google Scholar] [CrossRef]
- Bay, A.; Başpınar, O.; Leblebisatan, G.; Yalçın, A.S.; Irdem, A. Detection of Left Ventricular Regional Function in Asymptomatic Children with beta-Thalassemia Major by Longitudinal Strain and Strain Rate Imaging. Turk. J. Haematol. 2013, 30, 283–289. [Google Scholar] [CrossRef]
- Kapoor, A.; Gupta, A.; Phadke, S.; Sinha, A.; Kashyap, S.; Khanna, R.; Kumar, S.; Garg, N.; Tewari, S.; Goel, P. Use of strain, strain rate, tissue velocity imaging, and endothelial function for early detection of cardiovascular involvement in patients with beta-thalassemia. Ann. Pediatr. Cardiol. 2017, 10, 158–166. [Google Scholar] [CrossRef]
- Soltanpour, M.S.; Davari, K. The Correlation of Cardiac and Hepatic Hemosiderosis as Measured by T2*MRI Technique with Ferritin Levels and Hemochromatosis Gene Mutations in Iranian Patients with Beta Thalassemia Major. Oman Med. J. 2018, 33, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Fragasso, A.; Ciancio, A.; Mannarella, C.; Gaudiano, C.; Scarciolla, O.; Ottonello, C.; Francone, M.; Nardella, M.; Peluso, A.; Melpignano, A.; et al. Myocardial iron overload assessed by magnetic resonance imaging (MRI)T2* in multi-transfused patients with thalassemia and acquired anemias. Eur. J. Intern. Med. 2011, 22, 62–65. [Google Scholar] [CrossRef]
- Kolnagou, A.; Economides, C.; Eracleous, E.; Kontoghiorghes, G.J. Low serum ferritin levels are misleading for detecting cardiac iron overload and increase the risk of cardiomyopathy in thalassemia patients. The importance of cardiac iron overload monitoring using magnetic resonance imaging T2 and T2*. Hemoglobin 2006, 30, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Steen, H.; Matheis, P.; Westkott, M.; Breuninger, K.; Sander, Y.; Kammerer, R.; Galuschky, C.; Giannitsis, E.; Korosoglou, G.; et al. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J. Cardiovasc. Magn. Reson. 2015, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.; Lewandowski, A.J.; Lazdam, M.; Rai, A.; Francis, J.; Myerson, S.; Noble, A.; Becher, H.; Neubauer, S.; Petersen, S.E.; et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: Comparison with tagging and relevance of gender. J. Cardiovasc. Magn. Reson. 2013, 15, 8. [Google Scholar] [CrossRef] [PubMed]







| T2* < 10 ms (n = 9) | T2* 11–20 ms (n = 10) | T2* 21–30 ms (n = 8) | T2* > 30 ms (n = 22) | All pts (n = 49) | Controls (n = 18) | p Value | |
|---|---|---|---|---|---|---|---|
| Age (yr) | 27 ± 4.6 | 30 ± 6.9 | 26 ± 0 | 30 ± 12.2 | 29 ±9.6 | 30 ± 7.8 | 0.04 |
| Gender | m:5 f:4 | m:6 f:4 | m:0 f:8 | m:6 f:16 | m:17 f:32 | m:10 f:8 | 0.02 |
| Weight (kg) | 62 ± 20 | 63 ± 10 | 56 ± 7 | 59 ± 10 | 60 ± 12 | 67 ± 3 | 0.01 |
| BSA (m2) | 1.7 ± 0.3 | 1.7 ± 0.2 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.2 | 1.8 ± 0.1 | 0.21 |
| Ejection fraction (%) | 55 ± 4 | 56 ± 6 | 57 ± 4 | 58 ± 4 | 57 ± 5 | 65 ± 0.7 | 0.06 |
| Pre-transfusion Hgb for year of MR (g/dL) | 10.0 ± 1.2 | 9.5 ± 1.3 | 8.8 ± 0.9 | 9.7 ± 1.0 | 9.5 ± 1.1 | 0.1 | |
| Transfusion volume for year of MRI (ml/kg/year) | 111.0 ± 53.0 | 87.8 ± 69.5 | 101.7 ± 93.9 | 111.0 ± 101.7 | 104 ± 79.8 | 0.9 | |
| Ferritin (µg/L) | 6006 ± 4412 | 1903 ± 1221 | 2127 ± 1296 | 1310 ± 834 | 2678 ± 2817 | <0.01 | |
| Cardiac T2* (ms) | 8.4 ± 1.6 | 16.7 ± 2.9 | 26.5 ± 2.6 | 36.2 ± 3.1 | 19.1 ± 8.0 | <0.01 | |
| Presence of a spleen (n) | 3 (33%) | 4 (40%) | 4 (50%) | 8 (36%) | 19(39%) | 0.9 | |
| Chelator Medication (n) | |||||||
| Deferasirox | 6 (66%) | 7 (70%) | 5 (63%) | 14 (64%) | 32 (65%) | 0.39 | |
| Desferal | 4 (44%) | 3 (30%) | 1 (13%) | 2 (9%) | 10 (20%) | 0.25 | |
| Deferiprone | 3 (33%) | 2 (20%) | 2 (25%) | 3 (14%) | 10 (20%) | 0.67 | |
| T2* < 10 ms (n = 9) | T2* 11–20 ms (n = 10) | T2* 21–30 ms (n = 8) | T2* > 30 ms (n = 22) | p Value | ||
|---|---|---|---|---|---|---|
| Global circumferential strain (%) | −26.8 ± 2.7 | −31.5 ± 4.3 | −32.5 ± 3.3 | −32.4 ± 3.1 | <0.01 | |
| Global longitudinal strain (%) | −22.8 ± 3.9 | −22.9 ± 2.8 | −24.8 ± 3.0 | −25.0 ± 3.4 | 0.23 | |
| Global circumferential strain rate (s−1) | systole | −1.5 ± 0.3 | −1.8 ± 0.4 | −1.9 ± 0.6 | −1.8 ± 0.2 | 0.17 |
| diastole | 1.7 ± 0.4 | 2.1 ± 0.6 | 2.4 ± 0.6 | 2.3 ± 0.4 | <0.01 | |
| Global longitudinal strain rate (s−1) | systole | −1.3 ± 0.5 | −1.2 ± 0.2 | −1.4 ± 0.6 | −1.3 ± 0.3 | 0.95 |
| diastole | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.7 ± 0.4 | 1.7 ± 0.3 | 0.31 | |
| Patient Data v. Control Data | Controls (n = 18) | T2* < 10 ms (n = 9) | T2* 11–20 ms (n = 10) | T2* 21–30 ms (n = 8) | T2* > 30 ms (n = 22) | |
|---|---|---|---|---|---|---|
| Pair-Wise Comparison to Controls (p Values) | ||||||
| Global circumferential strain (%) | −31.4 ± 3.9 | <0.013 | 0.52 | 0.27 | 0.05 | |
| Global longitudinal strain (%) | −26.2 ± 3.6 | 0.04 | 0.02 | 0.37 | 0.29 | |
| Global circumferential strain rate (s−1) | systole | −1.5 ± 0.3 | 0.82 | 0.02 | 0.16 | <0.013 |
| diastole | 1.5 ± 0.2 | 0.11 | <0.013 | <0.013 | <0.013 | |
| Global longitudinal strain rate (s−1) | systole | −1.2 ± 0.3 | 0.49 | 0.12 | 0.10 | <0.013 |
| diastole | 1.1 ± 0.2 | <0.013 | <0.013 | <0.013 | <0.013 | |
| T2* < 10 ms (n = 9) | T2* 11–20 ms (n = 10) | T2* 21–30 ms (n = 8) | T2* >30 ms (n = 22) | All T2* Groups | ||
|---|---|---|---|---|---|---|
| Transfusion volume | ||||||
| Global circumferential strain (%) | 0.48 (0.31) | −0.47 (0.28) | −0.14 (0.56) | −0.27 (0.37) | −0.12 (>0.64) | |
| Global longitudinal strain (%) | 0.35 (0.30) | −0.76 (0.03) | −0.37 (0.38) | −0.24 (0.53) | −0.15 (0.68) | |
| Global circumferential strain rate (s−1) | systole | 0.35 (0.39) | −0.73 (0.04) | −0.21 (0.69) | −0.14 (0.68) | −0.22 (0.22) |
| diastole | 0.42 (0.29) | −0.59 (0.12) | 0.01 (0.98) | −0.43 (0.19) | −0.20 (0.26) | |
| Global longitudinal strain rate (s−1) | systole | 0.25 (0.53) | −0.72 (0.04) | 0.20 (0.31) | −0.51 (0.11) | −0.24 (0.18) |
| diastole | 0.29 (0.49) | −0.89 (<0.01) | 0.49 (0.31) | −0.35 (0.28) | −0.16 (0.37) | |
| Ferritin | ||||||
| Global circumferential strain (%) | 0.41 (0.31) | 0.41 (0.81) | −0.07 (0.81) | −0.09 (0.73) | −0.38 (0.01) | |
| Global longitudinal strain (%) | 0.15 (0.62) | 0.15 (0.65) | 0.07 (0.84) | −0.2 (0.64) | −0.12 (0.58) | |
| Global circumferential strain rate (s−1) | systole | 0.22 (0.50) | 0.22 (0.57) | 0.01 (0.98) | 0.27 (0.31) | −0.19 (0.21) |
| diastole | 0.17 (0.65) | 0.18 (0.65) | 0.36 (0.38) | 0.32 (0.22) | −0.24 (0.12) | |
| Global longitudinal strain rate (s−1) | systole | −0.03 (0.92) | −0.03 (0.92) | 0.23 (0.58) | 0.14 (0.60) | −0.03 (0.82) |
| diastole | −0.21 (0.56) | −0.21 (0.59) | 0.46 (0.26) | 0.25 (0.36) | −0.14 (0.37) | |
| Hemoglobin | ||||||
| Global circumferential strain (%) | −0.51 (0.18) | 0.51 (0.14) | 0.51 (0.29) | 0.53 (0.03) | −0.02 (0.85) | |
| Global longitudinal strain (%) | −0.38 (0.33) | 0.60 (0.08) | 0.60 (0.11) | −0.25 (0.36) | −0.12 (0.47) | |
| Global circumferential strain rate (s−1) | systole | 0.33 (0.34) | 0.33 (0.34) | −0.46 (0.24) | −0.09 (0.70) | −0.16 (0.29) |
| diastole | 0.24 (0.49) | 0.24 (0.50) | 0.07 (0.86) | −0.13 (0.60) | −0.15 (0.33) | |
| Global longitudinal strain rate (s−1) | systole | 0.28 (0.42) | 0.28 (0.42) | −0.13 (0.76)) | −0.40 (0.11) | −0.10 (0.52) |
| diastole | 0.22 (0.52) | 0.22 (0.52) | 0.35 (0.40) | −0.50 (0.04) | −0.10 (0.48) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansah, D.; Husain, N.; Ruh, A.; Berhane, H.; Smith, A.; Thompson, A.; De Freitas, A.; Rigsby, C.K.; Robinson, J.D. Cardiac Magnetic Resonance Strain in Beta Thalassemia Major Correlates with Cardiac Iron Overload. Children 2023, 10, 271. https://doi.org/10.3390/children10020271
Ansah D, Husain N, Ruh A, Berhane H, Smith A, Thompson A, De Freitas A, Rigsby CK, Robinson JD. Cardiac Magnetic Resonance Strain in Beta Thalassemia Major Correlates with Cardiac Iron Overload. Children. 2023; 10(2):271. https://doi.org/10.3390/children10020271
Chicago/Turabian StyleAnsah, Deidra, Nazia Husain, Alexander Ruh, Haben Berhane, Anthony Smith, Alexis Thompson, Andrew De Freitas, Cynthia K. Rigsby, and Joshua D. Robinson. 2023. "Cardiac Magnetic Resonance Strain in Beta Thalassemia Major Correlates with Cardiac Iron Overload" Children 10, no. 2: 271. https://doi.org/10.3390/children10020271
APA StyleAnsah, D., Husain, N., Ruh, A., Berhane, H., Smith, A., Thompson, A., De Freitas, A., Rigsby, C. K., & Robinson, J. D. (2023). Cardiac Magnetic Resonance Strain in Beta Thalassemia Major Correlates with Cardiac Iron Overload. Children, 10(2), 271. https://doi.org/10.3390/children10020271







