Ferumoxytol-Enhanced Cardiac Magnetic Resonance Angiography and 4D Flow: Safety and Utility in Pediatric and Adult Congenital Heart Disease
Abstract
1. Introduction
2. Evolution of CMR/MRA Contrast Agents
3. Repurposing of Ferumoxytol for Off-Label Diagnostic Use and Its Safety
4. Ferumoxytol-Enhanced MRA (FE-MRA) and 4D Phase Contrast MRI (4D Flow) in Congenital Heart Disease
4.1. Pulmonary Venous Anatomy
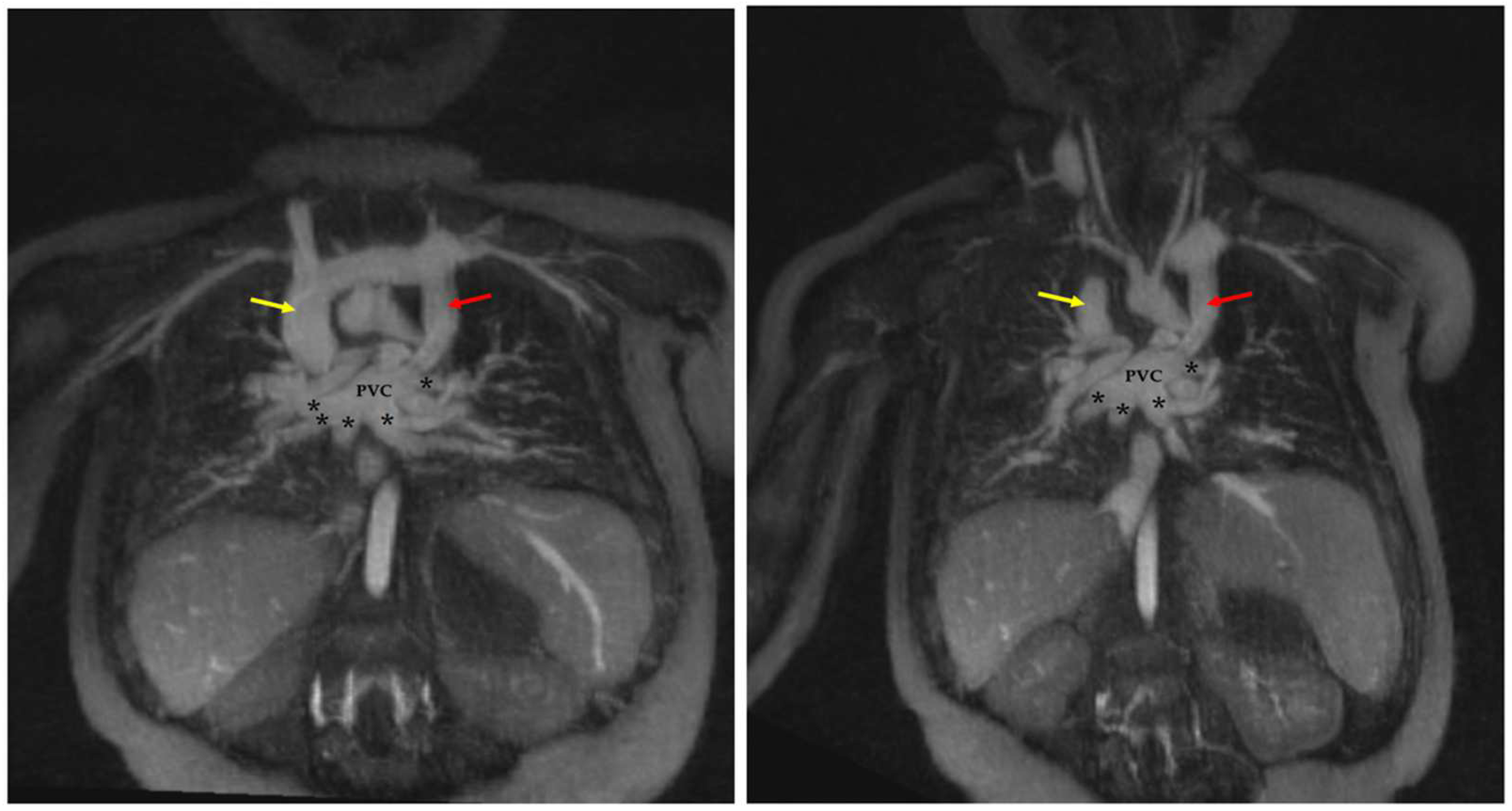
4.1.1. Total Anomalous Pulmonary Venous Connection (TAPVC)
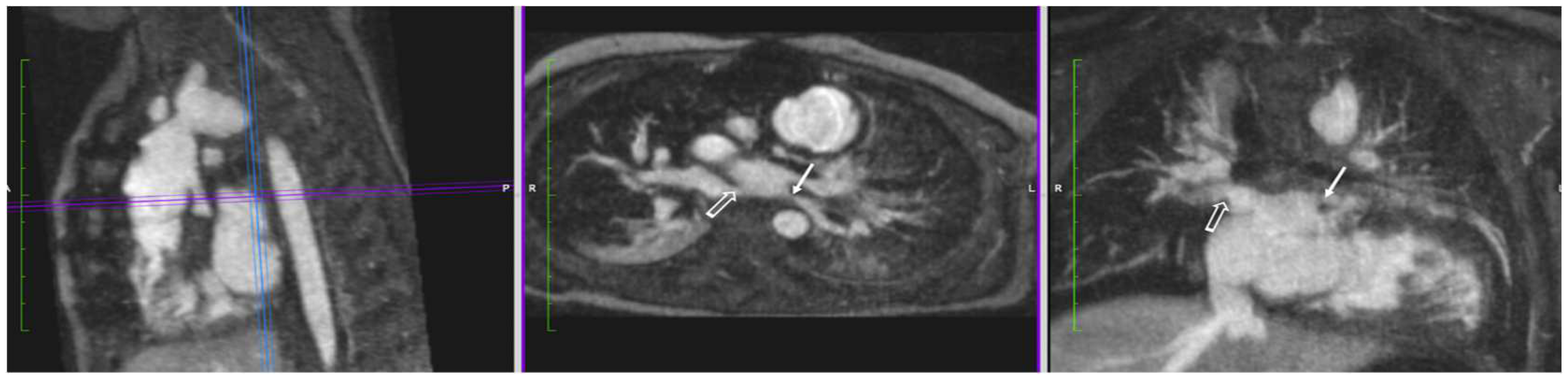
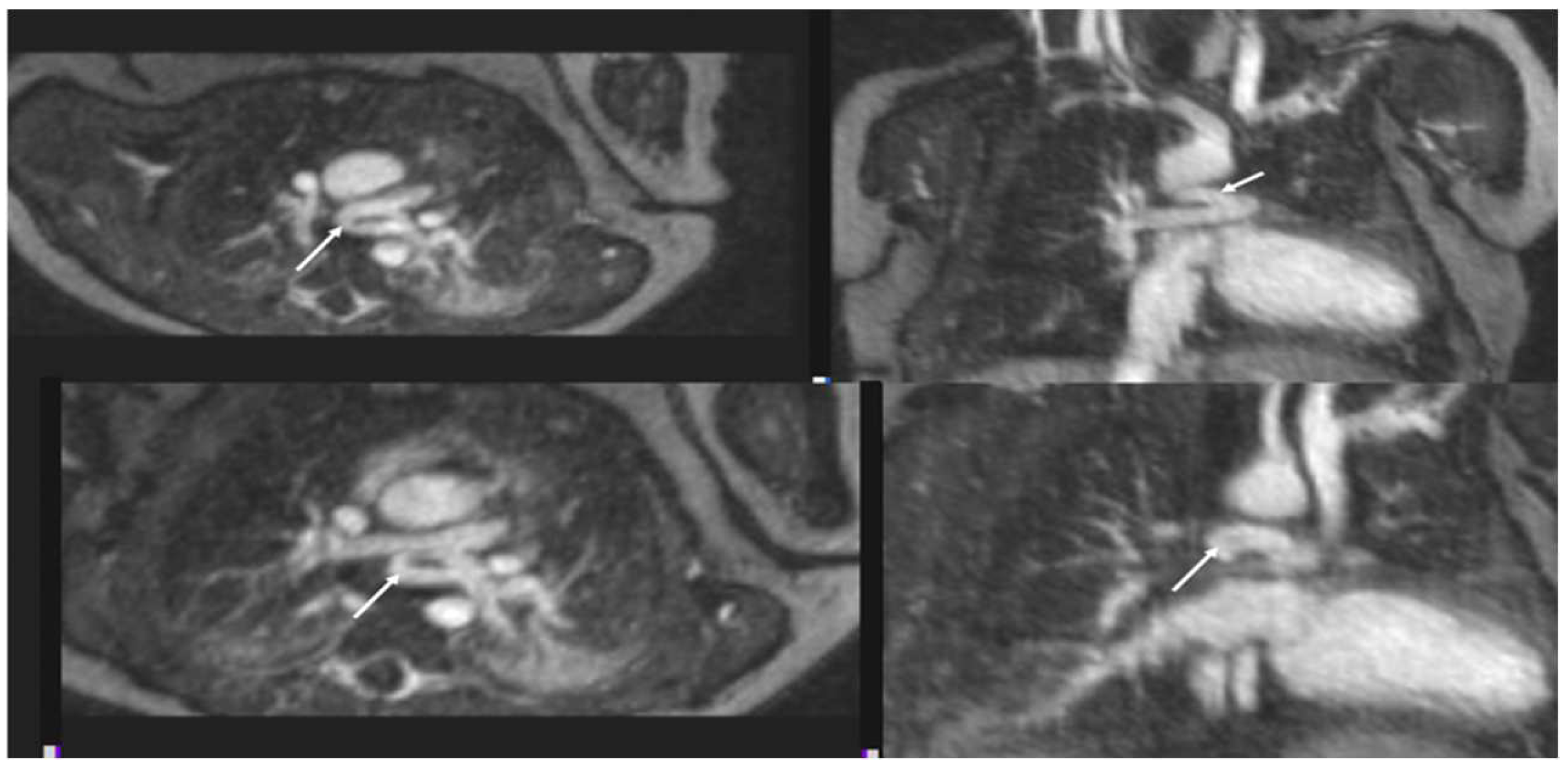
4.1.2. Pulmonary Vein Stenosis
4.2. Systemic Venous Anatomy
Total Cavopulmonary Anastomosis (Fontan Circulation)
4.3. Arterial Pathology
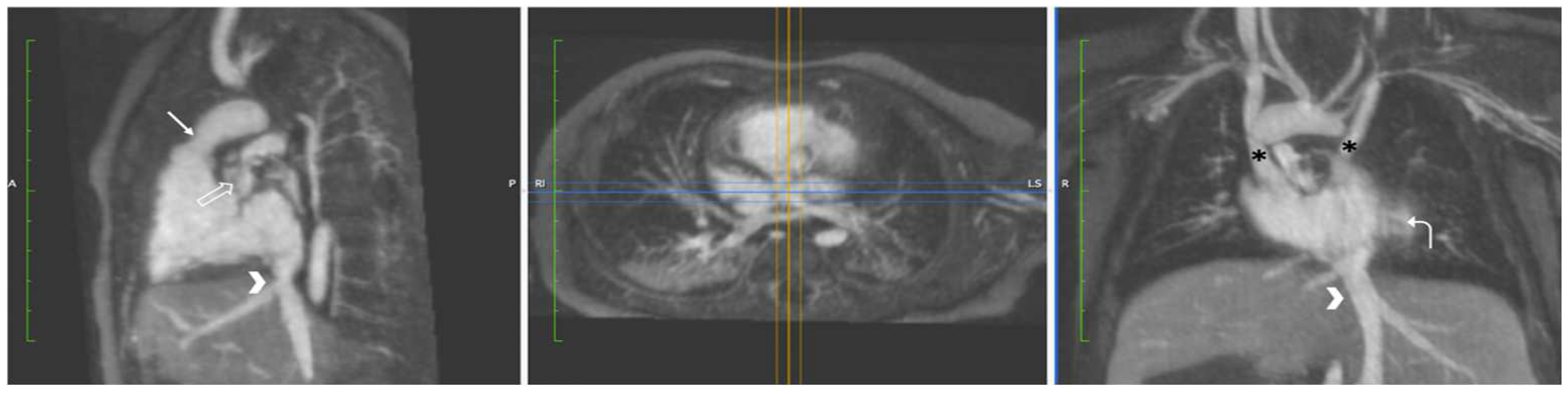
4.3.1. Coarctation of the Aorta
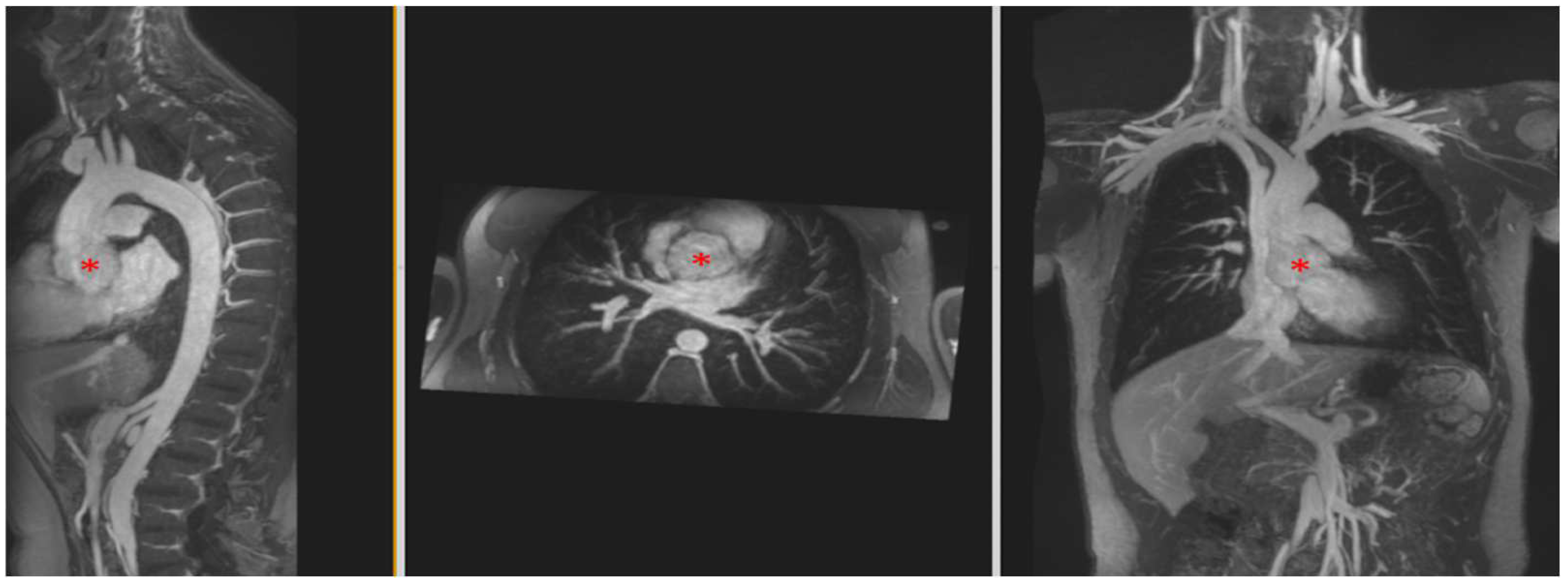
4.3.2. Aortic Aneurysm in Connective Tissue Disease
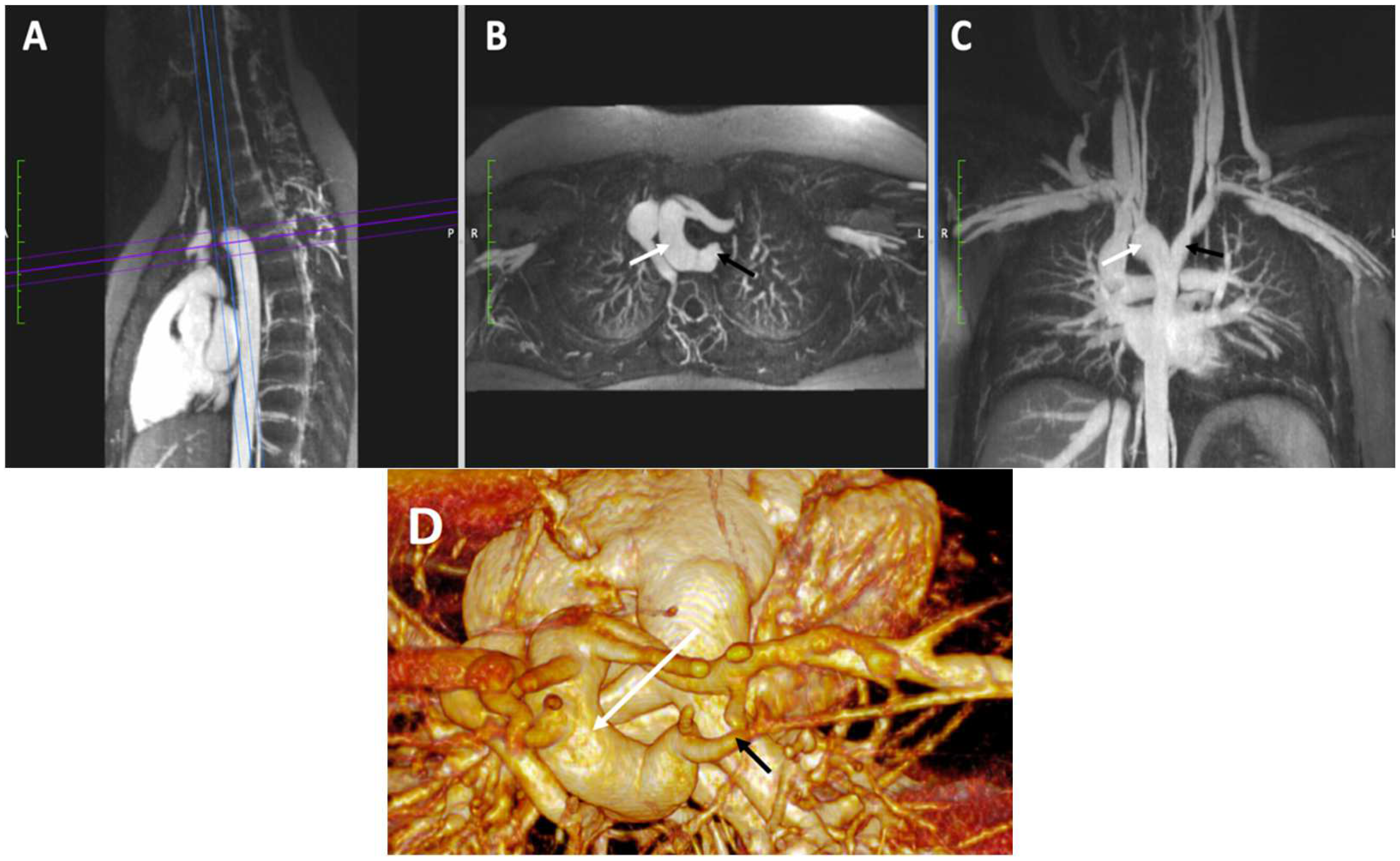
4.3.3. Vascular Ring
4.3.4. Pulmonary Atresia in Tetralogy of Fallot
4.3.5. Heterotaxy Syndrome and Double Outlet Right Ventricle
4.4. Intracardiac Anatomy
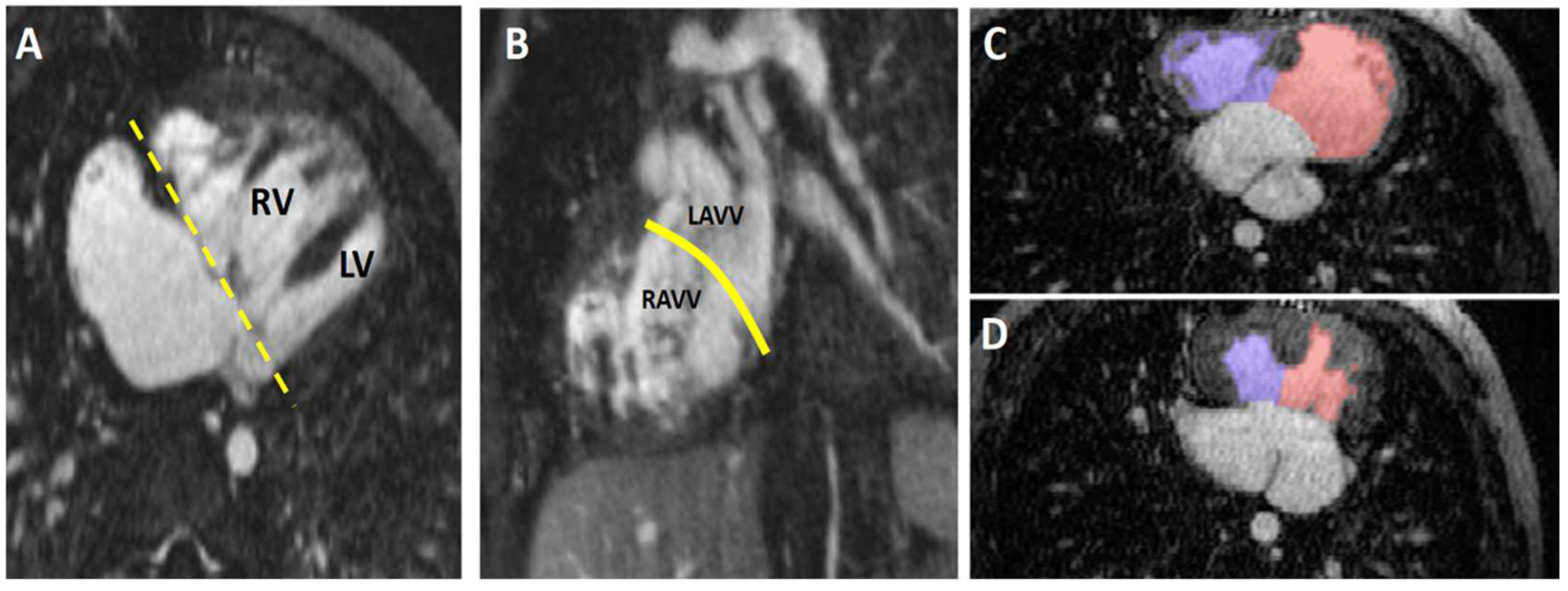
4.4.1. Unbalanced Atrioventricular Canal Defect
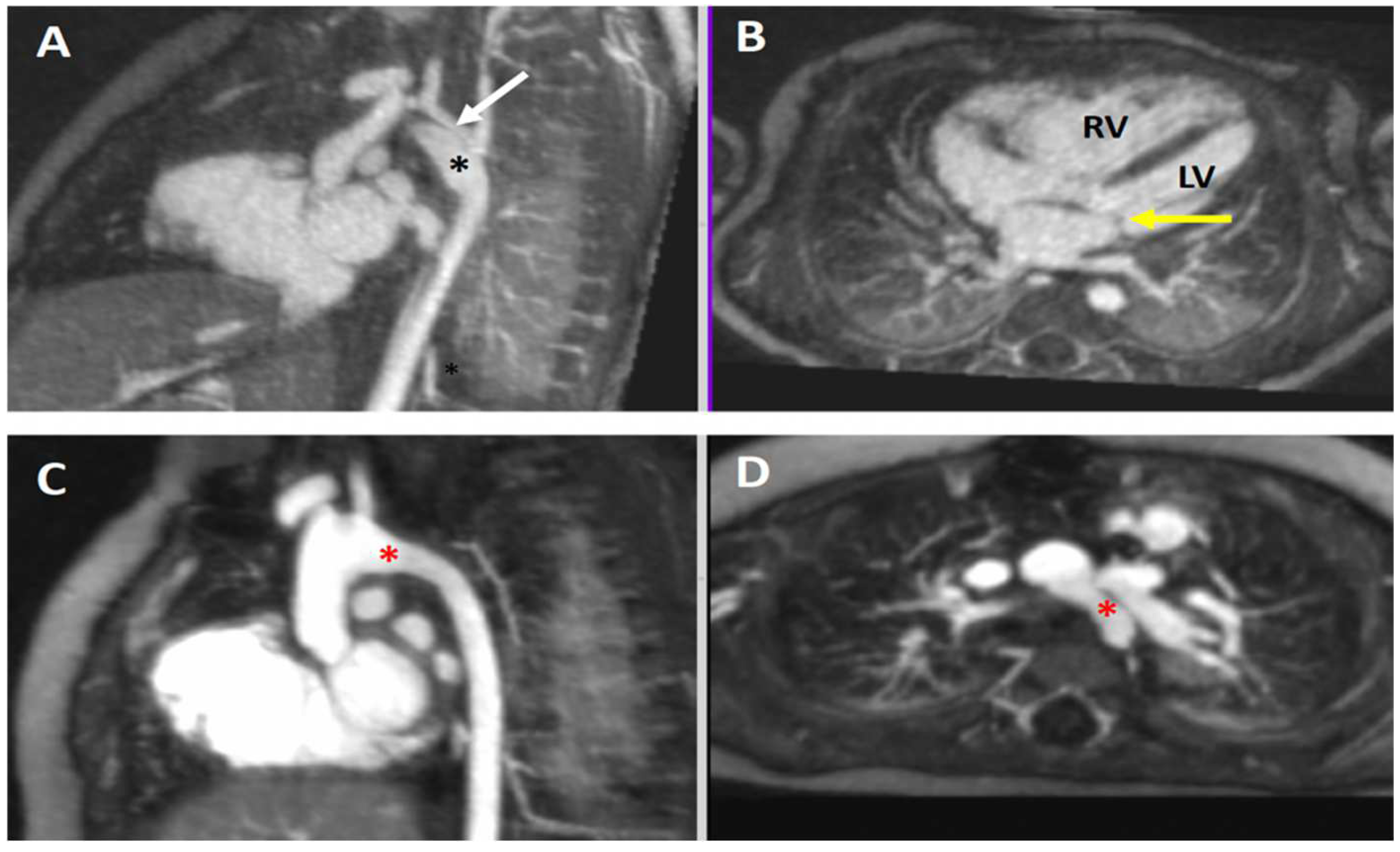
4.4.2. Hypoplastic Left Heart Complex
4.4.3. Coronary Artery Anatomy
4.5. Lymphatic Imaging
5. Future Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seetharam, K.; Lerakis, S. Cardiac magnetic resonance imaging: The future is bright. F1000Research 2019, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Busse, A.; Rajagopal, R.; Yucel, S.; Beller, E.; Oner, A.; Streckenbach, F.; Cantre, D.; Ince, H.; Weber, M.A.; Meinel, F.G. Cardiac MRI-Update 2020. Radiologe 2020, 60, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Demirkiran, A.; Everaars, H.; Amier, R.P.; Beijnink, C.; Bom, M.J.; Gotte, M.J.W.; van Loon, R.B.; Selder, J.L.; van Rossum, A.C.; Nijveldt, R. Cardiovascular magnetic resonance techniques for tissue characterization after acute myocardial injury. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Prosperi, S.; Fanisio, F.; Birtolo, L.I.; Costi, B.; Netti, L.; Chimenti, C.; Lavalle, C.; Maestrini, V.; et al. Myocardial Tissue Characterization in Heart Failure with Preserved Ejection Fraction: From Histopathology and Cardiac Magnetic Resonance Findings to Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 7650. [Google Scholar] [CrossRef]
- Patel, A.R.; Kramer, C.M. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10, 1180–1193. [Google Scholar] [CrossRef]
- Fogel, M.A.; Anwar, S.; Broberg, C.; Browne, L.; Chung, T.; Johnson, T.; Muthurangu, V.; Taylor, M.; Valsangiacomo-Buechel, E.; Wilhelm, C. Society for Cardiovascular Magnetic Resonance/European Society of Cardiovascular Imaging/American Society of Echocardiography/Society for Pediatric Radiology/North American Society for Cardiovascular Imaging Guidelines for the Use of Cardiac Magnetic Resonance in Pediatric Congenital and Acquired Heart Disease: Endorsed by The American Heart Association. Circ. Cardiovasc. Imaging 2022, 15, e014415. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults with Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e637–e697. [Google Scholar] [CrossRef]
- Renella, P.L.; Lai, W.W.; Prakash, A. The Role of Multimodality Cardiovascular Imaging. In Echocardiography in Pediatric and Congenital Heart Disease: From Fetus to Adult, 3rd ed.; Lai, W.W., Mertens, L.L.C., Geva, T., Eds.; Wiley-Blackwell: Oxford, UK, 2022; pp. 1011–1030. [Google Scholar]
- Yerly, J.; Gubian, D.; Knebel, J.F.; Schenk, A.; Chaptinel, J.; Ginami, G.; Stuber, M. A phantom study to determine the theoretical accuracy and precision of radial MRI to measure cross-sectional area differences for the application of coronary endothelial function assessment. Magn. Reson. Med. 2018, 79, 108–120. [Google Scholar] [CrossRef]
- Goldberg, A.; Jha, S. Phase-contrast MRI and applications in congenital heart disease. Clin. Radiol. 2012, 67, 399–410. [Google Scholar] [CrossRef]
- Ntsinjana, H.N.; Hughes, M.L.; Taylor, A.M. The role of cardiovascular magnetic resonance in pediatric congenital heart disease. J. Cardiovasc. Magn. Reson. 2011, 13, 51. [Google Scholar] [CrossRef]
- Valsangiacomo Buechel, E.R.; Grosse-Wortmann, L.; Fratz, S.; Eichhorn, J.; Sarikouch, S.; Greil, G.F.; Beerbaum, P.; Bucciarelli-Ducci, C.; Bonello, B.; Sieverding, L.; et al. Indications for cardiovascular magnetic resonance in children with congenital and acquired heart disease: An expert consensus paper of the Imaging Working Group of the AEPC and the Cardiovascular Magnetic Resonance Section of the EACVI. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.R.; Bhatti, L.; Marin, D.; Nelson, R.C. Emerging applications for ferumoxytol as a contrast agent in MRI. J. Magn. Reson. Imaging 2015, 41, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.P. Contrast-enhanced MR Angiography without Gadolinium. Radiology 2020, 297, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Daldrup-Link, H.E.; Theruvath, A.J.; Rashidi, A.; Iv, M.; Majzner, R.G.; Spunt, S.L.; Goodman, S.; Moseley, M. How to stop using gadolinium chelates for magnetic resonance imaging: Clinical-translational experiences with ferumoxytol. Pediatr. Radiol. 2022, 52, 354–366. [Google Scholar] [CrossRef]
- Nguyen, K.L.; Yoshida, T.; Han, F.; Ayad, I.; Reemtsen, B.L.; Salusky, I.B.; Satou, G.M.; Hu, P.; Finn, J.P. MRI with ferumoxytol: A single center experience of safety across the age spectrum. J. Magn. Reson. Imaging 2017, 45, 804–812. [Google Scholar] [CrossRef]
- Lai, L.M.; Cheng, J.Y.; Alley, M.T.; Zhang, T.; Lustig, M.; Vasanawala, S.S. Feasibility of ferumoxytol-enhanced neonatal and young infant cardiac MRI without general anesthesia. J. Magn. Reson. Imaging 2017, 45, 1407–1418. [Google Scholar] [CrossRef]
- Nguyen, K.L.; Yoshida, T.; Kathuria-Prakash, N.; Zaki, I.H.; Varallyay, C.G.; Semple, S.I.; Saouaf, R.; Rigsby, C.K.; Stoumpos, S.; Whitehead, K.K.; et al. Multicenter Safety and Practice for Off-Label Diagnostic Use of Ferumoxytol in MRI. Radiology 2019, 293, 554–564. [Google Scholar] [CrossRef]
- Ruangwattanapaisarn, N.; Hsiao, A.; Vasanawala, S.S. Ferumoxytol as an off-label contrast agent in body 3T MR angiography: A pilot study in children. Pediatr. Radiol. 2015, 45, 831–839. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, F.; Rapacchi, S.; Nguyen, K.L.; Brunengraber, D.Z.; Kim, G.J.; Finn, J.P.; Hu, P. Accelerated ferumoxytol-enhanced 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) cardiovascular MRI: Validation in pediatric congenital heart disease. NMR Biomed. 2017, 30, e3663. [Google Scholar] [CrossRef]
- Nguyen, K.L.; Han, F.; Zhou, Z.; Brunengraber, D.Z.; Ayad, I.; Levi, D.S.; Satou, G.M.; Reemtsen, B.L.; Hu, P.; Finn, J.P. 4D MUSIC CMR: Value-based imaging of neonates and infants with congenital heart disease. J. Cardiovasc. Magn. Reson. 2017, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhou, Z.; Han, E.; Gao, Y.; Nguyen, K.L.; Finn, J.P.; Hu, P. Self-gated 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) using rotating cartesian K-space (ROCK): Validation in children with congenital heart disease. Magn. Reson. Med. 2017, 78, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.L.; Ghosh, R.M.; Griffin, L.M.; Yoshida, T.; Bedayat, A.; Rigsby, C.K.; Fogel, M.A.; Whitehead, K.K.; Hu, P.; Finn, J.P. Four-dimensional Multiphase Steady-State MRI with Ferumoxytol Enhancement: Early Multicenter Feasibility in Pediatric Congenital Heart Disease. Radiology 2021, 300, 162–173. [Google Scholar] [CrossRef]
- Hartung, M.P.; Grist, T.M.; Francois, C.J. Magnetic resonance angiography: Current status and future directions. J. Cardiovasc. Magn. Reson. 2011, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Harvey, H.B.; Gowda, V.; Cheng, G. Gadolinium Deposition Disease: A New Risk Management Threat. J. Am. Coll. Radiol. 2020, 17, 546–550. [Google Scholar] [CrossRef]
- Choi, J.W.; Moon, W.J. Gadolinium Deposition in the Brain: Current Updates. Korean J. Radiol. 2019, 20, 134–147. [Google Scholar] [CrossRef]
- Finn, J.P.; Nguyen, K.L.; Hu, P. Ferumoxytol vs. Gadolinium agents for contrast-enhanced MRI: Thoughts on evolving indications, risks, and benefits. J. Magn. Reson. Imaging 2017, 46, 919–923. [Google Scholar] [CrossRef]
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-Based Contrast Agent Use, Their Safety, and Practice Evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef]
- Wagner, B.; Drel, V.; Gorin, Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am. J. Physiol. Renal. Physiol. 2016, 311, F1–F11. [Google Scholar] [CrossRef]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef]
- Lange, S.; Medrzycka-Dabrowska, W.; Zorena, K.; Dabrowski, S.; Slezak, D.; Malecka-Dubiela, A.; Rutkowski, P. Nephrogenic Systemic Fibrosis as a Complication after Gadolinium-Containing Contrast Agents: A Rapid Review. Int. J. Environ. Res. Public Health 2021, 18, 3000. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Osawa, M.; Oba, H.; Toyoda, K.; Kotoku, J.; Haruyama, T.; Takeshita, K.; Furui, S. High Signal Intensity in Dentate Nucleus on Unenhanced T1-weighted MR Images: Association with Linear versus Macrocyclic Gadolinium Chelate Administration. Radiology 2015, 275, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Huang, D.Q.; Shih, G.; Prince, M.R. Signal Change in the Dentate Nucleus on T1-Weighted MR Images After Multiple Administrations of Gadopentetate Dimeglumine Versus Gadobutrol. AJR Am. J. Roentgenol. 2016, 206, 414–419. [Google Scholar] [CrossRef] [PubMed]
- McCullough, B.J.; Kolokythas, O.; Maki, J.H.; Green, D.E. Ferumoxytol in clinical practice: Implications for MRI. J. Magn. Reson. Imaging 2013, 37, 1476–1479. [Google Scholar] [CrossRef] [PubMed]
- Layne, K.A.; Dargan, P.I.; Archer, J.R.H.; Wood, D.M. Gadolinium deposition and the potential for toxicological sequelae—A literature review of issues surrounding gadolinium-based contrast agents. Br. J. Clin. Pharmacol. 2018, 84, 2522–2534. [Google Scholar] [CrossRef] [PubMed]
- Vasanawala, S.S.; Nguyen, K.L.; Hope, M.D.; Bridges, M.D.; Hope, T.A.; Reeder, S.B.; Bashir, M.R. Safety and technique of ferumoxytol administration for MRI. Magn. Reson. Med. 2016, 75, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Broch, L.; Wester, S.; Haslund, A.; Edland, A. Anaphylactic reaction to MRI contrast agent. Tidsskr. Nor. Laegeforen 2020, 140, 1–4. [Google Scholar] [CrossRef]
- Han, F.; Rapacchi, S.; Khan, S.; Ayad, I.; Salusky, I.; Gabriel, S.; Plotnik, A.; Finn, J.P.; Hu, P. Four-dimensional, multiphase, steady-state imaging with contrast enhancement (MUSIC) in the heart: A feasibility study in children. Magn. Reson. Med. 2015, 74, 1042–1049. [Google Scholar] [CrossRef]
- Muehe, A.M.; Feng, D.; von Eyben, R.; Luna-Fineman, S.; Link, M.P.; Muthig, T.; Huddleston, A.E.; Neuwelt, E.A.; Daldrup-Link, H.E. Safety Report of Ferumoxytol for Magnetic Resonance Imaging in Children and Young Adults. Investig. Radiol. 2016, 51, 221–227. [Google Scholar] [CrossRef]
- Ning, P.; Zucker, E.J.; Wong, P.; Vasanawala, S.S. Hemodynamic safety and efficacy of ferumoxytol as an intravenous contrast agents in pediatric patients and young adults. Magn. Reson. Imaging 2016, 34, 152–158. [Google Scholar] [CrossRef]
- Fishbane, S.; Ungureanu, V.D.; Maesaka, J.K.; Kaupke, C.J.; Lim, V.; Wish, J. The safety of intravenous iron dextran in hemodialysis patients. Am. J. Kidney Dis. 1996, 28, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- FDA Drug Safety Communication: FDA Strengthens Warnings and Changes Prescribing Instructions to Decrease the Risk of Serious Allergic Reactions with Anemia Drug Feraheme (Ferumoxytol). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-warnings-and-changes-prescribing-instructions-decrease (accessed on 19 July 2022).
- Keenan, N.G.; Captur, G.; McCann, G.P.; Berry, C.; Myerson, S.G.; Fairbairn, T.; Hudsmith, L.; O’Regan, D.P.; Westwood, M.; Greenwood, J.P. Regional variation in cardiovascular magnetic resonance service delivery across the UK. Heart 2021, 107, 1974–1979. [Google Scholar] [CrossRef]
- Gathman, G.E.; Nadas, A.S. Total anomalous pulmonary venous connection: Clinical and physiologic observations of 75 pediatric patients. Circulation 1970, 42, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Latson, L.A.; Prieto, L.R. Congenital and acquired pulmonary vein stenosis. Circulation 2007, 115, 103–108. [Google Scholar] [CrossRef]
- Vyas, H.V.; Greenberg, S.B.; Krishnamurthy, R. MR imaging and CT evaluation of congenital pulmonary vein abnormalities in neonates and infants. Radiographics 2012, 32, 87–98. [Google Scholar] [CrossRef]
- Jalili, M.H.; Yu, T.; Hassani, C.; Prosper, A.E.; Finn, J.P.; Bedayat, A. Contrast-enhanced MR Angiography without Gadolinium-based Contrast Material: Clinical Applications Using Ferumoxytol. Radiol. Cardiothorac. Imaging 2022, 4, e210323. [Google Scholar] [CrossRef]
- Shepherd, B.; Abbas, A.; McParland, P.; Fitzsimmons, S.; Shambrook, J.; Peebles, C.; Brown, I.; Harden, S. MRI in adult patients with aortic coarctation: Diagnosis and follow-up. Clin. Radiol. 2015, 70, 433–445. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileau, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef]
- Dorfman, A.L.; Geva, T.; Samyn, M.M.; Greil, G.; Krishnamurthy, R.; Messroghli, D.; Festa, P.; Secinaro, A.; Soriano, B.; Taylor, A.; et al. SCMR expert consensus statement for cardiovascular magnetic resonance of acquired and non-structural pediatric heart disease. J. Cardiovasc. Magn. Reson. 2022, 24, 44. [Google Scholar] [CrossRef]
- Bakker, D.A.; Berger, R.M.; Witsenburg, M.; Bogers, A.J. Vascular rings: A rare cause of common respiratory symptoms. Acta Paediatr. 1999, 88, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, C.; Dubois, J.; Rypens, F.; Raboisson, M.J.; Dery, J. Tetralogy of Fallot: Preoperative assessment with MR and CT imaging. Diagn. Interv. Imaging 2016, 97, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Wortmann, L.; Yun, T.J.; Al-Radi, O.; Kim, S.; Nii, M.; Lee, K.J.; Redington, A.; Yoo, S.J.; van Arsdell, G. Borderline hypoplasia of the left ventricle in neonates: Insights for decision-making from functional assessment with magnetic resonance imaging. J. Thorac. Cardiovasc. Surg. 2008, 136, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Tchervenkov, C.I.; Jacobs, J.P.; Weinberg, P.M.; Aiello, V.D.; Beland, M.J.; Colan, S.D.; Elliott, M.J.; Franklin, R.C.; Gaynor, J.W.; Krogmann, O.N.; et al. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol. Young 2006, 16, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Temel, M.T.; Coskun, M.E.; Baspinar, O.; Demiryurek, A.T. Prevalence and characteristics of coronary artery anomalies in children with congenital heart disease diagnosed with coronary angiography. Turk. Kardiyol. Dern. Ars. 2017, 45, 527–532. [Google Scholar] [CrossRef]
- Gleich, S.; Latham, G.J.; Joffe, D.; Ross, F.J. Perioperative and Anesthetic Considerations in Pulmonary Atresia with Intact Ventricular Septum. Semin. Cardiothorac. Vasc. Anesth. 2018, 22, 256–264. [Google Scholar] [CrossRef]
- Kawel-Boehm, N.; Hetzel, S.J.; Ambale-Venkatesh, B.; Captur, G.; Francois, C.J.; Jerosch-Herold, M.; Salerno, M.; Teague, S.D.; Valsangiacomo-Buechel, E.; van der Geest, R.J.; et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 87. [Google Scholar] [CrossRef]
- Dori, Y.; Keller, M.S.; Fogel, M.A.; Rome, J.J.; Whitehead, K.K.; Harris, M.A.; Itkin, M. MRI of lymphatic abnormalities after functional single-ventricle palliation surgery. AJR Am. J. Roentgenol. 2014, 203, 426–431. [Google Scholar] [CrossRef]
- Mackie, A.S.; Veldtman, G.R.; Thorup, L.; Hjortdal, V.E.; Dori, Y. Plastic Bronchitis and Protein-Losing Enteropathy in the Fontan Patient: Evolving Understanding and Emerging Therapies. Can. J. Cardiol. 2022, 38, 988–1001. [Google Scholar] [CrossRef]
- Fratz, S.; Chung, T.; Greil, G.F.; Samyn, M.M.; Taylor, A.M.; Buechel, E.R.V.; Yoo, S.-J.; Powell, A.J. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J. Cardiovasc. Magn. Reson. 2013, 15, 51. [Google Scholar] [CrossRef]
- Arafati, A.; Hu, P.; Finn, J.P.; Rickers, C.; Cheng, A.L.; Jafarkhani, H.; Kheradvar, A. Artificial intelligence in pediatric and adult congenital cardiac MRI: An unmet clinical need. Cardiovasc. Diagn. Ther. 2019, 9, S310–S325. [Google Scholar] [CrossRef] [PubMed]
- Zucker, E.J. Compact pediatric cardiac magnetic resonance imaging protocols. Pediatr. Radiol. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Busch, S.; Johnson, T.R.; Wintersperger, B.J.; Minaifar, N.; Bhargava, A.; Rist, C.; Reiser, M.F.; Becker, C.; Nikolaou, K. Quantitative assessment of left ventricular function with dual-source CT in comparison to cardiac magnetic resonance imaging: Initial findings. Eur. Radiol. 2008, 18, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Chen, J.J.; Zhou, B.; Finn, J.P.; Hu, P.; Nguyen, K.L. Ferumoxytol-enhanced 4D multiphase, steady-state imaging with magnetic resonance in congenital heart disease: Ventricular volume and function across 2D and 3D software platforms. Quant. Imaging Med. Surg. 2022, 12, 4377–4389. [Google Scholar] [CrossRef] [PubMed]
- Hajhosseiny, R.; Bustin, A.; Munoz, C.; Rashid, I.; Cruz, G.; Manning, W.J.; Prieto, C.; Botnar, R.M. Coronary Magnetic Resonance Angiography: Technical Innovations Leading Us to the Promised Land? JACC Cardiovasc. Imaging 2020, 13, 2653–2672. [Google Scholar] [CrossRef]
- Rezkalla, J.; Raymundo, S.A.; Renella, P. Right ventricle-dependent coronary circulation diagnosed by non-invasive ferumoxytol-enhanced 4D cardiac magnetic resonance angiography in pulmonary atresia with intact ventricular septum. Cardiol. Young 2022, 1–3. [Google Scholar] [CrossRef]


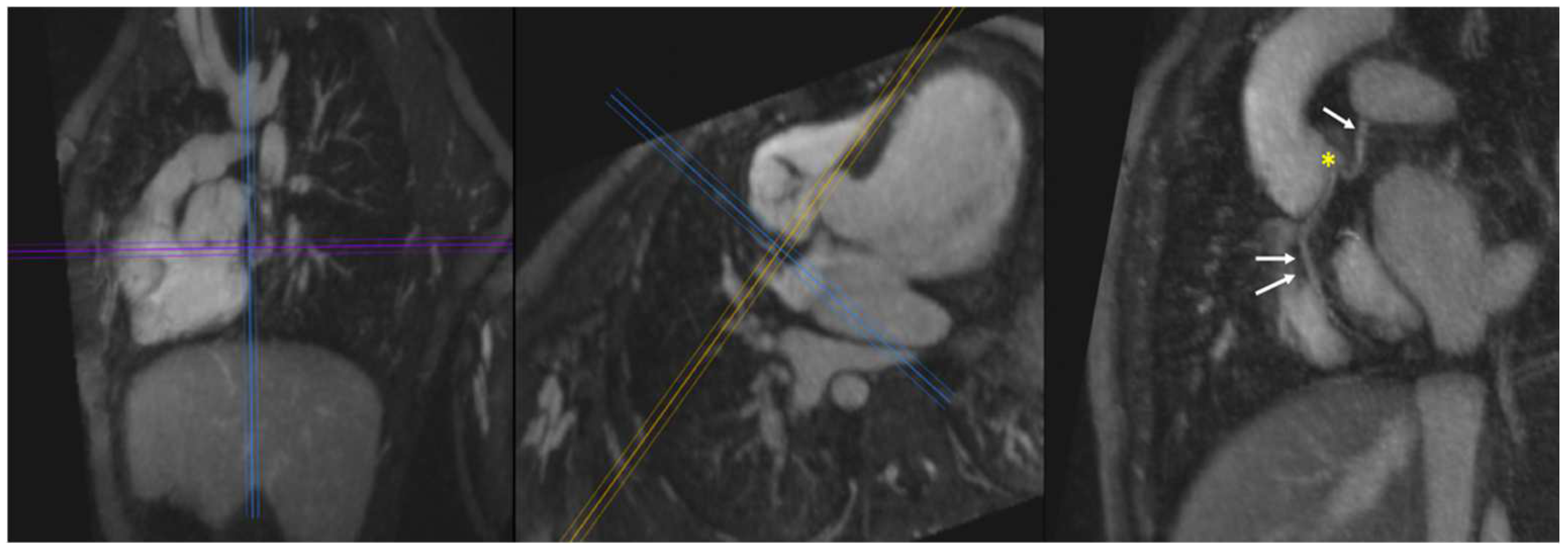

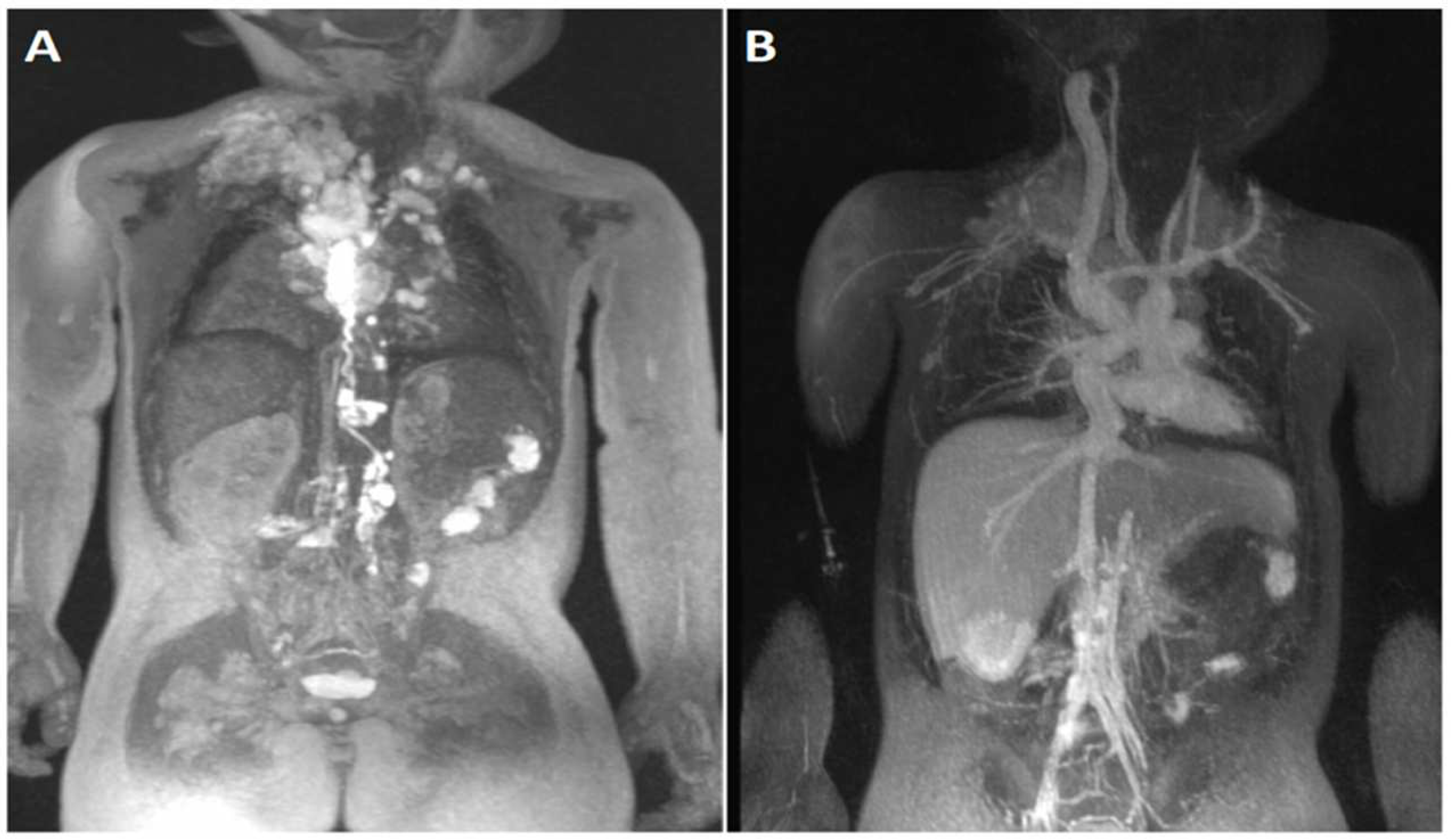
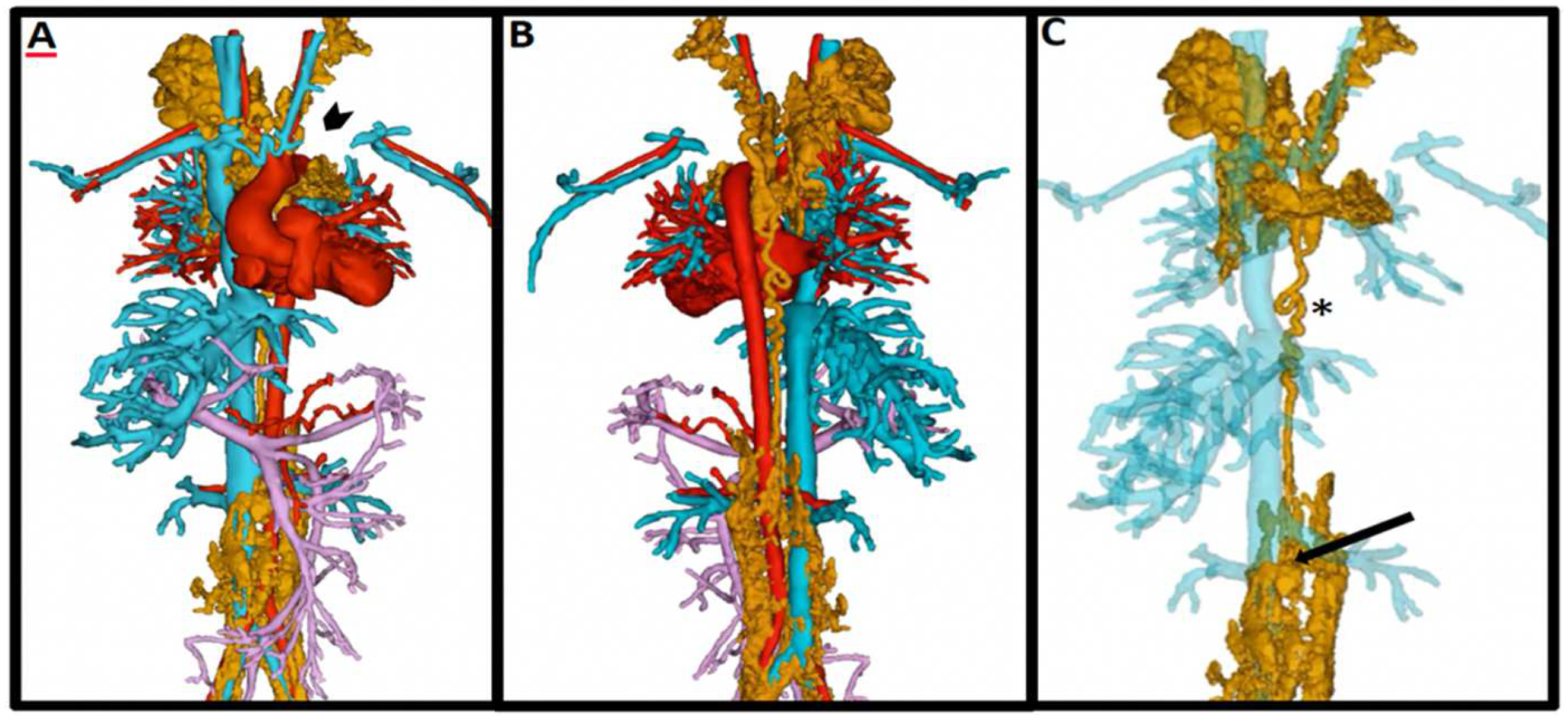
| General MRI Contrast Agents (Ferumoxytol vs. Gadolinium) | |||
|---|---|---|---|
| Author/Year | Type of Article | Summary | |
| Bashir et al., 2015 [13] | Review | Ferumoxytol shows potential to be used as a GBCA alternative in various applications of MRI, as well as in new in novel techniques due to its distribution within macrophages. | |
| Finn et al., 2017 [14] | Review | Ferumoxytol has several potential diagnostic applications and should be further investigated to define parameters for its safety and efficacy. | |
| Finn et al., 2020 [15] | Editorial | Obstacles to use of ferumoxytol for vascular imaging include: availability, expense, and off-label status. | |
| Daldrup-Link et al., 2022 [16] | Review | Ferumoxytol has long lasting blood pool enhancement and is useful in patients with renal insufficiency; however, it is contraindicated in patients with iron overload. | |
| Safety | Population | Purpose | Outcomes |
| Nguyen et al., 2017 N = 217 [17] | Patients (ages 3–94 years) at single center | Compare effects of ferumoxytol on monitored physiologic indices in patients under anesthesia with those of gadofosveset trisodium | No serious AEs with diagnostic use of ferumoxytol across wide spectrum of age, renal function, and indications. |
| Lai et al., 2017 N = 21 [18] | Neonates and young infants (1 day–11 weeks) | Evaluate feasibility of ferumoxytol-enhanced anesthesia-free cardiac MRI with rapid two-sequence protocol (4D flow and MRA) in complex CHD | One patient of 21 required additional imaging, one out of 13 with operative confirmation had a minor discrepancy. 4D flow was superior to MRA for evaluation of systemic arteries, valves, ventricular trabeculae, and overall quality. |
| Nguyen et al., 2019 N = 3215 [19] | Patients at 9 U.S. and 2 U.K. urban academic medical centers registered via FeraSafe multicenter MRI registry | Investigate incidence of acute adverse events for diagnostic ferumoxytol injection and describe registry practice pattern | No serious adverse events were recorded, minor infusion reactions were rare (<2%). Registry data revealed a lower rate of adverse events compared to post-marketing surveillance data for therapeutic use, correlating with different methods (lower total dose, slower average infusion rate, careful monitoring before/after). |
| Congenital Heart Disease | Population | Purpose | Outcomes |
| Ruangwattanapaisarn et al., 2015, N = 23 [20] | Pediatric patients (3 days–18 years) | Determine feasibility of ferumoxytol use in pediatric cardiac and vascular imagine (abdominal and cardiac MRA) | FE-MRA can achieve high image quality (high SNR) in abdominal cases and good blood pool to myocardium delineation for cardiac cases. Ferumoxytol dose of 1.5 or 3 mg Fe/kg were possible for venography |
| Zhou et al., 2017, N = 13 [21] | Pediatric CHD patients (4 days–13 years) | Validation of parallel imaging and compressed sensing combined reconstruction method for the 4D non-breath-held, multiphase, steady-state imaging technique (MUSIC) | CS-PI MUSIC reduced imaging time by approximately 50% while maintaining highly comparable image quality to the original MUSIC, with good reconstruction time (5 min). |
| Nguyen et al., 2017, N = 40 [22] | Pediatric patients (2 days–2 years) | Evaluate diagnostic performance and clinical value of 4D MUSIC in neonates/infants with CHD | FE-MUSIC provided accurate, high-quality images of cardiac and vascular anatomy. Findings on MUSIC, surgery, correlative imaging, and autopsy had excellent correspondence. |
| Han et al., 2017, N = 10 [23] | Pediatric patients with complex CHD (1 month–8 years) | Validate cardiac-respiratory self-gating (ROCK) strategy for multiphase steady-state imaging with MUSIC technique | ROCK-MUSIC provided equal or superior image quality and increased efficiency (40% scan time reduction) compared with original MUSIC. |
| Nguyen et al., 2021, N = 60 [24] | Pediatric patients, 20 each from 3 sites | Evaluate feasibility of 4D MUSIC MRI in pediatric CHD in a multicenter study | 4D MUSIC MRI is feasible in a multicenter setting, reduces image acquisition time, and simplifies the acquisition protocol. |
| Trait | Gadolinium | Ferumoxytol |
|---|---|---|
| Familiarity | Most commonly used MR contrast agents FDA-approved (including in children) | More recently employed as an MR contrast agent Off-label use for imaging purposes |
| Safety | Does not occur naturally in the body Very low rate of anaphylaxis | Iron is an essential element for physiologic function Very low rate of anaphylaxis Low incidence of mild, self-limiting, infusion reactions (especially in children, approximately 1–2%) Requires monitoring for 30 min after the infusion |
| Imaging Protocol | Requires precise bolus timing Separate arterial and venous phases Allows for myocardial perfusion and late enhancement imaging | No need for timing bolus Steady-state imaging Blood pool agent |
| Performance | High T1 relaxivity and signal-to-noise ratio Rapid blood transit | Superior T1 relaxivity and signal-to-noise ratio Stable blood concentration (supports high-resolution multidimensional imaging with uniform vessel signal) |
| Cost | In general, is less expensive than ferumoxytol preparations | Historically more expensive, but recent generic formulation has reduced the cost |
| Diagnosis | Advantages of Ferumoxytol over GBCAs |
|---|---|
| Aortic Aneurysm | Genetic syndromes linked to aortic aneurysms and dissection include (among others): Marfan’s, Loeys–Dietz, vascular Ehlers–Danlos, and Turner’s. Bicuspid aortic valve also confers a higher risk. High-resolution imaging of the entire aorta and its branches can be readily achieved with FE steady-state imaging. |
| Congenital Coronary Anomalies | Imaging of these very small structures is facilitated with longer sequences focused on high spatial resolution. Also crucial to adequate coronary artery imaging in smaller patients is a bright and evenly enhanced blood pool, which is made more feasible using ferumoxytol. |
| Fontan circulation (for example, tricuspid atresia or HLHS) | The characteristic slow flow of the Fontan circulation can make precise MRA timing with GBCAs challenging given their relatively rapid vascular transit time. Both the superior limb (Glenn shunt) and the inferior limb (Fontan conduit), as well as the branch pulmonary arteries and collateral vessels, can be uniformly opacified during steady-state imaging. |
| Lymphatic Imaging | Overlay of FE MRA and contrast-enhanced MRL images has allowed high-resolution comprehensive mapping of the vascular tree as it relates to abnormal lymphatic connections in patients with conditions such as chylothorax, protein losing enteropathy, and plastic bronchitis. These imaging techniques have facilitated the development of novel transcatheter treatments. |
| TAPVC | FE steady-state imaging allows both the individual pulmonary veins and the abnormal systemic venous connection(s) to be visualized simultaneously in a single acquisition without the need for a timing bolus. |
| Tetralogy of Fallot | FE MRA can facilitate visualization of both the central and distal pulmonary artery branches, which are at risk for hypoplasia or atresia. In more severe forms of Tetralogy of Fallot, the pulmonary arteries can be replaced by aortopulmonary collateral arteries. Simultaneous visualization and precise mapping of these collaterals, and their relative contribution to regional lung perfusion as compared to the native pulmonary arteries, are critical to preoperative planning. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renella, P.; Li, J.; Prosper, A.E.; Finn, J.P.; Nguyen, K.-L. Ferumoxytol-Enhanced Cardiac Magnetic Resonance Angiography and 4D Flow: Safety and Utility in Pediatric and Adult Congenital Heart Disease. Children 2022, 9, 1810. https://doi.org/10.3390/children9121810
Renella P, Li J, Prosper AE, Finn JP, Nguyen K-L. Ferumoxytol-Enhanced Cardiac Magnetic Resonance Angiography and 4D Flow: Safety and Utility in Pediatric and Adult Congenital Heart Disease. Children. 2022; 9(12):1810. https://doi.org/10.3390/children9121810
Chicago/Turabian StyleRenella, Pierangelo, Jennifer Li, Ashley E. Prosper, J. Paul Finn, and Kim-Lien Nguyen. 2022. "Ferumoxytol-Enhanced Cardiac Magnetic Resonance Angiography and 4D Flow: Safety and Utility in Pediatric and Adult Congenital Heart Disease" Children 9, no. 12: 1810. https://doi.org/10.3390/children9121810
APA StyleRenella, P., Li, J., Prosper, A. E., Finn, J. P., & Nguyen, K.-L. (2022). Ferumoxytol-Enhanced Cardiac Magnetic Resonance Angiography and 4D Flow: Safety and Utility in Pediatric and Adult Congenital Heart Disease. Children, 9(12), 1810. https://doi.org/10.3390/children9121810






