The Role of the Gut Microbiome in Youth with Polycystic Ovary Syndrome: A Systematic Review
Abstract
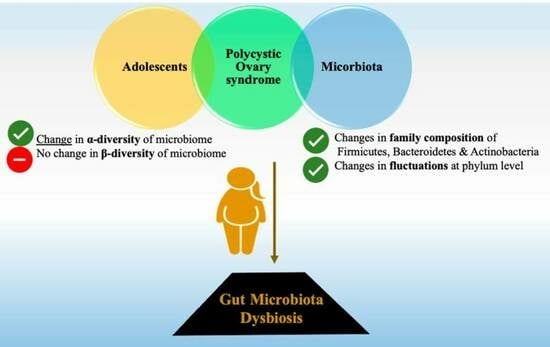
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Information Sources
2.2. Study Population Rationale and Eligibility Criteria
2.3. Study Outcomes
2.4. Screening and Data Collection
2.5. Quality Assessment of Included Studies
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wolf, W.M.; Wattick, R.A.; Kinkade, O.N.; Olfert, M.D. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int. J. Environ. Res. Public Health 2018, 15, 2589. [Google Scholar] [CrossRef] [PubMed]
- Naz, M.S.G.; Tehrani, F.R.; Majd, H.A.; Ahmadi, F.; Ozgoli, G.; Fakari, F.R.; Ghasemi, V. The prevalence of polycystic ovary syndrome in adolescents: A systematic review and meta-analysis. Int. J. Reprod. Biomed. (IJRM) 2019, 17, 533–542. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J. Clin. Endocrinol. Metabolism. 2021, 106, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.S.; Witchel, S.F.; Hoeger, K.M.; Oberfield, S.E.; Vogiatzi, M.G.; Misso, M.; Garad, R.; Dabadghao, P.; Teede, H. Adolescent polycystic ovary syndrome according to the international evi-dence-based guideline. BMC Med. 2020, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; Oberfield, S.E.; Witchel, S.; Auchus, R.J.; Chang, R.J.; Codner, E.; Dabadghao, P.; Darendeliler, F.; Elbarbary, N.S.; Gambineri, A.; et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm. Res. Paediatr. 2017, 88, 371–395. [Google Scholar] [CrossRef]
- Uysal, M.; Arslan, S. Treatment approach in ovarian pathologies in children: A single center’s experience. J. Clin. Trials Exp. Investig. 2022, 1, 86–91. [Google Scholar]
- Peigné, M.; Dewailly, D. Long term complications of polycystic ovary syndrome (PCOS). Ann. D’endocrinologie 2014, 75, 194–199. [Google Scholar] [CrossRef]
- Meczekalski, B.; Niwczyk, O.; Kostrzak, A.; Maciejewska-Jeske, M.; Bala, G.; Szeliga, A. PCOS in Adolescents—Ongoing Riddles in Diagnosis and Treatment. J. Clin. Med. 2023, 12, 1221. [Google Scholar] [CrossRef]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome–Brain Alliance: A Landscape View into Mental and Gastroin-testinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef]
- Jeffery, I.B.; Claesson, M.J.; O’Toole, P.W.; Shanahan, F. Categorization of the gut microbiota: Enterotypes or gradients? Nat. Rev. Microbiol. 2012, 10, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Matute, S.P.; Iyavoo, S. Exploring the gut microbiota: Lifestyle choices, disease associations, and personal genomics. Front. Nutr. 2023, 10, 1225120. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W. (Dis)Trust your gut: The gut microbiome in age-related inflammation, health, and disease. Microbiome 2017, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.; Gober, H.J.; Leung, W.T.; Huang, Z.; Pan, X.; Li, C.; Zhang, N.; Wang, L. Alterations in the intestinal microbiome associated with PCOS affect the clinical phe-notype. Biomed. Pharmacother. 2021, 133, 110958. [Google Scholar] [CrossRef] [PubMed]
- He, F.-F.; Li, Y.-M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Su, X. Elucidating the Beta-Diversity of the Microbiome: From Global Alignment to Local Alignment. mSystems 2021, 6, e0036321. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Liang, H.; Ye, L.; Lan, L.; Lu, F.; Wang, Q.; Lei, T.; Yang, X.; Cui, P.; et al. Differences in Alpha Diversity of Gut Microbiota in Neurological Diseases. Front. Neurosci. 2022, 16, 879318. [Google Scholar] [CrossRef]
- Li, P.; Shuai, P.; Shen, S.; Zheng, H.; Sun, P.; Zhang, R.; Lan, S.; Lan, Z.; Jayawardana, T.; Yang, Y.; et al. Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Med. 2023, 21, 302. [Google Scholar] [CrossRef]
- United Nations Definition of Youth. Available online: https://www.un.org/esa/socdev/documents/youth/fact-sheets/youth-definition.pdf (accessed on 24 November 2023).
- United Nations Population Fund (UNFPA). Available online: https://www.unfpa.org/emergencies/manual/8.htm (accessed on 24 November 2023).
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, N.D.; Guzelce, E.C.; Acikgoz, A.; Uyanik, E.; Bjørndal, B.; Berge, R.K.; Svardal, A.; Yildiz, B.O. Circulating gut microbiota metabolite trimethylamine N-oxide and oral contraceptive use in polycystic ovary syndrome. Clin. Endocrinol. 2019, 91, 810–815. [Google Scholar] [CrossRef]
- Eyupoglu, N.D.; Ergunay, K.; Acikgoz, A.; Akyon, Y.; Yilmaz, E.; Yildiz, B.O. Gut microbiota and oral contraceptive use in overweight and obese patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2020, 105, e4792–e4800. [Google Scholar] [CrossRef] [PubMed]
- Mammadova, G.; Ozkul, C.; Isikhan, S.Y.; Acikgoz, A.; Yildiz, B.O. Characterization of gut microbiota in polycystic ovary syndrome: Findings from a lean population. Eur. J. Clin. Investig. 2021, 51, e13417. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, C.; Malpique, R.; Carbonetto, B.; González-Torres, P.; Henares, D.; Brotons, P.; Muñoz-Almagro, C.; López-Bermejo, A.; de Zegher, F.; Ibáñez, L. Gut microbiota in adolescent girls with polycystic ovary syndrome: Effects of randomized treatments. Pediatr. Obes. 2021, 16, e12734. [Google Scholar] [CrossRef] [PubMed]
- Jobira, B.; Frank, D.N.; Pyle, L.; Silveira, L.J.; Kelsey, M.M.; Garcia-Reyes, Y.; E Robertson, C.; Ir, D.; Nadeau, K.J.; Cree-Green, M. Obese Adolescents With PCOS Have Altered Biodiversity and Relative Abundance in Gastrointestinal Microbiota. J. Clin. Endocrinol. Metab. 2020, 105, e2134–e2144. [Google Scholar] [CrossRef]
- Witchel, S.F.; Azziz, R.; Oberfield, S.E. History of Polycystic Ovary Syndrome, Premature Adrenarche, and Hyperandrogenism in Pediatric Endocrinology. Horm. Res. Paediatr. 2022, 95, 557–567. [Google Scholar] [CrossRef]
- Calcaterra, V.; Rossi, V.; Massini, G.; Casini, F.; Zuccotti, G.; Fabiano, V. Probiotics and Polycystic Ovary Syndrome: A Per-spective for Management in Adolescents with Obesity. Nutrients 2023, 15, 3144. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Yan, H.; Qin, Q.; Yan, S.; Chen, J.; Yang, Y.; Li, T.; Gao, X.; Ding, S. Comparison Of The Gut Microbiota In Different Age Groups In China. Front. Cell. Infect. Microbiol. 2022, 12, 877914. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Ochoa, S.; Cervantes-Guevara, G.; Cervantes-Pérez, L.A.; Cervantes-Cardona, G.A.; Cervantes-Pérez, G.; Gómez-Sánchez, E.; Cervantes-Pérez, E. The potential effects of metabolic surgery on gut microbiota: Novel insights. Cirugía Y Cir. 2023, 91, 719–720. [Google Scholar]
- Baars, A.; Oosting, A.; Lohuis, M.; Koehorst, M.; El Aidy, S.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef]
- Jaggar, M.; Rea, K.; Spichak, S.; Dinan, T.G.; Cryan, J.F. You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan. Front. Neuroendocr. 2019, 56, 100815. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Arnoriaga-Rodríguez, M.; Luque-Córdoba, D.; Priego-Capote, F.; Pérez-Brocal, V.; Moya, A.; Burokas, A.; Maldonado, R.; Fernández-Real, J.M. Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: Influences of obesity and menopausal status. Microbiome 2020, 8, 138. [Google Scholar] [CrossRef]
- Giampaolino, P.; Foreste, V.; Di Filippo, C.; Gallo, A.; Mercorio, A.; Serafino, P.; Improda, F.P.; Verrazzo, P.; Zara, G.; Buonfantino, C.; et al. Microbiome and PCOS: State-of-art and future aspects. Int. J. Mol. Sci. 2021, 22, 2048. [Google Scholar] [CrossRef]
- Yurtdaş, G.; Akdevelioğlu, Y. A New Approach to Polycystic Ovary Syndrome: The Gut Microbiota. J. Am. Coll. Nutr. 2020, 39, 371–382. [Google Scholar] [CrossRef]
- Zhou, L.; Ni, Z.; Yu, J.; Cheng, W.; Cai, Z.; Yu, C. Correlation Between Fecal Metabolomics and Gut Microbiota in Obesity and Polycystic Ovary Syndrome. Front. Endocrinol. 2020, 11, 628. [Google Scholar] [CrossRef]
- Barber, T.M.; McCarthy, M.I.; Wass, J.A.H.; Franks, S. Obesity and polycystic ovary syndrome. Clin. Endocrinol. (Oxf) 2006, 65, 137–145. [Google Scholar] [CrossRef]
- Lim, S.; Davies, M.; Norman, R.; Moran, L. Overweight, obesity and central obesity in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2012, 18, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. the gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Lai, Z.; Sun, L.; Zhang, Z.; Yang, J.; Li, Z.; Lin, J.; Zhang, Z. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): A pilot study. Res. Microbiol. 2019, 170, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic Bifidobacterium lactis V9 Regulates the Secretion of Sex Hormones in Polycystic Ovary Syndrome Patients through the Gut-Brain Axis. mSystems 2019, 4, e00017-19. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.J.; Siakowska, M.; Banaszewska, B.; Pawelczyk, L.; Duleba, A.J.; Kelley, S.T.; Thackray, V.G. Gut Microbial Diversity in Women with Polycystic Ovary Syndrome Correlates with Hyperandrogenism. J. Clin. Endocrinol. Metab. 2018, 103, 1502–1511. [Google Scholar] [CrossRef]
- Liang, Z.; Di, N.; Li, L.; Yang, D. Gut microbiota alterations reveal potential gut–brain axis changes in polycystic ovary syndrome. J. Endocrinol. Investig. 2021, 44, 1727–1737. [Google Scholar] [CrossRef]
- Hua, X.; Cao, Y.; Morgan, D.M.; Miller, K.; Chin, S.M.; Bellavance, D.; Khalili, H. Longitudinal analysis of the impact of oral contraceptive use on the gut microbiome. J. Med. Microbiol. 2022, 71, 001512. [Google Scholar] [CrossRef]
- Papadakis, G.; Kandaraki, E.A.; Garidou, A.; Koutsaki, M.; Papalou, O.; Diamanti-Kandarakis, E.; Peppa, M. Tailoring treatment for PCOS phenotypes. Expert Rev. Endocrinol. Metab. 2021, 16, 9–18. [Google Scholar] [CrossRef]
- Dambrova, M.; Latkovskis, G.; Kuka, J.; Strele, I.; Konrade, I.; Grinberga, S.; Hartmane, D.; Pugovics, O.; Erglis, A.; Liepinsh, E. Diabetes is Associated with Higher Trimethylamine N-oxide Plasma Levels. Exp. Clin. Endocrinol. Diabetes 2016, 124, 251–256. [Google Scholar] [CrossRef]
- Huang, J.; Liu, L.; Chen, C.; Gao, Y. PCOS without hyperandrogenism is associated with higher plasma Trimethylamine N-oxide levels. BMC Endocr. Disord. 2020, 20, 3. [Google Scholar] [CrossRef]
- Jobira, B.; Frank, D.N.; Silveira, L.J.; Pyle, L.; Kelsey, M.M.; Garcia-Reyes, Y.; Robertson, C.E.; Ir, D.; Nadeau, K.J.; Cree-Green, M. Hepatic steatosis relates to gastrointestinal microbiota changes in obese girls with polycystic ovary syndrome. PLoS ONE 2021, 16, e0245219. [Google Scholar] [CrossRef] [PubMed]
- Peters, B.A.; Shapiro, J.A.; Church, T.R.; Miller, G.; Trinh-Shevrin, C.; Yuen, E.; Friedlander, C.; Hayes, R.B.; Ahn, J. A taxonomic signature of obesity in a large study of American adults. Sci. Rep. 2018, 8, 9749. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]

| Author, Year | Country | Type of Study |
|---|---|---|
| Garcia-Beltran et al., 2020 [28] | Spain | Randomized controlled trial |
| Mammadova et al., 2020 [27] | Turkey | Cross-sectional |
| Jobira et al., 2020 [29] | U.S.A | Cross-sectional |
| Eyupoglu et al., 2020 [23] | Turkey | Cross-sectional |
| Author, Year | PCOS Group | Control Group | ||||
|---|---|---|---|---|---|---|
| n | Age | BMI (kg/m2) | n | Age | BMI (kg/m2) | |
| Adolescents | ||||||
| Garcia-Beltran et al., 2020 [28] | 30 | 15.8 (15.5–16.1) | 25.0 ± 1.0 | 31 | 15.9 (15.7–16.1) | 22.0 ± 0.0 |
| Jobira et al., 2020 [29] | 37 | 16.1 (15.8–16.4) | 36.0 (32.9, 39.7) | 21 | 14.5 (14.1–14.9) | 35.0 (30.7, 39.3) |
| Young people | ||||||
| Mammadova et al., 2020 [27] | 24 | 19.5 (19–22.5) | 22.9 ± 3.2 | 22 | 23.0 (22.0–24.3) | 22.5 ± 2.5 |
| Author, Year | PCOS Phyla Differences |
|---|---|
| Adolescents | |
| Garcia-Beltran et al., 2020 [28] | ↑ Family ΧΙ (Firmicutes) ↓ Prevotellaceae (Bacteroidetes) ↓ Prevotella (Firmicutes) ↓ Senegalimassilia (Actinobacteria) |
| Jobira et al., 2020 [29] | ↑ Actinobacteria ↓ Bacteroidetes |
| Young people | |
| Mammadova et al., 2020 [27] | ↑ Proteobacteria ↑ Gammaproteobacteria ↑ Erysipelotrichia ↑ Verrucomicrobia ↓ Roseburia ↓ Clostridium sensy stricto |
| Eyupoglu et al., 2020 [23] | ↑ Ruminococcaceae (Firmicutes) |
| Author, Year | α Diversity | Β Diversity |
|---|---|---|
| Adolescents | ||
| Garcia-Beltran et al., 2020 [28] | Significantly lower α diversity in PCOS patients compared to controls [Pielou’s Evenness Index (p = 0.03) and Shannon’s Index (p = 0.04)] | Significant differences in dispersion of dissimilarity matrices [Jaccard ANOSIM test (p = 0.001), Permanova test (p = 0.01), Permdisp test (p = 0.19), Bray–Curtis ANOSIM test (p = 0.001), Permanova test (p = 0.002), and Permdisp test (p= 0.0001)] |
| Jobira et al., 2020 [29] | Significantly lower α biodiversity in PCOS compared to controls [Evenness (p = 0.0052) and Shannon diversity (p = 0.045)] | Significant β diversity, reflecting overall gut microbial community composition [p < 0.001] |
| Young people | ||
| Mammadova et al., 2020 [27] | No difference between PCOS patients and controls (p = 0.784) | Not significant difference between PCOS patients and controls (p = 0.937). |
| Eyupoglu et al., 2020 [23] | No difference between PCOS patients and controls [Faith PD (p = 0.27), Pielou evenness Index (p= 0.79), and Shannon Index (p = 0.97)] | Not significant difference between PCOS patients and controls [Bray–Curtis (p = 0.58), Jaccard index (p = 0.99), and UniFrac distances (p = 0.76)] |
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall | |
|---|---|---|---|---|---|---|---|---|
| Study | ||||||||
| Mammadova et al., 2020 [27] | 🟢 | 🟢 | 🟢 | 🟡 | 🟢 | 🟢 | 🟡 | 🟢 |
| Jobira et al., 2020 [29] | 🟢 | 🟢 | 🟡 | 🟢 | 🟡 | 🟡 | 🟢 | 🟢 |
| Eyupoglu et al., 2020 [23] | 🟢 | 🟢 | 🟢 | 🟡 | 🟡 | 🟢 | 🟢 | 🟢 |
| Domains: | ||||||||
| D1: Bias due to confounding | 🟢 Low risk of bias | |||||||
| D2: Bias due to selection of participants | 🟡 Moderate risk of bias | |||||||
| D3: Bias in classification of interventions | 🔴 Serious risk of bias | |||||||
| D4: Bias due to deviations from intended interventions | ⚫ Critical risk of bias | |||||||
| D5: Bias due to missing data | 🔵 No information | |||||||
| D6: Bias in measurement of outcomes | ||||||||
| D7: Bias in selection of the reported results |
| D1 | D2 | D3 | D4 | D5 | Overall | |
|---|---|---|---|---|---|---|
| Study | ||||||
| Garcia-Beltran et al., 2020 [28] | 🟡 | 🟢 | 🟢 | 🟡 | 🟢 | 🟢 |
| Domains: | ||||||
| D1: Bias due to randomization | 🟢 Low | |||||
| D2: Bias due to deviations from intended interventions | 🟡 Some concerns | |||||
| D3: Bias due to missing data | 🔴 High | |||||
| D4: Bias due to outcome measurement | ||||||
| D5: Bias in selection of the reported results | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsinopoulou, V.-R.; Kotanidou, E.P.; Athanasiadis, N.; Sapountzi, E.; Bacopoulou, F.; Ntzani, E.; Galli-Tsinopoulou, A.; Christoforidis, A. The Role of the Gut Microbiome in Youth with Polycystic Ovary Syndrome: A Systematic Review. Children 2023, 10, 1872. https://doi.org/10.3390/children10121872
Tsinopoulou V-R, Kotanidou EP, Athanasiadis N, Sapountzi E, Bacopoulou F, Ntzani E, Galli-Tsinopoulou A, Christoforidis A. The Role of the Gut Microbiome in Youth with Polycystic Ovary Syndrome: A Systematic Review. Children. 2023; 10(12):1872. https://doi.org/10.3390/children10121872
Chicago/Turabian StyleTsinopoulou, Vasiliki-Rengina, Eleni P. Kotanidou, Nikolaos Athanasiadis, Evdoxia Sapountzi, Flora Bacopoulou, Evangelia Ntzani, Assimina Galli-Tsinopoulou, and Athanasios Christoforidis. 2023. "The Role of the Gut Microbiome in Youth with Polycystic Ovary Syndrome: A Systematic Review" Children 10, no. 12: 1872. https://doi.org/10.3390/children10121872
APA StyleTsinopoulou, V.-R., Kotanidou, E. P., Athanasiadis, N., Sapountzi, E., Bacopoulou, F., Ntzani, E., Galli-Tsinopoulou, A., & Christoforidis, A. (2023). The Role of the Gut Microbiome in Youth with Polycystic Ovary Syndrome: A Systematic Review. Children, 10(12), 1872. https://doi.org/10.3390/children10121872