Combining Cardiorespiratory Signals and Video-Based Actigraphy for Classifying Preterm Infant Sleep States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data and Annotation
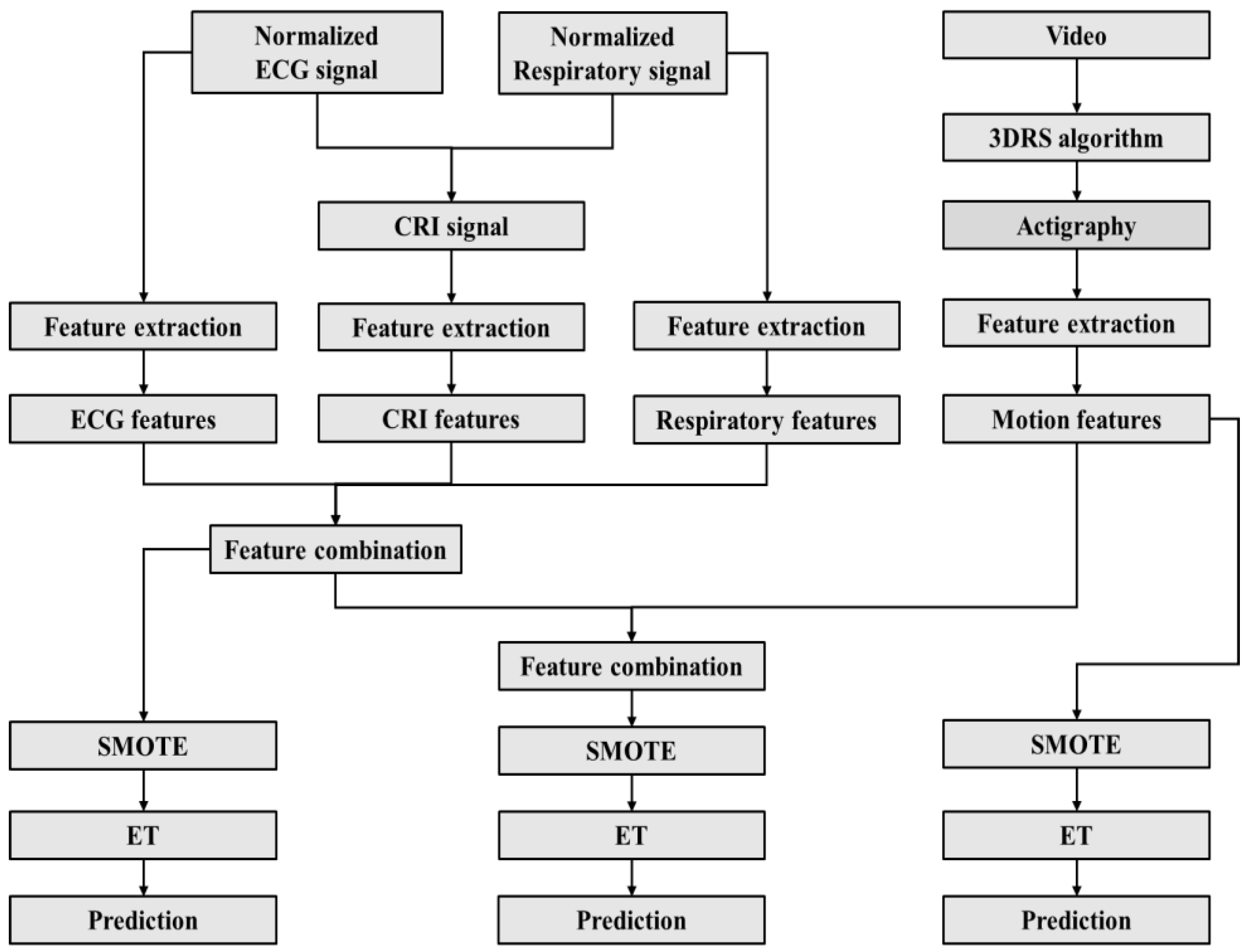
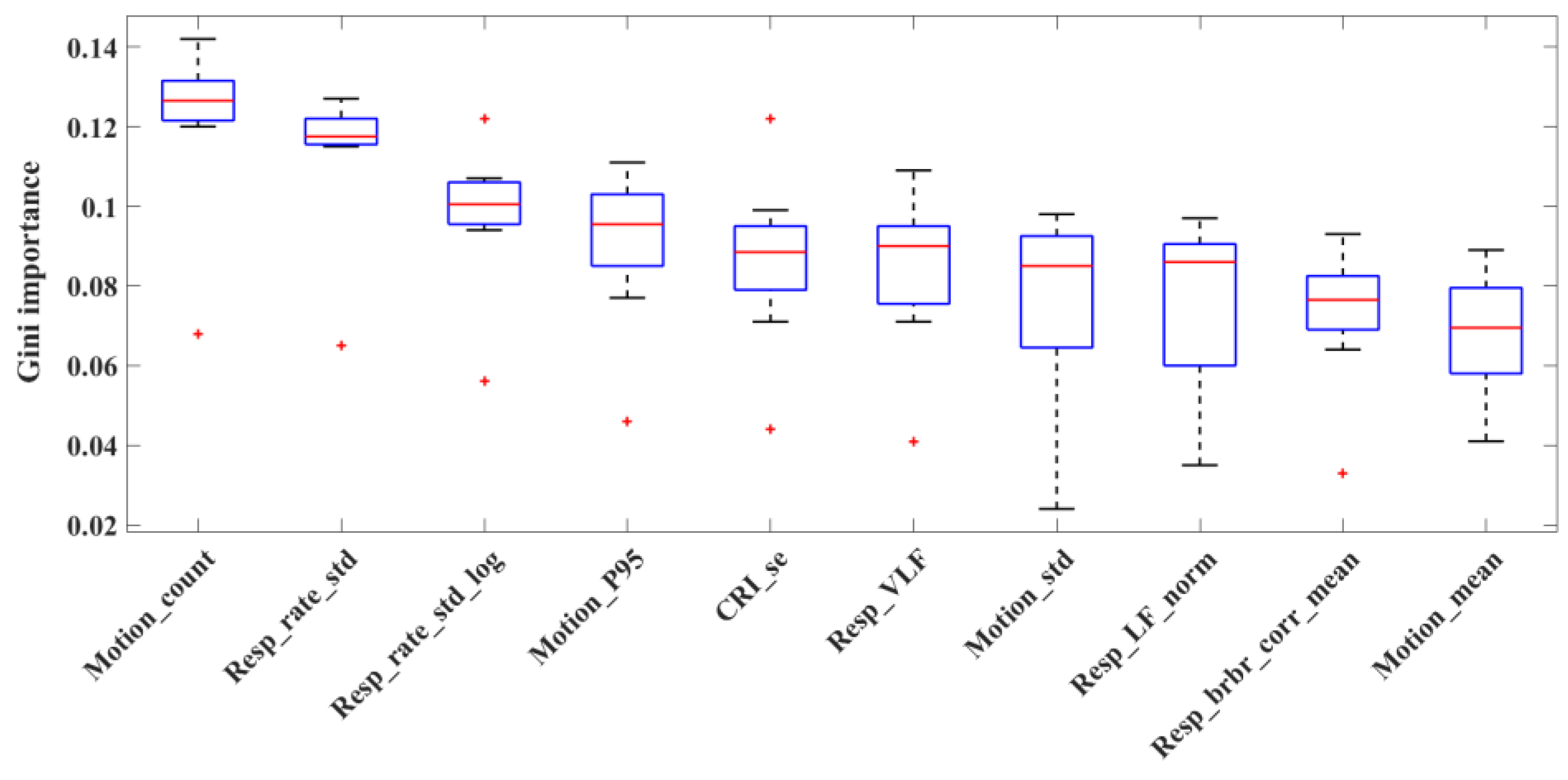
2.2. Feature Extraction
2.3. Feature Preprocessing
2.4. Classification Algorithms
- The motion features from video-based actigraphy (Motion).
- The ECG, respiration, and CRI features (ECG-Resp-CRI).
- The ECG, respiration, CRI, and motion features (ECG-Resp-CRI-Motion).
2.5. Model Evaluation
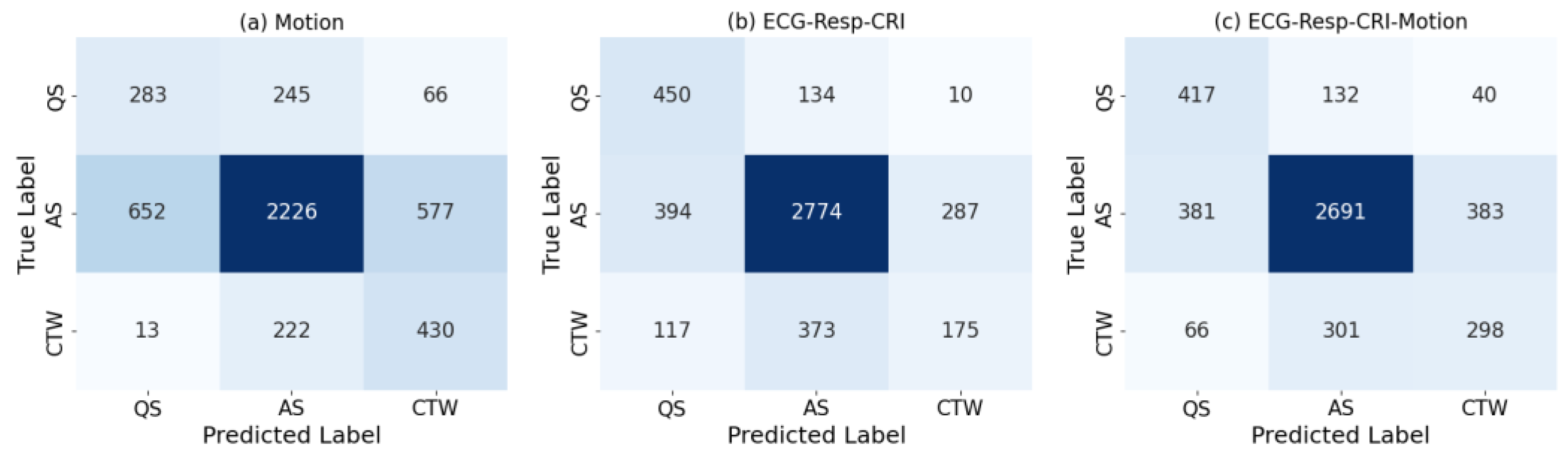
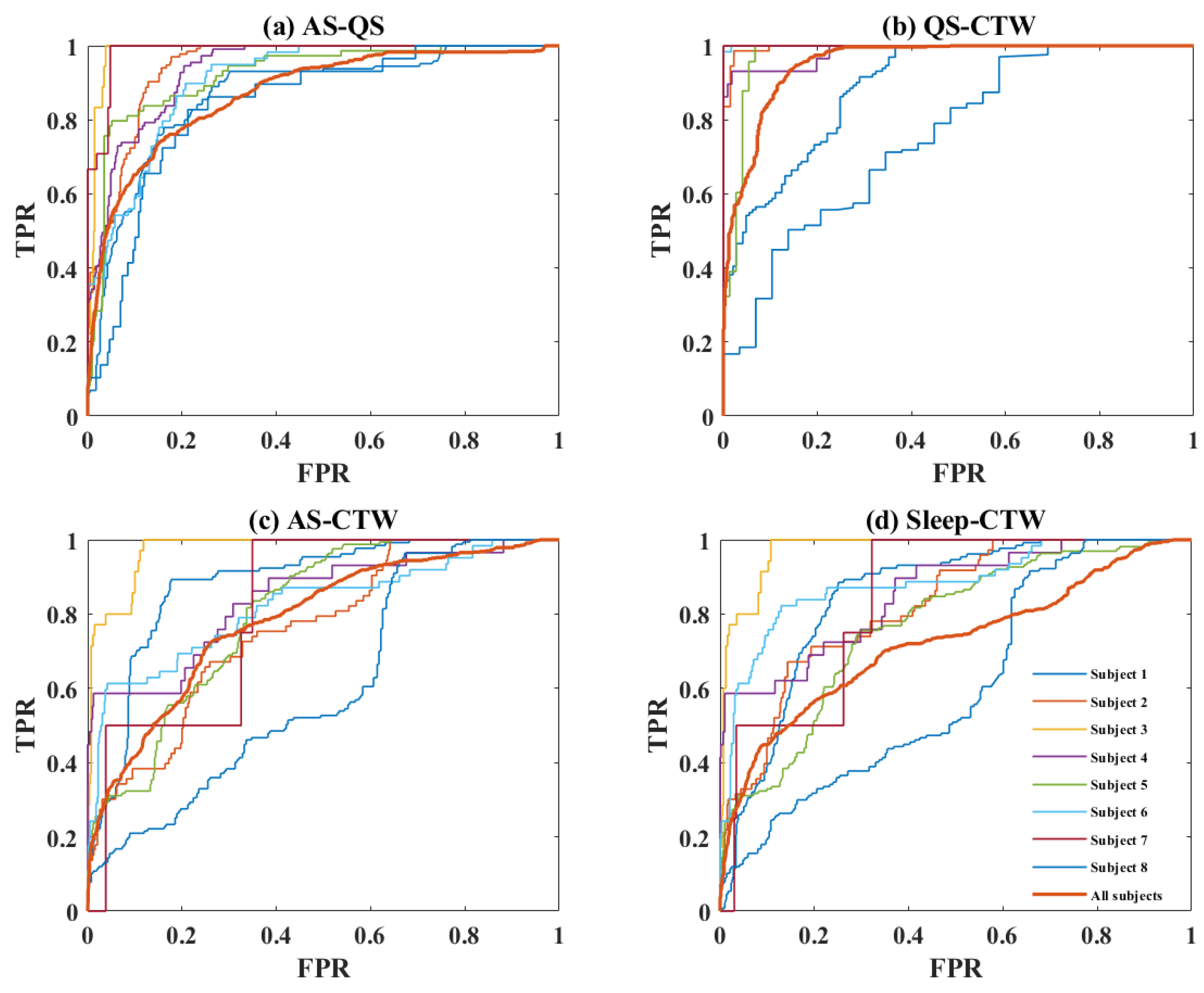
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dereymaeker, A.; Pillay, K.; Vervisch, J.; De Vos, M.; Van Huffel, S.; Jansen, K.; Naulaers, G. Review of Sleep-EEG in Preterm and Term Neonates. Early Hum. Dev. 2017, 113, 87–103. [Google Scholar] [CrossRef]
- Kapsi, S.; Katsantoni, S.; Drigas, A. The Role of Sleep and Impact on Brain and Learning. Int. J. Recent Contrib. Eng. Sci. IT 2020, 8, 59. [Google Scholar] [CrossRef]
- Huang, Q.; Lai, X.; Liao, J.; Tan, Y. Effect of Non-Pharmacological Interventions on Sleep in Preterm Infants in the Neonatal Intensive Care Unit. Medicine 2021, 100, e27587. [Google Scholar] [CrossRef] [PubMed]
- Uchitel, J.; Vanhatalo, S.; Austin, T. Early Development of Sleep and Brain Functional Connectivity in Term-Born and Preterm Infants. Pediatr. Res. 2022, 91, 771–786. [Google Scholar] [CrossRef]
- Shellhaas, R.A.; Burns, J.W.; Hassan, F.; Carlson, M.D.; Barks, J.D.; Chervin, R.D. Neonatal Sleep–Wake Analyses Predict 18-Month Neurodevelopmental Outcomes. Sleep 2017, 40, zsx144. [Google Scholar] [CrossRef]
- Stangenes, K.M.; Fevang, S.K.; Grundt, J.; Donkor, H.M.; Markestad, T.; Hysing, M.; Elgen, I.B.; Bjorvatn, B. Children Born Extremely Preterm Had Different Sleeping Habits at 11 Years of Age and More Childhood Sleep Problems than Term-born Children. Acta Paediatr. 2017, 106, 1966–1972. [Google Scholar] [CrossRef]
- Yiallourou, S.R.; Wallace, E.M.; Whatley, C.; Odoi, A.; Hollis, S.; Weichard, A.J.; Muthusamy, J.S.; Varma, S.; Cameron, J.; Narayan, O.; et al. Sleep: A Window Into Autonomic Control in Children Born Preterm and Growth Restricted. Sleep 2017, 40, zsx048. [Google Scholar] [CrossRef]
- Shepherd, K.L.; Wong, F.Y.; Odoi, A.; Yeomans, E.; Horne, R.S.C.; Yiallourou, S.R. Prone Sleeping Affects Cardiovascular Control in Preterm Infants in NICU. Pediatr. Res. 2021, 90, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Parikka, V.; Lehtonen, L.; Soukka, H. Parent–Infant Skin-to-Skin Contact Reduces the Electrical Activity of the Diaphragm and Stabilizes Respiratory Function in Preterm Infants. Pediatr. Res. 2022, 91, 1163–1167. [Google Scholar] [CrossRef]
- Vandormael, C.; Schoenhals, L.; Hüppi, P.S.; Filippa, M.; Borradori Tolsa, C. Language in Preterm Born Children: Atypical Development and Effects of Early Interventions on Neuroplasticity. Neural Plast. 2019, 2019, 6873270. [Google Scholar] [CrossRef]
- Feld, G.B.; Born, J. Sculpting Memory during Sleep: Concurrent Consolidation and Forgetting. Curr. Opin. Neurobiol. 2017, 44, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, K.L.; Yiallourou, S.R.; Odoi, A.; Yeomans, E.; Willis, S.; Horne, R.S.C.; Wong, F.Y. When Does Prone Sleeping Improve Cardiorespiratory Status in Preterm Infants in the NICU? Sleep 2020, 43, zsz256. [Google Scholar] [CrossRef] [PubMed]
- Burtchen, N.; Myers, M.M.; Lucchini, M.; Ordonez Retamar, M.; Rodriguez, D.; Fifer, W.P. Autonomic Signatures of Late Preterm, Early Term, and Full Term Neonates during Early Postnatal Life. Early Hum. Dev. 2019, 137, 104817. [Google Scholar] [CrossRef]
- Myers, M.M.; Elliott, A.J.; Odendaal, H.J.; Burd, L.; Angal, J.; Groenewald, C.; Nugent, J.D.; Yang, J.S.; Isler, J.R.; Dukes, K.A.; et al. Cardiorespiratory Physiology in the Safe Passage Study: Protocol, Methods and Normative Values in Unexposed Infants. Acta Paediatr. 2017, 106, 1260–1272. [Google Scholar] [CrossRef]
- Hoffman, S.B.; Govindan, R.B.; Johnston, E.K.; Williams, J.; Schlatterer, S.D.; du Plessis, A.J. Autonomic Markers of Extubation Readiness in Premature Infants. Pediatr. Res. 2023, 93, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Otte, R.A.; Long, X.; Westerink, J. A Behavioral Approach to Annotating Sleep in Infants: Building on the Classic Framework. Physiol. Rep. 2022, 10, e15178. [Google Scholar] [CrossRef]
- Gulia, K.K.; Aswathy, B.S.; Kumar, V.M. Developmental Aspects of Sleep. In Pediatric Sleep Medicine; Springer International Publishing: Cham, Switzerland, 2021; pp. 115–122. [Google Scholar] [CrossRef]
- Georgoulas, A.; Jones, L.; Laudiano-Dray, M.P.; Meek, J.; Fabrizi, L.; Whitehead, K. Sleep–Wake Regulation in Preterm and Term Infants. Sleep 2021, 44, zsaa148. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, H.; Yeo, W.H. Recent Advances in Wearable Sensors and Portable Electronics for Sleep Monitoring. iScience 2021, 24, 102461. [Google Scholar] [CrossRef]
- Werth, J.; Atallah, L.; Andriessen, P.; Long, X.; Zwartkruis-Pelgrim, E.; Aarts, R.M. Unobtrusive Sleep State Measurements in Preterm Infants—A Review. Sleep Med. Rev. 2017, 32, 109–122. [Google Scholar] [CrossRef]
- Kim, J.; Gueye-Ndiaye, S.; Mauer, E.; Modi, V.K.; Perlman, J.; Veler, H. Polysomnography Use in Complex Term and Preterm Infants to Facilitate Evaluation and Management in the Neonatal Intensive Care Unit. J. Clin. Sleep Med. 2021, 17, 1653–1663. [Google Scholar] [CrossRef]
- Barbeau, D.Y.; Weiss, M.D. Sleep Disturbances in Newborns. Children 2017, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Sunshine, J.E.; Gollakota, S. Contactless Infant Monitoring Using White Noise. In Proceedings of the 25th Annual International Conference on Mobile Computing and Networking, Los Cabos, Mexico, 21–25 October 2019; ACM: New York, NY, USA, 2019; pp. 1–16. [Google Scholar] [CrossRef]
- Beltrão, G.; Stutz, R.; Hornberger, F.; Martins, W.A.; Tatarinov, D.; Alaee-Kerahroodi, M.; Lindner, U.; Stock, L.; Kaiser, E.; Goedicke-Fritz, S.; et al. Contactless Radar-Based Breathing Monitoring of Premature Infants in the Neonatal Intensive Care Unit. Sci. Rep. 2022, 12, 5150. [Google Scholar] [CrossRef] [PubMed]
- De Groot, E.R.; Knoop, M.S.; van den Hoogen, A.; Wang, X.; Long, X.; Pillen, S.; Benders, M.; Dudink, J. The Value of Cardiorespiratory Parameters for Sleep State Classification in Preterm Infants: A Systematic Review. Sleep Med. Rev. 2021, 58, 101462. [Google Scholar] [CrossRef]
- Sentner, T.; Wang, X.; de Groot, E.R.; van Schaijk, L.; Tataranno, M.L.; Vijlbrief, D.C.; Benders, M.J.N.L.; Bartels, R.; Dudink, J. The Sleep Well Baby Project: An Automated Real-Time Sleep–Wake State Prediction Algorithm in Preterm Infants. Sleep 2022, 45, zsac143. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.K.; Shetty, M.; Siriwardhana, L.S.; Wong, F.Y.; Walter, L.M.; Horne, R.S.C. Autonomic Cardiovascular Control Is Altered by Intermittent Hypoxia in Preterm Infants. Acta Paediatr. 2023, 112, 2359–2367. [Google Scholar] [CrossRef]
- Holditch-Davis, D.; Scher, M.; Schwartz, T.; Hudson–Barr, D. Sleeping and Waking State Development in Preterm Infants. Early Hum. Dev. 2004, 80, 43–64. [Google Scholar] [CrossRef]
- Joseph, V.; Bairam, A.; Carroll, J.L. Control of Breathing During Sleep and Wakefulness in the Fetus, Newborn, and Child. In Pediatric Sleep Medicine; Springer International Publishing: Cham, Switzerland, 2021; pp. 19–31. [Google Scholar] [CrossRef]
- Hathorn, M.K.S. The Rate and Depth of Breathing in New-Born Infants in Different Sleep States. J. Physiol. 1974, 243, 101–113. [Google Scholar] [CrossRef]
- Zhang, D.; Long, X.; Xu, L.; Werth, J.; Wijshoff, R.; Aarts, R.M.; Andriessen, P. Characterizing Cardiorespiratory Interaction in Preterm Infants across Sleep States Using Visibility Graph Analysis. J. Appl. Physiol. 2021, 130, 1015–1024. [Google Scholar] [CrossRef]
- Dijk, D.J.; Landolt, H.P. Sleep Physiology, Circadian Rhythms, Waking Performance and the Development of Sleep-Wake Therapeutics. In Sleep-Wake Neurobiology and Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 441–481. [Google Scholar] [CrossRef]
- Selvaraju, V.; Spicher, N.; Wang, J.; Ganapathy, N.; Warnecke, J.M.; Leonhardt, S.; Swaminathan, R.; Deserno, T.M. Continuous Monitoring of Vital Signs Using Cameras: A Systematic Review. Sensors 2022, 22, 4097. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Choi, S.H. Smart Technologies toward Sleep Monitoring at Home. Biomed. Eng. Lett. 2019, 9, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, K.; Müller-Putz, G.R.; Urlesberger, B.; Müller, W.; Pfurtscheller, G. Relationship between Slow-Wave EEG Bursts and Heart Rate Changes in Preterm Infants. Neurosci. Lett. 2005, 385, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Espina, J.; Otte, R.A.; Wang, W.; Aarts, R.M.; Andriessen, P. Video-based Actigraphy Is an Effective Contact-free Method of Assessing Sleep in Preterm Infants. Acta Paediatr. 2021, 110, 1815–1816. [Google Scholar] [CrossRef] [PubMed]
- Werth, J.; Serteyn, A.; Andriessen, P.; Aarts, R.M.; Long, X. Automated Preterm Infant Sleep Staging Using Capacitive Electrocardiography. Physiol. Meas. 2019, 40, 055003. [Google Scholar] [CrossRef] [PubMed]
- Boe, A.J.; McGee Koch, L.L.; O’Brien, M.K.; Shawen, N.; Rogers, J.A.; Lieber, R.L.; Reid, K.J.; Zee, P.C.; Jayaraman, A. Automating Sleep Stage Classification Using Wireless, Wearable Sensors. NPJ Digit. Med. 2019, 2, 131. [Google Scholar] [CrossRef]
- Redmond, S.J.; de Chazal, P.; O’Brien, C.; Ryan, S.; McNicholas, W.T.; Heneghan, C. Sleep Staging Using Cardiorespiratory Signals. Somnologie-Schlafforschung Schlafmed. 2007, 11, 245–256. [Google Scholar] [CrossRef]
- Long, X.; Foussier, J.; Fonseca, P.; Haakma, R.; Aarts, R.M. Analyzing Respiratory Effort Amplitude for Automated Sleep Stage Classification. Biomed. Signal Process. Control 2014, 14, 197–205. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological Time-Series Analysis Using Approximate Entropy and Sample Entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Chen, H.Y.; Chen, J.R. Unobtrusive Sleep Monitoring Using Movement Activity by Video Analysis. Electronics 2019, 8, 812. [Google Scholar] [CrossRef]
- Fernández, A.; García, S.; Galar, M.; Prati, R.C.; Krawczyk, B.; Herrera, F. Learning from Imbalanced Data Sets; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Geurts, P.; Ernst, D.; Wehenkel, L. Extremely Randomized Trees. Mach. Learn. 2006, 63, 3–42. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Müller, A.; Nothman, J.; Louppe, G.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Koul, A.; Becchio, C.; Cavallo, A. Cross-Validation Approaches for Replicability in Psychology. Front. Psychol. 2018, 9, 1117. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.J.; Subramaniam, P.; Davis, P.G. Continuous Distending Pressure for Respiratory Distress in Preterm Infants. Cochrane Database Syst. Rev. 2015, 7, CD002271. [Google Scholar] [CrossRef]
- Long, X.; Fonseca, P.; Foussier, J.; Haakma, R.; Aarts, R.M. Sleep and Wake Classification With Actigraphy and Respiratory Effort Using Dynamic Warping. IEEE J. Biomed. Health Inform. 2014, 18, 1272–1284. [Google Scholar] [CrossRef]
- Karlen, W.; Mattiussi, C.; Floreano, D. Improving Actigraph Sleep/Wake Classification with Cardio-Respiratory Signals. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 5262–5265. [Google Scholar] [CrossRef]
- Ülgen, Ö.; Barış, H.E.; Aşkan, Ö.Ö.; Akdere, S.K.; Ilgın, C.; Özdemir, H.; Bekiroğlu, N.; Gücüyener, K.; Özek, E.; Boran, P. Sleep Assessment in Preterm Infants: Use of Actigraphy and AEEG. Sleep Med. 2023, 101, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.; Kalil Neto, F.; Radaelli, G.; Nunes, M.L. Effects of Non-Invasive Respiratory Support on Sleep in Preterm Infants Evaluated by Actigraphy. Sleep Sci. 2021, 14, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Hyde, M.; O’driscoll, D.M.; Binette, S.; Galang, C.; Tan, S.K.; Verginis, N.; Davey, M.J.; Horne, R.S.C. Validation of Actigraphy for Determining Sleep and Wake in Children with Sleep Disordered Breathing. J. Sleep Res. 2007, 16, 213–216. [Google Scholar] [CrossRef]
- Sadeh, A.; Acebo, C.; Seifer, R.; Aytur, S.; Carskadon, M.A. Activity-Based Assessment of Sleep-Wake Patterns during the 1st Year of Life. Infant Behav. Dev. 1995, 18, 329–337. [Google Scholar] [CrossRef]
- Bourel-Ponchel, E.; Hasaerts, D.; Challamel, M.J.; Lamblin, M.D. Behavioral-State Development and Sleep-State Differentiation during Early Ontogenesis. Neurophysiol. Clin. 2021, 51, 89–98. [Google Scholar] [CrossRef]
| Subject | Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean | SD | |
| GA (wk) | 30.4 | 33.8 | 27.4 | 31.7 | 29.3 | 27.0 | 33.3 | 27.4 | 30.0 | 2.7 |
| PMA (wk) | 31.3 | 34.4 | 29.0 | 34.9 | 30.6 | 29.1 | 34.7 | 35.9 | 32.5 | 2.8 |
| Weight (g) | 1606 | 2410 | 1160 | 1845 | 1110 | 755 | 2080 | 2480 | 1680.8 | 634.3 |
| Subject | Kappa Score | ||
|---|---|---|---|
| Motion | ECG-Resp-CRI | ECG-Resp-CRI-Motion | |
| 1 | 0.48 | 0.41 | 0.33 |
| 2 | 0.15 | 0.39 | 0.41 |
| 3 | 0.30 | 0.21 | 0.45 |
| 4 | 0.26 | 0.49 | 0.51 |
| 5 | 0.27 | 0.43 | 0.35 |
| 6 | 0.13 | 0.29 | 0.44 |
| 7 | 0.41 | 0.27 | 0.46 |
| 8 | 0.12 | 0.14 | 0.14 |
| Mean | 0.26 | 0.33 | 0.39 |
| SD | 0.12 | 0.11 | 0.11 |
| Performance Metric | Feature Set | ||
|---|---|---|---|
| Motion | ECG-Resp-CRI | ECG-Resp-CRI-Motion | |
| Accuracy | 0.64 ± 0.12 | 0.70 ± 0.13 | 0.72 ± 0.12 |
| Kappa | 0.26 ± 0.12 | 0.33 ± 0.11 | 0.39 ± 0.11 |
| Sensitivity AS | 0.64 ± 0.18 | 0.77 ± 0.15 | 0.74 ± 0.20 |
| Precision AS | 0.82 ± 0.12 | 0.85 ± 0.10 | 0.87 ± 0.11 |
| Sensitivity QS | 0.46 ± 0.30 | 0.74 ± 0.24 | 0.69 ± 0.30 |
| Precision QS | 0.35 ± 0.28 | 0.51 ± 0.16 | 0.53 ± 0.18 |
| Sensitivity CTW | 0.60 ± 0.32 | 0.29 ± 0.28 | 0.51 ± 0.15 |
| Precision CTW | 0.38 ± 0.21 | 0.51 ± 0.41 | 0.40 ± 0.16 |
| Feature Set | Sleep States | Subject | Statistic | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Mean | SD | ||
| Motion | AS vs. QS | 0.32 | 0.29 | 0.01 | 0.30 | 0.37 | 0.07 | 0.34 | −0.02 | 0.21 | 0.15 |
| QS vs. CTW | 0.63 | 0.60 | 0.53 | 0.67 | 0.47 | 0.75 | 0.63 | 0.21 | 0.56 | 0.15 | |
| AS vs. CTW | 0.63 | 0.15 | 0.49 | 0.36 | 0.23 | 0.59 | −0.03 | 0.13 | 0.32 | 0.22 | |
| Sleep vs. CTW | 0.57 | 0.20 | 0.47 | 0.30 | 0.30 | 0.59 | −0.03 | 0.14 | 0.32 | 0.20 | |
| ECG-Resp-CRI | AS vs. QS | 0.31 | 0.57 | 0.47 | 0.54 | 0.56 | 0.42 | 0.76 | 0.38 | 0.50 | 0.13 |
| QS vs. CTW | 0.80 | 0.62 | 1.00 | 0.91 | 0.89 | 0.30 | 1.00 | 0.04 | 0.69 | 0.33 | |
| AS vs. CTW | 0.00 | 0.00 | 0.00 | 0.27 | 0.36 | 0.18 | 0.01 | 0.00 | 0.10 | 0.04 | |
| Sleep vs. CTW | 0.01 | 0.00 | 0.04 | 0.24 | 0.31 | 0.00 | 0.00 | 0.00 | 0.07 | 0.12 | |
| ECG-Resp-CRI-Motion | AS vs. QS | 0.15 | 0.57 | 0.43 | 0.56 | 0.59 | 0.41 | 0.76 | 0.37 | 0.48 | 0.17 |
| QS vs. CTW | 0.59 | 0.94 | 1.00 | 0.88 | 0.94 | 0.87 | 1.00 | 0.21 | 0.80 | 0.25 | |
| AS vs. CTW | 0.34 | 0.20 | 0.46 | 0.50 | 0.28 | 0.56 | 0.07 | 0.09 | 0.31 | 0.17 | |
| Sleep vs. CTW | 0.29 | 0.39 | 0.50 | 0.52 | 0.34 | 0.55 | 0.06 | 0.10 | 0.34 | 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Peng, Z.; Van Pul, C.; Overeem, S.; Chen, W.; Dudink, J.; Andriessen, P.; Aarts, R.M.; Long, X. Combining Cardiorespiratory Signals and Video-Based Actigraphy for Classifying Preterm Infant Sleep States. Children 2023, 10, 1792. https://doi.org/10.3390/children10111792
Zhang D, Peng Z, Van Pul C, Overeem S, Chen W, Dudink J, Andriessen P, Aarts RM, Long X. Combining Cardiorespiratory Signals and Video-Based Actigraphy for Classifying Preterm Infant Sleep States. Children. 2023; 10(11):1792. https://doi.org/10.3390/children10111792
Chicago/Turabian StyleZhang, Dandan, Zheng Peng, Carola Van Pul, Sebastiaan Overeem, Wei Chen, Jeroen Dudink, Peter Andriessen, Ronald M. Aarts, and Xi Long. 2023. "Combining Cardiorespiratory Signals and Video-Based Actigraphy for Classifying Preterm Infant Sleep States" Children 10, no. 11: 1792. https://doi.org/10.3390/children10111792
APA StyleZhang, D., Peng, Z., Van Pul, C., Overeem, S., Chen, W., Dudink, J., Andriessen, P., Aarts, R. M., & Long, X. (2023). Combining Cardiorespiratory Signals and Video-Based Actigraphy for Classifying Preterm Infant Sleep States. Children, 10(11), 1792. https://doi.org/10.3390/children10111792










