Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer
Abstract
1. Introduction
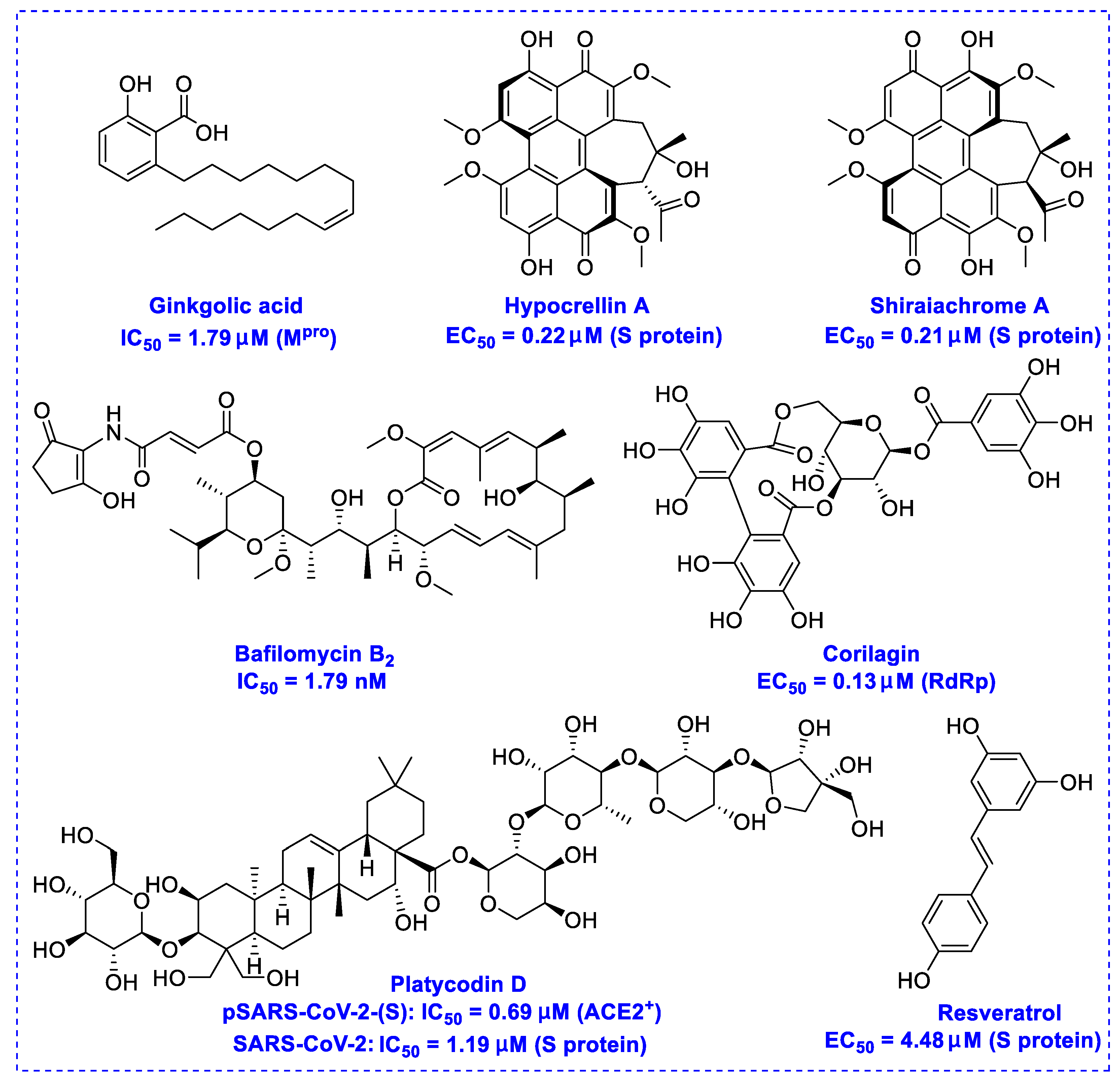
2. Natural Products as Monotherapy for the Treatment of SARS-CoV-2
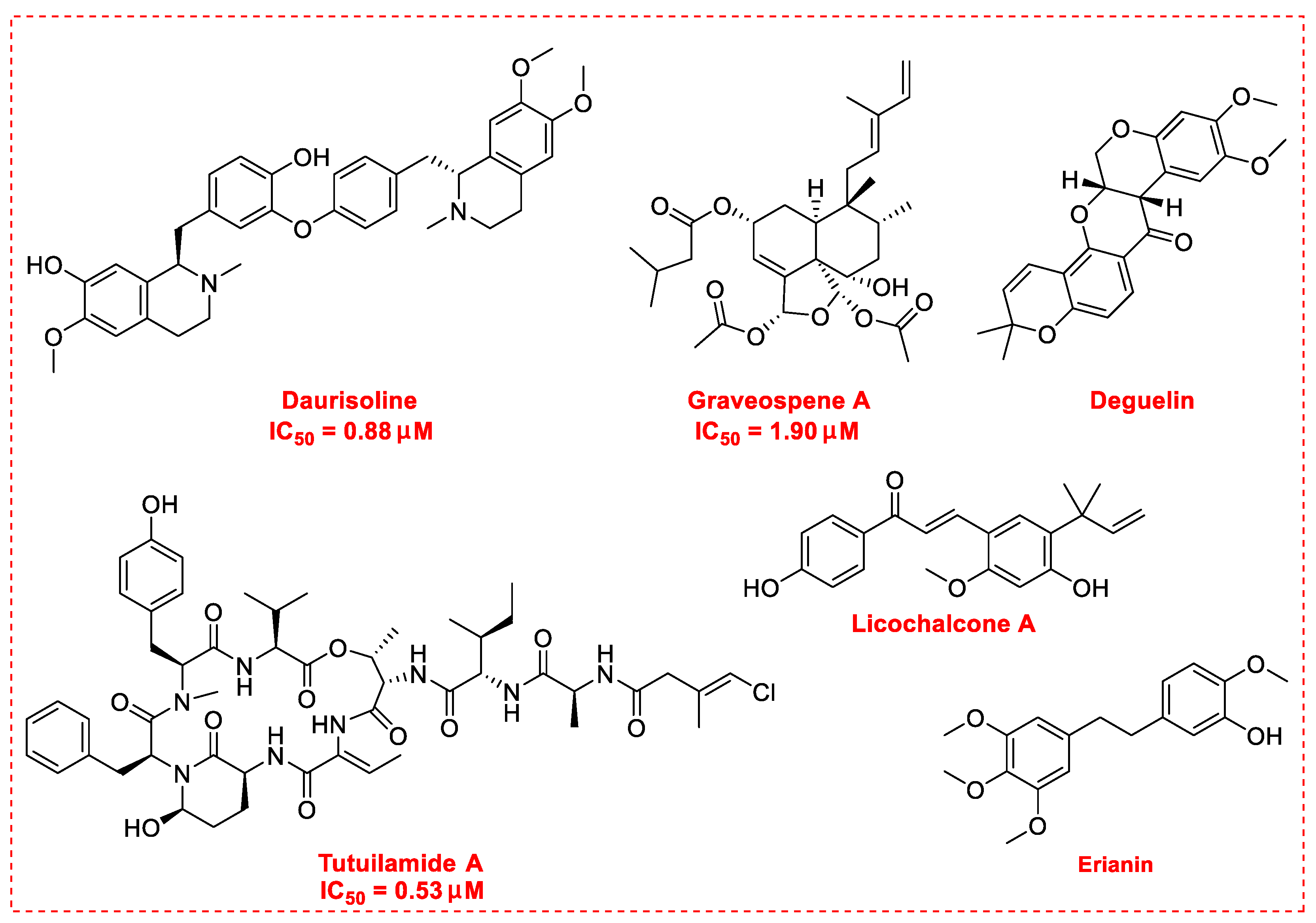
3. Natural Products as Monotherapy for the Treatment of Lung Cancer
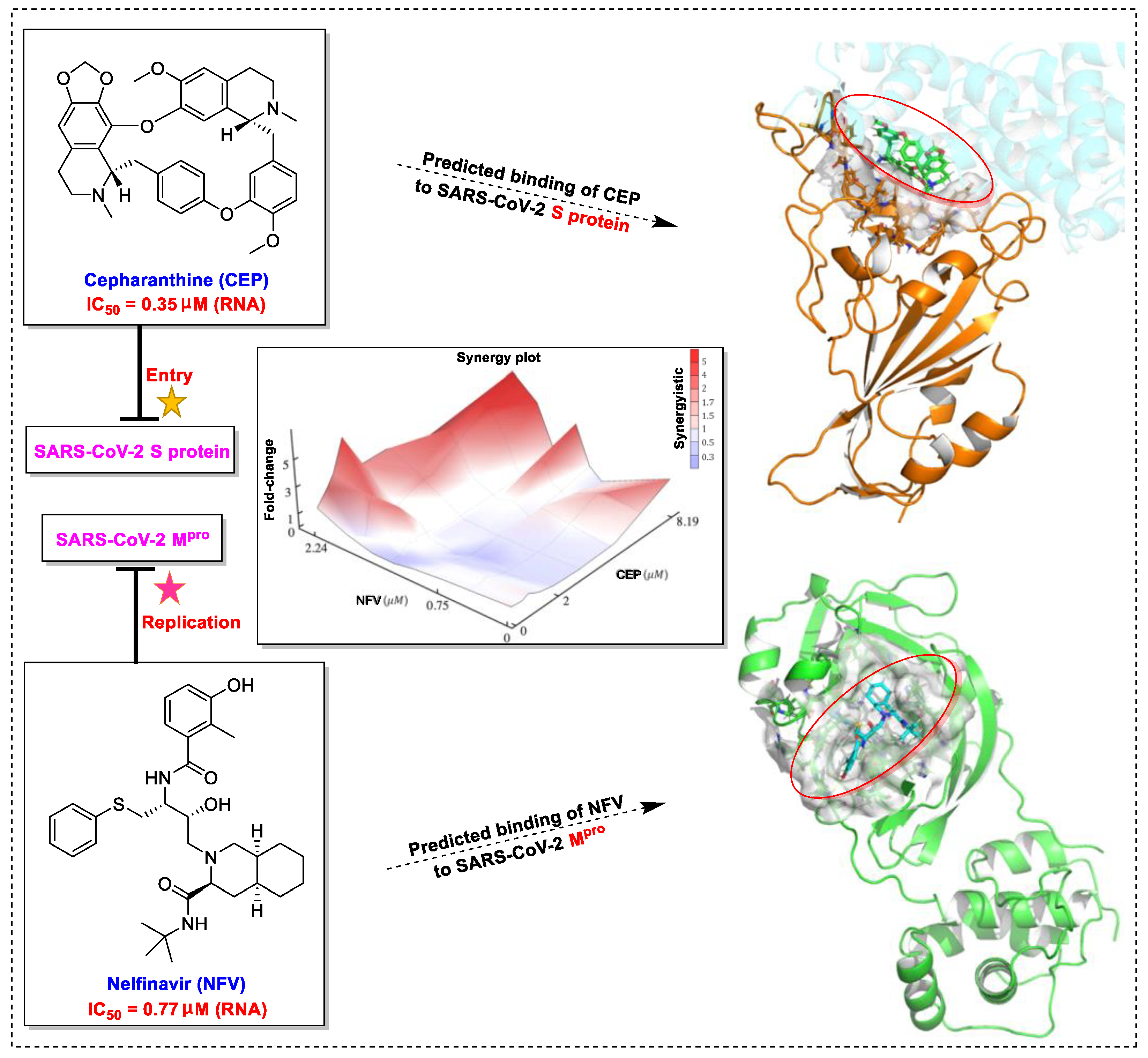
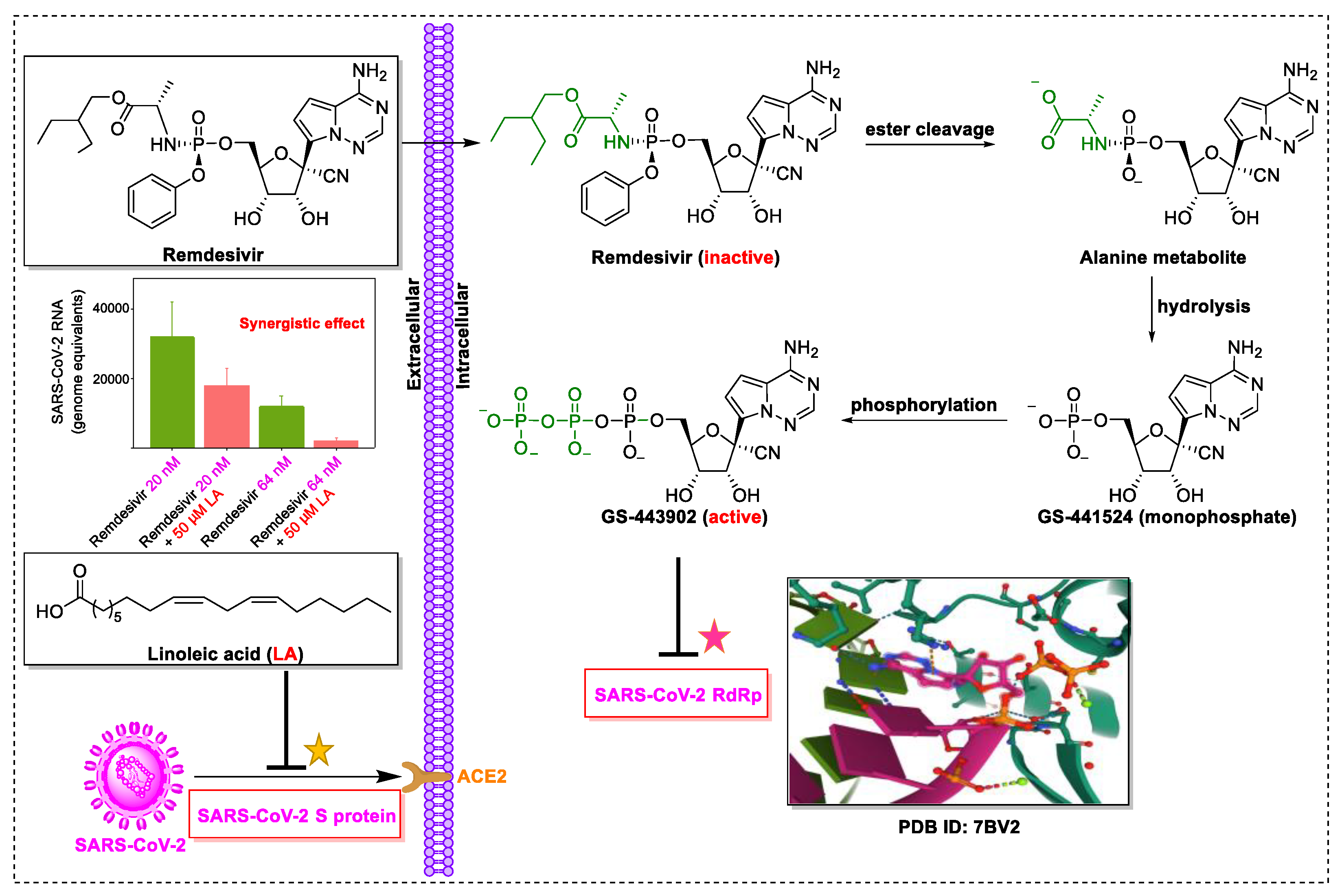
4. Natural Products in Combination with the FDA-Approved Drugs Inhibit SARS-CoV-2
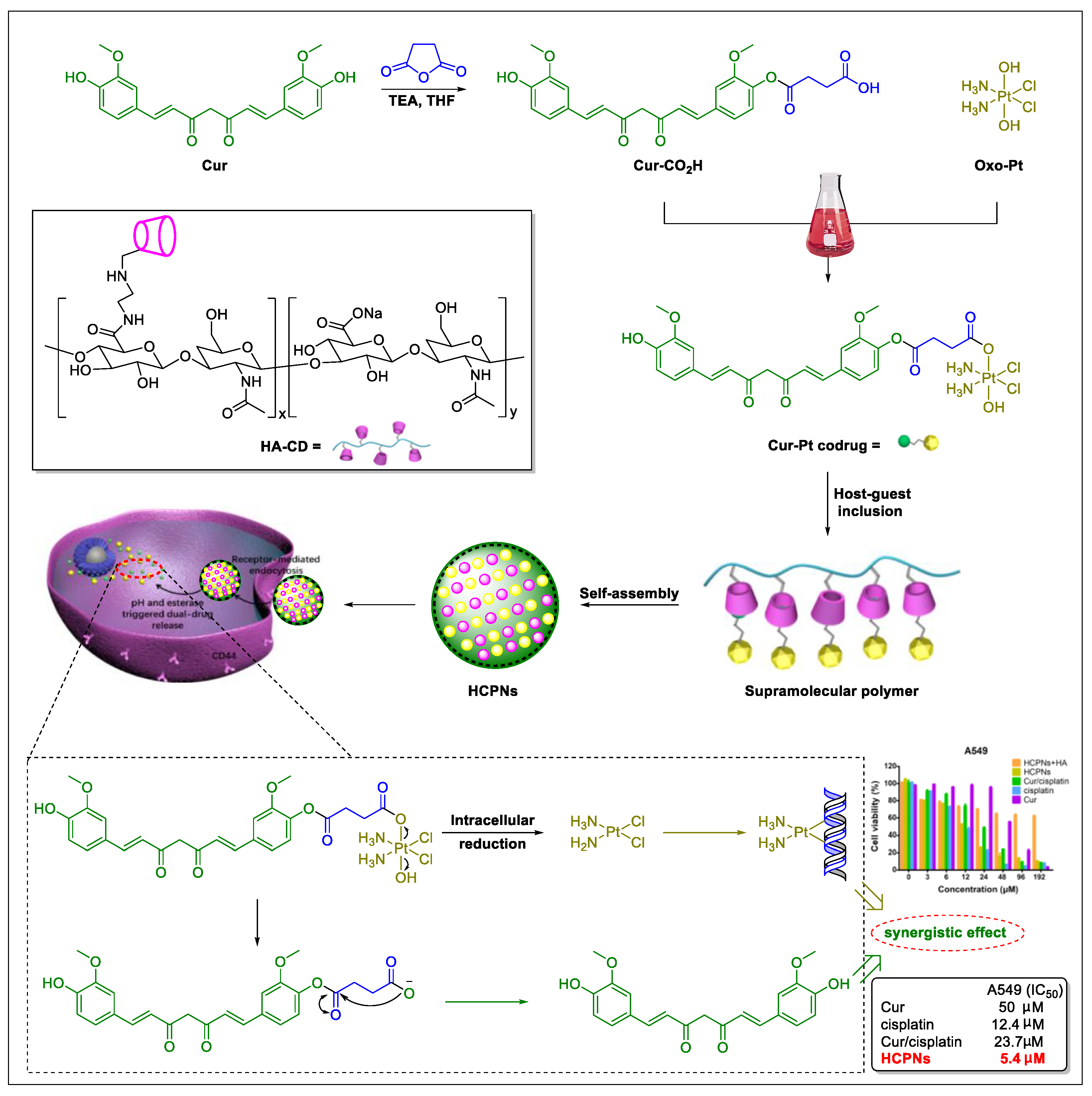
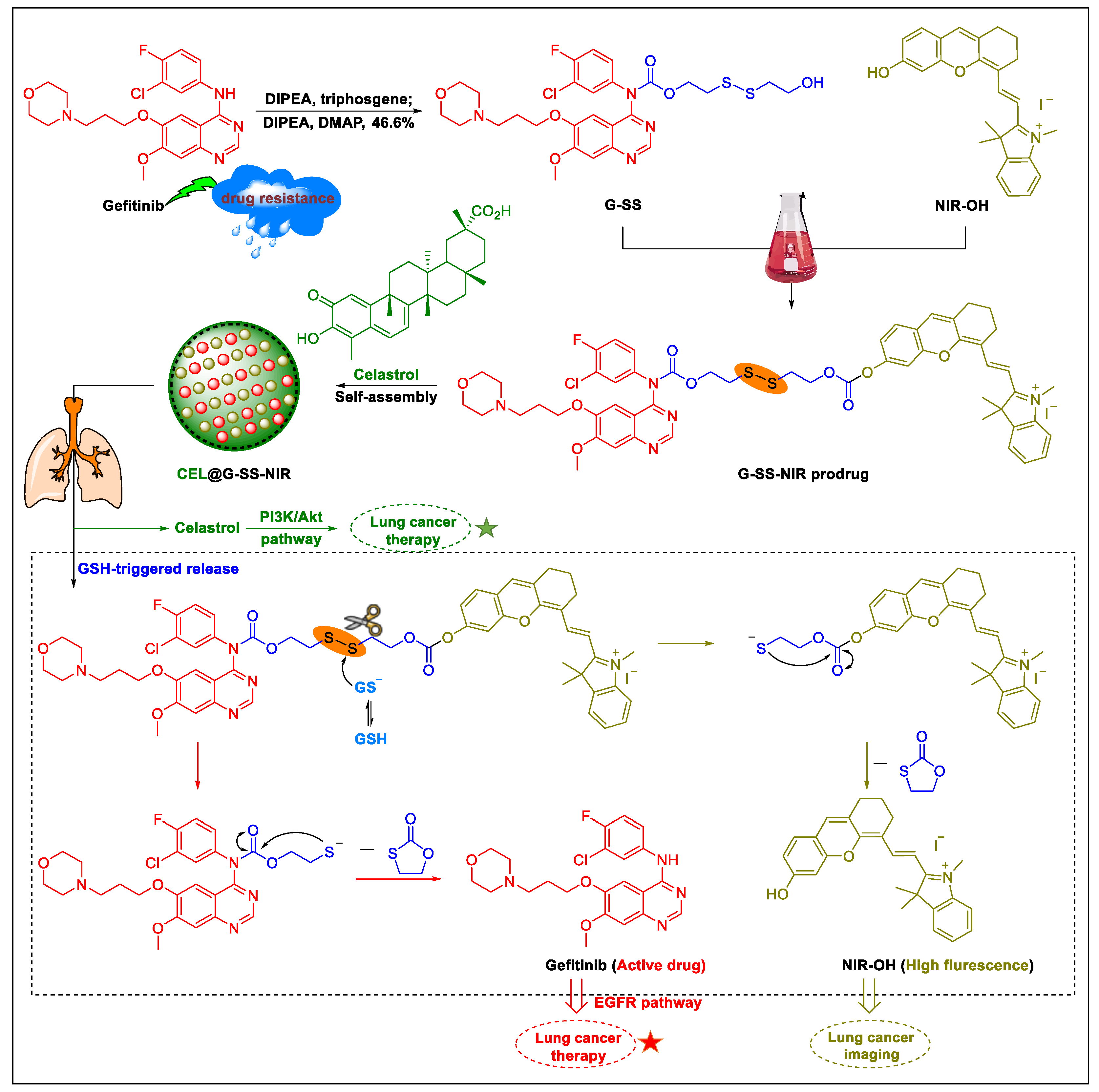
5. Natural Products in Combination with the FDA-Approved Anti-Lung Cancer Drugs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE2 | angiotensin-converting enzyme 2 |
| ACE2+ | overexpression of ACE2 |
| AKT | protein kinase B |
| ATP | adenosine triphosphate |
| β-CD | β-cyclodextrin |
| 3CLpro | 3C-Like protease |
| COVID-19 | coronavirus disease 2019 |
| cryo-EM | cryo-electron microscopy |
| EBOV | Ebola virus |
| EC50 | half-maximal effective concentration |
| EGFR | epidermal growth factor receptor |
| EMT | epithelial-mesenchymal transition |
| ERK | extracellular signal-regulated kinase |
| FDA | US Food and Drug Administration |
| GSH | glutathione |
| HA | hyaluronic acid |
| HCoV-229E | human coronavirus 229E |
| HCPNs | curcumin and cisplatin nanoparticles |
| HIV-1 | human immunodeficiency virus type 1 |
| IC50 | half-maximal inhibitory concentration |
| MAPK | mitogen-activated protein kinase |
| Mcl-1 | myeloid cell leukemia sequence-1 |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| MMPs | matrix-degrading metalloproteinases |
| Mpro | main protease |
| mTOR | mammalian target of rapamycin |
| NSCLC | non-small cell lung cancer |
| PDB | Protein Data Bank |
| PD-L1 | programmed death ligand-1 |
| PI3K | phosphoinositide 3-kinase |
| PLpro | papain-like protease |
| pSARS-CoV-2 | SARS-CoV-2 pseudovirus |
| QFPD | Qingfei Paidu decoction |
| RdRp | RNA-dependent RNA polymerase |
| ROS | reactive oxygen species |
| S protein | spike protein |
| SARS-CoV | severe acute respiratory syndrome coronavirus |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SHL | Shuanghuanglian oral liquid or injection |
| SI | selectivity index |
| TMPRSS2 | transmembrane protease serine 2 |
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Porras, G.; Chassagne, F.; Lyles, J.T.; Marquez, L.; Dettweiler, M.; Salam, A.M.; Samarakoon, T.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem. Rev. 2021, 121, 3495–3560. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Yao, Y.; Yin, W.; Ye, T. The role of natural products in the prevention and treatment of pulmonary fibrosis: A review. Food Funct. 2021, 12, 990–1007. [Google Scholar] [CrossRef]
- Wang, W.; Yao, Q.; Teng, F.; Cui, J.; Dong, J.; Wei, Y. Active ingredients from Chinese medicine plants as therapeutic strategies for asthma: Overview and challenges. Biomed. Pharmacother. 2021, 137, 111383. [Google Scholar] [CrossRef]
- He, Y.Q.; Zhou, C.C.; Yu, L.Y.; Wang, L.; Deng, J.L.; Tao, Y.L.; Zhang, F.; Chen, W.S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2021, 163, 105224. [Google Scholar] [CrossRef]
- Santoro, A.; Tomino, C.; Prinzi, G.; Cardaci, V.; Fini, M.; Macera, L.; Russo, P.; Maggi, F. Microbiome in chronic obstructive pulmonary disease: Role of natural products against microbial pathogens. Curr. Med. Chem. 2020, 27, 2931–2948. [Google Scholar] [CrossRef] [PubMed]
- Belchamber, K.B.R.; Donnelly, L.E. Targeting defective pulmonary innate immunity-A new therapeutic option? Pharmacol. Therapeut. 2020, 209, 107500. [Google Scholar] [CrossRef]
- Christy, M.P.; Uekusa, Y.; Gerwick, L.; Gerwick, W.H. Natural products with potential to treat RNA virus pathogens including SARS-CoV-2. J. Nat. Prod. 2021, 84, 161–182. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Song, L.; Hua, S. Perspectives and controversies regarding the use of natural products for the treatment of lung cancer. Cancer Med. 2021, 10, 2396–2422. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nature Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar]
- Eurosurveillance Editorial Team. Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Euro. Surveil. 2020, 25, 200131e. [Google Scholar]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.F.; Liu, J.L.; Yip, C.C.; Poon, R.W.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Phan, L.T.; Nguyen, T.V.; Luong, Q.C.; Nguyen, T.V.; Nguyen, H.T.; Le, H.Q.; Nguyen, T.T.; Cao, T.M.; Pham, Q.D. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N. Engl. J. Med. 2020, 382, 872–874. [Google Scholar] [CrossRef]
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, diagnostics and drug development. J. Hepatol. 2021, 74, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Singh, S.S.; Mattheolabakis, G.; Gu, X.; Withers, S.; Dahal, A.; Jois, S. A grafted peptidomimetic for EGFR heterodimerization inhibition: Implications in NSCLC models. Eur. J. Med. Chem. 2021, 216, 113312. [Google Scholar] [CrossRef]
- Dawkins, J.B.N.; Webster, R.M. The small-cell lung cancer drug market. Nat. Rev. Drug Discov. 2020, 19, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Twilley, D.; Esmear, T.; Oosthuizen, C.B.; Reid, A.M.; Nel, M.; Lall, N. Anti-SARS-CoV natural products with the potential to inhibit SARS-CoV-2 (COVID-19). Front. Pharmacol. 2020, 11, 561334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L. Turning the tide: Natural products and natural-product-inspired chemicals as potential counters to SARS-CoV-2 infection. Front. Pharmacol. 2020, 11, 1013. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Li, Y.S.; Zeng, R.; Liu, F.L.; Luo, R.H.; Huang, C.; Wang, Y.F.; Zhang, J.; Quan, B.; Shen, C.; et al. SARS-CoV-2 Mpro inhibitors with antiviral activity in a transgenic mouse model. Science 2021, 371, 1374–1378. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Feng, Z.; Liu, W.; Wang, Y.; Wang, G.; Yu, W.; Yang, G.; Yang, T.; Wang, Y.; Li, M. Exogenous hormone on episperm development and ginkgolic acid accumulation in Ginkgo biloba L. Ind. Crop. Prod. 2021, 160, 113140. [Google Scholar] [CrossRef]
- Chen, Z.; Cui, Q.; Cooper, L.; Zhang, P.; Lee, H.; Chen, Z.; Wang, Y.; Liu, X.; Rong, L.; Du, R. Ginkgolic acid and anacardic acid are specific covalent inhibitors of SARS-CoV-2 cysteine proteases. Cell Biosci. 2021, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Medina-Enríquez, M.M.; Lopez-León, S.; Carlos-Escalante, J.A.; Aponte-Torres, Z.; Cuapio, A.; Wegman-Ostrosky, T. ACE2: The molecular doorway to SARS-CoV-2. Cell Biosci. 2020, 10, 148. [Google Scholar] [CrossRef]
- Benton, D.J.; Wrobel, A.G.; Xu, P.; Roustan, C.; Martin, S.R.; Rosenthal, P.B.; Skehel, J.J.; Gamblin, S.J. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature 2020, 588, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Yang, C.; Wu, Y.; Lv, J.J.; Feng, X.; Tian, X.; Zhou, Z.; Pan, X.; Liu, S.; Tian, L.W. Axial chiral binaphthoquinone and perylenequinones from the stromata of hypocrella bambusae are SARS-CoV-2 entry inhibitors. J. Nat. Prod. 2021, 84, 436–443. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Mucci, L.A.; Antonarakis, E.S.; Nelson, P.S.; Kantoff, P.W. TMPRSS2 and COVID-19: Serendipity or opportunity for intervention? Cancer Discov. 2020, 10, 779–782. [Google Scholar] [CrossRef]
- Kim, T.Y.; Jeon, S.; Jang, Y.; Gotina, L.; Won, J.; Ju, Y.H.; Kim, S.; Jang, M.W.; Won, W.; Park, M.G.; et al. Platycodin D prevents both lysosome- and TMPRSS2-driven SARS-CoV-2 infection in vitro by hindering membrane fusion. Exp. Mol. Med. 2021. [Google Scholar] [CrossRef]
- Yang, M.; Wei, J.; Huang, T.; Lei, L.; Shen, C.; Lai, J.; Yang, M.; Liu, L.; Yang, Y.; Liu, G.; et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phytother. Res. 2021, 35, 1127–1129. [Google Scholar] [CrossRef]
- Ter Ellen, B.M.; Dinesh Kumar, N.; Bouma, E.M.; Troost, B.; van de Pol, D.P.I.; van der Ende-Metselaar, H.H.; Apperloo, L.; van Gosliga, D.; van den Berge, M.; Nawijn, M.C.; et al. Resveratrol and pterostilbene potently inhibit SARS-CoV-2 replication in vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, H.; Choi, H.Y.; Ma, Y.; Zhou, T.; Peng, Y.; Pang, K.; Shu, G.; Yang, X. Anti-esophageal cancer effect of corilagin extracted from Phmllanthi fructus via the mitochondrial and endoplasmic reticulum stress pathways. J. Ethnopharmacol. 2021, 269, 113700. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yi, D.; Lei, X.; Zhao, J.; Zhang, Y.; Cui, X.; Xiao, X.; Jiao, T.; Dong, X.; Zhao, X.; et al. Corilagin inhibits SARS-CoV-2 replication by targeting viral RNA-dependent RNA polymerase. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Loschwitz, J.; Jäckering, A.; Keutmann, M.; Olagunju, M.; Eberle, R.J.; Coronado, M.A.; Olubiyi, O.O.; Strodel, B. Novel inhibitors of the main protease enzyme of SARS-CoV-2 identified via molecular dynamics simulation-guided in vitro assay. Bioorg. Chem. 2021, 111, 104862. [Google Scholar] [CrossRef]
- Xie, X.; Lu, S.; Pan, X.; Zou, M.; Li, F.; Lin, H.; Hu, J.; Fan, S.; He, J. Antiviral bafilomycins from a feces-inhabiting Streptomyces sp. J. Nat. Prod. 2021, 84, 537–543. [Google Scholar] [CrossRef]
- Abdallah, H.; El-Halawany, A.; Sirwi, A.; El-Araby, A.; Mohamed, G.; Ibrahim, S.; Koshak, A.; Asfour, H.; Awan, Z.; Elfaky, M.A. Repurposing of Some Natural Product Isolates as SARS-COV-2 Main Protease Inhibitors via In Vitro Cell Free and Cell-Based Antiviral Assessments and Molecular Modeling Approaches. Pharmaceuticals 2021, 14, 213. [Google Scholar] [CrossRef] [PubMed]
- Sa-ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J.; et al. Anti-SARS-CoV-2 Aactivity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, M.; Qin, H.; Lin, H.; An, X.; Shi, Z.; Song, L.; Yang, X.; Fan, H.; Tong, Y. Artemether, artesunate, arteannuin B, echinatin, licochalcone B and andrographolide effectively inhibit SARS-CoV-2 and related viruses in vitro. Front. Cell. Infect. Microbiol. 2021. [Google Scholar] [CrossRef]
- Cao, R.; Hu, H.; Li, Y.; Wang, X.; Xu, M.; Liu, J.; Zhang, H.; Yan, Y.; Zhao, L.; Li, W.; et al. Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect. Dis. 2020, 6, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; Gilles, M.; Barral, K.; Nougairède, A.; Decroly, E.; de Lamballerie, X.; Coutard, B. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. Sci. Rep. 2020, 10, 13093. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Seo, S.H.; Woo, S.J.; Kwon, Y.; Song, M.; Ha, N.C. Epigallocatechin gallate inhibits the uridylate-specific endoribonuclease Nsp15 and efficiently neutralizes the SARS-CoV-2 strain. J. Agric. Food Chem. 2021. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Park, J.G.; Cho, K.H.; Choi, P.; Kim, T.; Ham, J.; Lee, J. Assessment of antiviral potencies of cannabinoids against SARSCoV-2 using computational and in vitro approaches. Int. J. Biol. Macromol. 2021, 168, 474–485. [Google Scholar] [CrossRef]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.; Chen, S.; Friesen, J.B.; Drayman, N.; Mohamed, A.; Dann, C.; Silva, D.; et al. Cannabidiol inhibits SARS-CoV-2 replication and promotes the host innate immune response. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, D.Y. Docking characterization and in vitro inhibitory activity of flavan-3-ols and dimeric proanthocyanidins against the main protease activity of SARS-CoV-2. Front. Plant Sci. 2020, 11, 601316. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Cooper, L.; Chen, Z.; Lee, H.; Rong, L.; Cui, Q. Discovery of chebulagic acid and punicalagin as novel allosteric inhibitors of SARS-CoV-2 3CLpro. Antivir. Res. 2021, 190, 105075. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Luo, R.; Zhang, M.; Wang, Y.; Song, T.; Tao, T.; Li, Z.; Jin, L.; Zheng, H.; Chen, W.; et al. A cross-talk between epithelium and endothelium mediates human alveolar-capillary injury during SARS-CoV-2 infection. Cell Death Dis. 2020, 11, 1042. [Google Scholar] [CrossRef]
- Du, A.; Zheng, R.; Disoma, C.; Li, S.; Chen, Z.; Li, S.; Liu, P.; Zhou, Y.; Shen, Y.; Liu, S.; et al. Epigallocatechin-3-gallate, an active ingredient of Traditional Chinese Medicines, inhibits the 3CLpro activity of SARS-CoV-2. Int. J. Biol. Macromol. 2021, 176, 1–12. [Google Scholar] [CrossRef]
- Choy, K.T.; Wong, A.Y.L.; Kaewpreedee, P.; Sia, S.F.; Chen, D.D.; Hui, K.P.Y.; Chu, D.K.W.; Chan, M.C.W.; Cheung, P.P.H.; Huang, X.; et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir. Res. 2020, 178, 104786. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Afsar, M.; Khandelwal, N.; Chander, Y.; Riyesh, T.; Dedar, R.K.; Gulati, B.R.; Pal, Y.; Barua, S.; Tripathi, B.N.; et al. Emetine suppresses SARS-CoV-2 replication by inhibiting interaction of viral mRNA with eIF4E. Antivir. Res. 2021, 189, 105056. [Google Scholar] [CrossRef]
- Ashhurst, A.; Tang, A.; Fajtova, P.; Yoon, M.; Aggarwal, A.; Stoye, A.; Larance, M.; Beretta, L.; Drelich, A.; Skinner, D.; et al. Potent in vitro anti-SARS-CoV-2 activity by gallinamide A and analogues via inhibition of cathepsin L. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tietjen, I.; Cassel, J.; Register, E.T.; Zhou, X.Y.; Messick, T.E.; Keeney, F.; Lu, L.D.; Beattie, K.D.; Rali, T.; Ertl, H.C.J.; et al. The natural stilbenoid (-)-hopeaphenol inhibits cellular entry of SARS-CoV-2 USA-WA1/2020, B.1.1.7 and B.1.351 variants. bioRxiv 2021. [Google Scholar] [CrossRef]
- O’Keefe, S.; Roboti, P.; Duah, K.B.; Zong, G.; Schneider, H.; Shi, W.Q.; High, S. Ipomoeassin-F inhibits the in vitro biogenesis of the SARS-CoV-2 spike protein and its host cell membrane receptor. J. Cell Sci. 2021, 134, jcs257758. [Google Scholar] [CrossRef] [PubMed]
- Gangadevi, S.; Badavath, V.N.; Thakur, A.; Yin, N.; Jonghe, S.D.; Acevedo, O.; Jochmans, D.; Leyssen, P.; Wang, K.; Neyts, J.; et al. Kobophenol A inhibits binding of host ACE2 receptor with Spike RBD domain of SARS-CoV-2, a lead compound for blocking COVID-19. J. Phys. Chem. Lett. 2021, 12, 1793–1802. [Google Scholar] [CrossRef]
- Kuzikov, M.; Costanzi, E.; Reinshagen, J.; Esposito, F.; Vangeel, L.; Wolf, M.; Ellinger, B.; Claussen, C.; Geisslinger, G.; Corona, A.; et al. Identification of inhibitors of SARS-CoV-2 3CL-Pro enzymatic activity using a small molecule in-vitro repurposing screen. ACS Pharmacol. Transl. Sci. 2021. [Google Scholar] [CrossRef]
- Xiao, T.; Cui, M.; Zheng, C.; Wang, M.; Sun, R.; Gao, D.; Bao, J.; Ren, S.; Yang, B.; Lin, J.; et al. Myricetin inhibit SARS-CoV-2 viral replication by targeting Mpro and ameliorates pulmonary inflammation. Front. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Clementi, N.; Scagnolari, C.; D’Amore, A.; Palombi, F.; Criscuolo, E.; Frasca, F.; Pierangeli, A.; Mancini, N.; Antonelli, G.; Clementi, M.; et al. Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol. Res. 2021, 163, 105255. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Ko, M.; Lee, J.; Choi, I.; Byun, S.Y.; Park, S.; Shum, D.; Kim, S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob. Agents Chemother. 2020, 64, e00819-20. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Jang, M.; Park, Y.I.; Cha, Y.E.; Park, R.; Namkoong, S.; Lee, J.I.; Park, J. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid. Based Compl. Alt. Med. 2020, 2020, 5630838. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.; Zhao, X.; Xu, D.; Zanin, M.; Chen, W.; Yang, Z.; Chen, J. Chinese Therapeutic Strategy for Fighting COVID-19 and Potential Small-Molecule Inhibitors against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J. Med. Chem. 2020, 63, 13205–13227. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 2021, 270, 113869. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, P.; Zhang, Z.; Youn, J.Y.; Zhang, H.; Cai, H.L. Traditional Chinese Medicine (TCM) in the treatment of viral infections: Efficacies and mechanisms. Pharm. Ther. 2021, 225, 107843. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of the People’s Republic of China. Notice on the Issunance of Guidelines of Diagnosis and Treatment for 2019-nCoV Infected Pneumonia (Version 7). 2020. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf (accessed on 3 March 2020).
- Xinhua Net. Academician Xiaolin Tong: The Total Effective Rate of Qingfeipaidu Formula was 97%, none Transfer from Mild to Severe Cases. 2020. Available online: http://www.kunlunce.com/ssjj/fl1/2020-03-18/141570.html (accessed on 18 March 2020).
- Su, H.X.; Yao, S.; Zhao, W.F.; Li, M.J.; Liu, J.; Shang, W.J.; Xie, H.; Ke, C.Q.; Hu, H.C.; Gao, M.N.; et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol. Sin. 2020, 41, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzym. Inhib. Med. Chemother. 2021, 36, 497–503. [Google Scholar] [CrossRef]
- Pooja, M.; Reddy, G.J.; Hema, K.; Dodoala, S.; Koganti, B. Unravelling high-affinity binding compounds towards transmembrane protease serine 2 enzyme in treating SARS-CoV-2 infection using molecular modelling and docking studies. Eur. J. Pharmacol. 2021, 890, 173688. [Google Scholar]
- Song, J.; Zhang, L.; Xu, Y.; Yang, D.; Zhang, L.; Yang, S.; Zhang, W.; Wang, J.; Tian, S.; Yang, S.; et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 2021, 183, 114302. [Google Scholar] [CrossRef]
- Ibrahim, M.A.A.; Mohamed, E.A.R.; Abdelrahman, A.H.M.; Allemailem, K.S.; Moustafa, M.F.; Shawky, A.M.; Mahzari, A.; Hakami, A.R.; Abdeljawaad, K.A.A.; Atia, M.A.M. Rutin and flavone analogs as prospective SARS-CoV-2 main protease inhibitors: In silico drug discovery study. J. Mol. Graph. Model. 2021, 105, 107904. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Musall, K.; Oo, A.; Cao, D.; Liang, B.; Hassandarvish, P.; Lan, S.; Slack, R.L.; Kirby, K.A.; Bassit, L.; et al. Baicalein and baicalin inhibit SARS-CoV-2 RNA-dependent-RNA polymerase. Microorganisms 2021, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Q. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharm. Sin. 2002, 23, 1086–1092. [Google Scholar]
- Huang, X.H.; Yan, X.; Zhang, Q.H.; Hong, P.; Zhang, W.X.; Liu, Y.P.; Xu, W.W.; Li, B.; He, Q.Y. Direct targeting of HSP90 with daurisoline destabilizes β-catenin to suppress lung cancer tumorigenesis. Cancer Lett. 2020, 489, 66–78. [Google Scholar] [CrossRef]
- Liu, F.; Ma, J.; Shi, Z.; Zhang, Q.; Wang, H.; Li, D.; Song, Z.; Wang, C.; Jin, J.; Xu, J.; et al. Clerodane diterpenoids isolated from the leaves of Casearia graveolens. J. Nat. Prod. 2020, 83, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Mittal, S.; Loka, M.; Aggarwal, V.; Aggarwal, D.; Masurkar, A.; Kaur, G.; Varol, M.; Sak, K.; Kumar, M.; et al. Deguelin targets multiple oncogenic signaling pathways to combat human malignancies. Pharmacol. Res. 2021, 166, 105487. [Google Scholar] [CrossRef]
- Gao, F.; Yu, X.; Li, M.; Zhou, L.; Liu, W.; Li, W.; Liu, H. Deguelin suppresses non-small cell lung cancer by inhibiting EGFR signaling and promoting GSK3β/FBW7-mediated Mcl-1 destabilization. Cell Death Dis. 2020, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Lusvarghi, S.; Hsiao, S.H.; Liu, T.C.; Li, Y.Q.; Huang, Y.H.; Hung, T.H.; Ambudka, S.V. Licochalcone A selectively resensitizes ABCG2-overexpressing multidrug-resistant cancer cells to chemotherapeutic drugs. J. Nat. Prod. 2020, 83, 1461–1472. [Google Scholar] [CrossRef]
- Luo, W.; Sun, R.; Chen, X.; Li, J.; Jiang, J.; He, Y.; Shi, S.; Wen, H. ERK activation-mediated autophagy induction resists licochalcone A-induced anticancer activities in lung cancer cells in vitro. OncoTargets Ther. 2020, 13, 13437–13450. [Google Scholar] [CrossRef]
- Yuan, L.W.; Jiang, X.M.; Xu, Y.L.; Huang, M.Y.; Chen, Y.C.; Yu, W.B.; Su, M.X.; Ye, Z.H.; Chen, X.; Wang, Y.; et al. Licochalcone A inhibits interferon-gamma-induced programmed death-ligand 1 in lung cancer cells. Phytomedicine 2021, 80, 153394. [Google Scholar] [CrossRef]
- Li, B.; Zhou, D.; Li, S.; Feng, Y.; Li, X.; Chang, W.; Zhang, J.; Sun, Y.; Qing, D.; Chen, G.; et al. Licochalcone A reverses NNK-induced ectopic miRNA expression to elicit in vitro and in vivo chemopreventive effects. Phytomedicine 2020, 76, 153245. [Google Scholar] [CrossRef]
- Gao, F.; Li, M.; Yu, X.; Liu, W.; Zhou, L.; Li, W. Licochalcone A inhibits EGFR signalling and translationally suppresses survivin expression in human cancer cells. J. Cell Mol. Med. 2021, 25, 813–826. [Google Scholar] [CrossRef]
- Yang, A.; Li, M.Y.; Zhang, Z.H.; Wang, J.Y.; Xing, Y.; Ri, M.; Jin, C.H.; Xu, G.H.; Piao, L.X.; Jin, H.L.; et al. Erianin regulates programmed cell death ligand 1 expression and enhances cytotoxic T lymphocyte activity. J. Ethnopharmacol. 2021, 273, 113598. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Q.; Feng, J.; Yan, L.; Sun, Y.; Liu, S.; Xiang, Y.; Zhang, M.; Pan, T.; Chen, X.; et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct. Tar. 2020, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Canuto, K.M.; Liu, C.; Suzuki, B.M.; Almaliti, J.; Sikandar, A.; Naman, C.B.; Glukhov, E.; Luo, D.; Duggan, B.M.; et al. Tutuilamides A-C: Vinyl-chloride-containing cyclodepsipeptides from marine cyanobacteria with potent elastase inhibitory properties. ACS Chem. Biol. 2020, 15, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Luo, D.; Seabra, G.M.; Luesch, H. Ahp-Cyclodepsipeptides as tunable inhibitors of human neutrophil elastase and kallikrein 7: Total synthesis of tutuilamide A, serine protease selectivity profile and comparison with lyngbyastatin 7. Bioorg. Med. Chem. 2020, 28, 115756. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.; Lang, S.J.; Gaafary, M.E.; Schmiech, M.; Simmet, T.; Syrovets, T. The cardenolide glycoside acovenoside A interferes with epidermal growth factor receptor (EGFR) trafficking in non-small cell lung cancer cells. Front. Pharmacol. 2021, 11, 611657. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Lee, S.; Han, S.; Lee, Y.J.; Kim, J.B.; Seo, E.K.; Jung, C.H. Triterpenoids from the leaves of Centella asiatica inhibit ionizing radiation-induced migration and invasion of human lung cancer cells. Evid. Based Compl. Alt. 2020, 2020, 3683460. [Google Scholar] [CrossRef]
- Zhang, X.; Ruan, Q.; Zhai, Y.; Lu, D.; Li, C.; Fu, Y.; Zheng, Z.; Song, Y.; Guo, J. Baicalein inhibits non-small-cell lung cancer invasion and metastasis by reducing ezrin tension in inflammation microenvironment. Cancer Sci. 2020, 111, 3802–3812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Nong, L.; Chen, M.; Gu, X.; Zhao, W.; Liu, M.; Cheng, W. Baicalein suppresses vasculogenic mimicry through inhibiting RhoA/ROCK expression in lung cancer A549 cell line. Acta Bioch. Bioph. Sin. 2020, 52, 1007–1015. [Google Scholar] [CrossRef]
- Li, J.; Yan, L.; Luo, J.; Tong, L.; Gao, Y.; Feng, W.; Wang, F.; Cui, W.; Li, S.; Sun, Z. Baicalein suppresses growth of non-small cell lung carcinoma by targeting MAP4K3. Biomed. Pharmacother. 2021, 133, 110965. [Google Scholar] [CrossRef]
- Sui, X.; Han, X.; Chen, P.; Wu, Q.; Feng, J.; Duan, T.; Chen, X.; Pan, T.; Yan, L.; Jin, T.; et al. Baicalin induces apoptosis and suppresses the cell cycle progression of lung cancer cells through downregulating Akt/mTOR signaling pathway. Front. Mol. Biosci. 2021, 7, 602282. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yao, L.; Sun, H.; Pang, S.; Kong, X.; Zhao, S.; Xu, S. Effects of wogonoside on invasion and migration of lung cancer A549 cells and angiogenesis in xenograft tumors of nude mice. J. Thorac. Dis. 2020, 12, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.Y.; Hsiao, Y.T.; Huang, Y.P.; Peng, S.F.; Huang, W.W.; Liu, K.C.; Hsia, T.C.; Way, T.D.; Chung, J.G. Casticin induces DNA damage and affects DNA repair associated protein expression in human lung cancer A549 cells. Molecules 2020, 25, 341. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Yang, G.; Ye, J.; Yao, Y.; Lu, G.; Chen, J.; Fang, L.; Lu, S.; Zhou, J. Dioscin elicits anti-tumour immunity by inhibiting macrophage M2 polarization via JNK and STAT3 pathways in lung cancer. J. Cell. Mol. Med. 2020, 24, 9217–9230. [Google Scholar] [CrossRef]
- Chen, A.; Jiang, P.; Zeb, F.; Wu, X.; Xu, C.; Chen, L.; Feng, Q. EGCG regulates CTR1 expression through its pro-oxidative property in non-small-cell lung cancer cells. J. Cell. Physiol. 2020, 235, 7970–7981. [Google Scholar] [CrossRef]
- Wei, R.; Wirkus, J.; Yang, Z.; Machuca, J.; Esparza, Y.; Mackenzie, G.G. EGCG sensitizes chemotherapeutic-induced cytotoxicity by targeting the ERK pathway in multiple cancer cell lines. Arch. Biochem. Biophys. 2020, 692, 108546. [Google Scholar] [CrossRef]
- Duan, J.; Li, Y.; Gao, H.; Yang, D.; He, X.; Fang, Y.; Zhou, G. Phenolic compound ellagic acid inhibits mitochondrial respiration and tumor growth in lung cancer. Food Funct. 2020, 11, 6332–6339. [Google Scholar] [CrossRef]
- Boonjing, S.; Pothongsrisit, S.; Wattanathamsan, O.; Sritularak, B.; Pongrakhananon, V. Erianthridin induces non-small cell lung cancer cell apoptosis through the suppression of extracellular signal-regulated kinase activity. Planta Med. 2021, 87, 283–293. [Google Scholar]
- Pothongsrisit, S.; Arunrungvichian, K.; Hayakawa, Y.; Sritularak, B.; Mangmool, S.; Pongrakhananon, V. Erianthridin suppresses non-small-cell lung cancer cell metastasis through inhibition of Akt/mTOR/p70 S6K signaling pathway. Sci. Rep. 2021, 11, 6618. [Google Scholar] [CrossRef]
- Choudhury, P.; Barua, A.; Roy, A.; Pattanayak, R.; Bhattacharyya, M.; Saha, P. Eugenol emerges as an elixir by targeting β-catenin, the central cancer stem cell regulator in lung carcinogenesis: An in vivo and in vitro rationale. Food Funct. 2021, 12, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gao, F.; Li, W.; Zhou, L.; Liu, W.; Li, M. Formononetin inhibits tumor growth by suppression of EGFR-Akt-Mcl-1 axis in non-small cell lung cancer. J. Exp. Clin. Canc. Res. 2020, 39, 62. [Google Scholar] [CrossRef]
- Yang, B.; Yang, N.; Chen, Y.; Maomao, Z.; Lian, Y.; Xiong, Z.; Wang, B.; Feng, L.; Jia, X. An integrated strategy for effective-component discovery of Astragali Radix in the treatment of lung cancer. Front. Pharmacol. 2021, 12, 580978. [Google Scholar] [CrossRef]
- Kang, D.Y.; Sp, N.; Jo, E.S.; Rugamba, A.; Hong, D.Y.; Lee, H.G.; Yoo, J.S.; Liu, Q.; Jang, K.J.; Yang, Y.M. The inhibitory mechanisms of tumor PD-L1 expression by natural bioactive gallic acid in non-small-cell lung cancer (NSCLC) cells. Cancers 2020, 12, 727. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bao, B. Gallic acid impedes non-small cell lung cancer progression via suppression of EGFR-dependent CARM1-PELP1 complex. Drug Des. Dev. Ther. 2020, 14, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Miao, L.; Huang, F.; Yu, Y.; Peng, Q.; Liu, Y.; Li, X.; Liu, H. Glochidiol, a natural triterpenoid, exerts its anti-cancer effects by targeting the colchicine binding site of tubulin. Invest. N. Drug. 2021, 39, 578–586. [Google Scholar] [CrossRef]
- Min, H.Y.; Pei, H.; Hyun, S.Y.; Boo, H.J.; Jang, H.J.; Cho, J.; Kim, J.H.; Son, J.; Lee, H.Y. Potent anticancer effect of the natural steroidal saponin gracillin is produced by inhibiting glycolysis and oxidative phosphorylation-mediated bioenergetics. Cancers 2020, 12, 913. [Google Scholar] [CrossRef]
- Yang, J.; Cao, L.; Li, Y.; Liu, H.; Zhang, M.; Ma, H.; Wang, B.; Yuan, X.; Liu, Q. Gracillin isolated from Reineckia carnea induces apoptosis of A549 Cells via the mitochondrial pathway. Drug Des. Dev. Ther. 2021, 2021, 233–243. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, W.; Li, T.; Jiang, L.; Lu, X.; Lin, J. Hispidulin exhibits potent anticancer activity in vitro and in vivo through activating ER stress in non-small-cell lung cancer cells. Oncol. Rep. 2020, 43, 1995–2003. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, Y.; Jiang, B.; Yin, J.; Lu, C.; Wang, J.; Zeng, J. Icaritin inhibits lung cancer-induced osteoclastogenesis by suppressing the expression of IL-6 and TNF-a and through AMPK/mTOR signaling pathway. Anti-Cancer Drug. 2020, 31, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, S.Y.; Hwang, W.; Sung, J.Y.; Shim, M.L.C.J.; Kim, Y.N.; Yoon, K. Isoharringtonine induces apoptosis of non-small cell lung cancer cells in tumorspheroids via the intrinsic pathway. Biomolecules 2020, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Fouzder, C.; Mukhuty, A.; Kundu, R. Kaempferol inhibits Nrf2 signalling pathway via downregulation of Nrf2 mRNA and induces apoptosis in NSCLC cells. Arch. Biochem. Biophys. 2021, 697, 108700. [Google Scholar] [CrossRef]
- Sheng, H.; Lv, W.; Zhu, L.; Wang, L.; Wang, Z.; Han, J.; Hu, J. Liriopesides B induces apoptosis and cell cycle arrest in human non-small cell lung cancer cells. Int. J. Mol. Med. 2020, 46, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Huang, M.Y.; Zhang, L.L.; Feng, Z.L.; Jiang, X.M.; Yuan, L.W.; Huang, R.Y.; Liu, B.; Yu, H.; Wang, Y.T.; et al. Nagilactone E increases PD-L1 expression through activation of c-Jun in lung cancer cells. Chin. J. Nat. Med. 2020, 18, 517–525. [Google Scholar] [CrossRef]
- Zhang, L.L.; Guo, J.; Jiang, X.M.; Chen, X.P.; Wang, Y.T.; Li, A.; Lin, L.G.; Li, H.; Lu, J.J. Identification of nagilactone E as a protein synthesis inhibitor with anticancer activity. Acta Pharmacol. Sin. 2020, 41, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Zhou, G.J.; Hou, Y.; Kong, Q.; Lu, J.J.; Zhang, Q.; Chen, X. Natural alkaloid 8-oxo-epiberberine inhibited TGF-β1-triggred epithelial-mesenchymal transition by interfering Smad3. Toxicol. Appl. Pharm. 2020, 404, 115179. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, R.; Li, M.; Zhu, Z.; Chen, Z.; Cui, L.; Luo, H.; Luo, L. Parthenolide inhibits the growth of non-small cell lung cancer by targeting epidermal growth factor receptor. Cancer Cell Int. 2020, 20, 561. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, W.; Wen, G.; Yu, B.; Xu, F.; Gan, X.; Tang, J.; Zeng, Q.; Zhu, L.; Chen, C.; et al. Parthenolide inhibits human lung cancer cell growth by modulating the IGF-1R/PI3K/Akt signaling pathway. Oncol. Rep. 2020, 44, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Liao, X.Z.; Zhang, Y.; He, Z.R.; Nie, S.Q.; Ke, B.; Shi, L.; Zhao, J.F.; Chen, W.H. Parthenolide augments the chemosensitivity of non-small-cell lung cancer to cisplatin via the PI3K/AKT signaling pathway. Front. Cell Dev. Biol. 2021, 8, 610097. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Zhang, R.; Liu, C.; Wang, Q.; Wang, X.; Guo, X.; Wang, P.; Sun, J. PDB-1 from potentilla discolor bunge suppresses lung cancer cell migration and invasion via FAK/Src and MAPK signaling pathways. Med. Chem. Res. 2020, 29, 887–896. [Google Scholar] [CrossRef]
- Wu, Y.; Si, Y.; Xiang, Y.; Zhou, T.; Liu, X.; Wu, M.; Li, W.; Zhang, T.; Xiang, K.; Zhang, L.; et al. Polyphyllin I activates AMPK to suppress the growth of non-small-cell lung cancer via induction of autophagy. Arch. Biochem. Biophys. 2020, 687, 108285. [Google Scholar] [CrossRef]
- Lai, L.; Shen, Q.; Wang, Y.; Chen, L.; Lai, J.; Wu, Z.; Jiang, H. Polyphyllin I reverses the resistance of osimertinib in non-small cell lung cancer cell through regulation of PI3K/Akt signaling. Toxicol. Appl. Pharm. 2021, 419, 115518. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thoracic Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, H.; Wang, A.; Ma, Y.; Gan, Y.; Li, G. Silibinin suppresses epithelial-mesenchymal transition in human non-small cell lung cancer cells by restraining RHBDD1. Cell. Mol. Biol. Lett. 2020, 25, 36. [Google Scholar] [CrossRef]
- Shen, K.H.; Hung, J.H.; Liao, Y.C.; Tsai, S.T.; Wu, M.J.; Chen, P.S. Sinomenine inhibits migration and invasion of human lung cancer cell through downregulating expression of miR-21 and MMPs. Int. J. Mol. Sci. 2020, 21, 3080. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wen, W.; Hou, X.; Wu, J.; Yi, L.; Zhi, Y.; Lv, Y.; Tan, X.; Liu, L.; Wang, P.; et al. Inhibitory effect of sinomenine on lung cancer cells via negative regulation of α7 nicotinic acetylcholine receptor. J. Leukocyte Biol. 2021, 109, 843–852. [Google Scholar] [CrossRef]
- Liu, W.; Yu, X.; Zhou, L.; Li, J.; Li, M.; Li, W.; Gao, F. Sinomenine inhibits non-small cell lung cancer via downregulation of hexokinases II-mediated aerobic glycolysis. OncoTargets Ther. 2020, 13, 3209–3221. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, F.Y.; Dai, S.Z.; Wang, J.Y.; Lin, Y.Y.; Sun, Y.; Tan, G.H.; Huang, Y.H. Toxicarioside O inhibits cell proliferation and epithelial-mesenchymal transition by downregulation of Trop2 in lung cancer cells. Front. Oncol. 2020, 10, 609275. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashed, S.; Baker, A.; Ahmad, S.S.; Syed, A.; Bahkali, A.H.; Elgorban, A.M.; Khan, M.S. Vincamine, a safe natural alkaloid, represents a novel anticancer agent. Bioorg. Chem. 2021, 107, 104626. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, M.; Zhou, L.; Liu, W.; Zuo, H.; Li, W. Xanthohumol targets the ERK1/2-Fra1 signaling axis to reduce cyclin D1 expression and inhibit non-small cell lung cancer. Oncol. Rep. 2020, 44, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C. Cepharanthine: An update of its mode of action, pharmacological properties and medical applications. Phytomedicine 2019, 62, 152956. [Google Scholar] [CrossRef]
- Okamoto, M.; Ono, M.; Baba, M. Potent inhibition of HIV type 1 replication by an antiinflammatory alkaloid, cepharanthine, in chronically infected monocytic cells. AIDS Res. Hum. Retrovir. 1998, 14, 1239–1245. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wang, Y.F.; Liu, X.J.; Lu, J.H.; Qian, C.W.; Wan, Z.Y. Antiviral activity of cepharanthine against severe acute respiratory syndrome coronavirus in vitro. Chin. Med. J. 2005, 118, 493–496. [Google Scholar]
- Kim, D.E.; Min, J.S.; Jang, M.S.; Lee, J.Y.; Shin, Y.S.; Song, J.H.; Kim, H.R.; Kim Se Jin, Y.H.; Kwon, S. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules 2019, 9, 696. [Google Scholar] [CrossRef]
- Ohashi, H.; Watashi, K.; Saso, W.; Shionoya, K.; Iwanami, S.; Hirokawa, T.; Shirai, T.; Kanaya, S.; Ito, Y.; Kim, K.S.; et al. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. Science 2021, 24, 102367. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Chen, Y.; Wang, L.; An, W.; An, X.; Song, L.; Tong, Y.; Fan, H.; Lu, C. Transcriptome analysis of cepharanthine against a SARS-CoV-2-related coronavirus. Brief. Bioinform. 2021, 22, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Bihani, S.C.; Hosur, M.V. Molecular basis for reduced cleavage activity and drug resistance in D30N HIV-1 protease. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jan, J.T.; Cheng, T.J.R.; Juang, Y.P.; Ma, H.H.; Wu, Y.T.; Yang, W.B.; Cheng, C.W.; Chen, X.; Chou, T.H.; Shie, J.J.; et al. Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2021579118. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Matsuyama, S.; Hoshino, T.; Yamamoto, N. Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Musarrat, F.; Chouljenko, V.; Dahal, A.; Nabi, R.; Chouljenko, T.; Jois, S.D.; Kousoulas, K.G. The anti-HIV drug nelfinavir mesylate (Viracept) is a potent inhibitor of cell fusion caused by the SARSCoV-2 spike (S) glycoprotein warranting further evaluation as an antiviral against COVID-19 infections. J. Med. Virol. 2020, 92, 2087–2095. [Google Scholar] [CrossRef]
- Foo, C.S.; Abdelnabi, R.; Kaptein, S.J.F.; Zhang, X.; ter Horst, S.; Mols, R.; Delang, L.; Rocha-Pereira, J.; Coelmont, L.; Leyssen, P.; et al. Nelfinavir markedly improves lung pathology in SARS-CoV-2-infected Syrian hamsters despite lack of an antiviral effect. bioRxiv 2021. [Google Scholar] [CrossRef]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, L.; Li, G.; Cong, F.; Li, Y.; Sun, J.; Luo, Y.; Chen, G.; Li, G.; Wang, P.; et al. Remdesivir metabolite GS-441524 effectively inhibits SARS-CoV-2 infection in mouse models. J. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Rubin, D.; Chan-Tack, K.; Farley, J.; Sherwat, A. FDA approval of remdesivir—A step in the right direction. N. Engl. J. Med. 2020, 383, 2598–2600. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.a.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9, eaal3653. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef]
- McMullan, L.K.; Flint, M.; Chakrabarti, A.; Guerrero, L.; Lo, M.K.; Porter, D.; Nichol, S.T.; Spiropoulou, C.F.; Albariño, C. Characterisation of infectious Ebola virus from the ongoing outbreak to guide response activities in the Democratic Republic of the Congo: A phylogenetic and in vitro analysis. Lancet Infect. Dis. 2019, 19, 1023–1032. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- De Wit, E.; Feldmann, F.; Cronin, J.; Jordan, R.; Okumura, A.; Thomas, T.; Scott, D.; Cihlar, T.; Feldmann, H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. USA 2020, 117, 6771–6776. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.K.; Feldmann, F.; Gary, J.M.; Jordan, R.; Bannister, R.; Cronin, J.; Patel, N.R.; Klena, J.D.; Nichol, S.T.; Cihlar, T.; et al. Remdesivir (GS-5734) protects African green monkeys from Nipah virus challenge. Sci. Transl. Med. 2019, 11, eaau9242. [Google Scholar] [CrossRef]
- Williamson, B.N.; Feldmann, F.; Schwarz, B.; Meade-White, K.; Porter, D.P.; Schulz, J.; van Doremalen, N.; Leighton, I.; Yinda, C.K.; Pérez-Pérez, L.; et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature 2020, 585, 273–276. [Google Scholar] [CrossRef]
- Jacobs, M.; Rodger, A.; Bell, D.J.; Bhagani, S.; Cropley, I.; Filipe, A.; Gifford, R.J.; Hopkins, S.; Hughes, J.; Jabeen, F.; et al. Late Ebola virus relapse causing meningoencephalitis: A case report. Lancet 2016, 388, 498–503. [Google Scholar] [CrossRef]
- Schwartz, I.S.; Heil, E.L.; McCreary, E.K. Remdesivir: A pendulum in a pandemic. BMJ 2020, 371, m4560. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- McCreary, E.K.; Angus, D.C. Efficacy of remdesivir in COVID-19. JAMA 2020, 324, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Chu, H.; Yang, D.; Sze, K.H.; Lai, P.M.; Yuan, S.; Shuai, H.; Wang, Y.; Kao, R.Y.T.; Chan, J.F.W.; et al. Characterization of lipidomic profile of human coronavirus -infected cells: Implications for lipid metabolism remodeling upon coronavirus replication. Viruses 2019, 11, 73. [Google Scholar] [CrossRef]
- Khalil, M.I.; Salih, M.A.; Mustafa, A.A. Broad beans (Vicia faba) and the potential to protect from COVID-19 coronavirus infection. Sudan J. Paediatr. 2020, 20, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P.; Serhan, C.N.; Bazinet, R.P. The need for precision nutrition, genetic variation and resolution in Covid-19 patients. Mol. Aspects Med. 2021, 77, 100943. [Google Scholar] [CrossRef]
- Toelzer, C.; Gupta, K.; Yadav, S.K.N.; Borucu, U.; Davidson, A.D.; Williamson, M.K.; Shoemark, D.K.; Garzoni, F.; Staufer, O.; Milligan, R.; et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science 2020, 370, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L. GS-5734: A potentially approved drug by FDA against SARS-CoV-2. N. J. Chem. 2020, 44, 12417–12429. [Google Scholar] [CrossRef]
- Yan, V.C.; Muller, F.L. Advantages of the parent nucleoside GS-441524 over remdesivir for Covid-19 treatment. ACS Med. Chem. Lett. 2020, 11, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Huang, Y.; Chan, L.; He, D.; Chen, T. Engineering EHD1-targeted natural borneol nanoemulsion potentiates therapeutic efficacy of gefitinib against non-small lung cancer. ACS Appl. Mater. Interfaces 2020, 12, 45714–45727. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.; Wang, S.; Gai, C.; Cui, X.; Xu, Z.; Li, W.; Zhang, W. Targeted delivery of quercetin by nanoparticles based on chitosan sensitizing paclitaxel-resistant lung cancer cells to paclitaxel. Mater. Sci. Eng. C 2021, 119, 111442. [Google Scholar] [CrossRef]
- Zang, H.; Qian, G.; Arbiser, J.; Owonikoko, T.K.; Ramalingam, S.S.; Fan, S.; Sun, S.Y. Overcoming acquired resistance of EGFR-mutant NSCLC cells to the third generation EGFR inhibitor, osimertinib, with the natural product honokiol. Mol. Oncol. 2020, 14, 882–895. [Google Scholar] [CrossRef]
- Dai, C.H.; Zhu, L.R.; Wang, Y.; Tang, X.P.; Du, Y.J.; Chen, Y.C.; Li, J. Celastrol acts synergistically with afatinib to suppress non-small cell lung cancer cell proliferation by inducing paraptosis. J. Cell. Physiol. 2021, 236, 4538–4554. [Google Scholar] [CrossRef]
- Silva, A.C.D.; Santos, P.D.D.F.; Silva, J.T.D.P.; Leimann, F.V.; Bracht, L.; Gonçalves, O.H. Impact of curcumin nanoformulation on its antimicrobial activity. Trends Food Sci. Tech. 2018, 72, 74–82. [Google Scholar] [CrossRef]
- Zhang, L.; Qiang, P.; Yu, J.; Miao, Y.M.; Chen, Z.Q.; Qu, J.; Zhao, Q.B.; Chen, Z.; Liu, Y.; Yao, X.; et al. Identification of compound CA-5f as a novel late-stage autophagy inhibitor with potent anti-tumor effect against non-small cell lung cancer. Autophagy 2019, 15, 391–406. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Najafi, M.; Makvandi, P.; Zarrabi, A.; Farkhondeh, T.; Samarghandian, S. Versatile role of curcumin and its derivatives in lung cancer therapy. J. Cell Physiol. 2020, 235, 9241–9268. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Kumar, S.; Kunwar, A.; Nath, S.; Sarma, H.D.; Tripathi, A.; Verma, G.; Chaudhari, D.P.; Aswal, V.K.; Melo, J.S. Structural and therapeutic properties of curcumin solubilized pluronic F127 micellar solutions and hydrogels. J. Mol. Liq. 2020, 314, 113591. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Zha, M.; Tian, T.; Xu, W.; Liu, S.; Jia, J.; Wang, L.; Yan, Q.; Li, N.; Yu, J.; Huang, L. The circadian clock gene Bmal1 facilitates cisplatin-induced renal injury and hepatization. Cell Death Dis. 2020, 11, 446. [Google Scholar] [CrossRef]
- Hazlitt, R.A.; Min, J.; Zuo, J. Progress in the development of preventative drugs for cisplatin-induced hearing loss. J. Med. Chem. 2018, 61, 5512–5524. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wei, S.; Zhang, B.; Li, W. Molecular mechanisms of cardiomyocyte death in drug-induced cardiotoxicity. Front. Cell Dev. Biol. 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, L.; Tao, H.; Kong, L.; Hu, Y. Ring finger protein 38 induces the drug resistance of cisplatin in non-small cell lung cancer. Cell Biol. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, H.; Chen, C.; Ren, K.; Xu, Y.; Liu, X.; He, L. Curcumin enhances cisplatin sensitivity of human NSCLC cell lines through influencing Cu-Sp1-CTR1 regulatory loop. Phytomedicine 2018, 48, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.M.; Chen, Y.; Chen, J.T.; Liu, Y. Targeted polysaccharide nanoparticle for adamplatin prodrug delivery. J. Med. Chem. 2013, 56, 9725–9736. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.P.; Chen, D.; Liu, C.F.; Zhuo, L.H.; Li, H.; Wang, C.; Bu, H.T.; Tian, W. β-Cyclodextrin-modified hyaluronic acid-based supramolecular self-assemblies for pH- and esterase- dual-responsive drug delivery. Carbohydr. Polym. 2020, 246, 116654. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Ma, X.; Zhang, L.; Yan, J.; Cui, H.; Zhang, Y.; Wang, D.; Zhang, H. Self-Assembled disulfide bond bearing paclitaxel-camptothecin prodrug nanoparticle for lung cancer therapy. Pharmaceutics 2020, 12, 1169. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Zhang, D.; Ling, H.; He, Z.; Sun, J.; Sun, M.; Liu, D. Pure redox-sensitive paclitaxel-maleimide prodrug nanoparticles: Endogenous albumin-induced size switching and improved antitumor efficiency. Acta Pharm. Sin. B 2021. [Google Scholar] [CrossRef]
- Wang, J.; Pei, Q.; Xia, R.; Liu, S.; Hu, X.; Xie, Z.; Jing, X. Comparison of redox responsiveness and antitumor capability of paclitaxel dimeric nanoparticles with different linkers. Chem. Mater. 2020, 32, 10719–10727. [Google Scholar] [CrossRef]
- Lan, J.S.; Liu, L.; Zeng, R.F.; Qin, Y.H.; Hou, J.W.; Xie, S.S.; Yue, S.; Yang, J.; Ho, R.J.Y.; Ding, Y.; et al. Tumor-specific carrier-free nanodrugs with GSH depletion and enhanced ROS generation for endogenous synergistic anti-tumor by a chemotherapy-photodynamic therapy. Chem. Eng. J. 2021, 407, 127212. [Google Scholar] [CrossRef]
- Venkatesha, S.H.; Dudics, S.; Astry, B.; Moudgil, K.D. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog. Dis. 2016, 74, ftw059. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, S.Y.; Lee, C. Axl is a novel target of celastrol that inhibits cell proliferation and migration, and increases the cytotoxicity of gefitinib in EGFR mutant non-small cell lung cancer cells. Mol. Med. Rep. 2019, 19, 3230–3236. [Google Scholar] [CrossRef]
- Nazim, U.M.; Yin, H.; Park, S.Y. Autophagy flux inhibition mediated by celastrol sensitized lung cancer cells to TRAIL-induced apoptosis via regulation of mitochondrial transmembrane potential and reactive oxygen species. Mol. Med. Rep. 2019, 19, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.; Chen, H.; You, J.; Peng, B.; Cao, F.; Zhang, X.; Chen, Q.; Uzan, G.; Xu, L.; et al. Celastrol improves the therapeutic efficacy of EGFR-TKIs for non-small-cell lung cancer by overcoming EGFR T790M drug resistance. Anti-Cancer Drug. 2018, 29, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Kanabar, D.D.; Patel, K.; Ayehunie, S.; Muth, A.; Gupta, V. Enhanced solubility, stability, permeation and anti-cancer efficacy of Celastrol-β-cyclodextrin inclusion complex. J. Mol. Liq. 2020, 318, 113936. [Google Scholar] [CrossRef]
- Cao, L.; Hong, W.; Cai, P.; Xu, C.; Bai, X.; Zhao, Z.; Huang, M.; Jin, J. Cryptotanshinone strengthens the effect of gefitinib against non-small cell lung cancer through inhibiting transketolase. Eur. J. Pharmacol. 2021, 890, 173647. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, G.; Jiang, L.; Zhang, X.; Xu, Z.; Yan, H.; Zhou, Z.; He, Q.; Yang, X.; Luo, P. Crosstalk between alveolar macrophages and alveolar epithelial cells/fibroblasts contributes to the pulmonary toxicity of gefitinib. Toxicol. Lett. 2021, 338, 1–9. [Google Scholar] [CrossRef]
- Xie, X.; Wang, X.; Wu, S.; Yang, H.; Liu, J.; Chen, H.; Ding, Y.; Ling, L.; Lin, H. Fatal toxic effects related to EGFR tyrosine kinase inhibitors based on 53 cohorts with 9569 participants. J. Thorac. Dis. 2020, 12, 4057–4069. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhan, C.; Wang, J.; Zeng, F.; Wu, S. An activatable nano-prodrug for treating tyrosine-kinase-inhibitor-resistant non-small cell lung cancer and for optoacoustic and fluorescent imaging. Small 2020, 16, 2003451. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, J.; Dong, X.; Zhao, W.; Zhu, Q. Fluorescent probe for sensitive discrimination of Hcy and Cys/GSH in living cells via dual-emission. Anal. Chim. Acta 2019, 1074, 123–130. [Google Scholar] [CrossRef]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Appendino, G.; Efferth, T.; Fürst, R.; Izzo, A.A.; Kayser, O.; Pezzuto, J.M.; Viljoen, A. Best practice in research—Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 2020, 246, 112230. [Google Scholar] [CrossRef] [PubMed]
| No. | Name | Structure | EC50 or IC50 (μM) | Strain | Refs |
|---|---|---|---|---|---|
| 1 | Acetoside |  | 0.043 | Vero E6 cells | [39] |
| 2 | Anacardic acid | 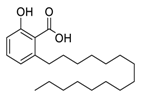 | 2.07 | USA-WA1/2020 | [26] |
| 3 | Andrographolide | 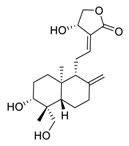 | 0.034 | Calu-3 cells | [40,41] |
| 4 | Apigenin-7-O-glucoside | 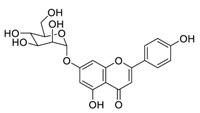 | 0.074 | Vero E6 cells | [39] |
| 5 | Artemisinin | 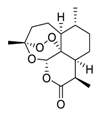 | 64.45 | Vero E6 cells | [41,42] |
| 6 | Azithromycin | 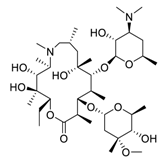 | 2.12 | Caco-2 cells | [43] |
| 7 | Baicalin | 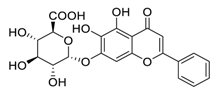 | 7.98 | Vero E6 cells | [44] |
| 8 | Cannabidiol | 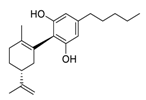 | 7.91 | Vero E6 cells | [45,46] |
| 9 | Catechin-3-O-gallate | 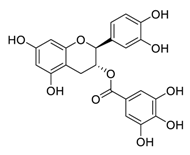 | 2.98 | Vero E6 cells | [47] |
| 10 | Chebulagic acid | 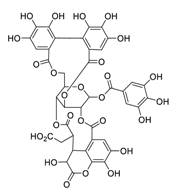 | 9.76 | Vero E6 cells | [48] |
| 11 | Daurisoline |  | 3.66 | Vero E6 cells | [49] |
| 12 | EGCG | 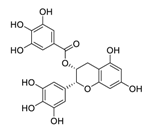 | 0.874 | Vero E6 cells | [44,50] |
| 13 | Emetine | 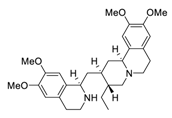 | 0.46 | Vero E6 cells | [51,52] |
| 14 | Epicatechin-3-O-gallate | 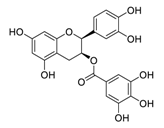 | 5.21 | Vero E6 cells | [47] |
| 15 | Gallinamide A | 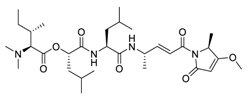 | 0.028 | Vero E6 cells | [53] |
| 16 | Gallocatechin-3-O-gallate | 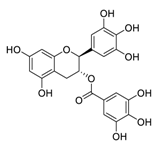 | 6.38 | Vero E6 cells | [47] |
| 17 | Hopeaphenol | 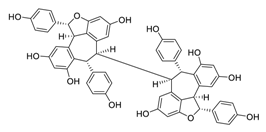 | 2.3 | B.1.351 | [54] |
| 18 | Ipomoeassin F | 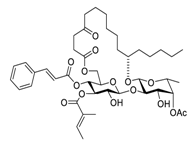 | semi-permeabilized mammalian cells | [55] | |
| 19 | Kobophenol A | 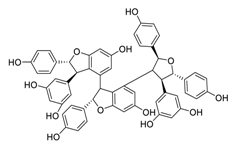 | 1.81 | Vero E6 cells | [56] |
| 20 | Myricetin | 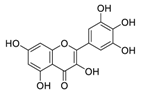 | 0.22 | Vero E6 cells | [57,58] |
| 21 | Naringenin | 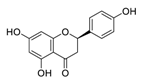 | 0.092 | Vero E6 cells | [39,59] |
| 22 | Osajin | 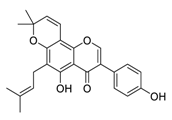 | 3.87 | Vero E6 cells | [60] |
| 23 | 2,3′,4,5′,6-Pentahydroxybenzophenone | 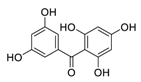 | 0.102 | Vero E6 cells | [39] |
| 24 | Procyanidin B2 | 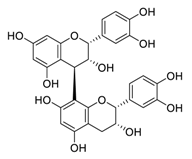 | 75.3 | Vero E6 cells | [47] |
| 25 | Punicalagin | 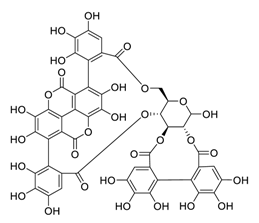 | 7.20 | Vero E6 cells | [48] |
| 26 | Sennoside B | 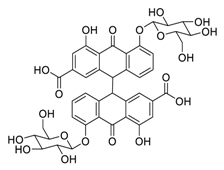 | 0.104 | Vero E6 cells | [39] |
| 27 | Shikonin | 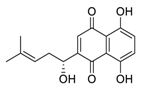 | 15.75 | Vero E6 cells | [61] |
| 28 | Δ9-Tetrahydrocannabinol | 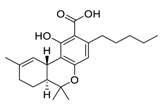 | 10.25 | Vero E6 cells | [45] |
| 29 | Tetrandrine | 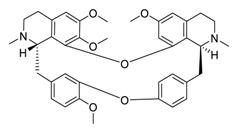 | 3.00 | Vero E6 cells | [60] |
| 30 | Theaflavin | 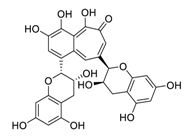 | 8.44 | HEK293T human embryonic kidney cells | [62] |
| Baicalein (The Active Ingredient of Huangqin) | Molecular Mechanisms of Baicalein | Herbal Formula Containing Huangqin | Registration Number | Sample Size of the Control Group |
|---|---|---|---|---|
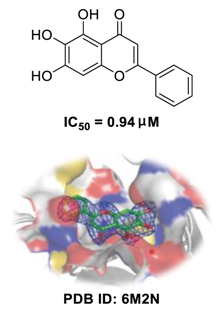 | RdRp inhibitor via noncovalent incorporation [73], potent antagonists against TMPRSS2 [70], improving respiratory function, decreasing IL-1β and TNF-α levels, and inhibiting cell infiltration [71,72]. | Qingfei Paidu decoction | ChiCTR2000029433 | 120 |
| ChiCTR2000030883 | 100 | |||
| ChiCTR2000032767 | 782 | |||
| Xinguan I decoction | ChiCTR2000029637 | 50 | ||
| Tanreqing capsules | ChiCTR2000029813 | 36 | ||
| Tanreqing injection | ChiCTR2000029432 | 72 | ||
| Kegan Liyan oral liquid | ChiCTR2000033720 | 240 | ||
| ChiCTR2000033745 | 240 | |||
| ChiCTR2000031982 | 240 | |||
| Shuanghuanglian oral liquid | ChiCTR2000033133 | 30 | ||
| ChiCTR2000029605 | 100 | |||
| Toujie Quwen granule | ChiCTR2000031888 | 150 |
| No. | Name | Structure | Mechanism of Anti-Lung Cancer | Refs |
|---|---|---|---|---|
| 1 | Acovenoside A | 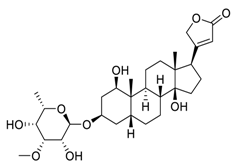 | Inhibit the adenosine triphosphate (ATP)-dependent Na+/K+ exchange through the Na+/K+-ATPase | [88] |
| 2 | Asiatic acid | 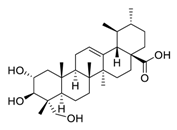 | Inhibited the ionizing radiation-induced migration and invasion | [89] |
| 3 | Baicalein | 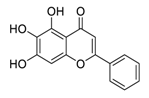 | Restrained ezrin tension by decreasing inducible nitric oxide synthase expression levels, suppress invasion, reduced vasculogenic mimicry formation | [90,91,92] |
| 4 | Baicalin | 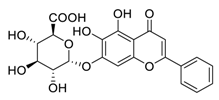 | Inhibited the invasion, migration, angiogenesis, and Akt/mTOR pathway | [93,94] |
| 5 | Casticin | 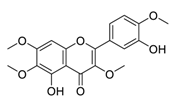 | Induced the expressions and nuclear translocation of phosphorylation of H2AX | [95] |
| 6 | Dioscin | 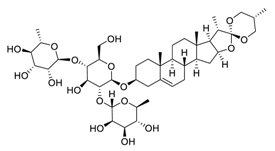 | Down-regulated signal transducer and activator of transcription 3 and c-Jun N-terminal kinase signaling pathways | [96] |
| 7 | EGCG | 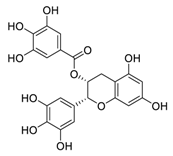 | Regulated CTR1 expression through the ERK1/2/NEAT1 signaling pathway | [97,98] |
| 8 | Ellagic acid | 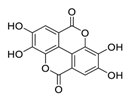 | Inhibited tumor growth, increased p-AMPK, and suppressed hypoxia-inducible factor 1α levels | [99] |
| 9 | Erianthridin | 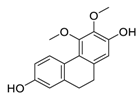 | Attenuated extracellular signal-regulated kinase activity and mediated apoptosis, matrix-degrading metalloproteinases (MMPs) expression | [100,101] |
| 10 | Eugenol | 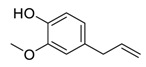 | Restriction of β-catenin nuclear transportation | [102] |
| 11 | Formononetin | 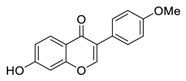 | Inhibited EGFR-Akt signaling, which in turn activates GSK3β and promotes Mcl-1 phosphorylation in NSCLC cells | [103,104] |
| 12 | Gallic Acid | 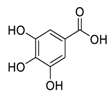 | Inhibited of EGFR activation and impairment, inhibition of phosphoinositide 3-kinase (PI3K) and AKT phosphorylation | [105,106] |
| 13 | Glochidiol | 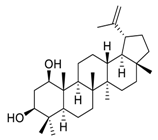 | Inhibited tubulin polymerization | [107] |
| 14 | Gracillin | 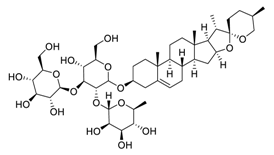 | Inhibited both glycolysis and mitochondria-mediated bioenergetics, induced apoptosis through the mitochondrial pathway | [108,109] |
| 15 | Hispidulin | 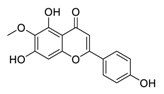 | Promoted apoptosis by hispidulin via increased generation of ROS | [110] |
| 16 | Icaritin | 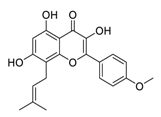 | Downregulated the immunosuppressive cytokine (TNF-α, IL10, IL6) and upregulated chemotaxis (CXCL9 and CXCL10) | [111] |
| 17 | Isoharringtonine | 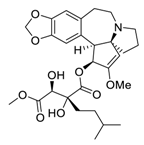 | Induced death tumor spheroids by activating the intrinsic apoptosis pathway | [112] |
| 18 | Kaempferol | 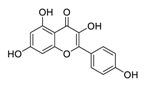 | Inhibitor of nuclear factor erythroid 2-related factor 2 | [113] |
| 19 | Liriopesides B | 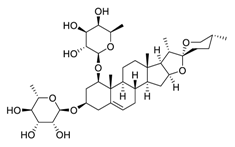 | Reduced proliferation, and induced apoptosis and cell cycle arrest, inhibited the progression of the cell cycle from the G1 to the S phase | [114] |
| 20 | Nagilactone E | 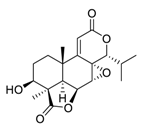 | Activated the c-Jun N-terminal kinases, increased the phosphorylation, and promoted the localization of c-Jun in the nucleus | [115,116] |
| 21 | 8-Oxo-epiberberine | 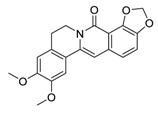 | Inhibited TGF-β1-induced epithelial-mesenchymal transition (EMT) possibly by interfering with Smad3 | [117] |
| 22 | Parthenolide | 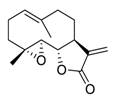 | Reduced the phosphorylation of EGFR and downstream signaling pathways mitogen-activated protein kinase (MAPK)/ERK, inhibited PI3K/Akt/FoxO3α signaling | [118,119,120] |
| 23 | PDB-1 | 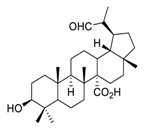 | Suppressed lung cancer cell migration and invasion via FAK/Src and MAPK signaling pathways | [121] |
| 24 | Polyphyllin I | 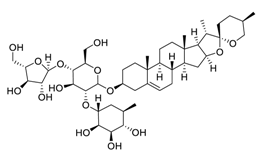 | Induced autophagy by activating AMPK and then inhibited mTOR signaling, promoted apoptosis, modulated the PI3K/Akt signaling | [122,123] |
| 25 | Quercetin | 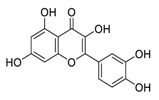 | Inhibited proliferation and induced apoptosis | [124] |
| 26 | Silibinin | 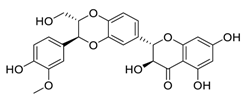 | Inhibited cell proliferation, migration, invasion, and EMT expression | [125] |
| 27 | Sinomenine | 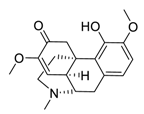 | Downregulated expression of MMPs and miR-21, suppressed α7 nicotinic acetylcholine receptors expression | [126,127,128] |
| 28 | Toxicarioside O | 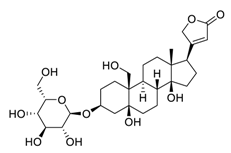 | Decreased the expression of trophoblast cell surface antigen 2, resulting in inhibition of the PI3K/Akt pathway and EMT program | [129] |
| 29 | Vincamine | 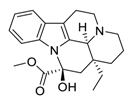 | Interaction with the apoptotic protein caspase-3 | [130] |
| 30 | Xanthohumol | 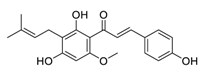 | Suppressed ERK1/2 signaling and reduced the protein levels of FOS-related antigen 1, decreased the mRNA level of cyclin D1 | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. https://doi.org/10.3390/biomedicines9060689
Yang L, Wang Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines. 2021; 9(6):689. https://doi.org/10.3390/biomedicines9060689
Chicago/Turabian StyleYang, Liyan, and Zhonglei Wang. 2021. "Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer" Biomedicines 9, no. 6: 689. https://doi.org/10.3390/biomedicines9060689
APA StyleYang, L., & Wang, Z. (2021). Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines, 9(6), 689. https://doi.org/10.3390/biomedicines9060689






