20-Hydroxyecdysone, from Plant Extracts to Clinical Use: Therapeutic Potential for the Treatment of Neuromuscular, Cardio-Metabolic and Respiratory Diseases
Abstract
1. Purpose
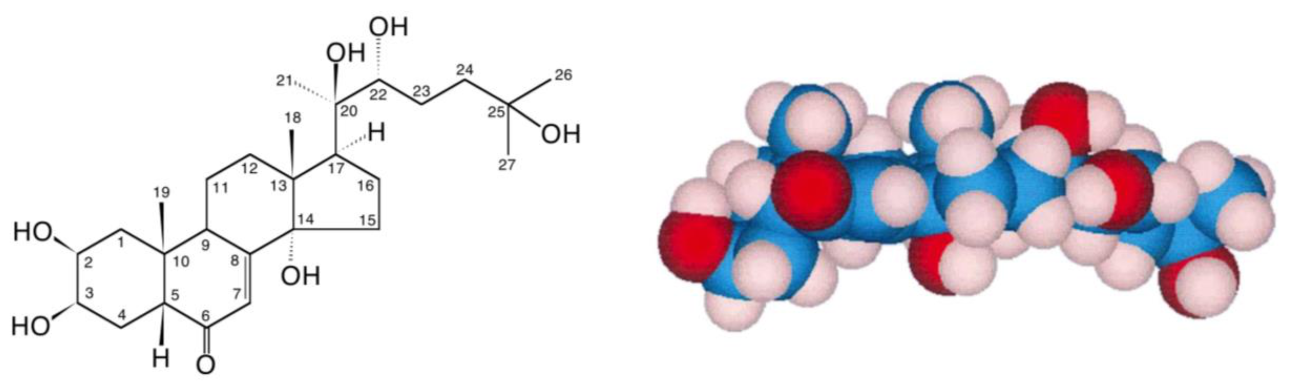
2. Ecdysteroids
3. A Traditional Medicinal Product
4. Pharmacologial Effects of 20E in Animals
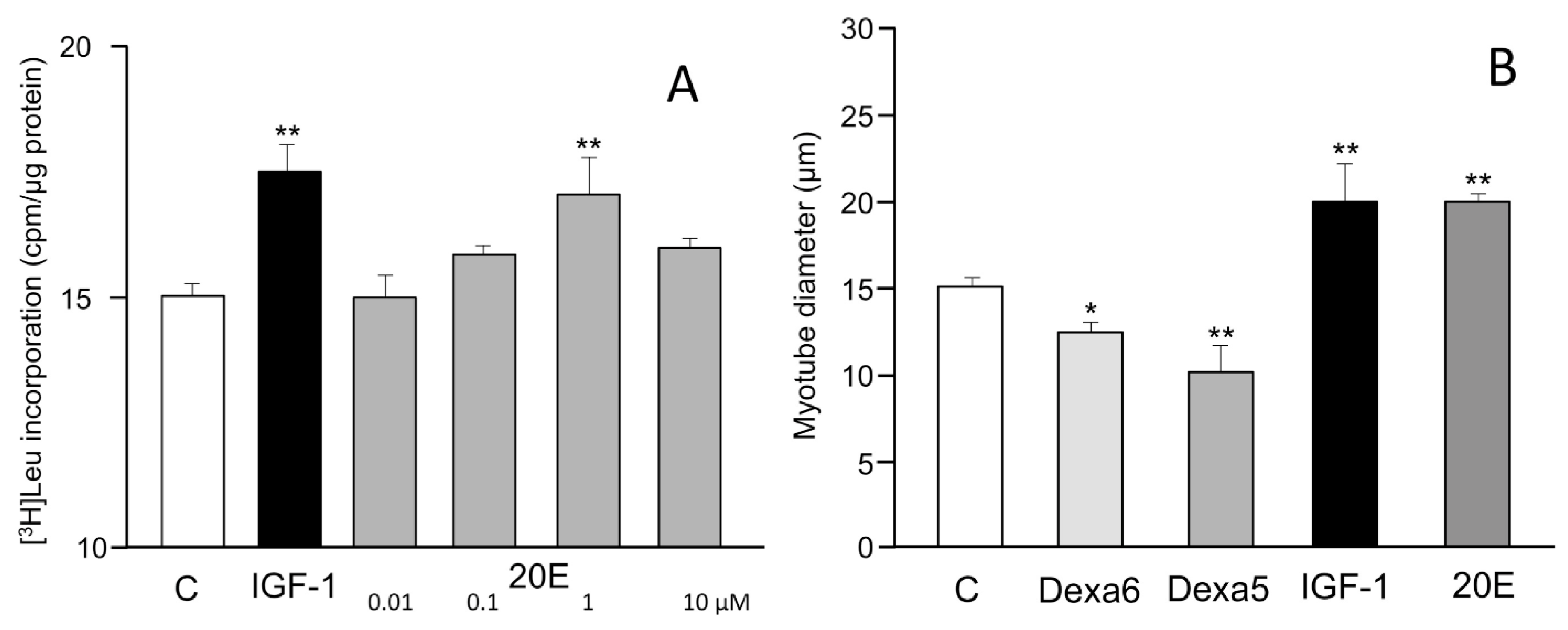
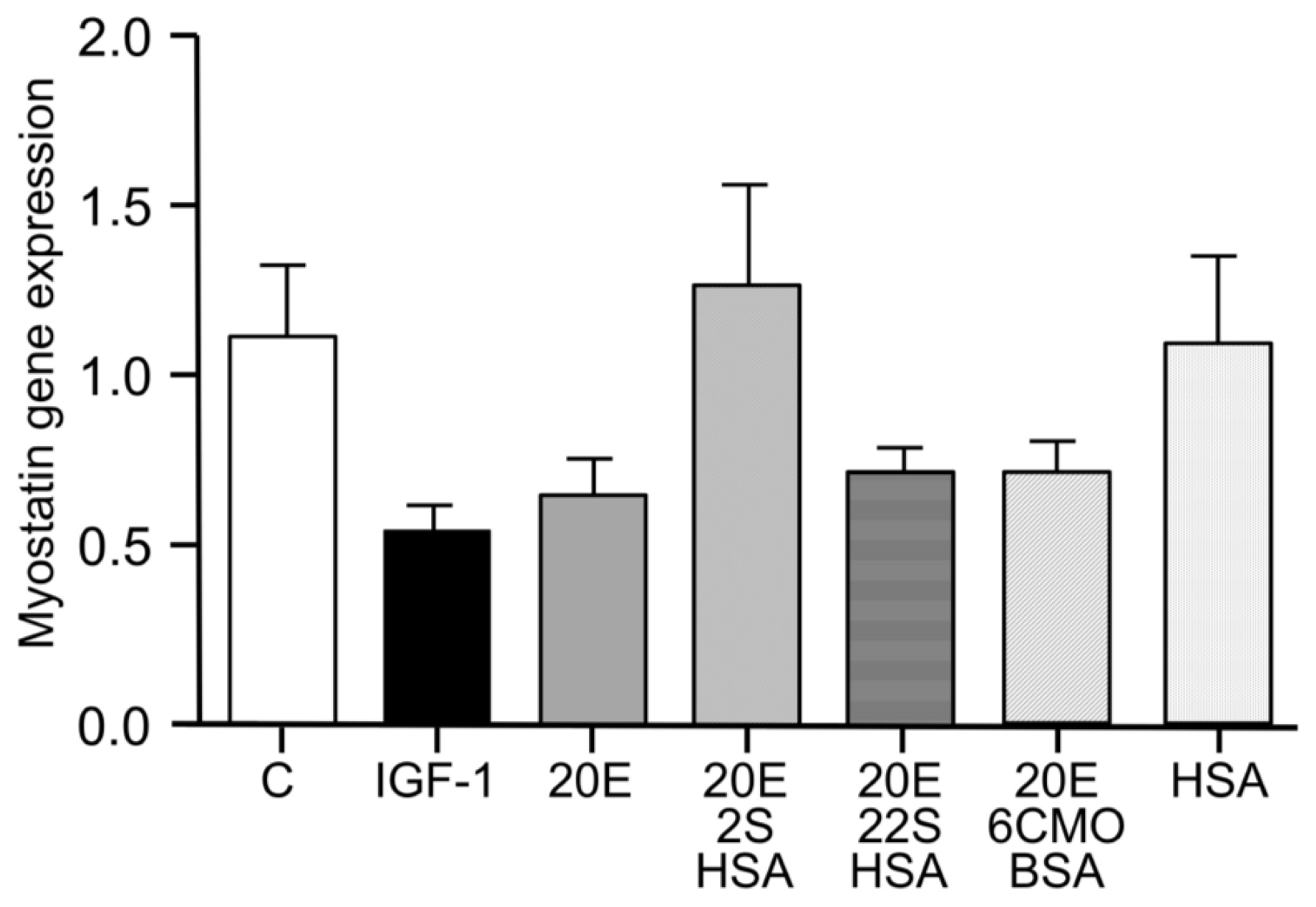
5. Protein Synthesis Stimulatory Effect In Vitro
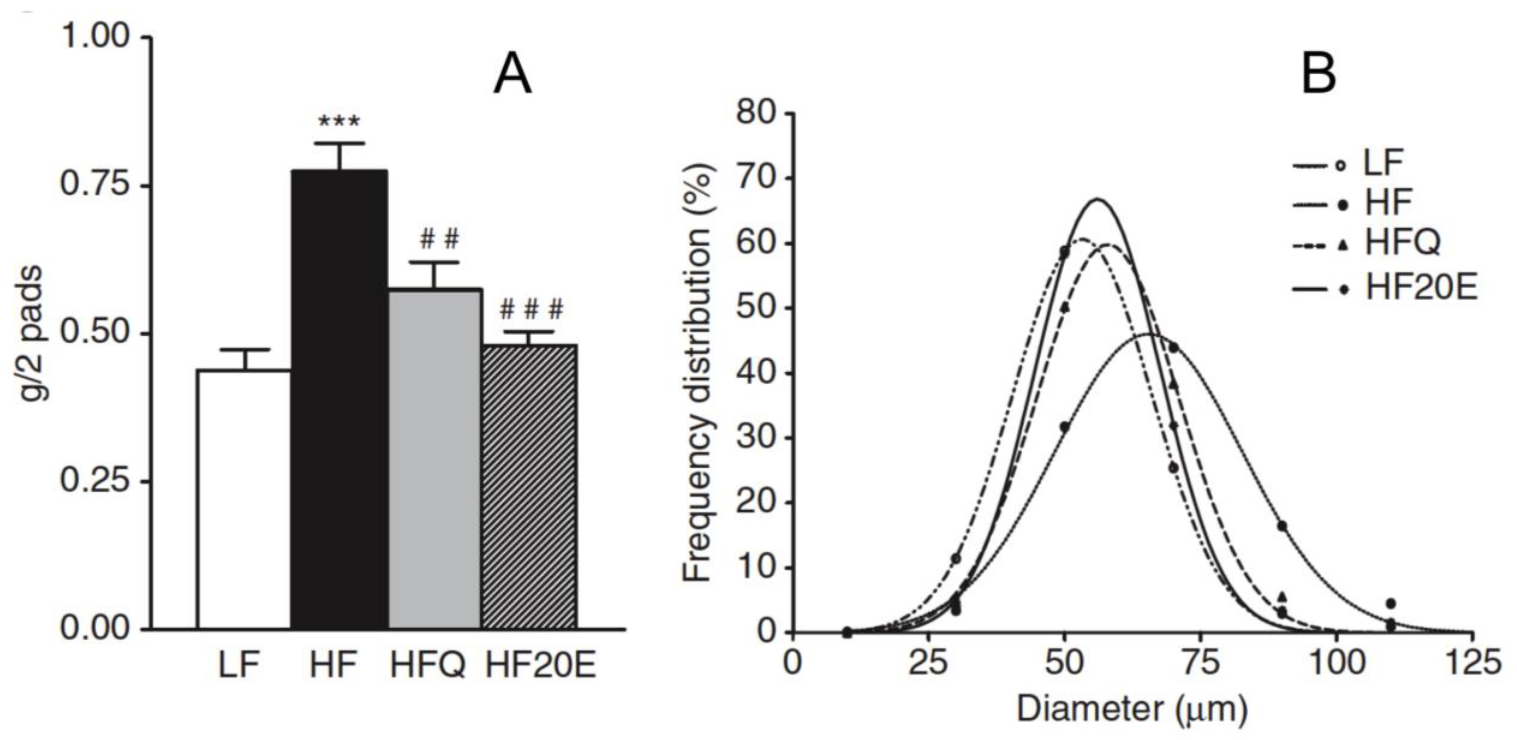
6. Anti-Obesity Effect
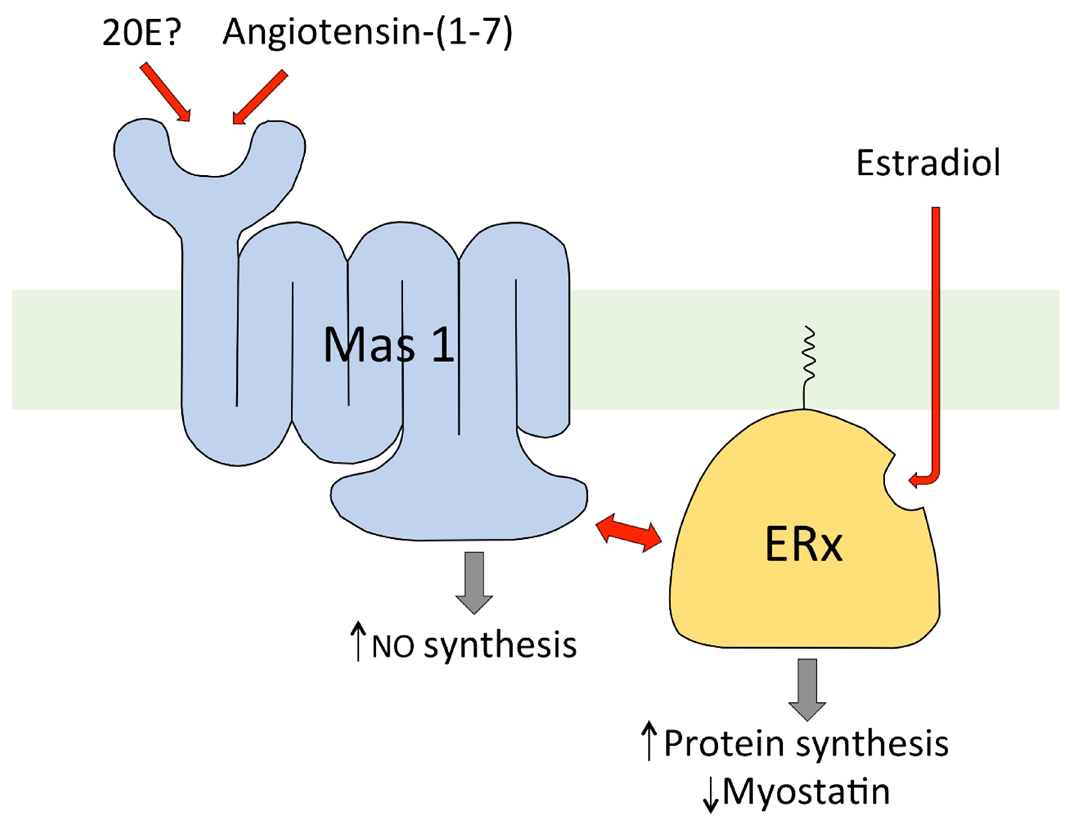
7. Mechanism of Action of 20E in Mammals
- Increases ATP synthesis in muscles [106]
- Stimulates production of erythrocytes [74]
- Decreases hyperglycemia in diabetic animals [63]
- Reduces plasma cholesterol levels [107]
- Decreases the activity of triglyceride lipase [108]
- Protects against experimental atherosclerosis in rabbits [109]
- Activates acetylcholinesterase in the brain [110]
- Activates glutamate decarboxylase (=GABA synthesis) in the brain [111]
- Possesses immunomodulatory activity [112].
8. Toxicity, Bioavailability, Pharmacokinetics and Metabolism in Animal Models
Toxicity
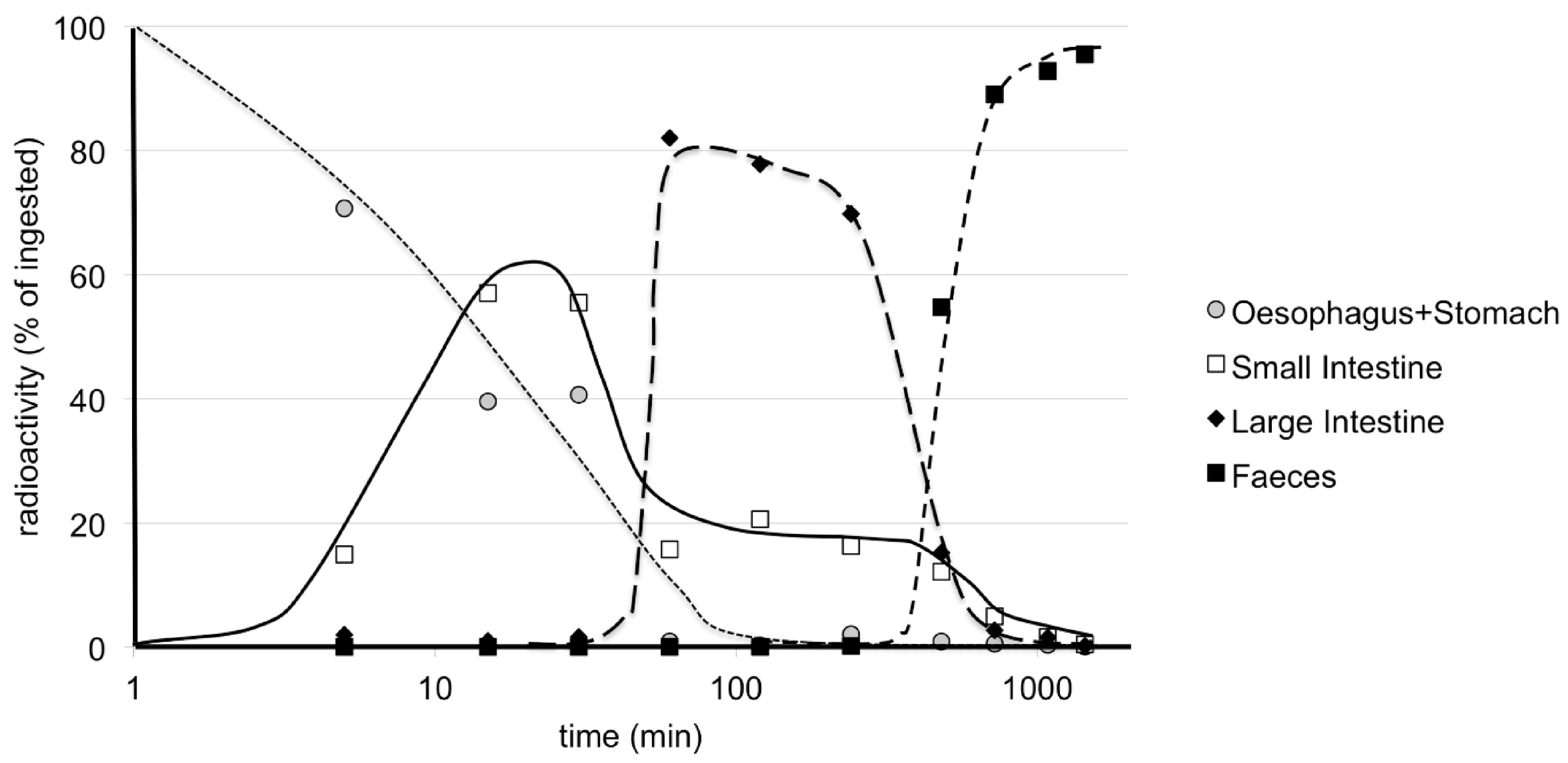
9. Pharmacokinetics and Metabolism in Mice—Early Studies
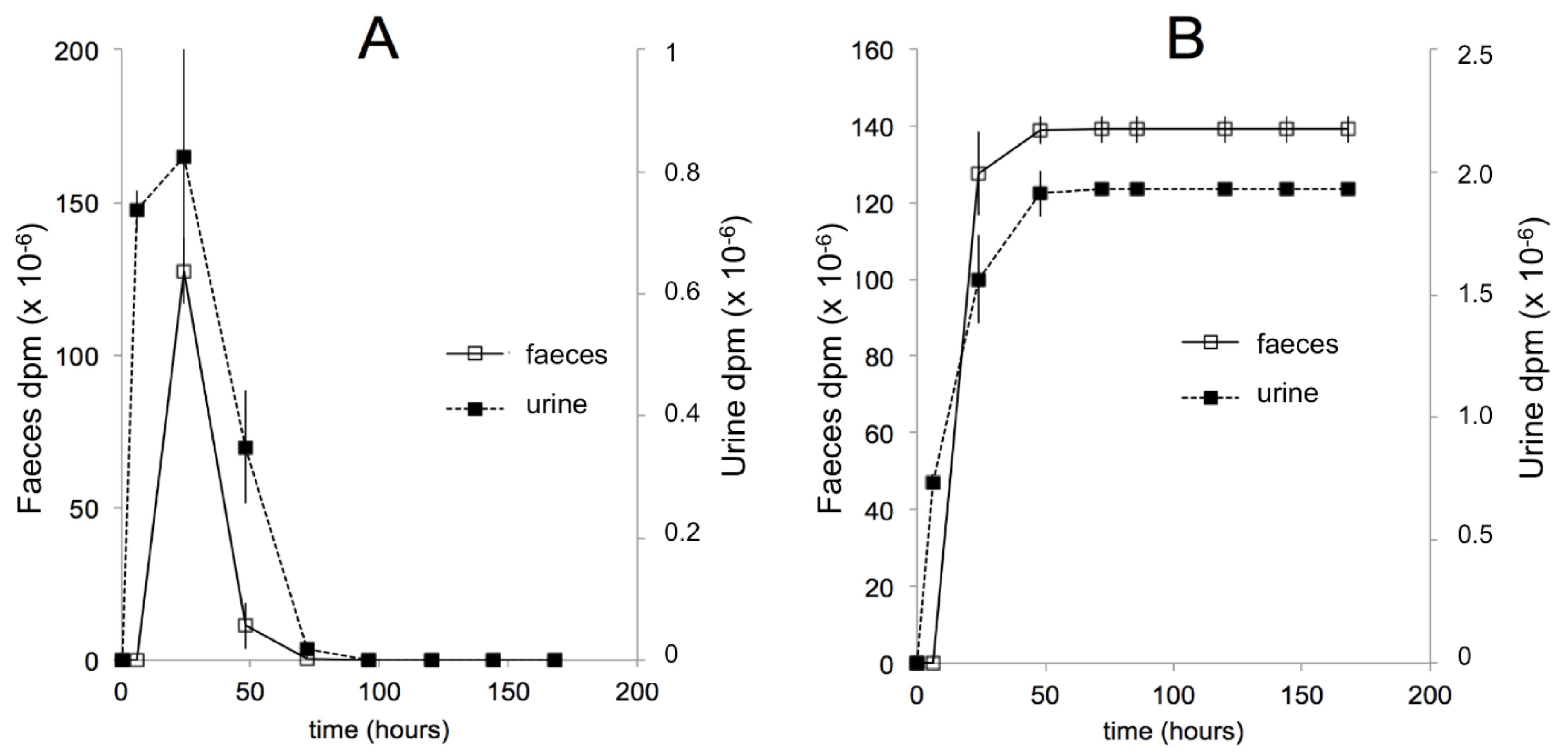
10. Pharmacokinetics after Oral Administration in Mice—More Recent Studies
11. Pharmacokinetics and Metabolism in Humans
11.1. Early Studies
11.2. Recent Studies
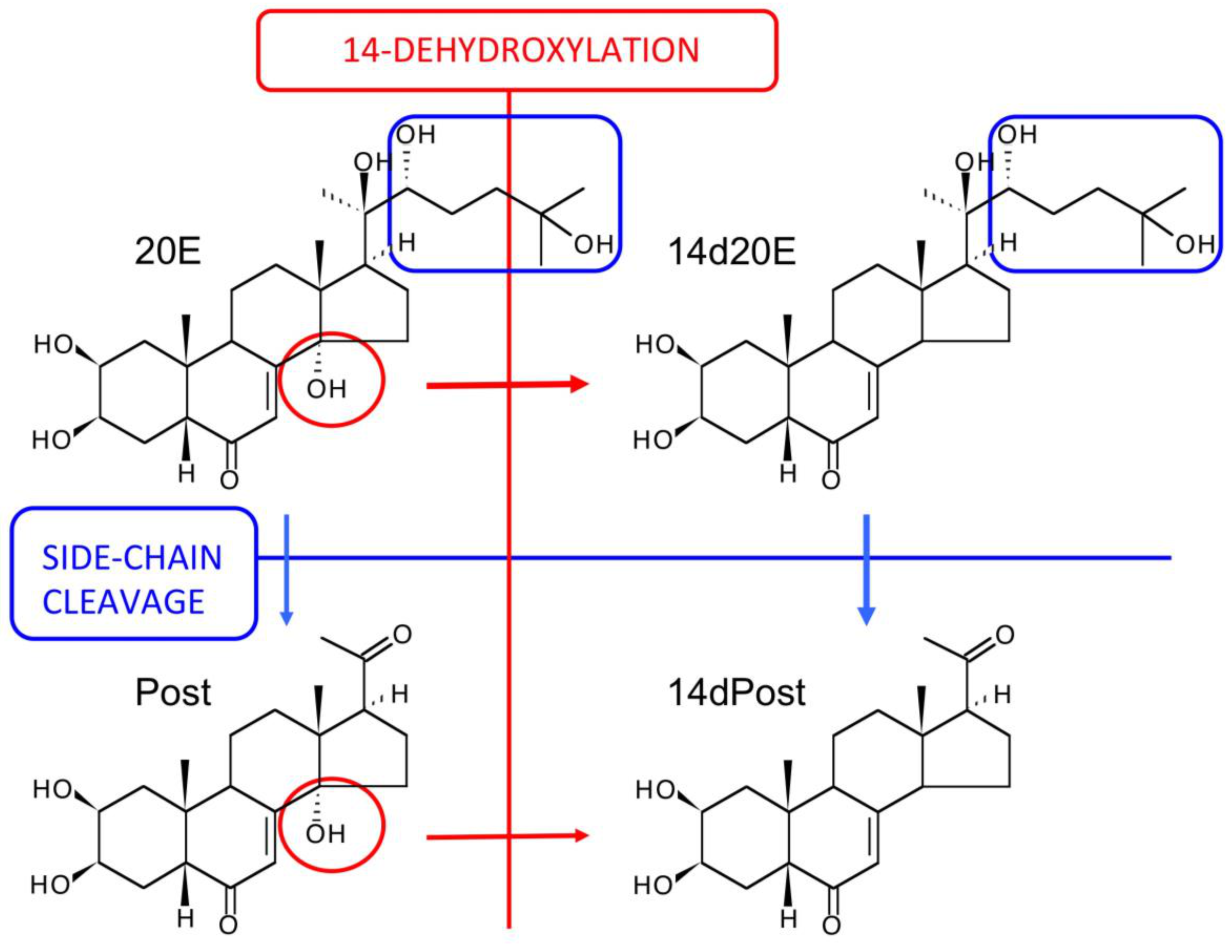
12. The Major Routes of 20E Metabolism in Rodents and Humans
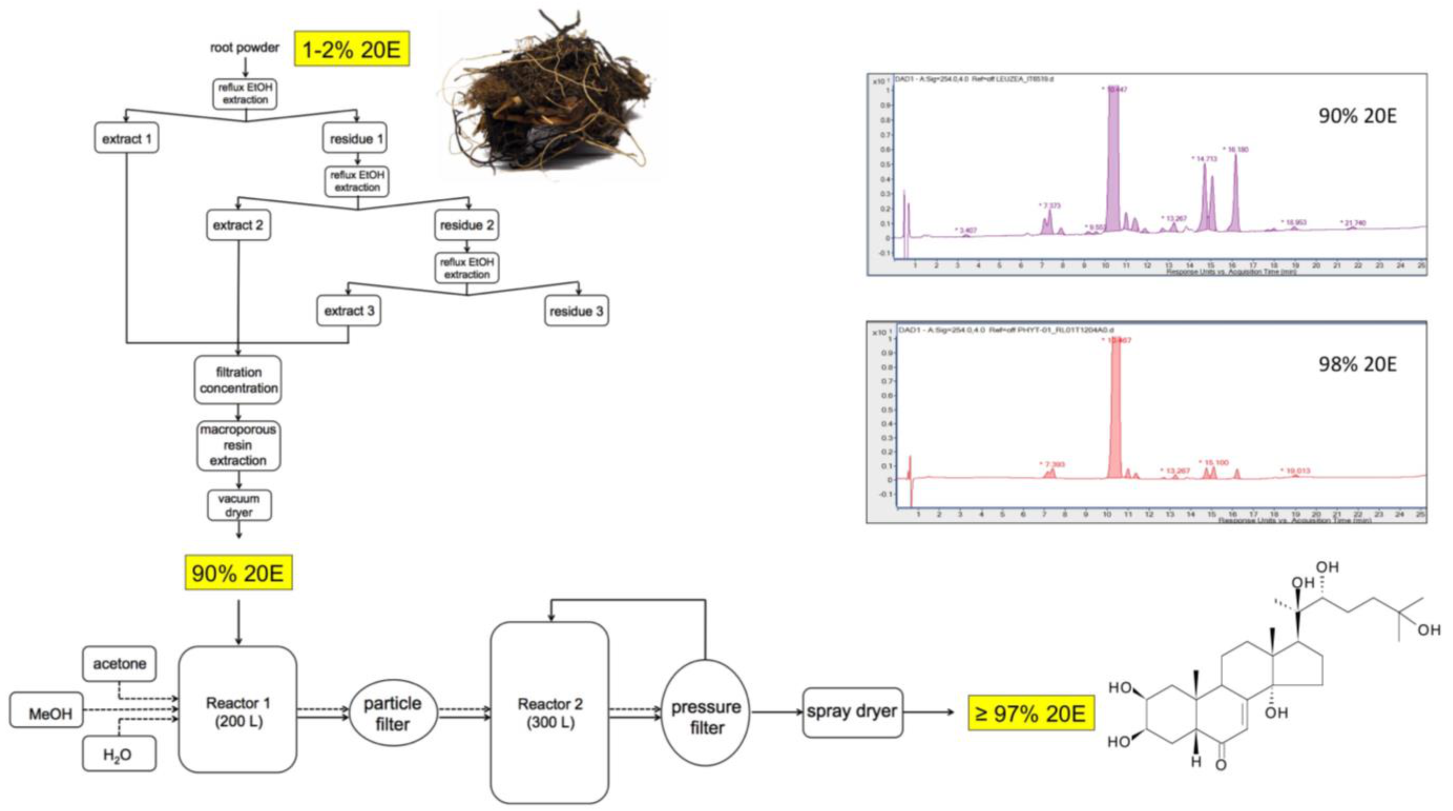
13. Large-Scale Production of Pure 20E for Drug Development
General Considerations
- Total chemical synthesis
- Isolation from the current plant source, e.g., Cyanotis sp. following cultivation and collection in China (Yunnan province)
- Cultivation of another species already identified as a good accumulator with a simple ecdysteroid profile (Rhaponticum, Serratula or Pfaffia sp.)
- Use plant cell or hairy-root cultures of an ecdysteroid-producing plant
- Generate recombinant yeasts using insect and/or plant enzymes of ecdysteroid biosynthesis.
- The plant should accumulate a high amount of 20E
- The plant should have a simple ecdysteroid profile (ideally just 20E)
- The plant should be easy and rapid to grow in accessible areas of the world
- The plant matrix should be amenable to the ready purification of ecdysteroids
- The purification and isolation of 20E should not involve expensive chromatographic methods
- The plant should not be susceptible to pests and diseases
- The species should not be rare or protected
- Culture, harvesting and processing costs should be low; initial processing should take place close to the culture site
14. Plants Species Presently Used to Produce Purified 20E
- Achyranthes (A. aspera, A. bidentata, A. fauriei, A. japonica): Perennial or HHA; warm temperate and sub-tropical plants; native to South-east Asia and/or Africa; all plant parts contain ecdysteroids with seeds containing the highest level (0.25%) in A. aspera and roots (1.74%) being the best source in A. bidentata;
- Cyanotis (C. arachnoidea, C. vaga): Perennial; tropical and sub-tropical plants; growing in tropical Africa, the Indian subcontinent and southern China; roots accumulate very high levels of 20E (up to 5.5% of the dw; Wang et al. [145]); simple ecdysteroid profile—major source for commercial 20E cultivated on a large scale in China;
- Pfaffia (P. glomerata, P. iresinoides): Perennial; tropical plants; native to South America; P. glomerata contains ecdysteroids throughout the plant; roots have a very simple ecdysteroid profile, consisting solely of 20E (0.9% of the dw);
- Rhaponticum (R. carthamoides): Perennial; sub-alpine plant; native to the Altai and Sayan Mountains in Central Asia; roots, flowers and seeds accumulate high levels of 20E (1–2% of dw); complex ecdysteroid profile, but >80% as 20E—used to prepare ECDYSTEN pills containing 5 mg of 20E (OPIH, Uzbekistan) [147];
- Serratula (S. centauroides, S. coronata, S. tinctoria, S. wolfii): Perennial; temperate plants; native to western and central Europe; S. tinctoria accumulates up to 2% of dw as ecdysteroids; the major ecdysteroids are 20E 3-Acetate, 20E and PolB; S. coronata is used to prepare SERPISTEN, a 8:1 mixture of 20E and 25S-inokosterone [148].
15. Purification Process
16. Quality Control and Stability
17. Regulatory Preclinical Studies
Preclinal Regulatory Requirements
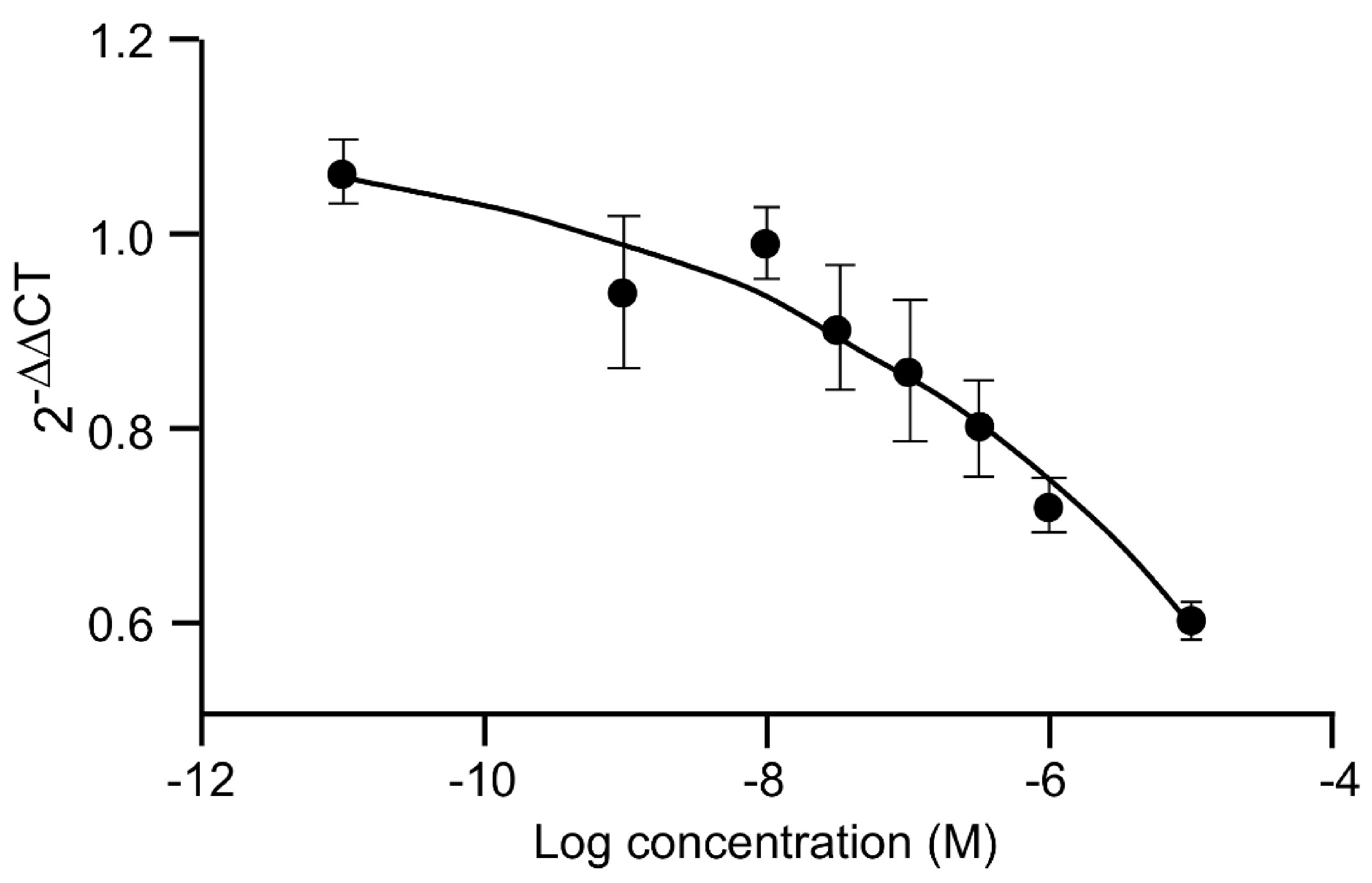
18. Regulatory Studies with 20E Metabolites
18.1. Some Drugability Calculations
18.2. SAR Studies
19. Clinical Studies
20. Neuro-Muscular Diseases
Sarcopenia
21. Cardio-Metabolic Diseases
21.1. Pre-Diabetes
21.2. Obesity
21.3. Menopause
21.4. Metabolic Syndrome
22. Respiratory Diseases
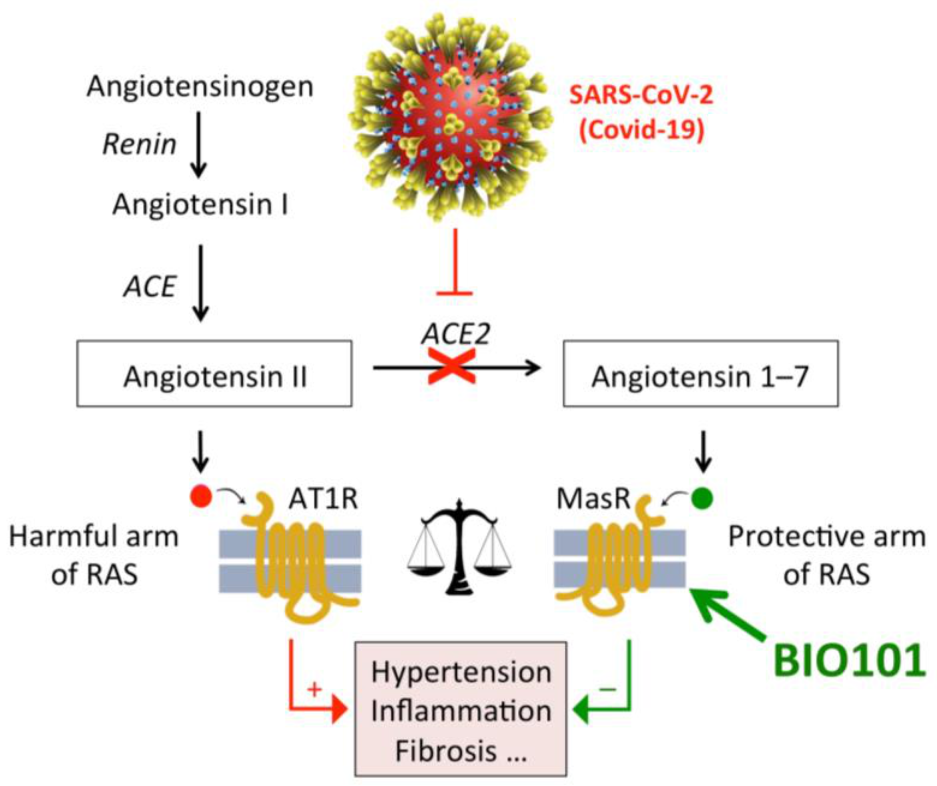
22.1. Respiratory Failure in COVID-19 Patients
22.2. COVID-19
23. Other Diseases
23.1. Hepatitis
23.2. Chronic Glomerulonephritis
23.3. Celiac Disease
23.4. Sexual Dysfunction
23.5. Parasitoses (Giardiasis, Hymenolepiasis, Lambliasis)
24. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| E | ecdysone |
| 20E | 20-hydroxyecdysone |
| 14d20E | 14-deoxy-20-hydroxyecdysone |
| Post | poststerone |
| 14dPost | 14-deoxypoststerone |
| 3-epi-Post | 3-epi-poststerone |
| 16αOHPost | 16α-hydroxypoststerone |
| 20R/SPost | 20-dihydropoststerone |
| 21OHPost | 21-hydroxypoststerone |
| 20,26E | 20,26-dihydroxyecdysone |
| PolB | polypodine B |
References
- Koolman, J. (Ed.) Ecdysone: From Chemistry of Mode of Action; Thieme Verlag: Stuttgart, Germany, 1989; 482p. [Google Scholar]
- Dinan, L.; Savchenko, T.; Whiting, P. On the distribution of phytoecdysteroids in plants. Cell Mol. Life Sci 2001, 58, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L.; Harmatha, J.; Volodin, V.; Lafont, R. Phytoecdysteroids: Diversity, biosynthesis and distribution. In Ecdysone: Structures and Functions; Smagghe, G., Ed.; Springer Science & Business Media B.V.: Berlin, Germany, 2009; pp. 3–45. [Google Scholar]
- Lafont, R.; Harmatha, J.; Marion-Poll, F.; Dinan, L.; Wilson, I.D. The Ecdysone Handbook, 3rd ed. 2002. continuously updated. Available online: http://ecdybase.org/ (accessed on 1 April 2021).
- Dinan, L.; Mamadalieva, N.; Lafont, R. Dietary Phytoecdysteroids. In Handbook of Dietary Phytochemicals; Xiao, J., Sarker, S.D., Asakawa, Y., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 1–54. [Google Scholar]
- Kametani, T.; Tsubuki, M. Strategies for the synthesis of ecdysteroids. In Ecdysone. From Chemistry to Mode of Action; Koolman, J., Ed.; Thieme Verlag: Stuttgart, Germany, 1989; pp. 74–96. [Google Scholar]
- Sláma, K.; Lafont, R. Insect hormones—ecdysteroids: Their presence and action in vertebrates. Eur J. Entomol 1995, 92, 355–377. [Google Scholar]
- Li, T.S.C. Taiwanese Native Medicinal Plants; Taylor & Francis: London, UK, 2006; 328p. [Google Scholar]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X. The genus Achyranthes: A review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2017, 203, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Lee, M.J.; Na, M.S.; Lee, M.Y.; Choi, D. Antioxidant properties of Achyranthis radix extract in rats. J. Ind. Eng. Chem. 2019, 15, 275–280. [Google Scholar] [CrossRef]
- Hsieh, W.T.; Liu, Y.T.; Lin, W.C. Anti-inflammatory properties of Ajuga bracteosa in vivo and in vitro study and their effects on mouse models of liver fibrosis. J. Ethnopharmacol. 2011, 135, 116–125. [Google Scholar] [CrossRef]
- Israili, Z.H.; Lyoussi, B. Ethnopharmacology of the plants of genus Ajuga. Pak. J. Pharm Sci 2009, 22, 425–462. [Google Scholar]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.E.; Balahbid, A.; El-Shazly, M.; Chamkhi, I. Ethnopharmacological use, phytochemistry, pharmacology, and toxicology of Ajuga iva (L.) Schreb. J. Ethnopharmacol. 2020, 258, 112875. [Google Scholar] [CrossRef]
- Cheng, D.M.; Yousef, G.G.; Grace, M.H.; Rogers, R.B.; Gorelick-Feldman, J.; Raskin, I.; Lila, M.A. In vitro production of metabolism-enhancing phytoecdysteroids from Ajuga turkestanica. Plant Cell Tiss Organ Cult. 2008, 93, 73–83. [Google Scholar] [CrossRef]
- Patil, K.S.; Bhaising, S.R. Ethnomedicinal uses, phytochemistry and pharmacological properties of the genus Boerhaavia. J. Ethnopharmacol. 2016, 182, 200–220. [Google Scholar] [CrossRef]
- Ibrahim, B.; Sowemimo, A.; van Rooyen, A.; Van de Venter, M. Antiinflammatory, analgesic and antioxidant activities of Cyathula prostrata (Linn.) Blume (Amaranthaceae). J. Ethnopharmacol. 2012, 141, 282–289. [Google Scholar] [CrossRef]
- Ajuogu, P.K.; Ere, R.; Nodu, M.B.; Nwachikwu, C.U.; Lgbere, O.O. The influence of graded levels of Cyathula prostrata (Linn.) Blume on semen quality characteristics of adult New Zealand white bucks. Transl. Anim. Sci. 2020, 4, 1134–1139. [Google Scholar] [CrossRef]
- Lee, S.; Xiao, C.; Pei, S. Ethnobotanical survey of medicinal plants at periodic market of Honhe Prefecture in Yunnan Province, SW China. J. Ethnopharmacol. 2008, 117, 362–377. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.; Zhou, J.; Wang, X.; Guo, L. Isolation and purification of three ecdysteroids from the stems of Diploclisia glaucescens by high-speed countercurrent chromatography and their anti-inflammatory activities in vitro. Molecules 2017, 22, 1310. [Google Scholar] [CrossRef]
- Schink, M.; Garcia-Käufer, M.; Bertrams, J.; Duckstein, S.M.; Müller, M.B.; Huber, R.; Stintzing, F.C.; Gründemann, C. Differential cytotoxic properties of Helleborus niger L. on tumour and immunocompetent cells. J. Ethnopharmacol. 2015, 159, 129–136. [Google Scholar] [CrossRef]
- Ho, H.; Teai, T.; Bianchini, J.P.; Lafont, R.; Raharivelomanana, P. Ferns: From traditional uses to pharmacological development, chemical identification of active principles. In Working with Ferns: Issues and Applications; Fernández, H., Ed.; Springer Science+Business Media B.V.: Berlin, Germany, 2011; pp. 321–346. [Google Scholar]
- Zhu, L.; Tan, J.; Wang, B.; Guan, L.; Liu, Y.; Zheng, C. In vitro antitumor activity and antifungal activity of Pennogenin steroidal saponins from Paris polyphylla var. yunnanensis. Iranian J. Pharm. Res. 2011, 10, 279–286. [Google Scholar]
- Franco, R.R.; de Almeida Takata, L.; Chagas, K.; Justino, A.B.; Saraiva, A.L.; Goulart, L.R.; de Melo Rodrigues Ávila, V.; Otoni, W.C.; Espindola, F.A.; da Silva, C.R. A 20-hydroxyecdysone-enriched fraction from Pfaffia glomerata (Spreng.) pedersen roots alleviates stress, anxiety, and depression in mice. J. Ethnopharmacol. 2021, 267, 113599. [Google Scholar]
- Abdillahi, H.S.; Finnie, J.F.; Van Staden, J. Anti-inflammatory, antioxidant, anti-tyrosinase and phenolic contents of four Podocarpus species used in traditional medicine in South Africa. J. Ethnopharmacol. 2011, 136, 496–503. [Google Scholar] [CrossRef]
- Gleńsk, M.; Dudek, M.K.; Ciach, M.; Wlodarczyk, M. Isolation and structural determination of flavan-3-ol derivatives from the Polypodium vulgare L. rhizomes water extract. Nat. Prod. Res. 2019, in press. [Google Scholar] [CrossRef]
- Kokoska, L.; Janikova, D. Chemistry and pharmacology of Rhaponticum carthamoides: A review. Phytochemistry 2009, 70, 842–855. [Google Scholar] [CrossRef]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal plants from the 14th edition of the Russia, Pharmacopoeia, recent updates. J. Ethnopharmacol. 2021, 268, 113685. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Yang, W.-Q.; Fan, C.-L.; Zhao, H.-N.; Huang, X.-J.; Wang, Y.; Ye, W.-C. New ecdysteroid and ecdysteroid glycosides from the roots of Serratula chinensis. J. Asian Nat. Prod. Res. 2017, 19, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Das, N.; Dinda, S.; Dinda, M.; SilSarma, I. The genus Sida, L. A traditional medicine: Its ethnopharmacological phytochemical and pharmacological data for commercial exploitation in herbal drug industry. J. Ethnopharmacol. 2015, 176, 135–176. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Pratap, K.; Verma, P.K.; Singh, B.; Padwad, Y. Validation of ethnopharmacological potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J. Ethnopharmacol. 2015, 175, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Dutt, V.; Kaur, N.; Mittal, A.; Dabur, R. Tinospora cordifolia protects from skeletal muscle atrophyby alleviating oxidative stress and inflammation induced by sciatic denervation. J. Ethnopharmacol. 2020, 254, 112720. [Google Scholar] [CrossRef]
- Suksamrarn, A.; Kumpun, S.; Yingyongnarongkul, B. Ecdysteroids from Vitex scabra bark. J. Nat. Prod. 2002, 65, 1690–1692. [Google Scholar] [CrossRef]
- Báthori, M. Phytoecdysteroids effects on mammalians, isolation and analysis. Mini Rev. Med. Chem. 2002, 2, 285–293. [Google Scholar] [CrossRef]
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: An update. J. Insect. Sci. 2003, 3/7, 30. [Google Scholar]
- Báthori, M.; Pongrácz, Z. Phytoecdysteroids—From isolation to their effects on Humans. Curr. Med. Chem. 2005, 12, 153–172. [Google Scholar] [CrossRef]
- Dinan, L.; Lafont, R. Effects and application of arthropod steroid hormones (ecdysteroids) in mammals. J. Endocrinol. 2006, 191, 1–8. [Google Scholar] [CrossRef]
- Zou, D.; Cao, L.; Chen, Q. Advances in pharmacological research on ecdysteroids. Zhongguo Xinyao Zazhi 2008, 17, 371–374. [Google Scholar]
- Báthori, M.; Tóth, N.; Hunyadi, A.; Márki, A.; Zador, E. Phytoecdysteroids and anabolic-androgenic steroids—Structure and effects on Humans. Curr. Med. Chem. 2008, 15, 75–91. [Google Scholar] [CrossRef]
- Lafont, R.; Dinan, L. Innovative and future applications of ecdysteroids. In Ecdysone: Structures and Functions; Smagghe, G., Ed.; Springer Science & Business Media B.V.: Berlin, Germany, 2009; pp. 551–578. [Google Scholar]
- Cahliková, L.; Macáková, K.; Chlebek, J.; Hošt’álková, A.; Kulkhánková, A.; Opletal, L. Ecdysterone and its activity on some degenerative diseases. Nat. Prod. Commun. 2011, 6, 707–718. [Google Scholar] [CrossRef]
- Lafont, R. Recent progress in ecdysteroid pharmacology. Theor Appl. Ecol. 2012, 6–12. [Google Scholar]
- Laekeman, G.; Vlietinck, A. Phytoecdysteroids: Phytochemistry and pharmacological activity. In Natural Products; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin, Germany, 2013; pp. 3827–3849. [Google Scholar]
- Bajguz, A.; Bakala, I.; Talarek, M. Ecdysteroids in plants and their pharmacological effects in vertebrates and Humans. In Studies in Natural Product Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier, B.V.: Amsterdam, The Netherlands, 2015; Volume 45, Chapter 5; pp. 121–145. [Google Scholar]
- Dinan, L.; Lafont, R. Phytoecdysteroids occurrence, distribution, biosynthesis, metabolism, mode of action and applications: Developments from 2005 to 2015. Pharm Bull. J. (Kazakhstan) 2015, 9–37. [Google Scholar]
- Das, N.; Mishra, S.K.; Bishayee, A.; Ali, E.S.; Bishayee, A. The phytochemical, biological, and medicinal attributes of phytoecdysteroids: An updated review. Acta Pharm. Sinica B 2020, in press. [Google Scholar] [CrossRef]
- Chermnykh, N.S.; Shimanovsky, N.L.; Shutko, G.V.; Syrov, V.N. Effects of methandrostenolone and ecdysterone on physical endurance of animals and protein metabolism in the skeletal muscles. Farmakol. Toksikol. 1988, 6, 57–62. [Google Scholar]
- Tóth, N.; Szabó, A.; Kacsala, P.; Héger, J.; Zádor, E. 20-Hydroxyecdysone increases fiber size in a muscle-specific fashion in rat. Phytomedicine 2008, 15, 691–698. [Google Scholar] [CrossRef]
- Syrov, V.N. Comparative experimental investigations of the anabolic activity of ecdysteroids and steranabols. Pharm. Chem. J. 2000, 34, 193–197. [Google Scholar] [CrossRef]
- Gorelick-Feldman, J.; MacLean, D.; Ilic, N.; Poulev, A.; Lila, M.A.; Cheng, D.; Raskin, I. Phytoecdysteroids increase protein synthesis in skeletal muscle cells. J. Agric. Food Chem. 2008, 56, 3532–3537. [Google Scholar] [CrossRef]
- Gorelick-Feldman, J.; Cohick, W.; Raskin, I. Ecdysteroids elicit a rapid Ca2+ flux leading to Akt activation and increased protein synthesis in skeletal muscle cells. Steroids 2010, 70, 632–637. [Google Scholar] [CrossRef]
- Goreclick-Feldman, J.I. Phytoecdysteroids: Understanding their Anabolic Activity. Ph.D. Thesis, Rutgers University, Newark, NJ, USA, 2009. Available online: https://rucore.libraries.rutgers.edu/rutgers-lib/25806/ (accessed on 1 April 2021).
- Lawrence, M.M. Ajuga turkestanica as a Countermeasure against Sarcopenia and Dynapenia. Master’s Thesis, Appalachian State University, Boone, NC, USA, 2012. [Google Scholar]
- Zubeldia, J.M.; Hernández-Santana, A.; Jiménez-del-Rio, M.; Pérez-López, V.; Pérez-Machín, R.; Garcia-Castellano, J.M. In vitro characterization of the efficacy and safety profile of a proprietary Ajuga turkestanica extract. Chinese Med. 2012, 3, 215–222. [Google Scholar] [CrossRef]
- Parr, M.K.; Zhao, P.; Haupt, O.; Tchoukouegno Ngueu, S.; Hengevoss, J.; Fritzemeier, K.H.; Piechotta, M.; Schlörer, N.; Muhn, P.; Zheng, W.Y.; et al. Estrogen receptor beta is involved in skeletal muscle hypertrophy induced by the phytoecdysteroid ecdysterone. Mol. Nutr. Food Res. 2014, 58, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Syrov, V.N.; Khushbaktova, Z.A.; Abzalova, M.K.; Sultanov, M.B. On the hypolipidemic and antiatherosclerotic action of phytoecdysteroids. Doklady Akademii Nauk Uzbekistan SSR 1983, 44–45. [Google Scholar]
- Kizelsztein, P.; Govorko, D.; Komarnytsky, S.; Evans, A.; Wang, Z.; Cefalu, W.T.; Raskin, I. 20-Hydroxyecdysone decreases weight and hyperglycemia in a diet-induced obesity mice model. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E433–E439. [Google Scholar] [CrossRef]
- Seidlova-Wuttke, D.; Ehrhardt, C.; Wuttke, W. Metabolic effects of 20-OH-ecdysone in ovariectomized rats. J. Steroid Biochem Mol. Biol 2010, 119, 121–126. [Google Scholar] [CrossRef]
- Syrov, V.N.; Khushbaktova, Z.A.; Nabiev, A.N. An experimental study of the hepatoprotective properties of phytoecdysteroids and nerobol in carbon tetrachloride—Induced liver injury. Eksp. Klin. Farmakol. 1992, 55, 61–65. [Google Scholar]
- Foucault, A.-S.; Mathé, V.; Lafont, R.; Even, P.; Dioh, W.; Veillet, S.; Tomé, D.; Huneau, D.; Hermier, D.; Quignard-Boulangé, A. Quinoa extract enriched in 20-hydroxyecdysone protects mice from diet-induced obesity and modulates adipokines expression. Obesity 2012, 20, 270–277. [Google Scholar] [CrossRef]
- Foucault, A.-S.; Even, P.; Lafont, R.; DIoh, W.; Veillet, S.; Tomé, D.; Huneau, J.F.; Hermier, D.; Quignard-Boulangé, A. Quinoa extract enriched in 20-hydroxyecdysone affects energy homeostasis and intestinal fat absorption in mice fed a high-fat diet. Physiol. Behav. 2014, 128, 226–231. [Google Scholar] [CrossRef]
- Agoun, H.; Semiane, N.; Mallek, A.; Bellahreche, Z.; Hammadi, S.; Madjerab, M.; Abdlalli, M.; Khalkhal, A.; Dahmani, Y. High-carbohydrate diet-induced metabolic disorders in Gerbillus tarabuli (a new model of non-alcoholic fatty liver disease). Protective effects of 20-hydroxyecdysone. Arch. Physiol. Biochem. 2019, 127, 127–135. [Google Scholar] [CrossRef]
- Buniam, J.; Chukijrungroat, N.; Rattanavichit, Y.; Surapongchai, J.; Weerachayaphom, J.; Bupha-Intr, T.; Saengsirisuwan, V. 20-Hydroxyecdysone ameliorates metabolic and cardiovascular dysfunction in high-fat-high-fructose-fed ovariectomized rats. BMC Complement. Med. Ther. 2020, 20, 140. [Google Scholar] [CrossRef]
- Yoshida, T.; Otaka, T.; Uchiyama, M.; Ogawa, S. Effect of ecdysterone on hyperglycemia in experimental animals. Biochem. Pharmacol. 1971, 20, 3263–3268. [Google Scholar] [CrossRef]
- Sundaram, R.; Naresh, R.; Shanthi, P.; Sachdanandam, P. Efficacy of 20-OH-ecdysone on hepatic key enzymes of carbohydrate metabolism in streptozotocin induced diabetic rats. Phytomedicine 2012, 19, 725–729. [Google Scholar] [CrossRef]
- Sundaram, R.; Naresh, R.; Shanthi, P.; Sachdanandam, P. Ameliorative effect of 20-OH ecdysone on streptozotocin induced oxidative stress and β-cell damage in experimental hyperglycemic rats. Process. Biochem. 2012, 47, 2072–2080. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, S.; Huang, M.; Ma, X.; Yang, J.; Deng, S.; Huang, Y.; Wen, Y.; Yang, X. β-edysterone from Cyanotis arachnoidea exerts hypoglycemic effects through activation of IRS-1/Akt/GLUT4 and IRS-1/Akt/GLUT2 signal pathways in KK-Ay mice. J. Funct. Foods 2017, 39, 123–132. [Google Scholar] [CrossRef]
- Hung, T.J.; Chen, W.M.; Liu, S.F.; Liao, T.N.; Lee, T.C.; Chuang, L.Y.; Guh, J.Y.; Hung, C.Y.; Hung, H.J.; Chen, P.Y.; et al. 20-Hydroxyecdysone attenuates TGF-β1-induced renal cellular fibrosis in proximal tubule cells. J. Diabetes Complicat. 2012, 26, 463–469. [Google Scholar] [CrossRef]
- Kurmukov, A.G.; Syrov, V.N. Anti-inflammatory properties of ecdysterone. Meditsinskii Zhurnal Uzb. 1988, 68–70. [Google Scholar]
- Song, G.; Xia, X.C.; Zhang, K.; Yu, R.; Li, B.; Li, M.; Yu, X.; Zhang, J.; Xue, S. Protective effect of 20-hydroxy-ecdysterone against lipopolysaccharides-induced acute lung injury in mice. J. Pharm. Drug Res. 2019, 2, 109–114. [Google Scholar]
- Cai, Y.J.; Dai, J.Q.; Fang, J.G.; Ma, L.P.; Hou, L.F.; Yang, L.; Liu, Z.L. Antioxidative and free radical scavenging effects of ecdysteroids from Serratula strangulata. Can. J. Physiol. Pharmacol. 2002, 80, 1187–1194. [Google Scholar] [CrossRef]
- Azizov, A.P. Effects of eleutherococcus, elton, leuzea, and leveton on the blood coagulation system during training in athletes. Eksp Klin Farmakol 1997, 60, 58–60. [Google Scholar]
- Xia, X.C.; Xue, S.P.; Wang, X.Y.; Liu, R. Effects of 20-hydroxyecdysone on expression of inflammatory cytokines in acute lung injury mice. Mod. Prev. Med. 2016, 5. [Google Scholar]
- Dongmo, A.B.; Nkeng-Efouet, P.A.; Devkota, K.P.; Wegener, J.W.; Sewald, N.; Wagner, H.; Vierling, W. Tetra-acetylajugasterone a new constituent of Vitex cienkowskii with vasorelaxant activity. Phytomed 2014, 21, 787–792. [Google Scholar] [CrossRef]
- Syrov, V.N.; Nasyrova, S.S.; Khushbaktova, Z.A. The results of experimental study of phytoecdysteroids as erythropoiesis stimulators in laboratory animals. Eksperimental’naia i Klin. Farmakol. 1997, 60, 41–44. [Google Scholar]
- Chen, Z.; Zhu, G.; Zhang, J.H.; Liu, Z.; Tang, W.; Feng, H. Ecdysterone-sensitive smooth muscle cell proliferation stimulated by conditioned medium of endothelial cells cultured with bloody cerebrospinal fluid. Acta Neurochir Suppl. 2008, 104, 183–187. [Google Scholar]
- Luo, H.; Yi, B.; Fan, W.; Chen, K.; Gui, L.; Chen, Z.; Li, L.; Feng, H.; Chi, L. Enhanced angiogenesis and astrocyte activation by ecdysterone treatment in a focal cerebral ischemia rat model. Acta Neurochirurgica Suppl. 2011, 110, 151–155. [Google Scholar]
- Kurmukov, A.G.; Ermishina, O.A. The effect of ecdysterone on experimental arrhythmias and changes in the hemodynamics and myocardial contractility induced by coronary artery occlusion. Farmakol. Toksikol. 1991, 54, 27–29. [Google Scholar] [PubMed]
- Korkach, Y.P.; Kotsyuruba, A.V.; Psryslazhnam, O.D.; Mohyl’nyts’ka, L.D.; Sahach, V.F. NO-dependent mechanisms of ecdysterone protective action on the heart and vessels in streptozotocin-induced diabetes mellitus in rats. Fiziol. Zhurnal 2007, 53, 3–8. [Google Scholar]
- Xia, X.; Zhang, Q.; Liang, G.; Lu, S.; Yang, Y.; Tian, Y. Role of 20-hydroxyecdysone in protecting rats against diabetic cardiomyopathy. Chln J. Geriatr Heart Brain Vessel Dis. 2013, 15, 412–415. [Google Scholar]
- Dilda, P.; Latil, M.; Didry-Barca, B.; On, S.; Serova, M.; Veillet, S.; Lafont, R. BIO101 demonstrates combined beneficial effects on skeletal muscle and respiratory functions in a mouse model of Duchenne muscular dystrophy. World Muscle Society WMS 2019, Copenhagen, Denmark (1-5/10/2019). Neuromuscul. Disord. 2019, 29, S158. [Google Scholar] [CrossRef]
- Latil, M.; Bézier, C.; Cottin, S.; Lafont, R.; Veillet, S.; Dilda, P.; Charbonnier, F.; Biondi, O. BIO101 demonstrates combined beneficial effects on muscle and motor neurons in a mouse model of severe spinal muscular atrophy. World Muscle Society WMS 2019, Copenhagen, (1-5/10/2019). Neuromuscul. Disord. 2019, 29, S189. [Google Scholar] [CrossRef]
- Luo, H.; Luo, C.; Zhang, Y.; Chi, L.; Li, L.; Chen, K. Effect of ecdysterone on injury of lipid peroxidation following focal cerebral ischemia in rats. Zhongguo Yaoye 2009, 18, 12–14. [Google Scholar]
- Liu, Z.; Chen, Y.; Chen, Z.; Tang, W.; Zhu, G.; Wang, X.; Feng, H. Effect of ecdysterone on the nervous lesions of rabbits acquired after subarachnoid hemorrhage. Med. J. Chin. People’s Lib. Army 2011, 36, 1351–1353. [Google Scholar]
- Hu, J.; Luo, C.X.; Chu, W.H.; Shen, Y.A.; Qian, Z.-M.; Zhu, G.; Yu, Y.B.; Feng, H. 20-Hydroxyecdysone protects against oxidative stress-induced neuronal injury by scavenging free radicals and modulating NF-kB and JNK pathways. PLoS ONE 2012, 7, e50764. [Google Scholar] [CrossRef]
- Shakhmurova, G.A.; Khushbaktova, Z.A.; Syrov, V.N. Estimation of hepatoprotective and immunocorrecting effects of the sum of phytoecdysteroids from Silene viridiflora in experimental animals treated with tetrachlormethan. O’zbekiston Biol. J. 2010, 16–20. [Google Scholar]
- Xia, X.; Zhang, Q.; Wang, Z.; Gui, G.; Liang, G.; Liu, R. Protective effect of 20-hydroxyecdysone on diabetic hepathopathy of rats. Xiandai Yufang Yixue 2013, 40, 4031–4034. [Google Scholar]
- Wu, X.; Jiang, Y.; Fan, S.; Wang, R.; Xiang, M.; Niu, H.; Li, T. Effects of ecdysterone on rat lung reperfusion injury. Chin. Pharm. Bull. 1998, 14, 256–258. [Google Scholar]
- Li, J.; Wu, X.; Zhang, J.; Wu, X.; Gao, D.; Shen, T.; Gu, C. Effect of ecdysterone on the expression of toll-like receptor 4 and surfactant protein A in lung tissue of rats with acute lung injury. Infect. Inflamm. Repair 2013, 22–26. [Google Scholar]
- Zou, D.; Xu, Z.; Cao, L.; Chen, Q. Effects of ecdysterone on early stage diabetic nephropathy in streptozotocin-induced diabetic rats. Chin. J. New Drugs Clin. Remedies 2010, 29, 842–846. [Google Scholar]
- Shakhmurova, G.A.; Syrov, V.N.; Khushbaktova, Z.A. Immunomodulating and antistress activity of ecdysterone and turkesterone under immobilization-induced stress conditions in mice. Pharm. Chem. J. 2010, 44, 7–9. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, X.; Liao, J.; Wu, C.; Zhang, Y.; Zhang, Z. Effect of ecdysterone on the healing of gastric ulcer in model rats. China Pharm. 2010, 21, 2332–2335. [Google Scholar]
- Gao, L.; Cai, G.; Shi, X. β-Ecdysterone induces osteogenic differentiation in mouse mesenchymal stem cells and relieves osteoporosis. Biol. Pharm. Bull. 2009, 31, 2245–2249. [Google Scholar] [CrossRef]
- Kapur, P.; Wuttke, W.; Jarry, H.; Seidlova-Wuttke, D. Beneficial effects of β-ecdysone on the joint, epiphyseal cartilage tissue and trabecular bone in ovariectomized rats. Phytomed 2010, 17, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Seidlova-Wuttke, D.; Christel, D.; Kapur, P.; Nguyen, B.T.; Jarry, H.; Wuttke, W. β-Ecdysone has bone protective but no estrogenic effects in ovariectomized rats. Phytomedicine 2010, 17, 884–899. [Google Scholar] [CrossRef]
- Dai, W.; Zhang, H.; Zhong, Z.A.; Jiang, L.; Chen, H.; Lay, Y.A.; Kot, A.; Ritchie, R.O.; Lane, N.E.; Yao, W. β-Ecdysone augments peak bone mass in mice of both sexes. Clin. Orthop Relat Res. 2015, 473, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Jiang, L.; Evan Lay, Y.-A.; Chen, H.; Jin, G.; Zhang, H.; Kot, A.; Ritchie, R.O.; Lane, N.E.; Yao, W. Prevention of glucocorticoid induced bone changes with beta-ecdysone. Bone 2015, 74, 48–57. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Zhang, W.; Xia, L. Protective effect of ecdysterone on rabbits chondrocytes that is injured by lipopolysaccharide. Tianjin Med J. 2015, 587–590. [Google Scholar]
- Wen, F.; Yu, J.; He, C.J.; Zhang, Z.W.; Yang, A.F. β-ecdysterone protects against apoptosis by promoting autophagy in nucleus pulposus cells and ameliorates disc degeneration. Mol. Med. Rep. 2019, 19, 2440–2448. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Z.; He, J. Effect of β-ecdysterone on the proliferation, differentiation and apoptosis of rat osteoblasts induced by high glucose. Chin. J. Clin. Pharmacol. 2021, 443–446. [Google Scholar]
- Detmar, M.; Dumas, M.; Bonté, F.; Meybeck, A.; Orfanos, C.E. Effects of ecdysterone on the differentiation of normal keratinocytes in vitro. Eur. J. Dermatol. 1994, 4, 558–562. [Google Scholar]
- Zhegn, G.Y.; Wu, X.; Li, Y.L.; Zfang, J.H.; Wang, W.J. Preparation and dose-effect analysis of ecdysterone cream for promoting wound healing. J. Southern Med. Univ. 2008, 28, 828–831. [Google Scholar]
- Ehrhardt, C.; Wessels, J.T.; Wuttke, W.; Seidlova-Wuttke, D. The effects of 20-hydroxyecdysone and 17β-estradiol on the skin of ovariectomized rats. Menopause 2011, 18, 323–327. [Google Scholar] [CrossRef]
- Dilda, P.; Foucault, A.S.; Serova, M.; On, S.; Raynal, S.; Veillet, S.; Dioh, W.; Lafont, R. BIO101, a drug candidate targeting Mas receptor for the treatment of age-reated muscle degeneration. From molecular target identification to clinical development. J. Cachexia Sarcopenia Muscle 2016, 7, 655. [Google Scholar]
- Antoshechkin, A.G. Selective plant extracts and their combination as nutritional therapeutic remedies. J. Nutr Ther. 2016, 5, 1–11. [Google Scholar] [CrossRef]
- Syrov, V.N.; Kurmukov, A.G. Anabolic activity of phytoecdysone—ecdysterone—isolated from Rhaponticum carthamoides (Will.) Illjin. Farmakol. Toksikol. 1967, 39, 690–693. [Google Scholar]
- Kholodova, I.D.; Tugai, V.A.; Zimina, V.P. Effect of vitamin D3 and 20-hydroxyecdysone on the content of ATP, creatine phosphate, carnosine and Ca2+ in skeletal muscles. Ukr. Biokhimicheskii Zhurnal 1997, 69, 3–9. [Google Scholar]
- Lupien, P.J.; Hinse, C.; Chaudhary, K.D. Ecdysterone as a hypoholesterolemic agent. Arch. Int. Physiol. Biochim. 1969, 77, 206–212. [Google Scholar]
- Catalan, R.E.; Martinez, A.M.; Aragones, M.D.; Miguel, B.G.; Robles, A.; Godoy, J.E. Alterations in rat lipid metabolism following ecdysterone treatment. Comp. Biochem. Physiol. 1985, 81b, 771–775. [Google Scholar] [CrossRef]
- Matsuda, H.; Kawaba, T.; Yamamoto, Y.; Ogawa, S. Effect of ecdysterone on experimental atherosclerosis in rabbits. Nippon Yakurigaku Zasshi 1974, 70, 325–339. [Google Scholar] [CrossRef]
- Catalan, R.E.; Aragones, M.D.; Godoy, J.E.; Martinez, A.M. Ecdysterone induces acetylcholinesterase in mammalian brain. Comp. Biochem. Physiol. 1984, 78c, 193–195. [Google Scholar] [CrossRef]
- Chaudhary, K.D.; Lupien, P.J.; Hinse, C. Effect of ecdysone on glutamic decarboxylase in rat brain. Experientia 1969, 25, 250–251. [Google Scholar] [CrossRef]
- Chiang, H.C.; Wang, J.J.; Wu, R.T. Immunomodulating effects of the hydrolysis products of formosamin C and β-ecdysone from Paris formosana Hayata. Anticancer Res. 1992, 12, 1475–1478. [Google Scholar]
- Lafont, R.; Raynal, S.; Serova, M.; Didry-Barca, B.; Guibout, L.; Dinan, L.; Latil, M.; Dilda, P.J.; Dioh, W.; Veillet, S. 20-Hydroxyecdysone activates the protective arm of the renin angiotensin system via Mas receptor. bioRxiv 2020. [Google Scholar] [CrossRef]
- Raynal, S.; Foucault, A.-S.; Ben Massoud, S.; Dioh, W.; Lafont, R.; Veillet, S. BIO101, a drug candidate targeting sarcopenic obesity through MAS receptor activation. J. Cachexia Sarcopenia Muscle 2015, 6, 429. [Google Scholar]
- Yoshida, T.; Galvez, S.; Tiwari, S.; Rezk, B.M.; Semprun-Prieto, L.; Higashi, Y.; Sukhanov, S.; Yablonka-Reuveni, Z.; Delafontaine, P. Angiotensin II inhibits satellite cell proliferation and prevents skeletal muscle regeneration. J. Biol. Chem. 2013, 288, 23823–23832. [Google Scholar] [CrossRef]
- Band, M.M.; Sumukadas, D.; Struthers, A.D.; Avenell, A.; Donnan, P.T.; Kemp, P.R.; Smith, K.T.; Hume, C.L.; Hapca, A.; Witham, M.D. Leucine and ACE inhibitors as therapies for sarcopenia (LACE trial): Study protocol for a randomised controlled trial. Trials 2018, 19, 6. [Google Scholar] [CrossRef]
- Höcht, C.; Mayer, M.; Taira, C.A. Therapeutic perspectives of angiotensin-(1-7) in the treatment of cardiovascular diseases. Open Pharmacol. J. 2009, 3, 21–31. [Google Scholar] [CrossRef][Green Version]
- Parr, M.K.; Botré, F.; Naß, A.; Hengevoss, J.; Diel, P.; Wolber, G. Ecdysteroids: A novel class of anabolic agents? Biol. Sport 2015, 32, 169–173. [Google Scholar] [CrossRef]
- Schreihofer, D.A.; Duong, P.; Cunningham, R.L. N-terminal truncations in sex steroid receptors and rapid steroid actions. Steroids 2018, 133, 15–20. [Google Scholar] [CrossRef]
- Sobrino, A.; Vallejo, S.; Novella, S.; Lázaro-Franco, M.; Mompeón, A.; Bueno-Betí, C.; Walther, T.; Sánchez-Ferrer, C.; Peiró, C. Mas receptor is involved in the estrogen-receptor induced nitric oxide-dependent vasorelaxation. Biochem. Pharmacol. 2017, 129, 67–72. [Google Scholar] [CrossRef]
- Ogawa, S.; Nishimoto, N.; Matsuda, H. Pharmacology of ecdysones un vertebrates. In Invertebrate Endocrinology and Hormonal Heterophylly; Burdette, W.J., Ed.; Springer: Berlin, Germany, 1974; pp. 341–344. [Google Scholar]
- Hikino, H.; Oizumi, Y.; Takemoto, T. Absorption, distribution, metabolism, and excretion of insect-metamorphosing hormone ecdysterone in mice. Chem. Pharm. Bull. (Tokyo) 1972, 20, 2454–2458. [Google Scholar] [CrossRef][Green Version]
- Lafont, R.; Girault, J.-P.; Kerb, U. Excretion and metabolism of injected ecdysone in the white mouse. Biochem. Pharmacol. 1988, 37, 1174–1177. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, S.; Ren, L.; Wang, R.; Bai, X.; Han, H.; Li, B.; Chen, H. Research on the relationship between tissue quantitative distribution of 3H-Achyranthes bidentata ecdysterone and channel-tropism of herbal drugs in mice. China J. Chin. Mater. Med. 2011, 36, 3018–3022. [Google Scholar]
- Girault, J.-P.; Lafont, R.; Kerb, U. Ecdysone catabolism in the white mouse. Drug Metab. Dispos. 1988, 16, 716–720. [Google Scholar] [PubMed]
- Kumpun, S.; Girault, J.-P.; Dinan, L.; Blais, C.; Maria, A.; Dauphin-Villemant, C.; Yingyongnarongkul, B.; Suksamrarn, A.; Lafont, R. The metabolism of 20-hydroxyecdysone in mice: Relevance to pharmacological effects and gene switch applications of ecdysteroids. J. Steroid Biochem. Mol. Biol. 2011, 126, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L.; Balducci, C.; Guibout, L.; Foucault, A.S.; Bakrim, A.; Kumpun, S.; Girault, J.-P.; Tourette, C.; Dioh, W.; Dilda, P.J.; et al. Ecdysteroid metabolism in mammals: The fate of ingested 20-hydroxyecdysone in mice and rats. J. Steroid Biochem. Mol. Biol. 2021, in press. [Google Scholar] [CrossRef]
- Balducci, C.; Dinan, L.; Guibout, L.; Foucault, A.S.; Carbonne, C.; Durand, J.-D.; Caradeux, C.; Bertho, G.; Girault, J.-P.; Lafont, R. The complex metabolism of poststerone in male rats. J. Steroid Biochem. Mol. Biol. 2021, in press. [Google Scholar] [CrossRef]
- Gharib, B.; Nugon-Baudon, L.; Lafont, R.; De Reggi, M. Ecdysteroids, a new pathological marker in man. In Biologie Prospective: Compte-rendus du 8è colloque de Pont-à-Mousson; John Libbey Eurotext: Arcueil, France, 1993; pp. 203–206. [Google Scholar]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arit, W.; Shackleton, C.H.L.; Storbeck, K.-H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef]
- Wells, J.E.; Hylemon, P.B. dentification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl. Environ. Microbiol. 2000, 66, 1107–1113. [Google Scholar] [CrossRef]
- Simon, P.; Koolman, J. Ecdysteroids in vertebrates: Pharmalogical aspects. In Ecdysone—from Chemistry to Mode of Action; Koolman, J., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 1989; pp. 254–259. [Google Scholar]
- Simon, P. Ecdysteroids in the mammalian organism and their detection as a means of diagnosis antihelmintic infections (Ecdysteroide im Säugerorganismus und ihr Nachweis als Möglichkeit der Diagnose helmintischer Infektionen). Ph.D. Thesis, University of Marburg, Marburg, Germany, 1988. [Google Scholar]
- Bolduc, T.M. Human Urinary Excretion Profiles after Exposure to ecdysterone. Master’s Thesis, University of Utah, Salt Lake City, UT, USA, 2008. [Google Scholar]
- Brandt, W. Pharmakokinetik und Metabolismus des 20-Hydroxyecdysons im Menschen. Ph.D. Thesis, Marburg, Germany, 2003. [Google Scholar]
- Dioh, W.; Del Signore, S.; Dupont, P.; Dilda, P.; Lafont, R.; Veillet, S. SARA-PK: A Single and Multiple Ascending Oral Doses Study to Assess the Safety and Evaluate the Pharmacokinetics of BIO101 in Healthy Young and Older Volunteers; ICFSR: Barcelona, Spain, 2017. [Google Scholar]
- Dioh, W.; Tourette, C.; Del Signore, S.; Daudigny, L.; Balducci, C.; Dupont, P.; Dilda, P.; Agus, S.; Lafont, R.; Veillet, S. A phase I, combined study of the safety and pharmacokinetics of BIO101 (20-hydroxyecdysone) in healthy young and elderly adult volunteers after single ascending and multiple ascending oral doses for 14 days. 2021. Manuscript in preparation. [Google Scholar]
- Thiem, B.; Kikowska, M.; Malinski, M.P.; Kruszka, D.; Napierala, M.; Florek, E. Ecdysteroids: Production in plant in vitro cultures. Phytochem. Rev. 2017, 16, 603–622. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Ohyama, K.; Nomura, K.; Hyodo, R.; Takahashi, K.; Yamada, J.; Morisaki, M. Biosynthesis of sterols and ecdysteroids in Ajuga hairy roots. Lipids 2000, 35, 279–288. [Google Scholar] [CrossRef]
- Chen, R.; Yang, S.; Zhang, L.; Zhou, Y.J. Advanced strategies for the production of natural products in yeast. iScience 2000, 23, 100879. [Google Scholar] [CrossRef]
- Yan, X.; Yan, Y.; Wei, W.; Wang, P.; Liu, Q.; Wei, Y.; Zhang, L.; Zhao, G.; Yue, J.; Zhou, Z. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res. 2014, 24, 770–773. [Google Scholar] [CrossRef]
- Duport, C.; Spagnoli, R.; Degryse, E.; Pompon, D. Self-sufficient biosynthesis of pregnenolone and progesterone in engineered yeast. Nat. Biotechnol. 1998, 16, 186–189. [Google Scholar] [CrossRef]
- Szczebara, F.M.; Chandelier, C.; Villeret, C.; Masurel, A.; Bourot, S.; Duport, C.; Blanchard, S.; Groisillier, A.; Testet, E.; Costaglioli, P.; et al. Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat. Biotechnol. 2003, 21, 143–149. [Google Scholar] [CrossRef]
- Dinan, L.; Balducci, C.; Guibout, L.; Lafont, R. Small-scale analysis of phytoecdysteroids in seeds by HPLC-DAD-MS for the identification and quantification of specific analogues, dereplication and chemotaxonomy. Phytochem. Anal. 2020, 31, 643–661. [Google Scholar] [CrossRef]
- Wang, J.-L.; Ruan, D.-C.; Cheng, Z.-Y.; Yang, C.-R. The dynamic variations of 20-hydroxyecdysone in Cyanotis arachnoidea. Acta Bot. Yunnanica 1996, 18, 459–464. [Google Scholar]
- Bandara, B.M.R.; Jayasinghe, L.; Karunaratne, V.; Wanningama, G.P.; Bokel, M.; Kraus, W. Ecdysterone from stem of Diploclisia glaucescens. Phytochemistry 1989, 28, 1073–1075. [Google Scholar] [CrossRef]
- Ramazonov, N.S.H.; Bobaev, I.D.; Syrov, V.N.; Sagdullaev, S.H.; Mamatkhanov, A.U. Chemistry, biology and production technology of phytoecdysteroids. Sci. Technol. Tashkent 2016, 258. (In Russian) [Google Scholar]
- Volodin, V.V.; Pchelenko, L.D.; Volodina, S.O.; Kudyasheva, A.G.; Shevchenko, O.G.; Zagorskaya, N.P. Pharmacological estimate of new ecdysteroid-containing substance “Serpisten”. Rastit Resur. 2006, 42, 113–130. [Google Scholar]
- Martnussen, I.; Volodin, V.; Volodina, S.; Uleberg, E. Effect of climate on plant growth and level od adaptogenic compounds in Maral root (Leuzea carthamoides Willd., DC), crowned saw-wort (Serratula coronata L.) and roseroot (Rhodiola rosea L.). Eur. J. Plant. Sci. Biotechnol. 2011, 5, 72–77. [Google Scholar]
- Dinan, L. Phytoecdysteroids: Biological aspects. Phytochem 2001, 57, 325–339. [Google Scholar] [CrossRef]
- Báthori, M.; Girault, J.P.; Kalasz, H.; Mathé, I.; Dinan, L.N.; Lafont, R. Complex phytoecdysteroid cocktail of Silene otites (Caryophyllaceae). Arch. Insect Biochem. Physiol. 1999, 41, 1–8. [Google Scholar] [CrossRef]
- Lobell, M.; Hendrix, M.; Hinzen, B.; Keldenich, J.; Meier, H.; Schmeck, C.; Schohe-Loop, R.; Wunberg, T.; Hillisch, A. In silico ADMET traffic lights as a tool for the prioritization of HTS hits. ChemMedChem 2006, 1, 1229–1236. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and developments settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Otaka, T.; Uchiyama, M.; Okui, S.; Takemoto, T.; Hikino, H.; Ogawa, S.; Nishimoto, N. Stimulatory effect of insect metamorphosing steroids from Achyranthes and Cyathula on protein synthesis in mouse liver. Chem. Pharm. Bull. 1968, 16, 2426–2429. [Google Scholar] [CrossRef][Green Version]
- Issaadi, H.M.; Csábi, J.; Hsieh, T.J.; Gáti, T.; Tóth, G.; Hunyadi, A. Side-chain cleaved phytoecdysteroid metabolites as activators of protein kinase B. Bioorg. Chem. 2019, 82, 405–413. [Google Scholar] [CrossRef]
- Novikov, V.S.; Shamarin, I.A.; Bortnovskii, V.N. A trial of the pharmacological correction of sleep disorders in sailors during a cruise. Voen Med. Zhurnal 1992, 47–49. [Google Scholar]
- Marina, T.F. Influence of CNS stimulators of plant origin on reflex activity of spinal cord. In: Stimulators of the Central Nervous System. Tomsk 1966, 31–36. [Google Scholar]
- Mirzaev, Y.R.; Syrov, V.N.; Krushev, S.A.; Iskanderova, S.D. Study of the effects of ecdysten on the sexual function under experimental and clinical conditions. Eksp. Klin. Farm. 2000, 63, 35–37. [Google Scholar]
- Syrov, V.N.; Khushbaktova, Z.A.; Komarin, A.S.; Abidov, A.B.; Pechenitsina, T.V.; Aripkhodzhaeva, F.A. Experimental and clinical evaluation of the efficacy of ecdysten in the treatment of hepatitis. Eksp. Klin. Farmakol. 2004, 67, 56–59. [Google Scholar] [PubMed]
- Mosharrof, A.H. Effects of extract from Rhaponticum carthamoides (Willd) Iljin (Leuzea) on learning and memory in rats. Acta Physiol. Pharmacol. Bulg. 1987, 13, 37–42. [Google Scholar]
- Opletal, L.; Sovova, M.; Dittrich, M.; Solich, P.; Dvorák, J.; Krátký, F.; Cerovský, J.; Hofbauer, J. Phytotherapeutic aspects of diseases of the circulatory system. Leuzea carthamoides (WILLD.) DC: The status of research and possible use of the taxon. Ceska Slov. Farm. 1997, 46, 247–255. [Google Scholar]
- Ambrosio, G.; Wirth, D.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Botrè, F.; Parr, M.K. How reliable is dietary supplement labelling?—Experiences from the analysis of ecdysterone supplements. J. Pharm. Biomed. Anal. 2020, 177, 112877. [Google Scholar] [CrossRef]
- Isenmann, E.; Ambrosio, G.; Joseph, J.F.; Mazzarino, M.; de la Torre, X.; Zimmer, P.; Kazlauskas, R.; Goebel, C.; Botrè, F.; Diel, P.; et al. Ecdysteroids as non-conventional anabolic agent: Performance enhancement by ecdysterone supplementation in humans. Arch. Toxicol. 2019, 93, 1807–1816. [Google Scholar] [CrossRef]
- Parr, M.K.; Ambrosio, G.; Wuest, B.; Mazzarino, M.; de la Torre, X.; Sibilia, F.; Joseph, J.F.; Diel, P.; Botré, F. Targeting the administration of ecdysterone in doping control samples. Forensic Toxicol. 2020, 38, 172–184. [Google Scholar] [CrossRef]
- Kibrik, N.D.; Reshetnyak, J.A. Therapeutical approaches to sexual disadaption. Eur. Neuropsychopharmacol. 1996, 6, 167. [Google Scholar] [CrossRef]
- Saatov, Z.; Agzamkhodjaeva, D.A.; Syrov, V.N. Distribution of phytoecdysteroids in plants of Uzbekistan and the possibility of using drugs based on them in nephrological practice. Chem. Nat. Comp. 1999, 35, 186–191. [Google Scholar] [CrossRef]
- Osipova, S.O.; Islamova, Z.I.; Syrov, V.N.; Badalova, N.S.; Khushbaktova, Z.A. Ecdysten in the treatment of giardiasis. Med. Parazitol. (Mosk) 2002, 29–33. [Google Scholar]
- Islamova, Z.I.; Syrov, V.N.; Khushbaktova, Z.A.; Osipova, S.O. The efficacy of ecdystene versus metronidazole in the treatment of lambliasis. Med. Parazitol. (Mosc.) 2010, 14–17. [Google Scholar]
- Makhmudova, L.B. Experience of using ecdisten in the treatment of hymenolepiasis. Med. Parazitol. (Mosc.) 2012, 45–47. [Google Scholar]
- Seidlova-Wuttke, D.; Wuttke, W. In a placebo-controlled study β-ecdysone (ECD) prevented the development of the metabolic syndrome. Planta Med. 2012, 78, CL37. [Google Scholar] [CrossRef]
- Wuttke, M.; Seidlova-Wuttke, D. Beta-ecdysone (Ecd) prevents visceral, bone marrow and joint fat accumulation and has positive effects on serum lipids, bone and joint cartilage. Planta Med. 2012, 78, PD68. [Google Scholar] [CrossRef]
- Wuttke, W.; Seidlova-Wuttke, D. Eine neue Alternative für die Prävention und Therapie postmenopausaler Erkrankungen, insbesondere des metabolischen Syndroms. J. Gynäkol. Endokrinol. 2015, 25, 6–12. [Google Scholar]
- Agus, S.; Dioh, W. A Double-blind, Placebo Controlled, Randomized INTerventional Clinical Trial (SARA-INT). Clinicaltrials.gov NCT03452488 2018. [Google Scholar]
- Thole, S.W. The Metabolic Syndrome: The Effects of β-ecdysone on Selected Body Parameters and Serum Lipids on the Metabolic Syndrome. Ph.D. Thesis, University of Göttingen, Göttingen, Germany, 2018. [Google Scholar]
- Rayas, A.L.F. Effect of phytoecdysterone administration in subjects ith prediabetes. Clinicaltrials.gov identifier NCT03906201 2019. [Google Scholar]
- Agus, S.; Dioh, W. Testing the Efficacy and Safety of BIO101 for the Prevention of Respiratory Deterioration in COVID-19 Patients (COVA). Clinicaltrials.gov NCT04472728 2020, ongoing. [Google Scholar]
- Dustmukhamedova, D.K.H.; Kamilovz, A.T. The characteristic of energy metabolism disorders and its correction in children with celiac disease. Am. J. Med. Medic. Sci. 2020, 10, 780–783. [Google Scholar]
- Kwan, P. Sarcopenia: Neurological point of view. J. Bone Metab. 2013, 24, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Drey, M.; Krieger, B.; Sieber, C.C.; Bauer, J.M.; Hettwer, S.; Bertsch, T.; DISARCO Study Group. Motoneuron loss is associated with sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Dioh, W.; Chabane, M.; Tourette, C.; Azbekyan, A.; Morelot-Panzini, C.; Hajjar, L.A.; Lins, M.; Nair, G.B.; Whitehouse, T.; Mariani, J.; et al. Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Latil, M.; Camelo, S.; Veillet, S.; Lafont, R.; Dilda, P.J. Developing new drugs that activate the protective arm of the renin-angiotensin system as a potential treatment for repiratory failure in COVID-19 patients. Drug Discov. Today 2021, in press. [Google Scholar] [CrossRef]
- WADA. Summary of Major Modifications and Explanatory Notes. 2020 prohibited list. Available online: https://www.wada-ama.org/sites/default/files/wada_2020_english_summary_of_modifications_.pdf (accessed on 1 April 2021).
| Scientific Name | Plant Part | Major Constituents | Claimed Therapeutical Value | World Area | References |
|---|---|---|---|---|---|
| Achyranthes bidentata | root | 20E, Inokosterone, polysaccharides | Anticancer, anti-inflammatory, diuretic, anti-osteoporotic | India, China Taiwan | [8,9] |
| Achyranthes japonica | root, leaf | Inokosterone, 20E, saponin, oleanolic acid, calcium oxalate | Antirheumatic, for amenorrhea, carbuncles, fever, dystocia, urinary ailments | Korea, Japan, China | [10] |
| Ajuga bracteosa A. decumbens | whole plant | 20E, ajugasterone C ajugalactone, cyasterone, kiransin, β-sitosterol, cerotic acid, palmitic acid, luteolin | Antitussive, antipyretic, anti-inflammatory, antiphlogistic, antibacterial; treats bladder ailments, diarrhea, bronchitis | Taiwan | [11] |
| Ajuga iva | aerial parts | 20E, cyasterone, ajugasterone C, apigenin, apigenin dihexoside, carvacrol | Diabetes, rheumatism, allergy, cancer, renal, metabolic disorders, digestive, cardiovascular, and respiratory disorders | Africa | [12,13] |
| Ajuga turkestanica | aerial parts | 20E, turkesterone, cyasterone, iridoids | Weight deficiency, ulcers, burns, wound healing, heart protective, hair growth | Uzbekistan, Tadzhikistan | [14] |
| Boerhaavia diffusa | root, aerial parts | 20E, flavonoids, rotenoids, punarnavoside | Immunomodulatory, anticancer, antidiabetic, anti-inflammatory, diuretic, hepatoprotective, antimicrobial, antifungal, anticonvulsant, antioxidant | Brazil, India, Iran, Angola, Ghana, Congo | [15] |
| Cyathula prostrata | leaf, root | 20E, cyasterone, terpenoids, flavonols, tannins | Laxative, antitoxic, analgesic, alleviates flu, cough, rheumatism, dysentery, syphilis | Tropical Africa, China, Australia | [8,16,17] |
| Cyanotis arachnoidea | aerial parts, root | 20E, ajugasterone C, poststerone, rubrosterone, daucosterol | Skin diseases, psoriasis, gastritis, tuberctulosis | China | [18] |
| Diploclisia glaucescens | stem, leaf | paristerone, 20E, capitasterone, oleanane glycosides | Rheumatism, snake venom, biliousness, venereal diseases | China | [19] |
| Helleborus niger | rhizomes, leaves | 20E, polypodine B, bufadienolides, | Stomachic, tonic, analgesic, antirheumatic | Romania | [20] |
| Microsorum membranifolium | fronds | 20E, ecdysone, 2-deoxy-20E, 2-deoxyecdysone, various conjugates | Asthma, purgative, antiemetic, healing of fractured bones | French Polynesia | [21] |
| Paris polyphylla | aerial parts | 20E, calonysterone, steroidal saponins, luteolin, quercetin | Antibiotic, anti-inflammatory, liver cancer | Southwest China | [22] |
| Pfaffia glomerata | root | 20E, pterosterone, polypodine B, ginsenosides | General stimulant, analgesic, anabolic, anti-inflammatory, immunostimulant, sedative, hypocholesterolemic | Brazil | [23] |
| Podocarpus macrophyllus var. nakaii | stem bark, leaf, root, fruit | 20E, ponasterone A, makisterones, pinene, camphene, cadinene, podocarpene, kaurene | Antihelminthic, blood disorders; tonic for heart, kidneys, lungs, stomach | South Africa | [8,24] |
| Polypodium vulgare | rhizome | 20E, polypodine B, polypodaurein, polypodosaponin, flavonoids | Expectorant, cough, pertussis, diuretic | Poland | [25] |
| Rhaponticum carthamoides | leaf, root | 20E, makisterones, triterpenes, sesquiterpene lactones, phenolic acids, flavonoids, thiophenes, lignans | Tonic, roborant, adaptogenic, antidepressive, antiparasitic | Eastern Europe | [26,27] |
| Serratula chinensis | roots | 20E glycosides, sphingolipids, cerebrosides | Pharyngitis, measles, anti-inflammatory, hypocholesterolemic, anti-cancer, | Southern China | [8,28] |
| Sida rhombifolia | root, seed | 20E, ecdysone, ecdysteroid glycosides, cyclopropenoid fatty acids | Enteritis, hepatitis, flu, pneumonia, improves blood circulation, resolves phlegm, alleviates pain | India | [8,29] |
| Tinospora cordifolia | aerial parts | 20E, polypodine B, alkaloids, diterpenoid lactones, sinapic acid | Anti-osteoporotic, anti-inflammatory, anti-stress, immuno-modulator, anti-spasmodic, chemo- and radio-protective, anti-anxiety, neuroprotective | India | [30,31] |
| Vitex scabra | bark, leaf | 20E, turkesterone, khainaoside, syringaresinol | Astringent, antihelminthic, gastrointestinal disorders, wound healing, sexual enhancer | Thailand | [32] |
| Effect | 20E and/or Other Ecdysteroid Preparations |
|---|---|
| Anabolic (muscle) | [46,47,48,49,50,51,52,53,54] |
| Fat-reducing/Hypolipidaemic | [55,56,57,58,59,60,61,62] |
| Anti-diabetic | [56,62,63,64,65,66] |
| Anti-fibrotic | [67] |
| Anti-inflammatory | [68,69] |
| Anti-oxidant | [70] |
| Anti-thrombotic | [71,72] |
| Vasorelaxant | [73] |
| Hematopoiesis stimulation | [74] |
| Angiogenic | [75,76] |
| Cardioprotective | [62,77,78,79] |
| Neuromuscular protective | [80,81] |
| Neuroprotective | [81,82,83,84] |
| Liver protective | [85,86] |
| Lung protective | [69,72,87,88] |
| Kidney protective | [58,67,89] |
| Gastric protective | [90,91] |
| Bone, cartilage protective | [92,93,94,95,96,97,98,99] |
| Skin protective/repairing | [100,101,102] |
| 5 min | 10 min | 30 min | 1 h | 3 h | 6 h | 12 h | 24 h | |
|---|---|---|---|---|---|---|---|---|
| Blood plasma | 0.061 | 0.057 | 0.052 | 0.047 | 0.032 | 0.023 | 0.015 | 0.011 |
| Urine | 0.482 | 0.921 | 1.534 | 0.281 | 0.102 | 0.096 | 0.087 | 0.046 |
| Feces | 0.015 | 0.019 | 0.035 | 0.068 | 0.099 | 0.931 | 0.312 | 0.041 |
| Bile | 0.421 | 0.456 | 1.042 | 0.901 | 0.301 | 0.209 | 0.198 | 0.094 |
| Liver | 0.312 | 0.251 | 0.213 | 0.112 | 0.078 | 0.061 | 0.056 | 0.046 |
| Heart | 0.062 | 0.052 | 0.035 | 0.033 | 0.031 | 0.029 | 0.023 | 0.022 |
| Spleen | 0.041 | 0.026 | 0.024 | 0.018 | 0.023 | 0.027 | 0.032 | 0.027 |
| Lungs | 0.116 | 0.069 | 0.057 | 0.053 | 0.049 | 0.045 | 0.036 | 0.028 |
| Kidney | 0.137 | 0.123 | 0.098 | 0.071 | 0.055 | 0.043 | 0.037 | 0.032 |
| Adrenals | 0.184 | 0.139 | 0.098 | 0.081 | 0.073 | 0.065 | 0.052 | 0.043 |
| Testis | 0.048 | 0.036 | 0.029 | 0.026 | 0.023 | 0.021 | 0.019 | 0.017 |
| Skeletal muscle | 0.021 | 0.023 | 0.028 | 0.017 | 0.015 | 0.013 | 0.011 | 0.010 |
| Spinal cord | 0.019 | 0.028 | 0.067 | 0.041 | 0.033 | 0.024 | 0.020 | 0.018 |
| Brain | 0.013 | 0.014 | 0.015 | 0.013 | 0.012 | 0.01 | 0.008 | 0.007 |
| Small intestine | 0.254 | 0.139 | 0.094 | 0.083 | 0.076 | 0.061 | 0.05 | 0.033 |
| Stomach | 0.034 | 0.047 | 0.075 | 0.061 | 0.053 | 0.041 | 0.032 | 0.023 |
| Large intestine | 0.011 | 0.023 | 0.029 | 0.031 | 0.035 | 0.048 | 0.038 | 0.023 |
| Bladder | 0.013 | 0.024 | 0.038 | 0.026 | 0.023 | 0.018 | 0.015 | 0.013 |
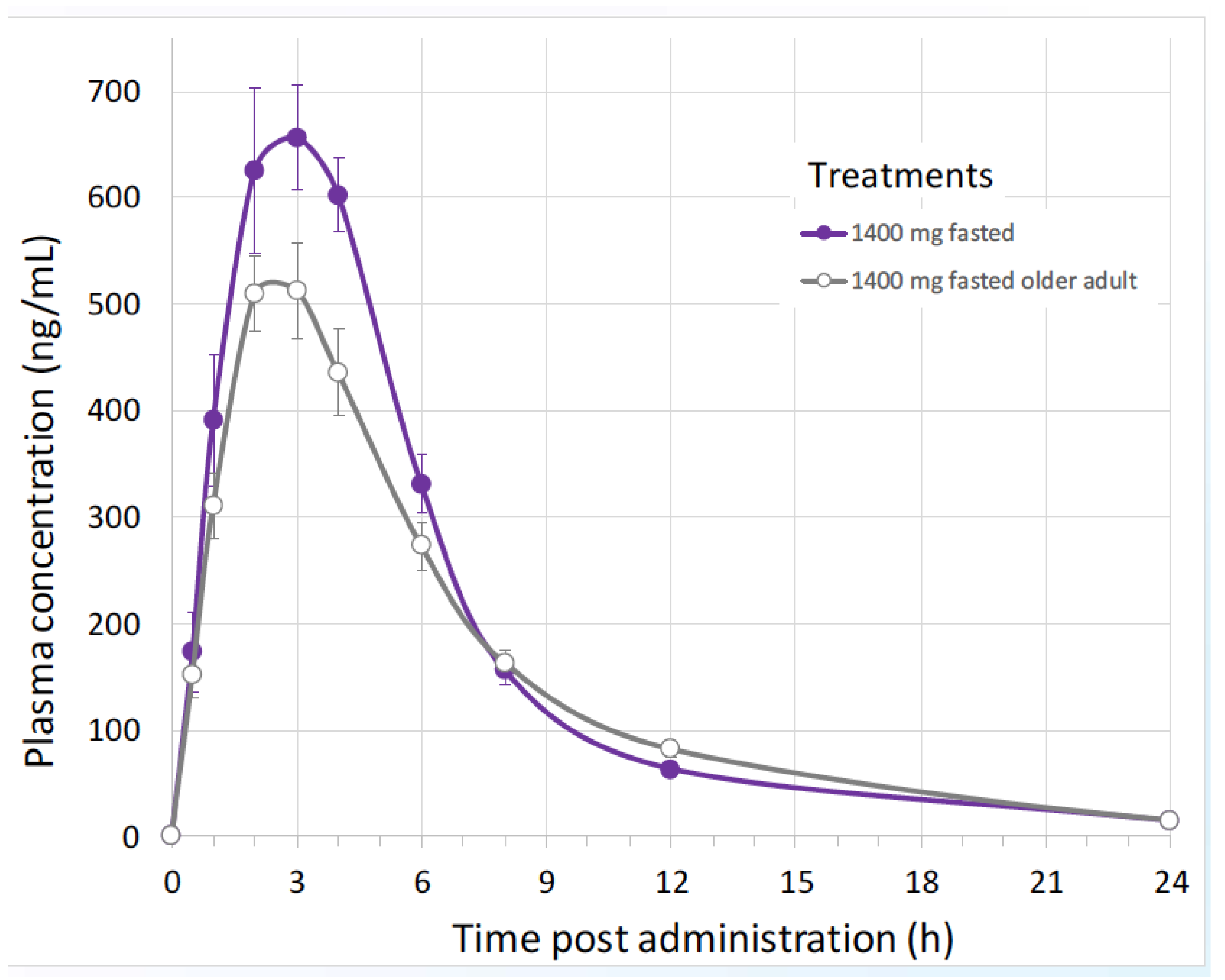
| B1O101 PK Parameter (Unit) | 100 mg | 350 mg | 700 mg | 1400 mg |
|---|---|---|---|---|
| Cmax (ng/mL) | 141 (16.6) | 317 (37.9) | 399 (24.7) | 710 (20.2) |
| tmax (h) | 2.03 (1.00–3.02) | 3.00 (1. 05–4.00) | 3.00 (2.00–4.02) | 3.50 (2.00–4.02) |
| AUC0-t (ng·h/mL) | 767 (31.1) | 1924 (40.1) | 2578 (22.9) | 4148 (15.9) |
| Non-Clinical Activities | Studies | Clinical Trial | ||
|---|---|---|---|---|
| Non Medicinal Drug Trial | Phase 1 | Phase 2 | ||
| Regulatory requirements | ||||
| Absorption | Pharmacokinetics | NA | ✓ | ✓ |
| Toxicokinetics | NA | ✓ | ✓ | |
| Transporters (Caco-2 cells) | NA | ✓ | ✓ | |
| Metabolism | Microsomal metabolism | NA | NA | ✓ |
| ADME $ study | NA | NA | ✓ | |
| CYP inhibition/induction | NA | ✓ | ✓ | |
| Metabolite identification | NA | ✓ | ✓ | |
| Toxicology | Phototoxicity | NA | ✓ | ✓ |
| Genotoxicity tests (Ames; micronuclei) | NA | ✓ | ✓ | |
| Repeated toxicology studies | NA | ✓ | ✓ | |
| Micronuclei tests | NA | ✓ | ✓ | |
| Distribution | Red blood cell partitioning | NA | ✓ | ✓ |
| Plasma protein binding | NA | ✓ | ✓ | |
| Pharmacology | In vitro studies | ✓ | ✓ | ✓ |
| In vivo studies | ✓ | ✓ | ✓ | |
| Safety pharmacology | Cardiovascular system | NA | ✓ | ✓ |
| Central nervous system and respiratory function | NA | ✓ | ✓ | |
| Documents required for submission dossier | ||||
| Investigational Medicinal Product Dossier (IMPD) | NA | ✓ | ✓ | |
| Investigation Brochure (IB) | NA | ✓ | ✓ | |
| Technical Product Dossier | ✓ | NA | NA | |
| Literature related to the product | ✓ | NA | NA | |
| Clinical Trial Protocol | ✓ | ✓ | ✓ | |
| Informed consent | ✓ | ✓ | ✓ | |
| Investigative New Drug (IND) Package for the FDA | NA | ✓ | ✓ | |
| Compound | MW | LogP | PSA (Å2) | Rotatable Bonds | H-Acceptors | H-Donors | TL Score |
|---|---|---|---|---|---|---|---|
| 20E | 480.30 | 1.36 | 138.45 | 5 | 7 | 6 | 3 |
| 20,26E | 496.64 | 0.35 | 158.67 | 6 | 8 | 7 | 4 |
| 14d20E | 464.64 | 2.30 | 118.21 | 5 | 6 | 5 | 1 |
| 6αOH20E | 482.66 | 1.54 | 141.60 | 5 | 7 | 7 | 4 |
| 6αOH14d20E | 466.66 | 2.49 | 121.37 | 5 | 6 | 6 | 3 |
| Post | 362.46 | 1.03 | 94.83 | 1 | 5 | 3 | 0 |
| 14dPost | 346.47 | 1.97 | 74.60 | 1 | 4 | 2 | 0 |
| 6αOHPost | 364.48 | 1.22 | 97.98 | 1 | 5 | 4 | 0 |
| 6αOH14dPost | 348.48 | 2.16 | 77.75 | 1 | 4 | 3 | 0 |
| Aim | Age | N | Dose | Duration | Output | Ref |
|---|---|---|---|---|---|---|
| Sexual disadaptation | 27–61 | 93 20F, 73M | 7.5–10 mg/day | 1 month | Improvement of libido and sexual activity in 75% of patients | [165] |
| Chonic glomerulonephritis | 35 ± 7 | 35 | 15 mg/day | 10 days | Improvement of kidney function and of microcirculation | [166] |
| Sexual function | N/A, M | 60 | 5 mg, 2×/day | 30 days | Improved sperm quality and copulative function in patients with disturbed spermatogenesis as a complication of urologic diseases Improvement of sexual function during recovery from myocardial infarction | [158] |
| 40–60 M | 48 | 5 mg 3×/day | ||||
| Giardiasis | N/A | 35 | 5 mg 3× or 4×/day | 10 days | Parasite elimination in 68.7% of patients | [167] |
| Hepatitis | N/A | N/A | 5 mg b.i.d. | 30 days | In case of hepatitis B, improvement of liver state | [159] |
| Lambliasis | N/A | N/A | 5 mg 4×/day | 10 days | Therapy eliminated most parasites within 10 days | [168] |
| Hymenolepiasis | N/A | 22 | 5 mg 3×/day | 2 weeks | Reduction in symptoms and parasitological efficacy of 36.4% | [169] |
| Metabolic syndrome | Overweight | 39 | 2 × 50 mg/day | 3 months? | Reduction in body weight (−1.3%) waist circumference (−3.2%), body fat (−7.6%), C-reactive protein (−38%), total cholesterol (−17%), triglycerides (−37%), muscle increase (+2.9%) | [170] |
| Menopause disorders | Overweight | N/A | 100 or 200 mg/day | 3 months | Prevention of metabolic syndrome and osteoporosis, reduction in body weight, reduction in plasma cholesterol and CRP; proposed for hormone replacement therapy | [171,172] |
| Sarcopenia | ≥65 | 231 | 175/350 mg b.i.d. | 6–9 months | Expected: change from baseline of gait speed (400MW test), appendicular lean mass and handgrip strength | [173] |
| Metabolic syndrome | >18 | 64 test 28 Control | 40 or 90 mg/day | 3–6–9 months | Reductions in body mass, proportion of body fat, waist cicumference and hsCRP, retention of muscle mass | [174] |
| Prediabetes | 30–60 | 34 | 300 mg/day | 12 weeks | Expected: changes in micronuclei, reduction in fasted glycemia, glycated hemoglobin | [175] |
| ARDS in COVID 19 | ≥55 | 310 | 350 mg b.i.d. | 28 days | Expected: Prevention of respiratory deterioration in severe COVID-19 patients | [176] |
| Celiac disease | Children 3–14 | 25 | 2.5 mg/kg/day | 14 days | Reduction in symptoms Improvement of energy metabolism | [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinan, L.; Dioh, W.; Veillet, S.; Lafont, R. 20-Hydroxyecdysone, from Plant Extracts to Clinical Use: Therapeutic Potential for the Treatment of Neuromuscular, Cardio-Metabolic and Respiratory Diseases. Biomedicines 2021, 9, 492. https://doi.org/10.3390/biomedicines9050492
Dinan L, Dioh W, Veillet S, Lafont R. 20-Hydroxyecdysone, from Plant Extracts to Clinical Use: Therapeutic Potential for the Treatment of Neuromuscular, Cardio-Metabolic and Respiratory Diseases. Biomedicines. 2021; 9(5):492. https://doi.org/10.3390/biomedicines9050492
Chicago/Turabian StyleDinan, Laurence, Waly Dioh, Stanislas Veillet, and Rene Lafont. 2021. "20-Hydroxyecdysone, from Plant Extracts to Clinical Use: Therapeutic Potential for the Treatment of Neuromuscular, Cardio-Metabolic and Respiratory Diseases" Biomedicines 9, no. 5: 492. https://doi.org/10.3390/biomedicines9050492
APA StyleDinan, L., Dioh, W., Veillet, S., & Lafont, R. (2021). 20-Hydroxyecdysone, from Plant Extracts to Clinical Use: Therapeutic Potential for the Treatment of Neuromuscular, Cardio-Metabolic and Respiratory Diseases. Biomedicines, 9(5), 492. https://doi.org/10.3390/biomedicines9050492





