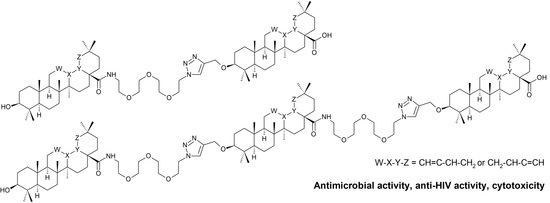
Triterpenoid–PEG Ribbons Targeting Selectivity in Pharmacological Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
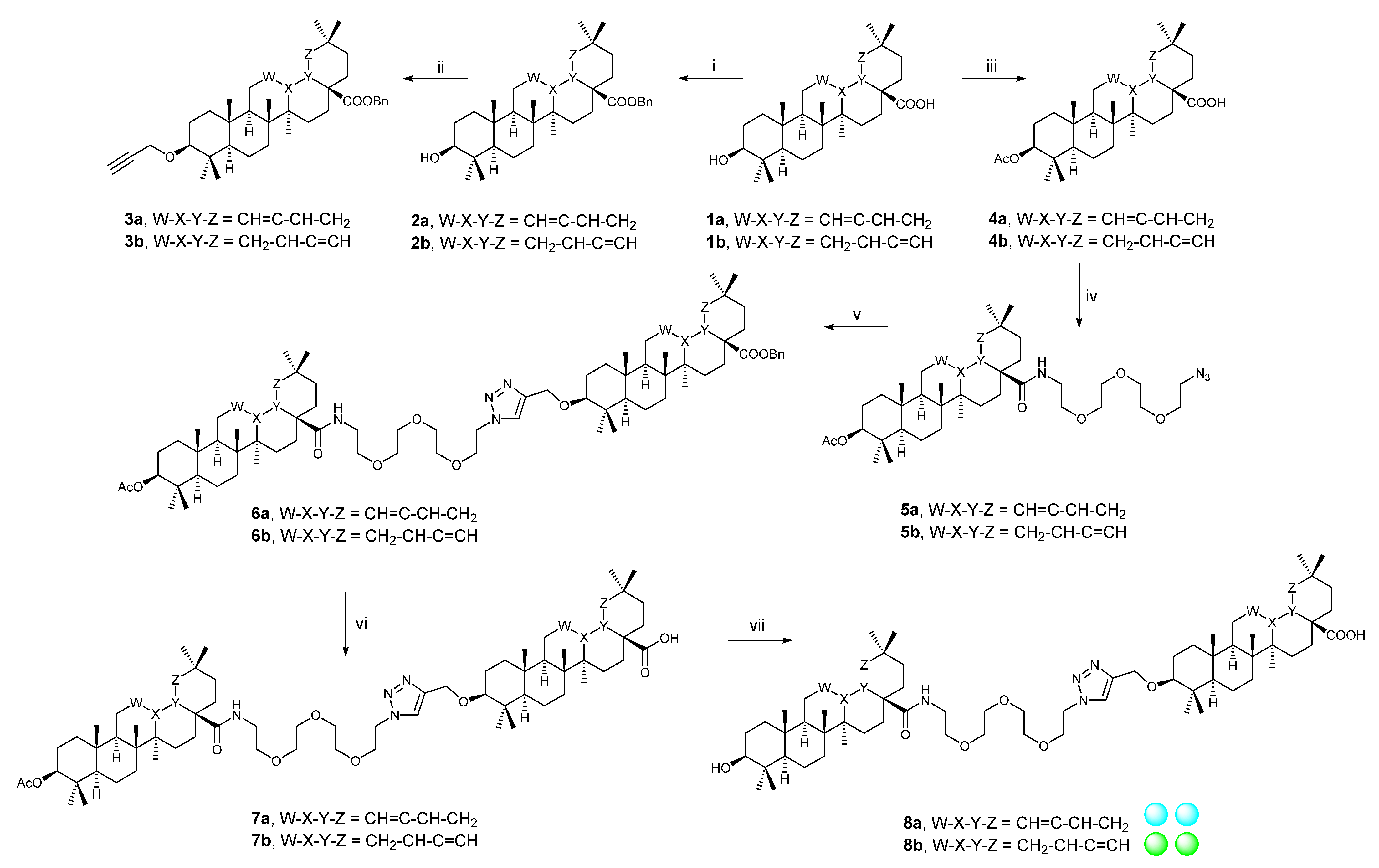
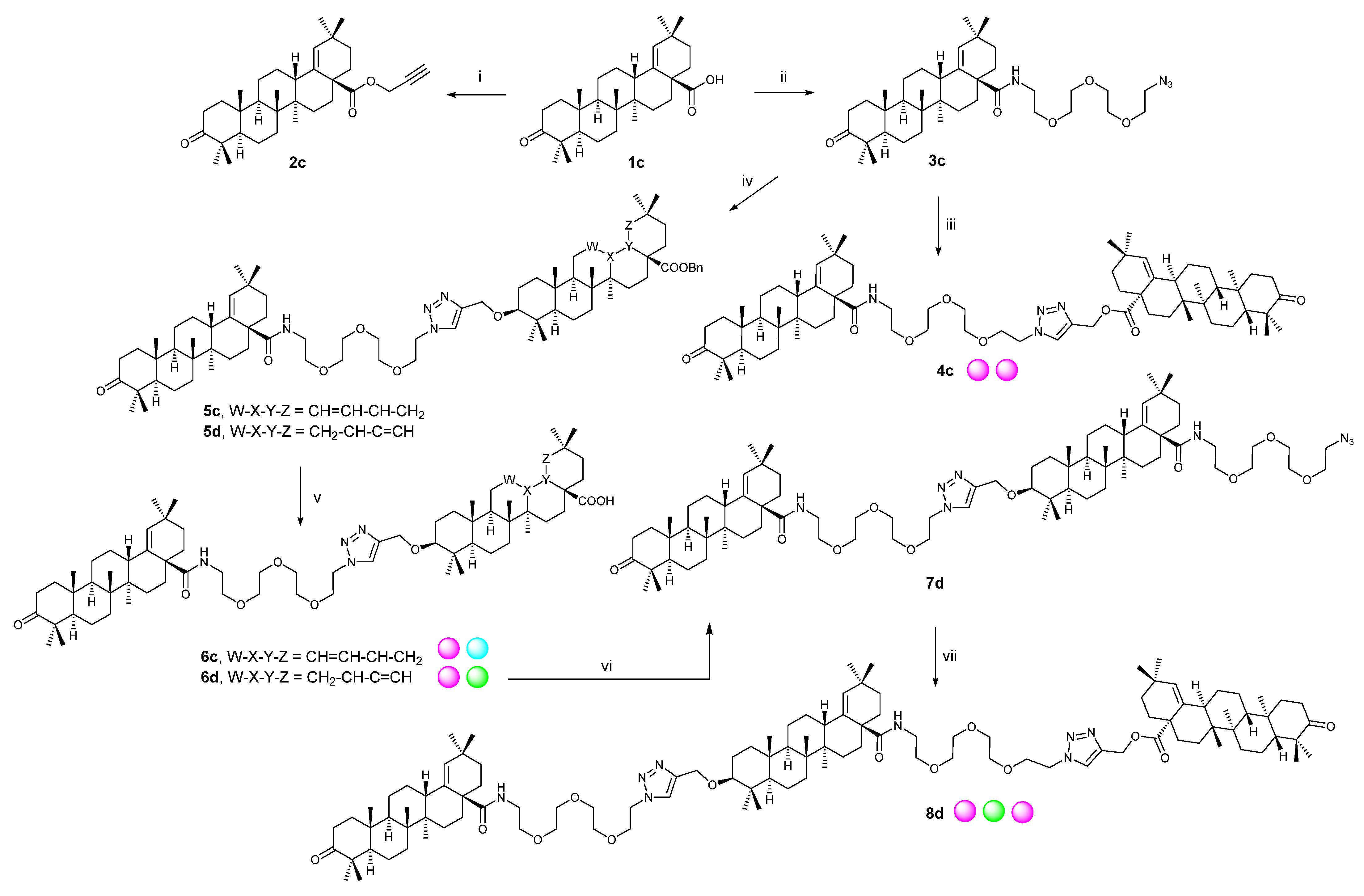
2.2. Synthetic Procedures
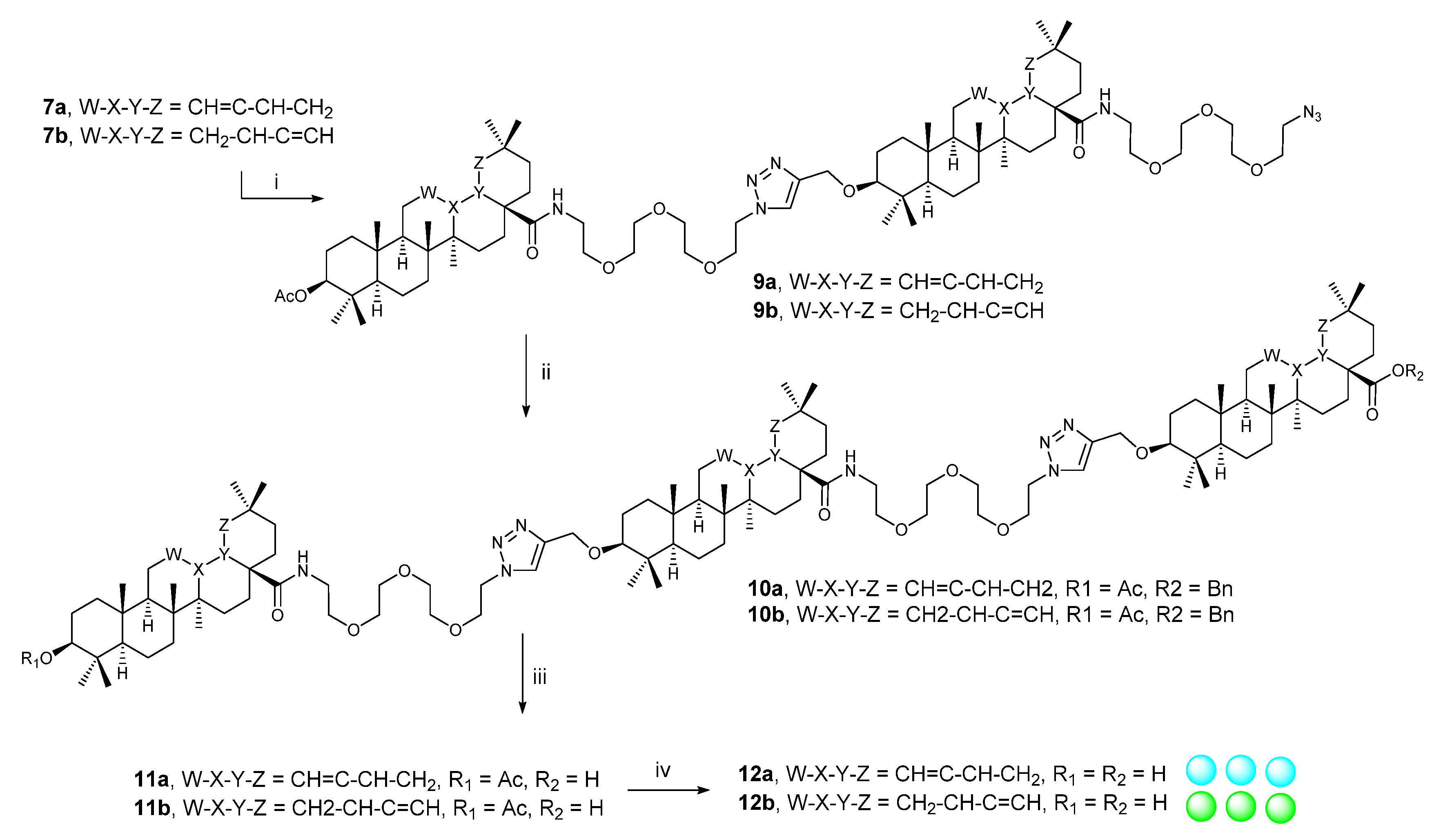
2.2.1. Procedure A
2.2.2. Procedure B
2.2.3. Procedure C
2.2.4. Procedure D
2.2.5. Procedure E
2.2.6. Procedure F
2.2.7. Procedure G
2.2.8. Procedure H
2.3. Antimicrobial Screening Tests
2.4. Cytotoxicity Screening Tests and Cell Cultures Used
2.5. Antiviral Tests
2.6. Supramolecular Self-Assembly Studies
2.6.1. Ability to Form Supramolecular Gel
2.6.2. UV-VIS Spectrometry
3. Results and Discussion
3.1. Synthetic Procedures
3.1.1. Procedure A
3.1.2. Procedure B
3.1.3. Procedure C
3.1.4. Procedure D
3.1.5. Procedure E
3.1.6. Procedure F
3.1.7. Procedure G
3.1.8. Procedure H
3.2. Cytotoxicity
3.3. Antimicrobial Activity
3.4. Antiviral Activity
3.5. Gel formation and Supramolecular Self-Assembly
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bag, B.G.; Majumdar, R. Self-assembly of renewable nano-sized triterpenoids. Chem. Rec. 2017, 17, 841–873. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef]
- Jaeger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwada, Y.; Wang, H.-K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L.M.; Kozuka, M.; Okabe, H.; et al. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar] [CrossRef]
- Mengoni, F.; Lichtner, M.; Battinelli, L.; Marzi, M.; Mastroianni, C.M.; Vullo, V.; Mazzanti, G. In vitro anti-HIV activity of oleanolic acid on infected human mononuclear cells. Planta Med. 2002, 68, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, H.; Ojha, D.; Bag, P.; Chandel, H.S.; Bhattacharyya, S.; Chatterjee, T.K.; Mukherjee, P.K.; Chakraborti, S.; Chattopadhyay, D. Anti-herpes virus activities of Achyranthes aspera: An Indian ethnomedicine, and its triterpene acid. Microbiol. Res. 2013, 168, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Woldemichael, G.M.; Singh, M.P.; Maiese, W.M.; Timmermann, B.N. Constituents of antibacterial extract of Caesalpinia paraguariensis Burk. Z. Naturforsch. C J. Biosci. 2003, 58, 70–75. [Google Scholar] [CrossRef]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szakiel, A.; Ruszkowski, D.; Grudniak, A.; Kurek, A.; Wolska, K.I.; Doligalska, M.; Janiszowska, W. Antibacterial and antiparasitic activity of oleanolic acid and its glycosides isolated from marigold (Calendula officinalis). Planta Med. 2008, 74, 1709–1715. [Google Scholar] [CrossRef]
- Bamuamba, K.; Gammon, D.W.; Meyers, P.; Dijoux-Franca, M.-G.; Scott, G. Anti-mycobacterial activity of five plant species used as traditional medicines in the Western Cape Province (South Africa). J. Ethnopharmacol. 2008, 117, 385–390. [Google Scholar] [CrossRef]
- Kuete, V.; Wabo, G.F.; Ngameni, B.; Mbaveng, A.T.; Metuno, R.; Etoa, F.-X.; Ngadjui, B.T.; Beng, V.P.; Meyer, J.J.M.; Lall, N. Antimicrobial activity of the methanolic extract, fractions and compounds from the stem bark of Irvingia gabonensis (Ixonanthaceae). J. Ethnopharmacol. 2007, 114, 54–60. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Q.; Yu, Y.; Zhang, Y.; Zhang, M.; Liu, Q.; Zhang, E.; Li, S.; Song, G. Oleanolic acid, a novel endothelin A receptor antagonist, alleviated high glucose-induced cardiomyocytes injury. Am. J. Chin. Med. 2018, 46, 1187–1201. [Google Scholar] [CrossRef]
- Kurukulasuriya, R.; Link, J.T.; Madar, D.J.; Pei, Z.; Richards, S.J.; Rohde, J.J.; Souers, A.J.; Szczepankiewicz, B.G. Potential drug targets and progress towards pharmacologic inhibition of hepatic glucose production. Curr. Med. Chem. 2003, 10, 123–153. [Google Scholar] [CrossRef] [PubMed]
- Klaman, L.D.; Boss, O.; Peroni, O.D.; Kim, J.K.; Martino, J.L.; Zabolotny, J.M.; Moghal, N.; Lubkin, M.; Kim, Y.-B.; Sharpe, A.H.; et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, T.; Zhang, L.; Alexander, T.; Yue, J.; Vranic, M.; Volchuk, A. Oleanolic acid enhances insulin secretion in pancreatic β-cells. FEBS Lett. 2008, 582, 1375–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.B.; Singh, S.; Bani, S.; Gupta, B.D.; Banerjee, S.K. Anti-inflammatory activity of oleanolic acid in rats and mice. J. Pharm. Pharmacol. 1992, 44, 456–458. [Google Scholar] [CrossRef]
- Akihisa, T.; Kamo, S.; Uchiyama, T.; Akazawa, H.; Banno, N.; Taguchi, Y.; Yasukawa, K. Cytotoxic activity of Perilla frutescens var. japonica leaf extract is due to high concentrations of oleanolic and ursolic acids. J. Nat. Med. 2006, 60, 331–333. [Google Scholar] [CrossRef]
- Ma, C.-M.; Wu, X.-H.; Masao, H.; Wang, X.-J.; Kano, Y. HCV protease inhibitory, cytotoxic and apoptosis-inducing effects of oleanolic acid derivatives. J. Pharm. Pharm. Sci. 2009, 12, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, Q.; Xiao, S.-L.; Yu, F.; Ye, M.; Zheng, Y.-X.; Zhao, C.-K.; Sun, D.-A.; Zhang, L.-H.; Zhou, D.-M. Elucidation of the pharmacophore of echinocystic acid, a new lead for blocking HCV entry. Eur. J. Med. Chem. 2013, 64, 160–168. [Google Scholar] [CrossRef]
- Kong, L.; Li, S.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X.; et al. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Brooks, C.J.W. Morolic acid, a triterpenoid sapogenin. J. Am. Chem. Soc. 1950, 72, 3314. [Google Scholar] [CrossRef]
- Hostettmann-Kaldas, M.; Nakanishi, K. Moronic acid, a simple triterpenoid keto acid with antimicrobial activity isolated from Ozoroa mucronata. Planta Med. 1979, 37, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, M.; Riese, U.; Graf, H.; Melzig, M.F.; Ciesielski, S.; Baumann, D.; Dittmann, K.; Wegner, C. Constituents in evening primrose oil with radical scavenging, cyclooxygenase, and neutrophil elastase inhibitory activities. J. Agric. Food Chem. 2002, 50, 5533–5538. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerzen, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef]
- Gehrke, I.T.S.; Neto, A.T.; Pedroso, M.; Mostardeiro, C.P.; Da Cruz, I.B.M.; Silva, U.F.; Ilha, V.; Dalcol, I.I.; Morel, A.F. Antimicrobial activity of Schinus lentiscifolius (Anacardiaceae). J. Ethnopharmacol. 2013, 148, 486–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Martinez, S.; Navarrete-Vazquez, G.; Estrada-Soto, S.; Leon-Rivera, I.; Rios, M.Y. Chemical constituents of the hemiparasitic plant Phoradendron brachystachyum DC Nutt (Viscaceae). Nat. Prod. Res. 2013, 27, 130–136. [Google Scholar] [CrossRef]
- Ramirez-Espinosa, J.J.; Rios, M.Y.; Lopez-Martinez, S.; Lopez-Vallejo, F.; Medina-Franco, J.L.; Paoli, P.; Camici, G.; Navarrete-Vazquez, G.; Ortiz-Andrade, R.; Estrada-Soto, S. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP-1B: In vitro, in silico, and in vivo approaches. Eur. J. Med. Chem. 2011, 46, 2243–2251. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Espinosa, J.J.; Garcia-Jimenez, S.; Rios, M.Y.; Medina-Franco, J.L.; Lopez-Vallejo, F.; Webster, S.P.; Binnie, M.; Ibarra-Barajas, M.; Ortiz-Andrade, R.; Estrada-Soto, S. Antihyperglycemic and sub-chronic antidiabetic actions of morolic and moronic acids, in vitro and in silico inhibition of 11β-HSD 1. Phytomedicine 2013, 20, 571–576. [Google Scholar] [CrossRef]
- Giner-Larza, E.M.; Manez, S.; Giner, R.M.; Recio, M.C.; Prieto, J.M.; Cerda-Nicolas, M.; Rios, J.L. Anti-inflammatory triterpenes from Pistacia terebinthus Galls. Planta Med. 2002, 68, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Flekhter, O.B.; Medvedeva, N.I.; Tolstikov, G.A.; Galin, F.Z.; Yunusov, M.S.; Mai, H.N.T.; Tien, L.V.; Savinova, I.V.; Boreko, E.I.; Titov, L.P.; et al. Synthesis of olean-18(19)-ene derivatives from botulin. Russ. J. Bioorg. Chem. 2009, 35, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, E.F.; Medvedeva, N.I.; Kazakov, D.V.; Kukovinets, O.S.; Lobov, A.N.; Suponitsky, K.Y.; Kazakova, O.B. An efficient synthesis of moronic and heterobetulonic acids from allobetulin. Tetrahedron Lett. 2016, 57, 148–151. [Google Scholar] [CrossRef]
- Chung, P.Y. Novel targets of pentacyclic triterpenoids in Staphylococcus aureus: A systematic review. Phytomedicine 2020, 73, 152933. [Google Scholar] [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial resistance and COVID-19: Intersections and implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.L.; Coghe, F.; Scano, A.; Carta, K.G.; Orru, G. Co-infection of Streptococcus pneumoniae in respiratory infections caused by SARS-CoV-2. Biointerface Res. Appl. Chem. 2021, 11, 12170–12177. [Google Scholar]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [Green Version]
- Le Devedec, F.; Fuentealba, D.; Strandman, S.; Bohne, C.; Zhu, X.X. Aggregation behavior of pegylated bile acid derivatives. Langmuir 2012, 28, 13431–13440. [Google Scholar] [CrossRef]
- Castillo, P.M.; de la Mata, M.; Casula, M.F.; Sanchez-Alcazar, J.A.; Zaderenko, A.P. PEGylated versus non-PEGylated magnetic nanoparticles as camptothecin delivery system. Beilstein J. Nanotechnol. 2014, 5, 1312–1319. [Google Scholar] [CrossRef]
- Zacchigna, M.; Cateni, F.; Drioli, S.; Procida, G.; Altieri, T. PEG–ursolic acid conjugate: Synthesis and in vitro release studies. Sci. Pharm. 2014, 82, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Martinez, A.; Martin-Fonseca, S.; Garcia-Granados, A.; Ferrer-Martin, R.M.; Lupianez, J.A.; Parra, A. Semi-synthesis and antiproliferative evaluation of PEGylated pentacyclic triterpenes. Eur. J. Med. Chem. 2016, 118, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Veronese, F.M. State of the art in PEGylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Vida, N.; Rárová, L.; Kolář, M.; Šaman, D.; Havlíček, L.; Drašar, P.; Wimmer, Z. Polyamine derivatives of betulinic acid and β-sitosterol: A comparative investigation. Steroids 2015, 100, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Malík, M.; Özdemir, Z.; Rárová, L.; Janovská, L.; Šlouf, M.; Šaman, D.; Šarek, J.; Nonappa, N.; Wimmer, Z. Spermine amides of selected triterpenoid acids: Dynamic supramolecular system formation influences the cytotoxicity of the drugs. J. Mater. Chem. B 2020, 8, 484–491. [Google Scholar] [CrossRef]
- Humpolíčková, J.; Weber, J.; Stárková, J.; Mašínová, E.; Günterová, J.; Flaisigová, I.; Konvalinka, J.; Majerová, T. Inhibition of the precursor and mature forms of HIV-1 protease as a tool for drug evaluation. Sci. Rep. 2018, 8, 10438. [Google Scholar] [CrossRef] [PubMed]
- Konč, J.; Tichý, M.; Pohl, R.; Hodek, J.; Džubák, P.; Hajdúch, M.; Hocek, M. Sugar modified pyrimido[4,5-b]indole nucleosides: Synthesis and antiviral activity. Med. Chem. Commun. 2017, 8, 1856–1862. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, Z.; Rybková, M.; Vlk, M.; Šaman, D.; Rárová, L.; Wimmer, Z. Synthesis and pharmacological effects of diosgenin-betulinic acid conjugates. Molecules 2020, 25, 3546. [Google Scholar] [CrossRef]
- Özdemir, Z.; Šaman, D.; Bertula, K.; Lahtinen, M.; Bednárová, L.; Pazderková, M.; Rárová, L.; Wimmer, Z. Rapid self-healing and thixotropic organogelation of amphiphilic oleanolic acid–spermine conjugates. Langmuir 2021, 37, 2693–2706. [Google Scholar] [CrossRef]
- Ha, W.; Zhao, X.-B.; Zhao, W.-H.; Tang, J.-J.; Shi, Y.P. A colon-targeted podophyllotoxin nanoprodrug: Synthesis, characterization, and supramolecular hydrogel formation for the drug combination. J. Mater. Chem. B 2021, 9, 3200–3209. [Google Scholar] [CrossRef]
- Keum, C.; Hong, J.; Kim, D.; Lee, S.-Y.; Kim, H. Lysozyme-instructed self-assembly of amino-acid-functionalized perylene diimide for multidrug-resistant cancer cells. ACS Appl. Mater. Interfaces 2021, 13, 14866–14874. [Google Scholar] [CrossRef]
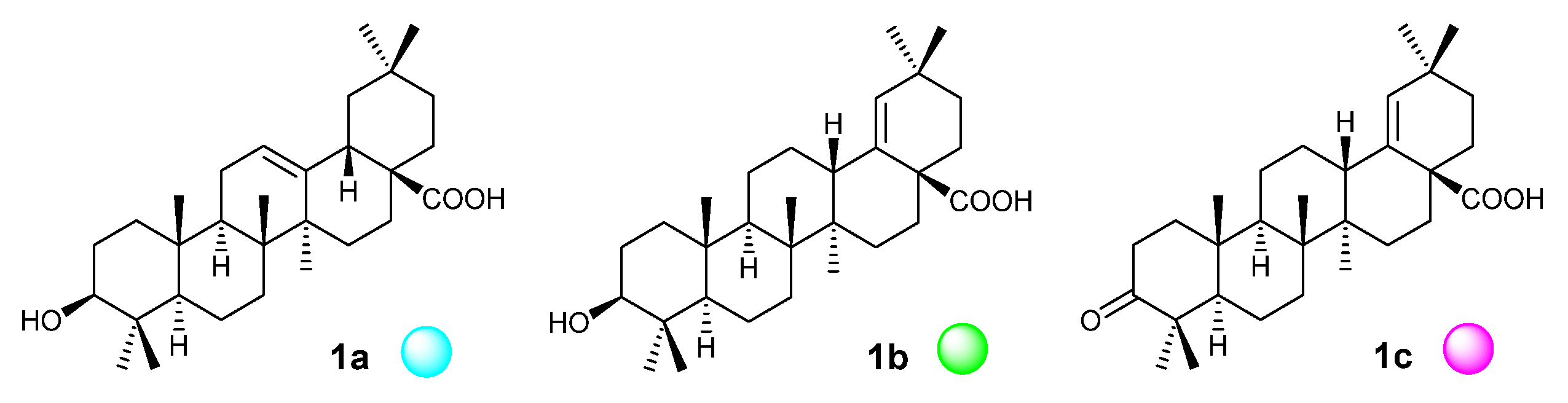
| Microorganism | Compound | Inhibition [%] (c = 25 µg·mL−1) c |
|---|---|---|
| Staphylococcus aureus (+) | 1a ● | – |
| Staphylococcus aureus | 8a ●● | 52 ± 7.8e |
| Staphylococcus aureus | 6c ●● | 13 ± 2.0 |
| Staphylococcus aureus | 12a ●●● | 28 ± 4.2 |
| Staphylococcus aureus | 1b ● | 21 ± 3.2 |
| Staphylococcus aureus | 8b ●● | – |
| Staphylococcus aureus | 6d ●● | 25 ± 3.8 |
| Staphylococcus aureus | 12b ●●● | 40 ± 6.0 e |
| Staphylococcus aureus | 1c ● | – |
| Staphylococcus aureus | 4c ●● | 11 ± 1.7 |
| Escherichia coli (+) | 1a ● | – |
| Escherichia coli | 8a ●● | 51 ± 7.7 e |
| Escherichia coli | 12a ●●● | – |
| Pseudomonas aeruginosa (−) | 1c ● | – |
| Pseudomonas aeruginosa | 4c ●● | 50 ± 7.5 e |
| Enterococcus faecalis (+) | 1a d● | 87 ± 13.0 |
| Enterococcus faecalis | 1b ● | 38 ± 5.7 |
| Enterococcus faecalis | 1c ● | – |
| Enterococcus faecalis | 4c ●● | 62 ± 9.3 e |
| Enterococcus faecalis | 8d ●●● | 38 ± 5.7 |
| Bacillus cereus (+) | 1a d ● | 76 ± 11.4 |
| Bacillus cereus | 1b d ● | 78 ± 11.7 |
| Bacillus cereus | 1c ● | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, Z.; Bildziukevich, U.; Čapková, M.; Lovecká, P.; Rárová, L.; Šaman, D.; Zgarbová, M.; Lapuníková, B.; Weber, J.; Kazakova, O.; et al. Triterpenoid–PEG Ribbons Targeting Selectivity in Pharmacological Effects. Biomedicines 2021, 9, 951. https://doi.org/10.3390/biomedicines9080951
Özdemir Z, Bildziukevich U, Čapková M, Lovecká P, Rárová L, Šaman D, Zgarbová M, Lapuníková B, Weber J, Kazakova O, et al. Triterpenoid–PEG Ribbons Targeting Selectivity in Pharmacological Effects. Biomedicines. 2021; 9(8):951. https://doi.org/10.3390/biomedicines9080951
Chicago/Turabian StyleÖzdemir, Zulal, Uladzimir Bildziukevich, Martina Čapková, Petra Lovecká, Lucie Rárová, David Šaman, Michala Zgarbová, Barbora Lapuníková, Jan Weber, Oxana Kazakova, and et al. 2021. "Triterpenoid–PEG Ribbons Targeting Selectivity in Pharmacological Effects" Biomedicines 9, no. 8: 951. https://doi.org/10.3390/biomedicines9080951
APA StyleÖzdemir, Z., Bildziukevich, U., Čapková, M., Lovecká, P., Rárová, L., Šaman, D., Zgarbová, M., Lapuníková, B., Weber, J., Kazakova, O., & Wimmer, Z. (2021). Triterpenoid–PEG Ribbons Targeting Selectivity in Pharmacological Effects. Biomedicines, 9(8), 951. https://doi.org/10.3390/biomedicines9080951