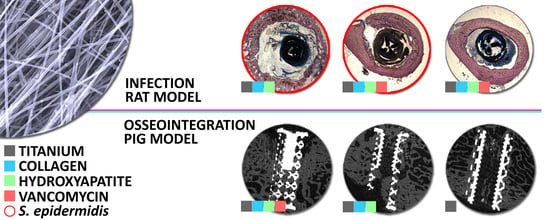
Vancomycin-Loaded Collagen/Hydroxyapatite Layers Electrospun on 3D Printed Titanium Implants Prevent Bone Destruction Associated with S. epidermidis Infection and Enhance Osseointegration
Abstract
1. Introduction
2. Materials and Methods
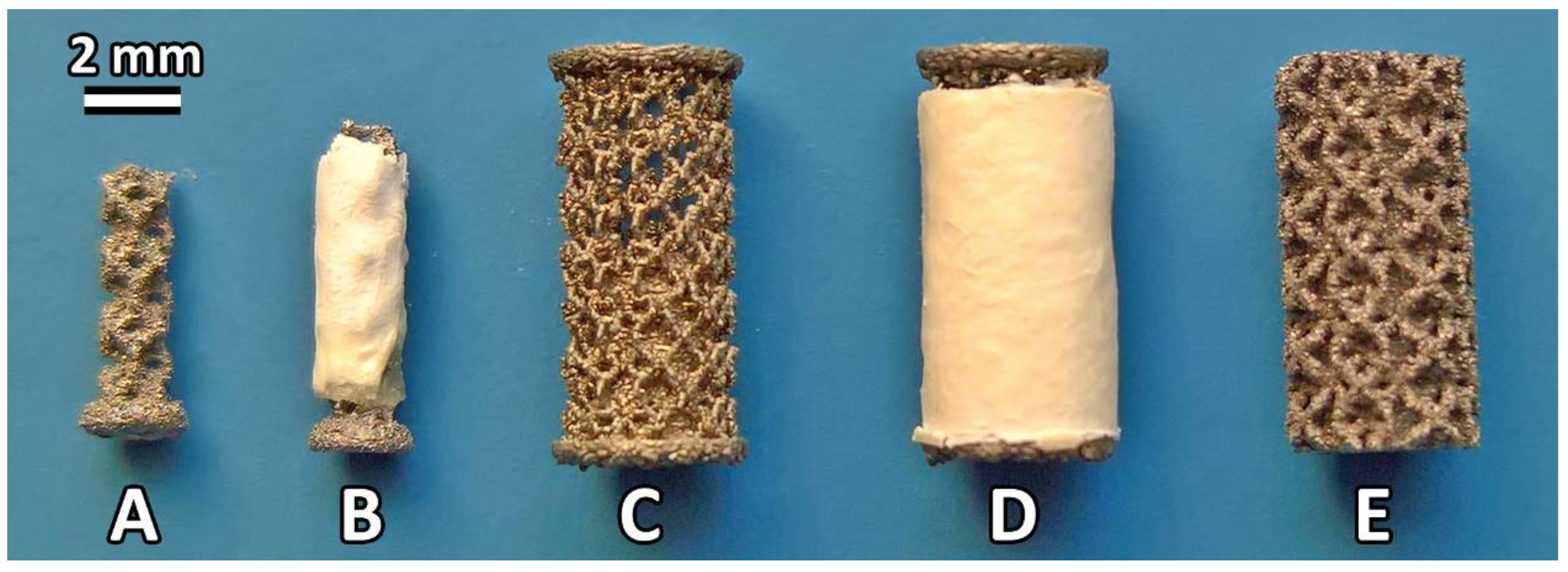
2.1. Preparation of the Collagen/Hydroxyapatite Electrospun Layers
2.2. Preparation of the Titanium Implants
2.3. Characterization of the Selected Strain of Staphylococcus epidermidis
2.4. The Experimental Rat Model Simulating the Clinically Relevant Introduction of Bacterial Infection to the Bone
2.5. Bone Histology of the Rat Model
2.6. Micro-CT Analysis of Rat Model
2.7. SEM and Elemental Analysis of Rat Model
2.8. Vancomycin Concentration in Blood Plasma of Rat Model
2.9. The Minipig Model for Analysis of Osseointegration of COLHA Layers
2.10. Bone Histology of Minipig Model
2.11. Micro-CT Analysis of Minipig Model
2.12. Statistical Analysis
3. Results
3.1. The Prevention of the Bone Infection Accompanied by Bone Structure Destruction in the Rat Model
3.1.1. The Infection Potential of S. epidermidis Impregnated Implants
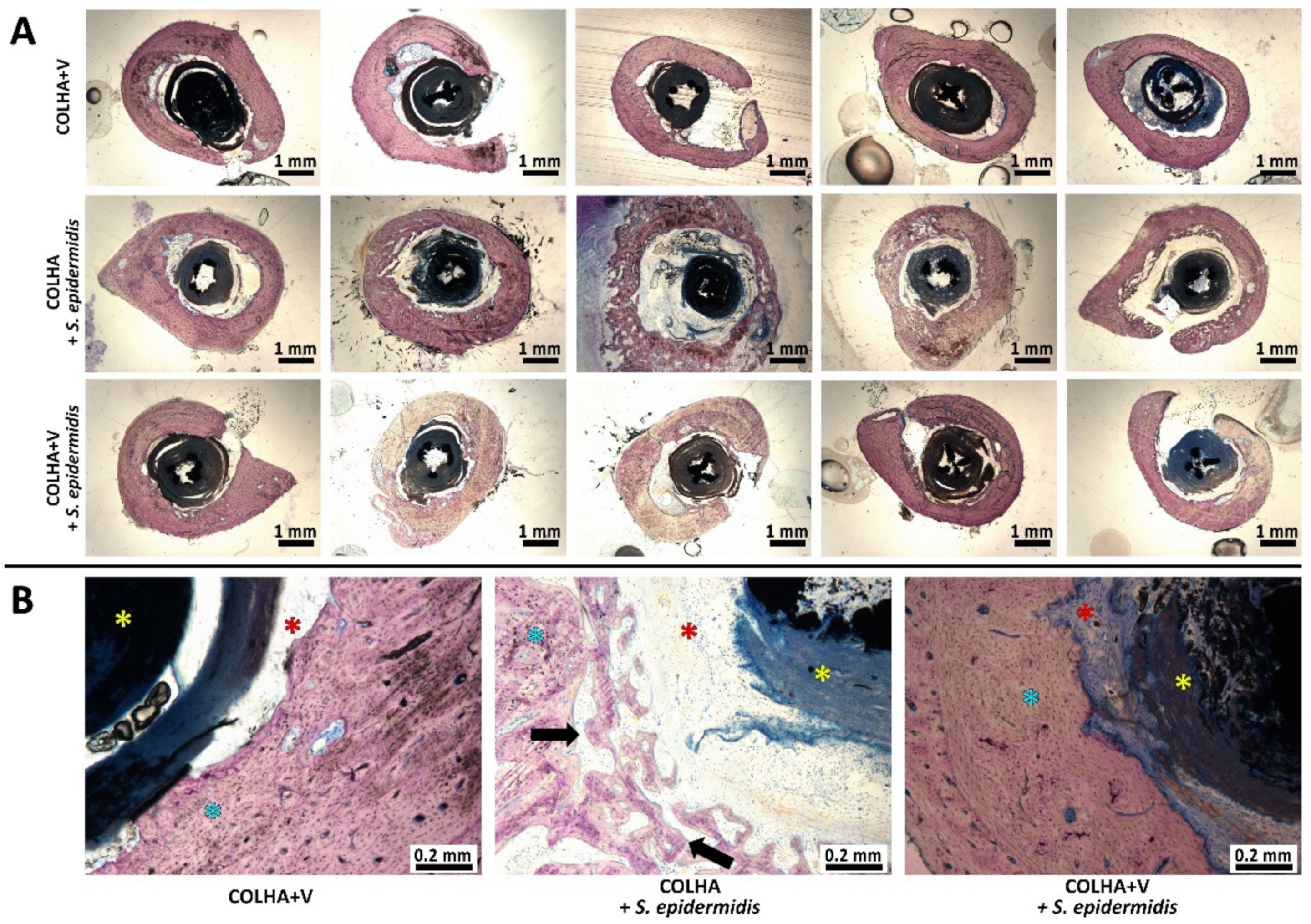
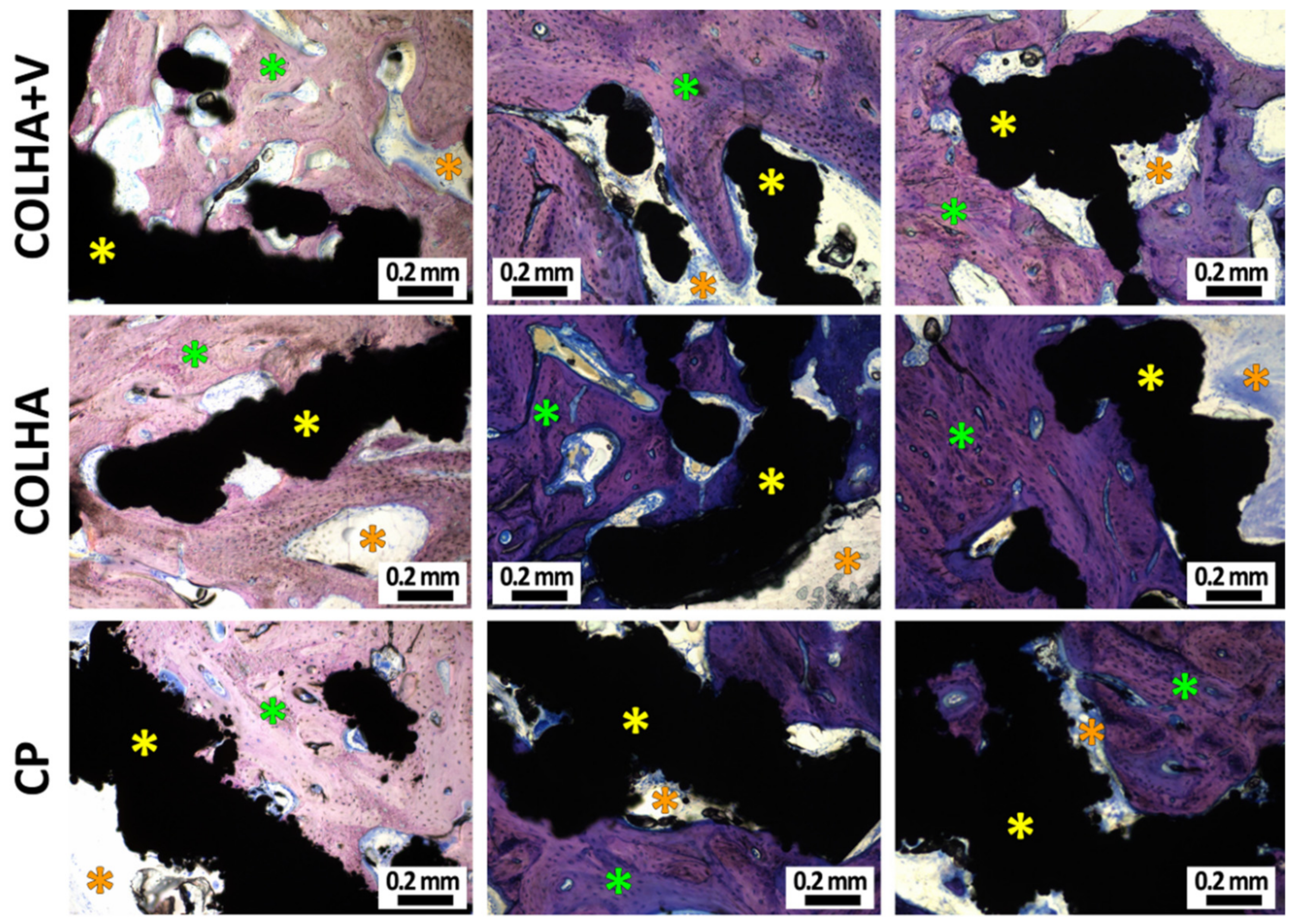
3.1.2. Bone Histology
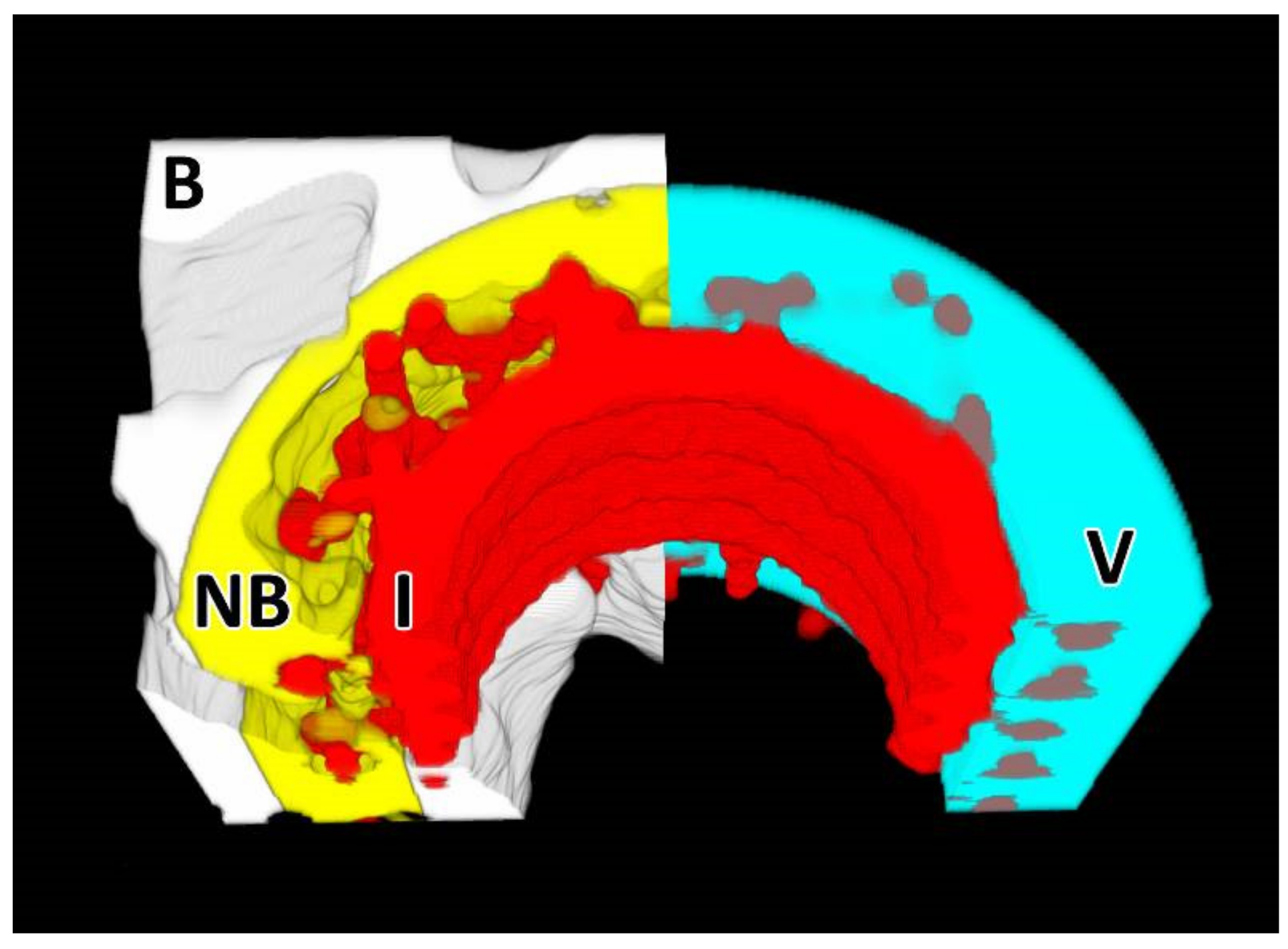
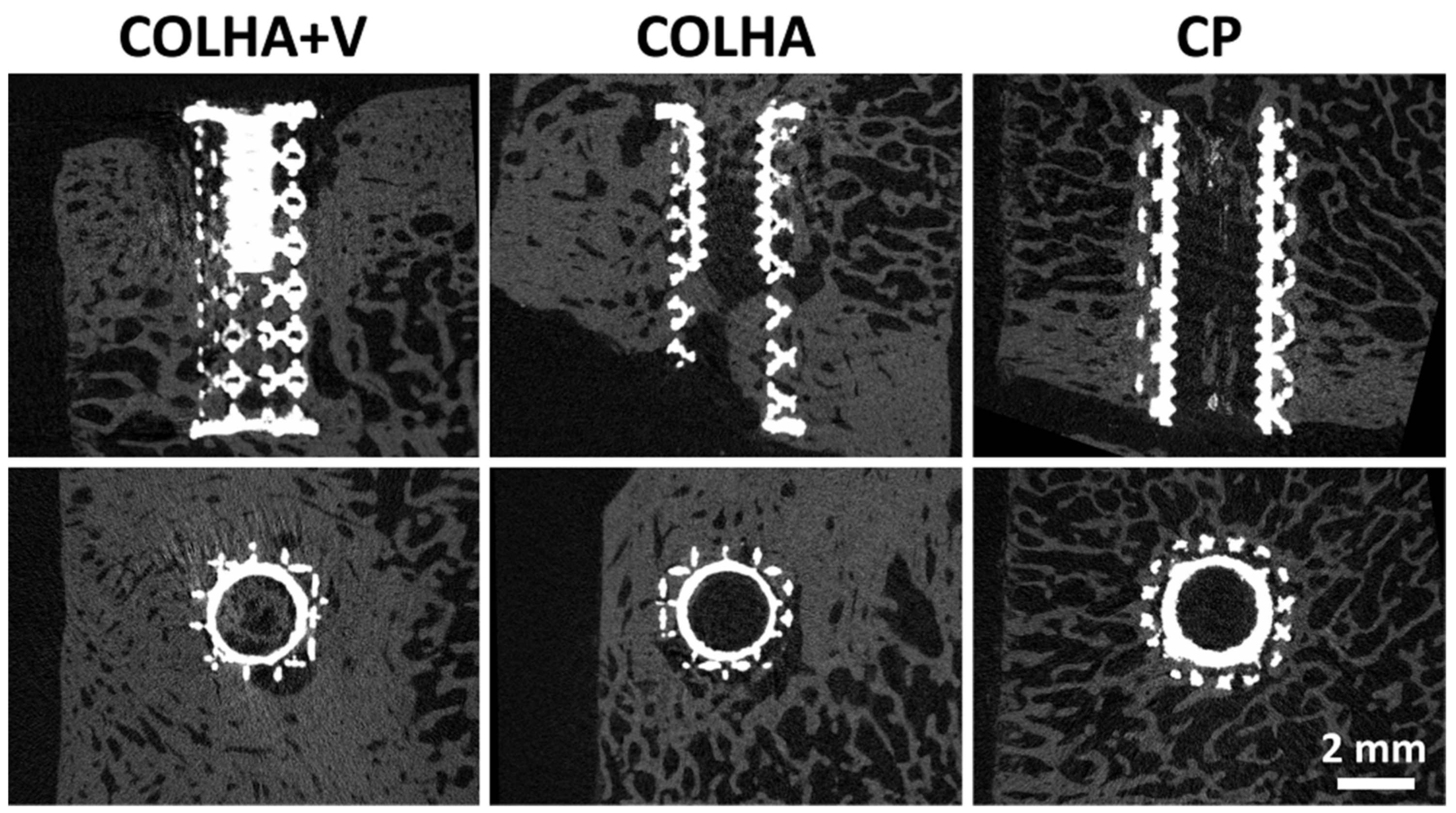
3.1.3. Micro-CT Analysis
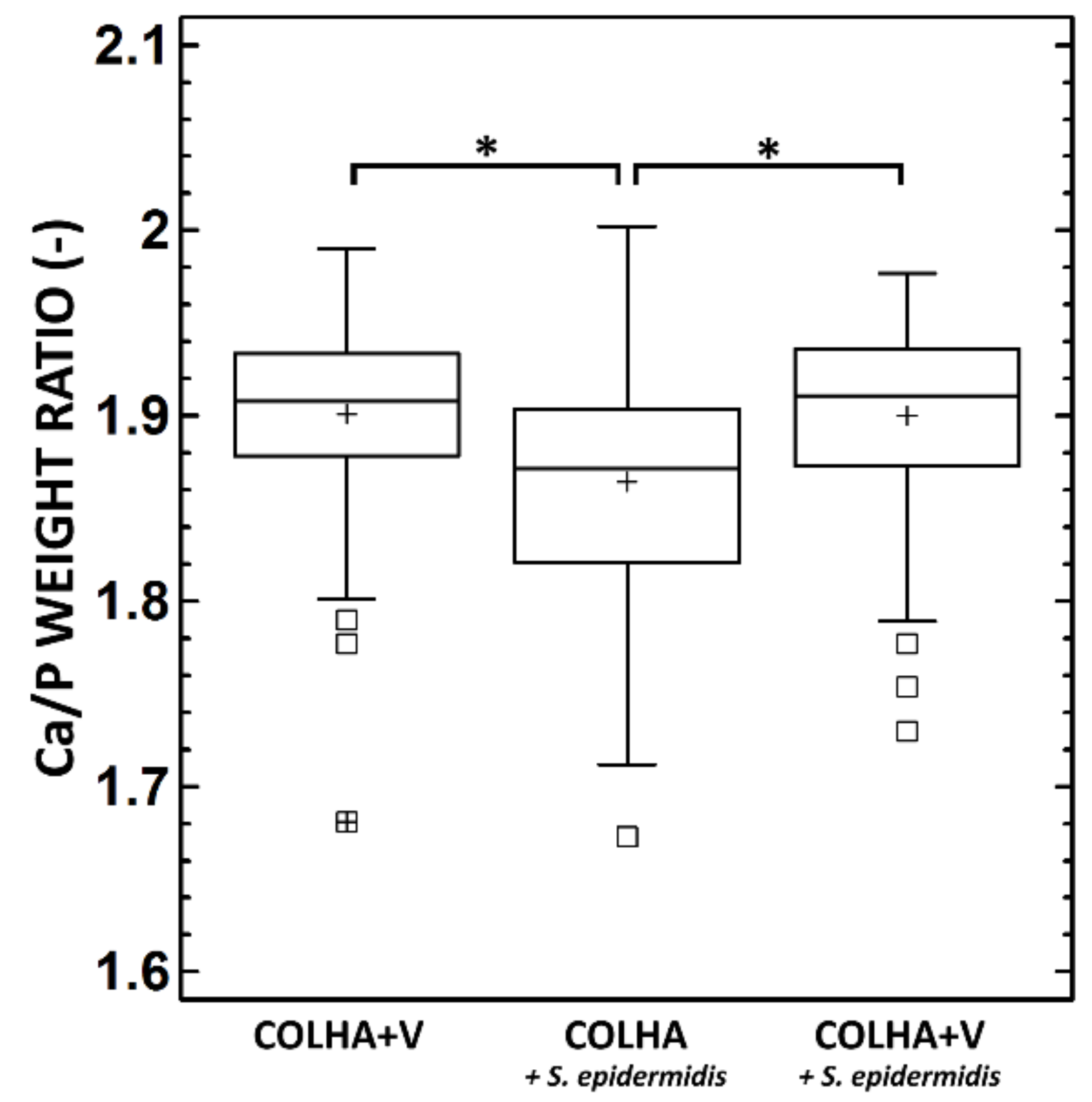
3.1.4. SEM and Elemental Analysis
3.1.5. Vancomycin Concentration in the Plasma
3.2. Evaluation of Osseointegration in the Minipig Model
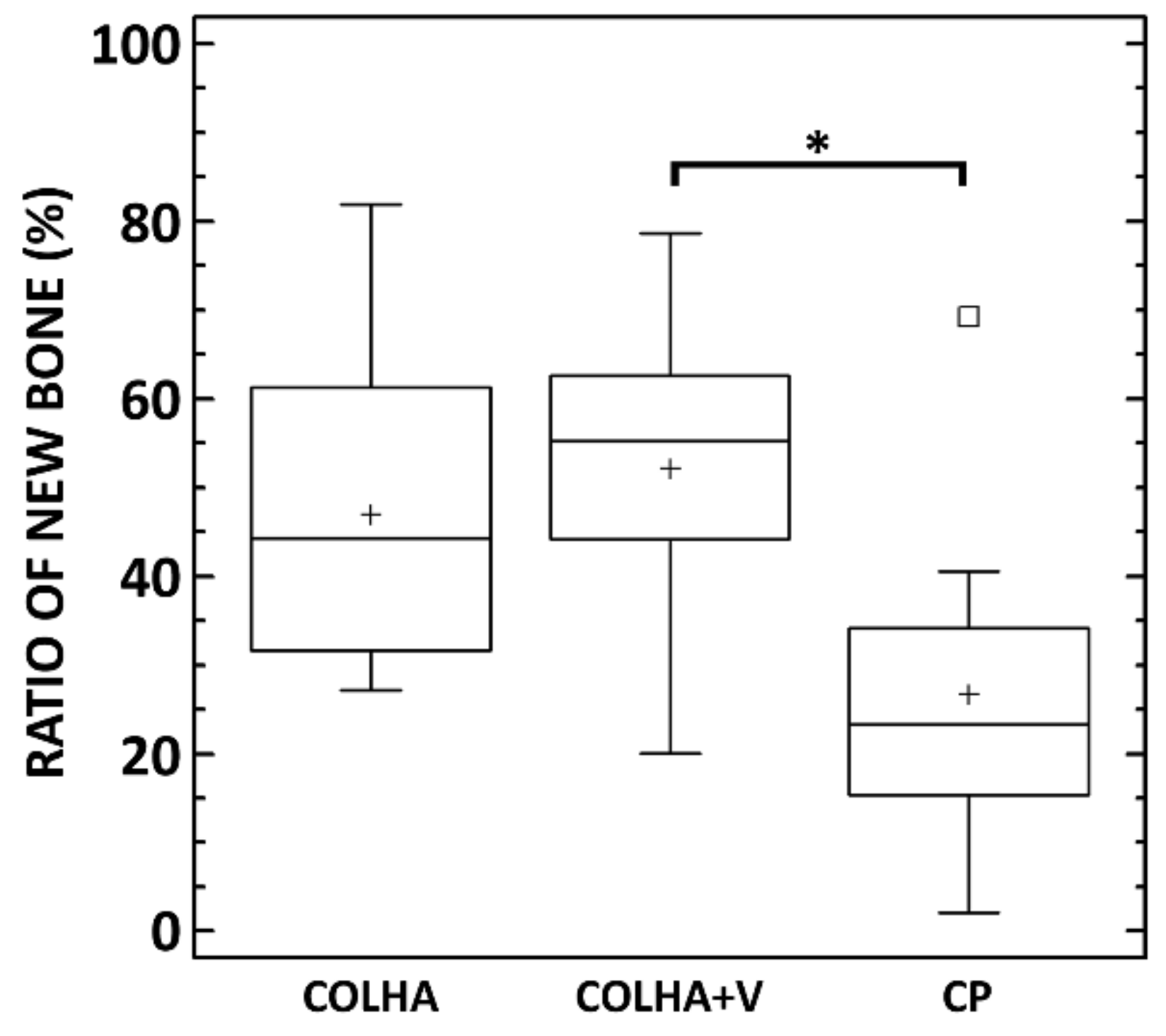
3.2.1. Bone Histology
3.2.2. Micro-CT Analysis
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ulrich, S.D.; Seyler, T.M.; Bennett, D.; Delanois, R.E.; Saleh, K.J.; Thongtrangan, I.; Kuskowski, M.; Cheng, E.Y.; Sharkey, P.F.; Parvizi, J.; et al. Total hip arthroplasties: What are the reasons for revision? Int. Orthop. 2008, 32, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef]
- Suchý, T.; Šupová, M.; Klapková, E.; Horný, L.; Rýglová, Š.; Žaloudková, M.; Braun, M.; Sucharda, Z.; Ballay, R.; Veselý, J.; et al. The sustainable release of vancomycin and its degradation products from nanostructured collagen/hydroxyapatite composite layers. J. Pharm. Sci. 2016, 105, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Geffers, M.; Ewald, A.; Lemm, M.; Nies, B.; Gbureck, U. Ready-to-use injectable calcium phosphate bone cement paste as drug carrier. Acta Biomater. 2013, 9, 9558–9567. [Google Scholar] [CrossRef]
- Antoci, V.; King, S.B.; Jose, B.; Parvizi, J.; Zeiger, A.R.; Wickstrom, E.; Freeman, T.A.; Composto, R.J.; Ducheyne, P.; Shapiro, I.M.; et al. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J. Orthop. Res. 2007, 25, 858–866. [Google Scholar] [CrossRef]
- Lawson, M.C.; Bowman, C.N.; Anseth, K.S. Vancomycin derivative photopolymerized to titanium kills S. epidermidis. Clin. Orthop. Relat. Res. 2007, 461, 96–105. [Google Scholar] [CrossRef]
- Parvizi, J.; Rothman, R.H.; Hozack, W.J.; Shapiro, I.M.; Adams, C.S.; Wickstrom, E.; Purtill, J.J.; Sharkey, P.F.; Hickok, N.J.; Zeiger, A.R. Frank stinchfield award: Titanium surface with biologic activity against infection. Clin. Orthop. Relat. Res. 2004, 429, 33–38. [Google Scholar] [CrossRef]
- Lawson, M.C.; Hoth, K.C.; Deforest, C.A.; Bowman, C.N.; Anseth, K.S. Inhibition of staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives. Clin. Orthop. Relat. Res. 2010, 468, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Antoci, V.; Adams, C.S.; Parvizi, J.; Ducheyne, P.; Shapiro, I.M.; Hickok, N.J. Covalently attached vancomycin provides a nanoscale antibacterial surface. Clin. Orthop. Relat. Res. 2007, 461, 81–87. [Google Scholar] [CrossRef]
- Edupuganti, O.P.; Antoci, V.; King, S.B.; Jose, B.; Adams, C.S.; Parvizi, J.; Shapiro, I.M.; Zeiger, A.R.; Hickok, N.J.; Wickstrom, E. Covalent bonding of vancomycin to Ti6Al4V alloy pins provides long-term inhibition of Staphylococcus aureus colonization. Bioorganic Med. Chem. Lett. 2007, 17, 2692–2696. [Google Scholar] [CrossRef]
- Klemm, K. Gentamicin-PMMA-beads in treating bone and soft tissue infections. Zent. Chir. 1979, 104, 934–942. [Google Scholar]
- Villalba-Rodriguez, A.M.; Parra-Saldivar, R.; Ahmed, I.; Karthik, K.; Malik, Y.S.; Dhama, K.; Iqbal, H.M.N. Bio-inspired biomaterials and their drug delivery perspectives—A review. Curr. Drug Metab. 2018, 18. [Google Scholar] [CrossRef]
- Geurts, J.; Chris Arts, J.J.; Walenkamp, G.H.I.M. Bone graft substitutes in active or suspected infection. Contra-indicated or not? Injury 2011, 42. [Google Scholar] [CrossRef]
- Uskokovic, V. Nanostructured platforms for the sustained and local delivery of antibiotics in the treatment of osteomyelitis. Crit. Rev. Drug Carr. Syst. 2014, 32, 1–59. [Google Scholar] [CrossRef]
- Alt, V.; Franke, J.; Schnettler, R. Local delivery of antibiotics in the surgical treatment of bone infections. Tech. Orthop. 2015, 30, 230–235. [Google Scholar] [CrossRef]
- Nair, M.; Krishnan, A. Antibiotic releasing biodegradable scaffolds for osteomyelitis. Curr. Drug Deliv. 2014, 11, 687–700. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, M.; Patel, S.; MacFarlane, R.J.; Haddad, F.S. Biodegradable antibiotic delivery systems. J. Bone Jt. Surg. Br. 2011, 93-B, 151–157. [Google Scholar] [CrossRef]
- Fleiter, N.; Walter, G.; Bösebeck, H.; Vogt, S.; Büchner, H.; Hirschberger, W.; Hoffmann, R. Clinical use and safety of a novel gentamicin-releasing resorbable bone graft substitute in the treatment of osteomyelitis/osteitis. Bone Jt. Res. 2014, 3, 223–229. [Google Scholar] [CrossRef]
- Manchon, A.; Prados-Frutos, J.; Rueda-Rodriguez, C.; Salinas-Goodier, C.; Alkhraisat, M.; Rojo, R.; Rodriguez-Gonzalez, A.; Berlanga, A.; Lopez-Cabarcos, E. Antibiotic release from calcium phosphate materials in oral and maxillofacial surgery. molecular, cellular and pharmaceutical aspects. Curr. Pharm. Biotechnol. 2017, 18, 52–63. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Lucke, M.; Wildemann, B.; Haas, N.P.; Raschke, M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: A review. Injury 2006, 37, S105–S112. [Google Scholar] [CrossRef] [PubMed]
- Ueng, S.W.N.; Yuan, L.J.; Lee, N.; Lin, S.S.; Liu, S.J.; Chan, E.C.; Weng, J.H. In vivo study of hot compressing molded 50:50 poly (DL-lactide-co-glycolide) antibiotic beads in rabbits. J. Orthop. Res. 2002, 20, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y.; Li, D.; Wei, B.; Xu, Y.; Gu, Q.; Wang, L. Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. Int. J. Nanomed. 2014, 3027–3036. [Google Scholar] [CrossRef]
- Ruszczak, Z.; Friess, W. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv. Drug Deliv. Rev. 2003, 55, 1679–1698. [Google Scholar] [CrossRef]
- Stinner, D.J.; Noel, S.P.; Haggard, W.O.; Watson, J.T.; Wenke, J.C. Local antibiotic delivery using tailorable chitosan sponges: The future of infection control? J. Orthop. Trauma 2010, 24, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.G.; Kwon, I.C.; Kim, Y.H.; Han, D.K.; Park, K.D.; Han, K.; Jeong, S.Y. Development of a local antibiotic delivery system using fibrin glue. J. Control. Release 1996, 39, 65–70. [Google Scholar] [CrossRef]
- Suchý, T.; Šupová, M.; Klapková, E.; Adamková, V.; Závora, J.; Žaloudková, M.; Rýglová, Š.; Ballay, R.; Denk, F.; Pokorný, M.; et al. The release kinetics, antimicrobial activity and cytocompatibility of differently prepared collagen/hydroxyapatite/vancomycin layers: Microstructure vs. nanostructure. Eur. J. Pharm. Sci. 2017, 100, 219–229. [Google Scholar] [CrossRef]
- Chen, D.W.; Hsu, Y.-H.; Liao, J.-Y.; Liu, S.-J.; Chen, J.-K.; Ueng, S.W.-N. Sustainable release of vancomycin, gentamicin and lidocaine from novel electrospun sandwich-structured PLGA/collagen nanofibrous membranes. Int. J. Pharm. 2012, 430, 335–341. [Google Scholar] [CrossRef]
- Lambert, L.; Novakova, M.; Lukac, P.; Cechova, D.; Sukenikova, L.; Hrdy, J.; Mlcek, M.; Chlup, H.; Suchy, T.; Grus, T. Evaluation of the immunogenicity of a vascular graft covered with collagen derived from the european carp (cyprinus carpio) and bovine collagen. Biomed Res. Int. 2019. [Google Scholar] [CrossRef]
- Suchy, T.; Supova, M.; Sauerova, P.; Kalbacova, M.H.; Klapkova, E.; Pokorny, M.; Horny, L.; Zavora, J.; Ballay, R.; Denk, F.; et al. Evaluation of collagen/hydroxyapatite electrospun layers loaded with vancomycin, gentamicin and their combination: Comparison of release kinetics, antimicrobial activity and cytocompatibility. Eur. J. Pharm. Biopharm. 2019, 140, 50–59. [Google Scholar] [CrossRef]
- Lian, X.; Liu, H.; Wang, X.; Xu, S.; Cui, F.; Bai, X. Antibacterial and biocompatible properties of vancomycin-loaded nano-hydroxyapatite/collagen/poly (lactic acid) bone substitute. Prog. Nat. Sci. Mater. Int. 2013, 23, 549–556. [Google Scholar] [CrossRef]
- Pon-On, W.; Charoenphandhu, N.; Teerapornpuntakit, J.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.-M. In vitro study of vancomycin release and osteoblast-like cell growth on structured calcium phosphate-collagen. Mater. Sci. Eng. C 2013, 33, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Ionita, D.; Bajenaru-Georgescu, D.; Totea, G.; Mazare, A.; Schmuki, P.; Demetrescu, I. Activity of vancomycin release from bioinspired coatings of hydroxyapatite or TiO2 nanotubes. Int. J. Pharm. 2017, 517, 296–302. [Google Scholar] [CrossRef]
- Lian, X.; Mao, K.; Liu, X.; Wang, X.; Cui, F. In vivo osteogenesis of vancomycin loaded nanohydroxyapatite/collagen/calcium sulfate composite for treating infectious bone defect induced by chronic osteomyelitis. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Coelho, C.C.; Sousa, S.R.; Monteiro, F.J. Heparinized nanohydroxyapatite/collagen granules for controlled release of vancomycin. J. Biomed. Mater. Res. Part A 2015, 103, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Faigle, G.; Bernstein, A.; Suedkamp, N.P.; Mayr, H.O.; Peters, F.; Huebner, W.D.; Seidenstuecker, M. Release behavior of VAN from four types of CaP-ceramic granules using various loading methods at two different degrees of acidity. J. Mater. Sci. Mater. Med. 2018, 29, 12. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.J.; Wang, X.M.; Cui, F.Z. In Vitro Antibacterial Properties of vancomycin-loaded nano-hydroxyapatite/collagen/calcium sulfate hemihydrates (VCM/nHAC/CSH) bone substitute. Mater. Sci. Forum 2013, 745–746, 6–12. [Google Scholar] [CrossRef]
- Mao, K.; Liu, J.; Lian, X.; Wang, Q.; Wang, X.; Mei, W.; Mao, K. Controlled release of rhbmp-2 and vancomycin from nhac/$α$-csh scaffold for treatment of chronic osteomyelitis. J. Biomater. Tissue Eng. 2015, 5, 294–300. [Google Scholar] [CrossRef]
- Pokorný, M.; Suchý, T.; Kotzianová, A.; Klemeš, J.; Denk, F.; Šupová, M.; Sucharda, Z.; Sedláček, R.; Horný, L.; Králík, V.; et al. Surface treatment of acetabular cups with a direct deposition of a composite nanostructured layer using a high electrostatic field. Molecules 2020, 25, 1173. [Google Scholar] [CrossRef]
- Pokorny, M.; Rassushin, V.; Wolfova, L.; Velebny, V. Increased production of nanofibrous materials by electroblowing from blends of hyaluronic acid and polyethylene oxide. Polym. Eng. Sci. 2016, 56, 932–938. [Google Scholar] [CrossRef]
- European committee for antimicrobial susceptibility testing (EUCAST) of the European society of clinical microbiology and infectious diseases (ESCMID) determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, 596–600. [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: http://www.eucast.org (accessed on 6 August 2020).
- Ruzicka, F.; Hola, V.; Votava, M.; Tejkalova, R.; Horvat, R.; Heroldova, M.; Woznicova, V. Biofilm detection and the clinical significance of Staphylococcus epidermidis isolates. Folia Microbiol. 2004, 49, 596–600. [Google Scholar] [CrossRef]
- Cannon, C.Z.; Kissling, G.E.; Goulding, D.R.; King-Herbert, A.P.; Blankenship-Paris, T. Analgesic effects of tramadol, carprofen or multimodal analgesia in rats undergoing ventral laparotomy. Lab Anim. 2011, 40, 85–93. [Google Scholar] [CrossRef]
- Babuska, V.; Moztarzadeh, O.; Kubikova, T.; Moztarzadeh, A.; Hrusak, D.; Tonar, Z. Evaluating the osseointegration of nanostructured titanium implants in animal models: Current experimental methods and perspectives (Review). Biointerphases 2016, 11, 30801. [Google Scholar] [CrossRef]
- Kubíková, T.; Bartoš, M.; Juhas, Š.; Suchý, T.; Sauerová, P.; Hubálek-Kalbáčová, M.; Tonar, Z. Comparison of ground sections, paraffin sections and micro-CT imaging of bone from the epiphysis of the porcine femur for morphometric evaluation. Ann. Anat. Anat. Anz. 2018, 220, 85–96. [Google Scholar] [CrossRef]
- Jiřík, M.; Bartoš, M.; Tomášek, P.; Malečková, A.; Kural, T.T.; Horáková, J.; Lukáš, D.; Suchý, T.; Kochová, P.; Hubálek Kalbáčová, M.; et al. Generating standardized image data for testing and calibrating quantification of volumes, surfaces, lengths, and object counts in fibrous and porous materials using X-ray microtomography. Microsc. Res. Tech. 2018, 81, 551–568. [Google Scholar] [CrossRef] [PubMed]
- Kourkoumelis, N.; Balatsoukas, I.; Tzaphlidou, M. Ca/P concentration ratio at different sites of normal and osteoporotic rabbit bones evaluated by Auger and energy dispersive X-ray spectroscopy. J. Biol. Phys. 2012, 38, 279–291. [Google Scholar] [CrossRef]
- Melichercik, P.; Klapkova, E.; Landor, I.; Judl, T.; Sibek, M.; Jahoda, D. The effect of Vancomycin degradation products in the topical treatment of osteomyelitis. Bratisl. Lek. Listy 2014, 115, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Roldán, J.C.; Detsch, R.; Schaefer, S.; Chang, E.; Kelantan, M.; Waiss, W.; Reichert, T.E.; Gurtner, G.C.; Deisinger, U. Bone formation and degradation of a highly porous biphasic calcium phosphate ceramic in presence of BMP-7, VEGF and mesenchymal stem cells in an ectopic mouse model. J. Cranio-Maxillofac. Surg. 2010, 38, 423–430. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Ripamonti, A.; Roveri, N.; Romanello, M.; Suarez, K.N.; Moro, L. Chemical and structural characterization of the mineral phase from cortical and trabecular bone. J. Inorg. Biochem. 1997, 68, 45–51. [Google Scholar] [CrossRef]
- Termine, J.D.; Eanes, E.D.; Greenfield, D.J.; Nylen, M.U.; Harper, R.A. Hydrazine-deproteinated bone mineral. Calcif. Tissue Res. 1973, 12, 73–90. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Noordin, S.; Masri, B.A.; Garbuz, D.S.; Duncan, C.P.; Hancock, R.E.W.; Wang, R. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 1344–1352. [Google Scholar] [CrossRef]
- Tang, T.; Ao, H.; Yang, S.; Wang, Y.; Lin, W.; Yu, Z.; Yang, Y. In vivo evaluation of the anti-infection potential of gentamicin-loaded nanotubes on titania implants. Int. J. Nanomed. 2016, 2223–2234. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, X.G.; Wang, J.W.; Zhou, H.; Dong, J. Treatment of osteomyelitis defects by a vancomycin-loaded gelatin/$β$-tricalcium phosphate composite scaffold. Bone Jt. Res. 2018, 7, 46–57. [Google Scholar] [CrossRef]
- Zaichick, S.; Zaichick, V. Neutron activation analysis of Ca, Cl, Mg, Na, and P content in human bone affected by osteomyelitis or osteogenic sarcoma. J. Radioanal. Nucl. Chem. 2012, 293, 241–246. [Google Scholar] [CrossRef]
- Fiore, E.; Levi, M.; Gianesella, M.; Benazzi, C.; Morgante, M.; Beltrame, A.; Vaccaro, C.; Gentile, A. Epiphysitis in fattening bulls: Radiological and pathologic findings. Large Anim. Rev. 2016, 22, 43–45. [Google Scholar]
- Henderson, B.; Nair, S.P. Hard labour: Bacterial infection of the skeleton. Trends Microbiol. 2003, 11, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.A.; Leid, J.G.; Calhoun, J.H.; Costerton, J.W.; Shirtliff, M.E. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol. Med. Microbiol. 2008, 52, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Esmonde-White, K.A.; Esmonde-White, F.W.L.; Holmes, C.M.; Morris, M.D.; Roessler, B.J. Alterations to bone mineral composition as an early indication of osteomyelitis in the diabetic foot. Diabetes Care 2013, 36, 3652–3654. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Wang, C.; Canden, A.; Burr, M.; Agarwal, J. Local intramedullary delivery of vancomycin can prevent the development of long bone Staphylococcus aureus infection. PLoS ONE 2016, 11, e0160187. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.S.B.; Heilshorn, S.C.S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Alghamdi, H.S.; van Oirschot, B.A.J.A.; Bosco, R.; van den Beucken, J.J.J.P.; Aldosari, A.A.F.; Anil, S.; Jansen, J.A. Biological response to titanium implants coated with nanocrystals calcium phosphate or type 1 collagen in a dog model. Clin. Oral Implant. Res. 2013, 24, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, S.; Li, Z.; Li, S.; Liu, J.; Zhang, C. Osseointegration effect of biomimetic intrafibrillarly mineralized collagen applied simultaneously with titanium implant: A pilot in vivo study. Clin. Oral Implant. Res. 2019, 30, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, L.; Cui, Y.; Song, T.-X.; Qiu, Z.-Y.; Wang, X.-M.; Tan, B.-S. Clinical evaluations of mineralized collagen in the extraction sites preservation. Regen. Biomater. 2016, 3, 41–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, C.; Zilm, M.; Wei, M. Fabrication of intrafibrillar and extrafibrillar mineralized collagen/apatite scaffolds with a hierarchical structure. J. Biomed. Mater. Res. A 2016, 104, 1153–1161. [Google Scholar] [CrossRef]
- Jang, C.H.; Lee, H.; Kim, M.; Kim, G.H. Accelerated osteointegration of the titanium-implant coated with biocomponents, collagen/hydroxyapatite/bone morphogenetic protein-2, for bone-anchored hearing aid. J. Ind. Eng. Chem. 2018, 63, 230–236. [Google Scholar] [CrossRef]
- Lee, S.-W.; Hahn, B.-D.; Kang, T.Y.; Lee, M.-J.; Choi, J.-Y.; Kim, M.-K.; Kim, S.-G. Hydroxyapatite and collagen combination-coated dental implants display better bone formation in the peri-implant area than the same combination plus bone morphogenetic protein-2–coated implants, hydroxyapatite only coated implants, and uncoated implants. J. Oral Maxillofac. Surg. 2014, 72, 53–60. [Google Scholar] [CrossRef]
- Hahn, B.-D.; Lee, J.-M.; Park, D.-S.; Choi, J.-J.; Ryu, J.; Yoon, W.-H.; Lee, B.-K.; Shin, D.-S.; Kim, H.-E. Mechanical and in vitro biological performances of hydroxyapatite–carbon nanotube composite coatings deposited on Ti by aerosol deposition. Acta Biomater. 2009, 5, 3205–3214. [Google Scholar] [CrossRef]
- Lucke, M.; Wildemann, B.; Sadoni, S.; Surke, C.; Schiller, R.; Stemberger, A.; Raschke, M.; Haas, N.P.; Schmidmaier, G. Systemic versus local application of gentamicin in prophylaxis of implant-related osteomyelitis in a rat model. Bone 2005, 36, 770–778. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ren, L.; Zhang, S.; Wei, X.; Yang, K.; Dai, K. Antibacterial effect of a copper-containing titanium alloy against implant-associated infection induced by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2021, 119, 472–484. [Google Scholar] [CrossRef]
- Søe, N.H.; Jensen, N.V.; Jensen, A.L.; Koch, J.; Poulsen, S.S.; Pier, G.B.; Johansen, H.K. Active and passive immunization against Staphylococcus aureus periprosthetic osteomyelitis in rats. In Vivo 2017, 31, 45–50. [Google Scholar] [CrossRef]
- Tran, P.A.; O’Brien-Simpson, N.; Palmer, J.A.; Bock, N.; Reynolds, E.C.; Webster, T.J.; Deva, A.; Morrison, W.A.; O’Connor, A.J. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant Staphylococcus aureus and epidermidis: In vitro and in vivo assessment. Int. J. Nanomed. 2019, 14, 4613–4624. [Google Scholar] [CrossRef]
- Gomes, F.; Teixeira, P.; Oliveira, R. Mini-review: Staphylococcus epidermidis as the most frequent cause of nosocomial infections: Old and new fighting strategies. Biofouling 2014, 30, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Viney, M.; Riley, E.M. The immunology of wild rodents: Current status and future prospects. Front. Immunol. 2017, 8, 1481. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; Romanò, C.L.; Bottagisio, M.; Monti, L.; De Vecchi, E.; Previdi, S.; Accetta, R.; Drago, L. Modeling staphylococcus epidermidis-induced non-unions: Subclinical and clinical evidence in rats. PLoS ONE 2016, 11, e0147447. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchý, T.; Vištejnová, L.; Šupová, M.; Klein, P.; Bartoš, M.; Kolinko, Y.; Blassová, T.; Tonar, Z.; Pokorný, M.; Sucharda, Z.; et al. Vancomycin-Loaded Collagen/Hydroxyapatite Layers Electrospun on 3D Printed Titanium Implants Prevent Bone Destruction Associated with S. epidermidis Infection and Enhance Osseointegration. Biomedicines 2021, 9, 531. https://doi.org/10.3390/biomedicines9050531
Suchý T, Vištejnová L, Šupová M, Klein P, Bartoš M, Kolinko Y, Blassová T, Tonar Z, Pokorný M, Sucharda Z, et al. Vancomycin-Loaded Collagen/Hydroxyapatite Layers Electrospun on 3D Printed Titanium Implants Prevent Bone Destruction Associated with S. epidermidis Infection and Enhance Osseointegration. Biomedicines. 2021; 9(5):531. https://doi.org/10.3390/biomedicines9050531
Chicago/Turabian StyleSuchý, Tomáš, Lucie Vištejnová, Monika Šupová, Pavel Klein, Martin Bartoš, Yaroslav Kolinko, Tereza Blassová, Zbyněk Tonar, Marek Pokorný, Zbyněk Sucharda, and et al. 2021. "Vancomycin-Loaded Collagen/Hydroxyapatite Layers Electrospun on 3D Printed Titanium Implants Prevent Bone Destruction Associated with S. epidermidis Infection and Enhance Osseointegration" Biomedicines 9, no. 5: 531. https://doi.org/10.3390/biomedicines9050531
APA StyleSuchý, T., Vištejnová, L., Šupová, M., Klein, P., Bartoš, M., Kolinko, Y., Blassová, T., Tonar, Z., Pokorný, M., Sucharda, Z., Žaloudková, M., Denk, F., Ballay, R., Juhás, Š., Juhásová, J., Klapková, E., Horný, L., Sedláček, R., Grus, T., ... Hrabák, J. (2021). Vancomycin-Loaded Collagen/Hydroxyapatite Layers Electrospun on 3D Printed Titanium Implants Prevent Bone Destruction Associated with S. epidermidis Infection and Enhance Osseointegration. Biomedicines, 9(5), 531. https://doi.org/10.3390/biomedicines9050531