Experimental Bioactive Glass-Containing Composites and Commercial Restorative Materials: Anti-Demineralizing Protection of Dentin
Abstract
:1. Introduction
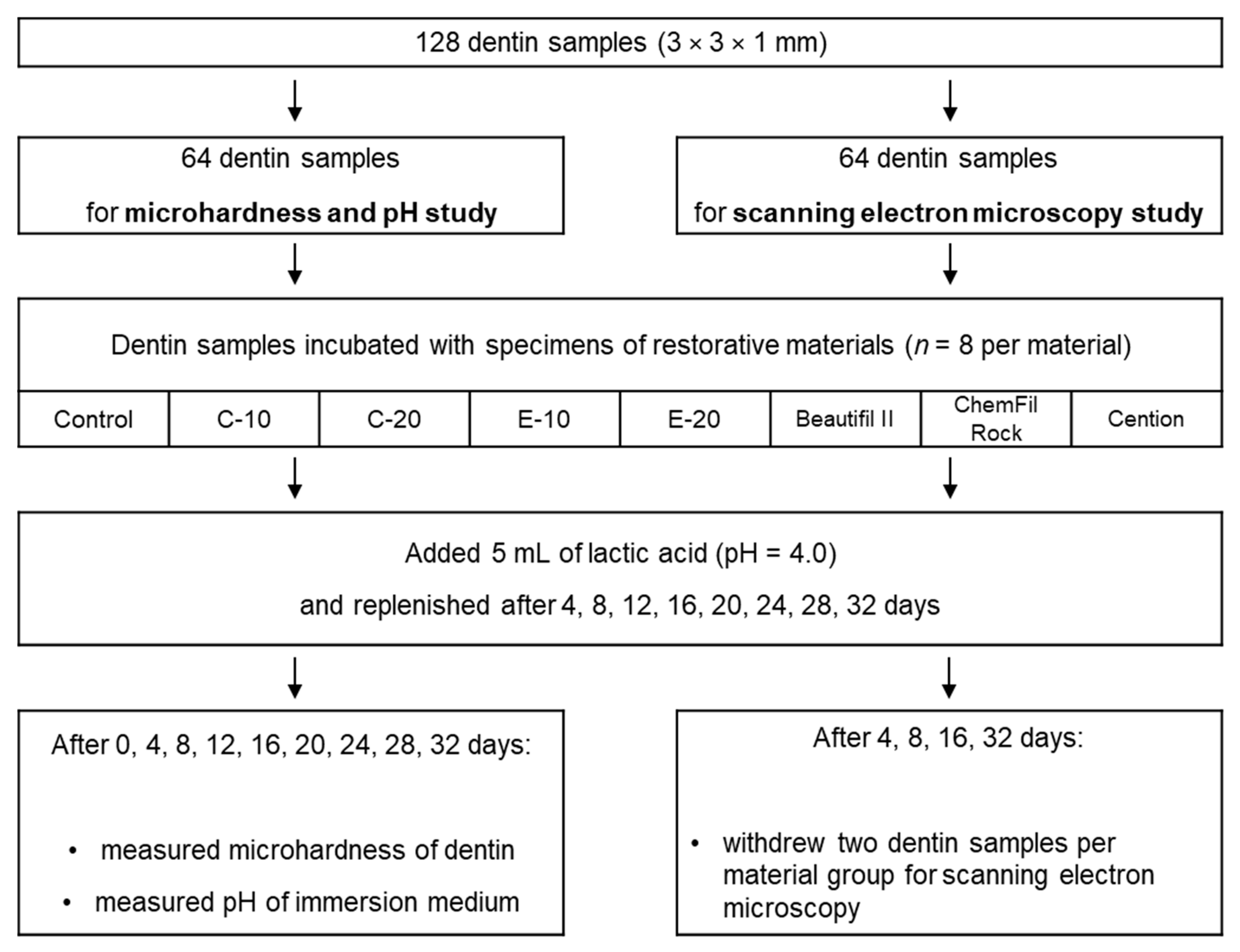
2. Materials and methods
2.1. Experimental Resin Composites
2.2. Dentin Samples
2.3. Restorative Material Specimens
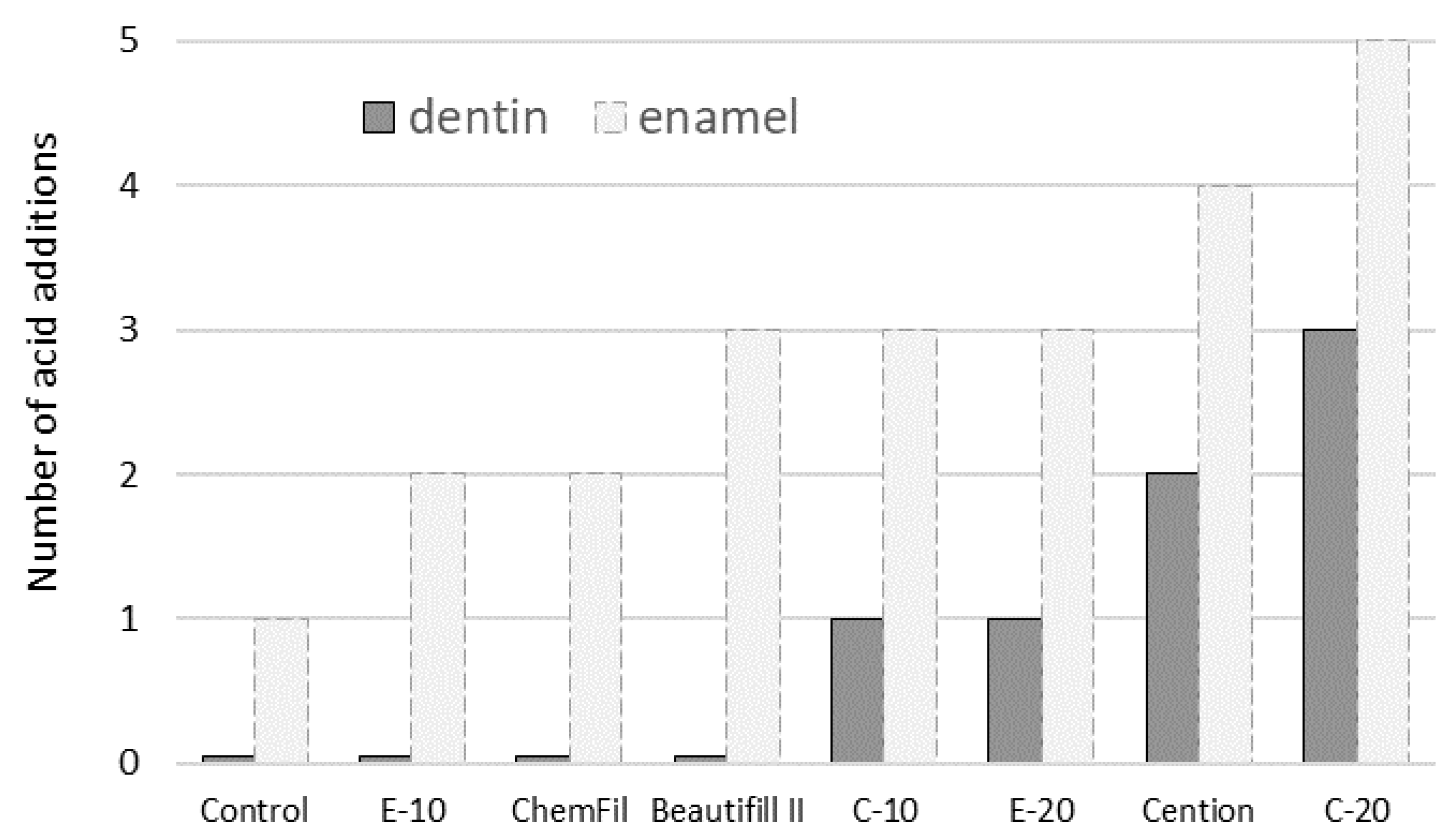
2.4. Immersion in Lactic Acid Solution
2.5. Microhardness Measurements
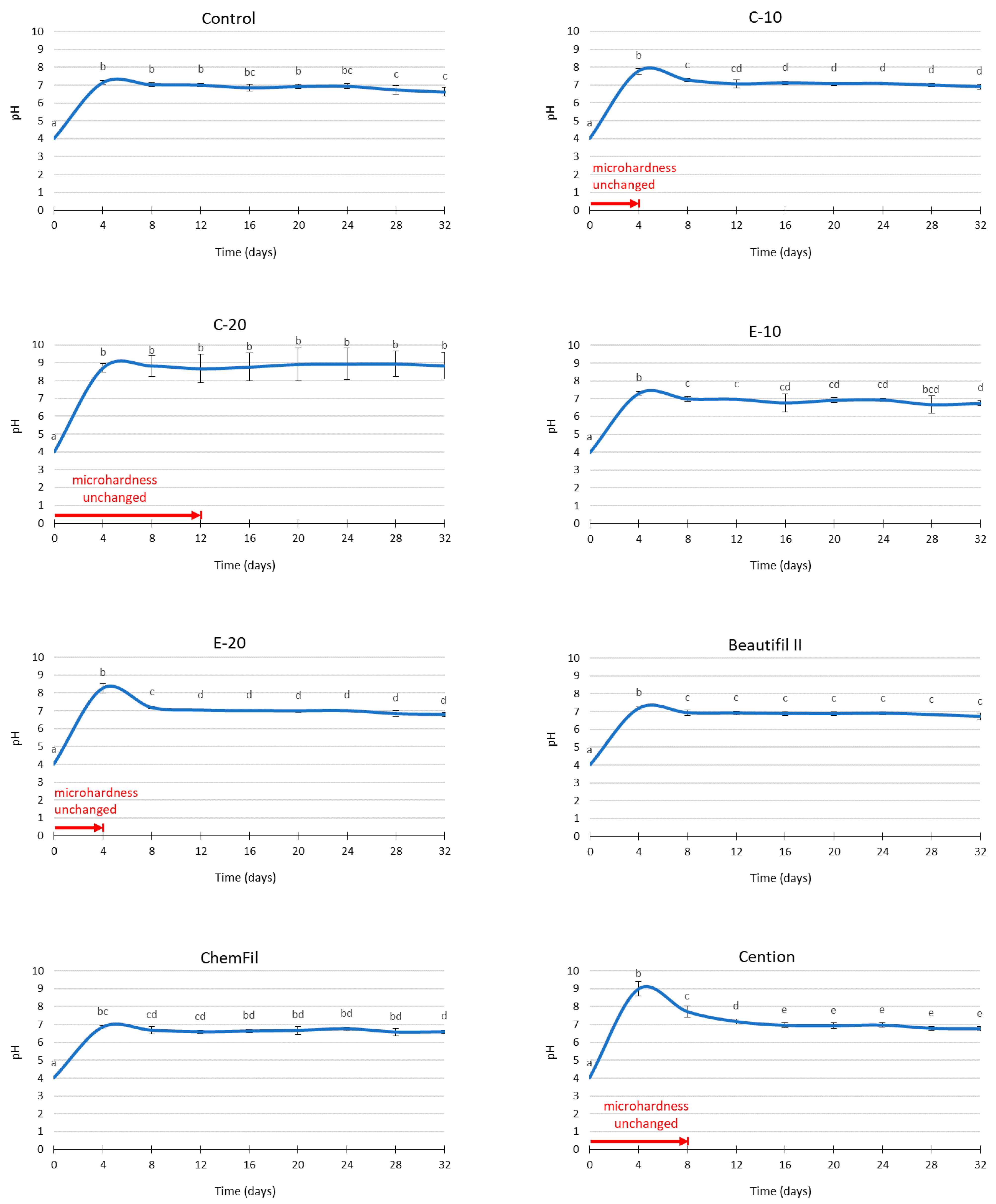
2.6. pH Measurements
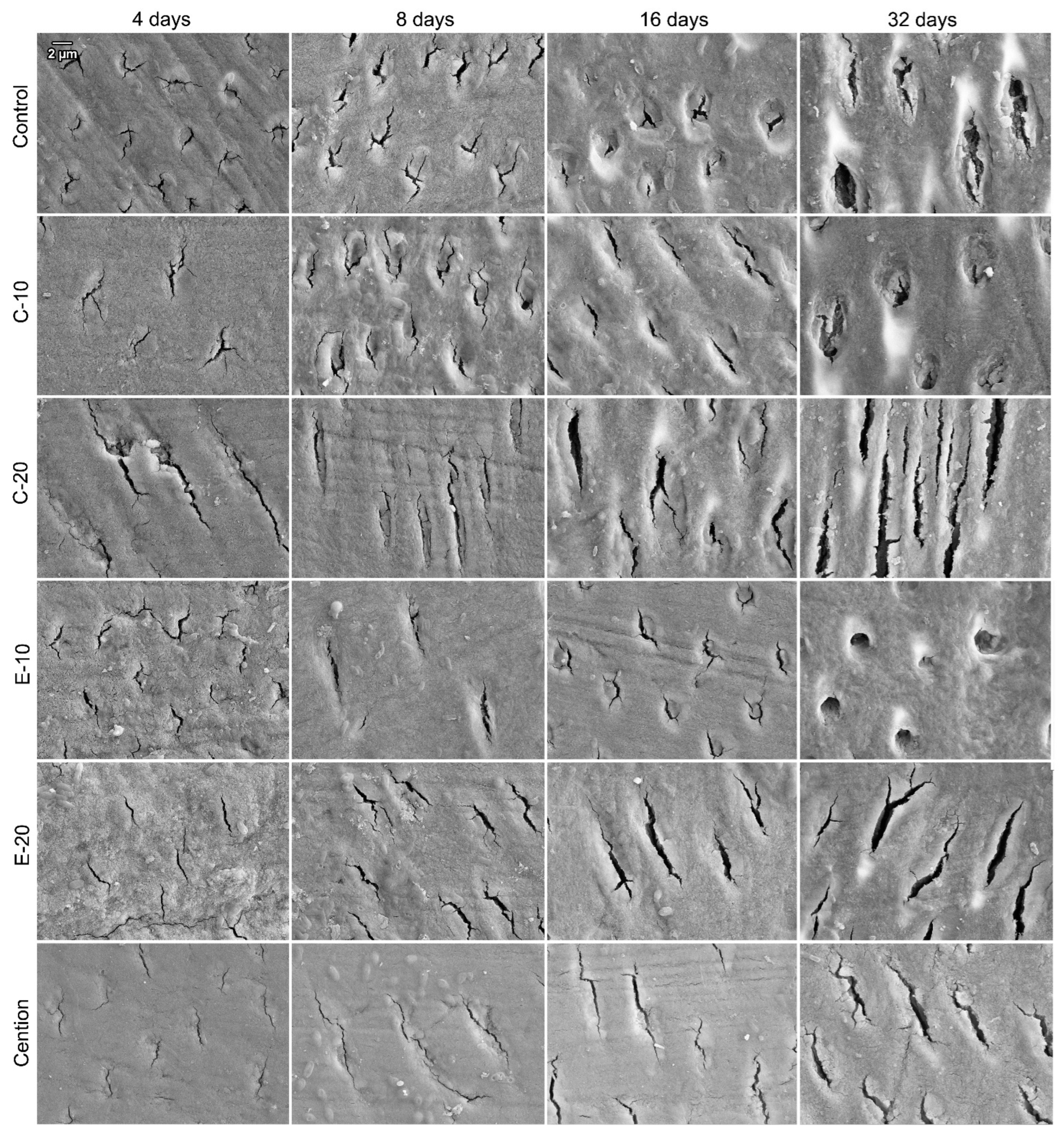
2.7. Scanning Electron Microscopy
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aminoroaya, A.; Neisiany, R.E.; Khorasani, S.N.; Panahi, P.; Das, O.; Madry, H.; Cucchiarini, M.; Ramakrishna, S. A Review of Dental Composites: Challenges, Chemistry Aspects, Filler Influences, and Future Insights. Compos. Part B Eng. 2021, 216, 108852. [Google Scholar] [CrossRef]
- Par, M.; Marovic, D.; Attin, T.; Tarle, Z.; Tauböck, T.T. Effect of Rapid High-Intensity Light-Curing on Polymerization Shrinkage Properties of Conventional and Bulk-Fill Composites. J. Dent. 2020, 101, 103448. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Marovic, D.; Attin, T.; Tarle, Z.; Tauböck, T.T. Rapid High-Intensity Light-Curing of Bulk-Fill Composites: A Quantitative Analysis of Marginal Integrity. J. Dent. 2021, 111, 103708. [Google Scholar] [CrossRef]
- Nedeljkovic, I.; De Munck, J.; Vanloy, A.; Declerck, D.; Lambrechts, P.; Peumans, M.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, K.L. Secondary Caries: Prevalence, Characteristics, and Approach. Clin. Oral Investig. 2020, 24, 683–691. [Google Scholar] [CrossRef]
- Askar, H.; Krois, J.; Göstemeyer, G.; Bottenberg, P.; Zero, D.; Banerjee, A.; Schwendicke, F. Secondary Caries: What Is It, and How It Can Be Controlled, Detected, and Managed? Clin. Oral Investig. 2020, 24, 1869–1876. [Google Scholar] [CrossRef]
- Luo, S.; Liu, F.; He, J. Preparation of Low Shrinkage Stress Dental Composite with Synthesized Dimethacrylate Oligomers. J. Mech. Behav. Biomed. Mater. 2019, 94, 222–228. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Z.; Guo, Y.; Zhang, H.; Qiu, Y.; Li, J.; Ma, D.; Li, Z.; Zhen, P.; Liu, B.; et al. Novel Core–Shell CHX/ACP Nanoparticles Effectively Improve the Mechanical, Antibacterial and Remineralized Properties of the Dental Resin Composite. Dent. Mater. 2021, 37, 636–647. [Google Scholar] [CrossRef]
- Par, M.; Attin, T.; Tarle, Z.; Tauböck, T.T. A New Customized Bioactive Glass Filler to Functionalize Resin Composites: Acid-Neutralizing Capability, Degree of Conversion, and Apatite Precipitation. J. Clin. Med. 2020, 9, 1173. [Google Scholar] [CrossRef]
- Jardim, R.N.; Rocha, A.A.; Rossi, A.M.; de Almeida Neves, A.; Portela, M.B.; Lopes, R.T.; Pires dos Santos, T.M.; Xing, Y.; Moreira da Silva, E. Fabrication and Characterization of Remineralizing Dental Composites Containing Hydroxyapatite Nanoparticles. J. Mech. Behav. Biomed. Mater. 2020, 109, 103817. [Google Scholar] [CrossRef]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive Glass Fillers Reduce Bacterial Penetration into Marginal Gaps for Composite Restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, M.; Zhu, X.X. Functional Fillers for Dental Resin Composites. Acta Biomater. 2021, 122, 50–65. [Google Scholar] [CrossRef]
- Al-eesa, N.A.; Johal, A.; Hill, R.G.; Wong, F.S.L. Fluoride Containing Bioactive Glass Composite for Orthodontic Adhesives—Apatite Formation Properties. Dent. Mater. 2018, 34, 1127–1133. [Google Scholar] [CrossRef]
- Al-Eesa, N.A.; Wong, F.S.L.; Johal, A.; Hill, R.G. Fluoride Containing Bioactive Glass Composite for Orthodontic Adhesives—Ion Release Properties. Dent. Mater. 2017, 33, 1324–1329. [Google Scholar] [CrossRef]
- Tauböck, T.T.; Zehnder, M.; Schweizer, T.; Stark, W.J.; Attin, T.; Mohn, D. Functionalizing a Dentin Bonding Resin to Become Bioactive. Dent. Mater. 2014, 30, 868–875. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Sauro, S.; Watson, T.F.; Toledano, M. Experimental Resin Cements Containing Bioactive Fillers Reduce Matrix Metalloproteinase–Mediated Dentin Collagen Degradation. J. Endod. 2012, 38, 1227–1232. [Google Scholar] [CrossRef]
- Odermatt, R.; Par, M.; Mohn, D.; Wiedemeier, D.B.; Attin, T.; Tauböck, T.T. Bioactivity and Physico-Chemical Properties of Dental Composites Functionalized with Nano- vs. Micro-Sized Bioactive Glass. J. Clin. Med. 2020, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Par, M.; Spanovic, N.; Mohn, D.; Attin, T.; Tauböck, T.T.; Tarle, Z. Curing Potential of Experimental Resin Composites Filled with Bioactive Glass: A Comparison between Bis-EMA and UDMA Based Resin Systems. Dent. Mater. 2020, 36, 711–723. [Google Scholar] [CrossRef]
- Par, M.; Mohn, D.; Attin, T.; Tarle, Z.; Tauböck, T.T. Polymerization Shrinkage Behaviour of Resin Composites Functionalized with Unsilanized Bioactive Glass Fillers. Sci. Rep. 2020, 10, 15237. [Google Scholar] [CrossRef]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tauböck, T.T. Anti-Demineralizing Protective Effects on Enamel Identified in Experimental and Commercial Restorative Materials with Functional Fillers. Sci. Rep. 2021, 11, 11806. [Google Scholar] [CrossRef]
- Par, M.; Prskalo, K.; Tauböck, T.T.; Skenderovic, H.; Attin, T.; Tarle, Z. Polymerization Kinetics of Experimental Resin Composites Functionalized with Conventional (45S5) and a Customized Low-Sodium Fluoride-Containing Bioactive Glass. Sci. Rep. 2021, 11, 21225. [Google Scholar] [CrossRef]
- Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Tauböck, T.T. Ion Release and Hydroxyapatite Precipitation of Resin Composites Functionalized with Two Types of Bioactive Glass. J. Dent. in press.
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Enamel Surface Remineralization Effect by Fluorinated Graphite and Bioactive Glass-Containing Orthodontic Bonding Resin. Materials 2019, 12, 1308. [Google Scholar] [CrossRef] [Green Version]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials 2019, 12, 1813. [Google Scholar] [CrossRef] [Green Version]
- Manfred, L.; Covell, D.A.; Crowe, J.J.; Tufekci, E.; Mitchell, J.C. A Novel Biomimetic Orthodontic Bonding Agent Helps Prevent White Spot Lesions Adjacent to Brackets. Angle Orthod. 2013, 83, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Langhorst, S.E.; O’Donnell, J.N.R.; Skrtic, D. In Vitro Remineralization of Enamel by Polymeric Amorphous Calcium Phosphate Composite: Quantitative Microradiographic Study. Dent. Mater. 2009, 25, 884–891. [Google Scholar] [CrossRef] [Green Version]
- Sauro, S.; Thompson, I.; Watson, T. Effects of Common Dental Materials Used in Preventive or Operative Dentistry on Dentin Permeability and Remineralization. Oper. Dent. 2011, 36, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Pashley, E.L.; Carvalho, R.M.; Tay, F.R. The Effects of Dentin Permeability on Restorative Dentistry. Dent. Clin. 2002, 46, 211–245. [Google Scholar] [CrossRef]
- Kelić, K.; Par, M.; Peroš, K.; Šutej, I.; Tarle, Z. Fluoride-Releasing Restorative Materials: The Effect of a Resinous Coat on Ion Release. Acta Stomatol. Croat. 2020, 54, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Drysdale, T.; Frank, R.M. Experimental Erosion of Dentin. Eur. J. Oral Sci. 1991, 99, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhang, W.; Pashley, D.H.; Breschi, L.; Mao, J.; Chen, J.; Tay, F.R. Biomimetic Remineralization of Dentin. Dent. Mater. 2014, 30, 77–96. [Google Scholar] [CrossRef] [Green Version]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Mechanical Properties Of Experimental Composites Containing Bioactive Glass After Artificial Aging In Water And Ethanol. Clin. Oral Investig. 2019, 23, 2733–2741. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Dentin Bond Strength Of Experimental Composites Containing Bioactive Glass. J. Adhes. Dent. 2018, 20, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Maruo, Y.; Sano, H.; Yoshida, Y.; Van Meerbeek, B. Bacterial Adhesion Not Inhibited By Ion-Releasing Bioactive Glass Filler. Dent. Mater. 2017, 33, 723–734. [Google Scholar] [CrossRef] [PubMed]
| Bioactive Glass 45S5 | Experimental Fluoride-Containing Bioactive Glass | Inert Barium Glass | Silica | |
|---|---|---|---|---|
| Particle size (d50) | 3 µm | 3 µm | 1 µm | 5–50 nm |
| Composition (wt%) | 45.0% SiO2 24.5% CaO 24.5% Na2O 6.0% P2O5 | 33.5% SiO2 33.0% CaO 10.5% Na2O 11.0% P2O5 12.0% CaF2 | 55.0% SiO2 25.0% BaO 10.0% Al2O3 10.0% B2O3 | >99.8% SiO2 |
| Silanization (wt%) | none | none | 3.2 | 4–6 |
| Manufacturer | Schott, Mainz, Germany | Schott, Mainz, Germany | Schott, Mainz, Germany | Evonik, Hanau, Germany |
| Product name/LOT | G018-144/M111473 | experimental batch | GM27884/Sil13696 | Aerosil R 7200/157020635 |
| Material Designation | Filler Composition (wt%) | Total Filler Ratio (wt%) | ||
|---|---|---|---|---|
| Bioactive Glass 45S5 | Experimental Fluoride-Containing Bioactive Glass | Reinforcing Fillers (Inert Barium Glass: Silica = 2:1) | ||
| Control | 0 | 0 | 70 | 70 |
| C-10 | 10 | 0 | 60 | 70 |
| C-20 | 20 | 0 | 50 | 70 |
| E-10 | 0 | 10 | 60 | 70 |
| E-20 | 0 | 20 | 50 | 70 |
| Time | No. of Acid Addition Cycles | Material | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | C-10 | C-20 | E-10 | E-20 | Beautifil II | ChemFil | Cention | ||
| Initial | 0 | 48.9 (1.5) a | 50.2 (4.2) a | 51.8 (2.7) a | 53.6 (2.3) a | 52.5 (2.4) a | 51.6 (3.5) a | 48.9 (4.4) a | 53.0 (3.8) a |
| 4 days | 1 | 42.6 (1.9) b | 45.7 (4.9) ab | 48.7 (2.1) ab | 46.9 (2.9) b | 47.7 (4.7) ab | 45.6 (3.5) b | 42.2 (3.8) b | 51.2 (4.5) a |
| 8 days | 2 | 36.7 (1.4) c | 39.4 (5.5) bc | 48.7 (2.3) ab | 44.0 (2.6) bc | 44.3 (4.3) b | 40.6 (4.0) c | 36.4 (3.9) c | 48.1 (4.8) ab |
| 12 days | 3 | 33.5 (2.6) c | 34.3 (5.3) c | 45.6 (3.6) abc | 40.8 (3.1) c | 38.9 (4.3) c | 36.1 (3.4) c | 32.1 (2.7) c | 43.3 (5.6) b |
| 16 days | 4 | 22.6 (2.9) d | 23.7 (5.0) d | 37.9 (6.4) cd | 26.7 (3.5) d | 27.3 (2.0) d | 23.8 (2.8) d | 22.0 (3.1) d | 32.6 (3.4) c |
| 20 days | 5 | 18.4 (2.4) e | 23.2 (5.1) d | 41.8 (5.4) bcd | 25.5 (3.6) d | 24.8 (3.5) d | 23.0 (2.8) d | 21.4 (1.9) d | 30.1 (4.6) cd |
| 24 days | 6 | 18.5 (3.1) e | 21.2 (4.6) de | 39.3 (6.2) cd | 23.3 (3.1) de | 24.5 (2.8) d | 21.1 (1.9) d | 20.0 (1.7) d | 28.0 (2.8) cd |
| 28 days | 7 | 17.4 (2.4) e | 17.0 (3.0) de | 34.8 (6.6) d | 20.0 (2.8) ef | 22.1 (1.8) de | 19.2 (2.6) de | 18.1 (2.4) de | 24.1 (2.5) de |
| 32 days | 8 | 15.1 (1.2) e | 15.7 (2.8) e | 34.3 (8.1) d | 16.6 (2.5) f | 17.0 (2.3) e | 15.8 (2.1) e | 14.5 (1.6) e | 21.6 (2.3) e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Par, M.; Gubler, A.; Attin, T.; Tarle, Z.; Tarle, A.; Tauböck, T.T. Experimental Bioactive Glass-Containing Composites and Commercial Restorative Materials: Anti-Demineralizing Protection of Dentin. Biomedicines 2021, 9, 1616. https://doi.org/10.3390/biomedicines9111616
Par M, Gubler A, Attin T, Tarle Z, Tarle A, Tauböck TT. Experimental Bioactive Glass-Containing Composites and Commercial Restorative Materials: Anti-Demineralizing Protection of Dentin. Biomedicines. 2021; 9(11):1616. https://doi.org/10.3390/biomedicines9111616
Chicago/Turabian StylePar, Matej, Andrea Gubler, Thomas Attin, Zrinka Tarle, Andro Tarle, and Tobias T. Tauböck. 2021. "Experimental Bioactive Glass-Containing Composites and Commercial Restorative Materials: Anti-Demineralizing Protection of Dentin" Biomedicines 9, no. 11: 1616. https://doi.org/10.3390/biomedicines9111616
APA StylePar, M., Gubler, A., Attin, T., Tarle, Z., Tarle, A., & Tauböck, T. T. (2021). Experimental Bioactive Glass-Containing Composites and Commercial Restorative Materials: Anti-Demineralizing Protection of Dentin. Biomedicines, 9(11), 1616. https://doi.org/10.3390/biomedicines9111616








