Abstract
Cancer therapy is a growing field, and annually, a high number of research is performed to develop novel antitumor drugs. Attempts to find new antitumor drugs continue, since cancer cells are able to acquire resistance to conventional drugs. Natural chemicals can be considered as promising candidates in the field of cancer therapy due to their multiple-targeting capability. The nobiletin (NOB) is a ubiquitous flavone isolated from Citrus fruits. The NOB has a variety of pharmacological activities, such as antidiabetes, antioxidant, anti-inflammatory, hepatoprotective, and neuroprotective. Among them, the antitumor activity of NOB has been under attention over recent years. In this review, we comprehensively describe the efficacy of NOB in cancer therapy. NOB induces apoptosis and cell cycle arrest in cancer cells. It can suppress migration and invasion of cancer cells via the inhibition of epithelial-to-mesenchymal transition (EMT) and EMT-related factors such as TGF-β, ZEB, Slug, and Snail. Besides, NOB inhibits oncogene factors such as STAT3, NF-κB, Akt, PI3K, Wnt, and so on. Noteworthy, onco-suppressor factors such as microRNA-7 and -200b undergo upregulation by NOB in cancer therapy. These onco-suppressor and oncogene pathways and mechanisms are discussed in this review.
1. Introduction
Recently, nutritionists have been interested in recommending plants and fruits in the treatment of different illnesses [1,2]. This suggestion is due to the presence of beneficial natural chemicals in plants and fruits and, also, their metabolites, which exert health-promoting effects after being absorbed in the body [3,4,5,6,7,8,9,10]. It has been demonstrated that the identification, isolation, and purification of these natural chemicals may be a milestone in the treatment of diseases, particularly in human malignancies [11,12,13,14,15,16,17,18,19,20]. A high number of studies have focused on cancer therapy using plant-derived natural compounds [6,13,14,15,16,17,18,19,20]. In this review, we demonstrate the potential of nobiletin (NOB) in cancer therapy based on the newly published articles.
2. Sources of NOB
The NOB, as a polymethoxyflavone (PMF), was named after Citrus nobilis [21]. NOB is a ubiquitous flavone extensively derived from the peel of Citrus fruits [22]. Interestingly, NOB can be isolated from a variety of Citrus fruits, including mandarin oranges (Citrus reticulate), sweet oranges or Valencia oranges (Citrus sinesis), Miaray mandarins (Citrus miaray), flat lemons or Hayata (Citrus depressa), tangerines (Citrus tangerine), bitter oranges (Citrus aurantium), Unshu Mikans or Satsuma mandarins (Citrus Unshiu arnicia indica), Cleopatra mandarins (Citrus reshni), mandarin oranges (Citrus tachibana), Koji oranges (Citrus leiocarpa), Natsu Mikans (Citrus tardira), Jimikan (Citrus succosa), kinokuni mandarins (Citrus kinokuni), Fukushu (Citrus erythrose), Supkat (Citrus sunki), and hybrids of mandarin orange with pomelo (Citrus deliciosa) [22,23,24,25,26,27,28]. This shows that NOB is abundantly found in nature, and using it in the treatment of diseases is a cost-effective approach. Among the aforementioned plants, Citrus tangerine has the highest concentration of NOB, leading to its application in disease therapy [29]. Several methods are applied to isolate PMF from orange peel, such as supercritical fluid extraction, microwave-assisted extraction, and the Soxhlet method, enabling us to obtain high contents of this extract [30]. At the final step of extraction, carbon dioxide and ethanol are used to concentrate bioactive compounds [31]. The highest yield of NOB is observed at a temperature of 80 °C, the pressure of 30 MPa, and an optimum sample particle size of 375 μm [32]. In addition to these conventional methods, NOB can be isolated by total synthesis of over eleven steps [33]. The NOB has a molecular weight of 402.39, and its chemical and molecular formula are 5,6,7,8,3/,4/-hexamethoxy flavone, and C21H22O8, respectively [34]. Chromene and arene rings of NOB are at the same plane. The C atoms of two methoxy groups in the arene ring are at the same plane. However, C atoms of four methoxy groups linking to a chromene ring may not necessarily be in parallel [35].
3. Bioavailability of NOB
Although studies exhibit that NOB is exclusively found in nature and various Citrus plants, some restrictions have reduced NOB potential. It has been demonstrated that NOB has poor solubility in water (1–5 μg/mL) and minimal oral bioavailability (˂1%), resulting in a decrease in its therapeutic and biological activities [36]. It is worth mentioning that, after ingestion, NOB undergoes many alterations to produce metabolites [37,38]. The kind of metabolite depends on the species of Citrus plant [22]. Three common metabolites of NOB include 3/-demethylnobiletin (3/-DMN), 4/-DMN, and 3/,4/-DMN [39,40]. A study has investigated the amount of aforementioned metabolites in mice after 20 weeks of daily feeding of 500 ppm NOB as 3.28 (3/-DMN), 24.13 (4/-DMN), and 12.03 (3/,4/-DMN) nmol/g. Interestingly, the bioavailability of NOB was reported as 2.03 nmol/g, which was lower compared to its metabolites [41]. This shows that NOB is immediately metabolized in the body into its metabolites. The metabolism of NOB comprises two phases, including phase I and phase II metabolism. The cytochrome P450 participates in phase I demethylation of NOB [42]. The CYP1A1, CYP1A2, CYP1B, and CYP3A5 are involved in the conversion of NOB into 3/DMN, while only CYP1A1 and CYP1A2 contribute to the transformation of 3/-DMN into 3/,4/-DMN [43]. The phase II metabolism of NOB occurs in the small intestine by sulfation or glucuronidation [44]. As a consequence of the rapid metabolism of NOB and its poor bioavailability, studies have focused on improving NOB bioavailability using various methods. Recently, an ionic liquid containing choline and geranic acid (CAGE) has been developed for promoting NOB bioavailability. The in vitro and in vivo experiments have demonstrated the capability of CAGE in enhancing NOB bioavailability. The enhanced bioavailability of NOB by CAGE is due to the multipoint hydrogen bonding between NOB and CAGE. The CAGE not only elevates the transdermal absorption of NOB but also increases the bioavailability of NOB after oral administration by 20 times [45]. The plant exine capsules can also be considered as a potential strategy in improving NOB bioavailability, since plant exine capsules have high loading capacity (770 ± 40 mg/g) and provide the prolonged release of NOB [46]. It is worth mentioning that nanostrategies are also promising candidates in enhancing NOB bioavailability. It is said that NOB-loaded nanoemulsions are able to enhance the therapeutic capacity of NOB [47]. Micelles are other nanoparticles that have been used in the delivery of NOB for bone loss treatment with excellent features such as low particle size (124 nm), high loading capacity (7.6%), and great entrapment efficiency (76.34%) [48]. However, we are at the beginning point of NOB delivery, and more studies are required to develop novel carriers for the delivery of NOB.
4. Therapeutic and Biological Activities of NOB
The interest directed towards NOB emanates from its efficacy in the treatment of different diseases. Studies have demonstrated that NOB has a variety of therapeutic and biological activities, including antidiabetic [49], antioxidant [50], osteoprotective [51], anti-inflammatory [52], hepatoprotective [53], cardioprotective [54,55], and neuroprotective [56], as well as improving metabolic disorders [57]. Notably, recent studies have shown the role of molecular signaling pathways involved in the protective effects of NOB in various diseases. Diabetes mellitus (DM) is a chronic metabolic disorder that glucose uptake undergoes impairment [58]. It is held that inflammatory factors participate in glucose uptake interference [59]. The administration of NOB (50 mg/kg) remarkably improves glucose resistance by inhibition of the NF-κB signaling pathway, as a factor involved in inflammation to suppress tumor necrosis factor (TNF)-mediated glucose uptake disruption [60]. Although iron is a vital element for physiological processes such as hemoglobin synthesis and DNA replication [61], increasing evidence shows that iron overload is associated with the elevated generation of reactive oxygen species (ROS) that, in turn, induce cell and tissue damages [62,63,64,65,66,67,68]. The NOB is suggested to be a potential agent in fighting against iron overload. The administration of NOB inhibits mitochondrial-mediated apoptosis via reducing ROS generation to attenuate vascular endothelium injury caused by iron overload [69]. Interleukin-21 (IL-21), produced by stimulated CD4+ T immune cells [70], plays a significant role in the progression of rheumatoid arthritis (RA) via induction of inflammatory factors and matrix metalloproteinases (MMPs) such as MMP-3 and MMP-13 [71]. A newly published article (2020) has examined the efficacy of NOB in RA therapy. It seems that IL-21 binds to its receptor to elevate ROS generation. On one hand, ROS induces mitochondrial dysfunction, and on the other hand, ROS stimulates the JAK1/STAT3 axis to stimulate MMP-3 and -13 and inflammatory factors including TNF-α and IL-6. The NOB disrupts the IL-6 attachment into its receptor to interfere with the aforementioned axis, leading to the alleviation of RA [72]. The NOB not only is beneficial in the management and treatment of DM, but also, it can be recommended for the prevention of DM, since it is capable of decreasing insulin resistance, obesity, dyslipidemia, and hepatic steatosis [73]. Nowadays, a high number of people are searching for promoting their longevity. The NOB is suggested to be beneficial in this case. An experiment on Caenorhabditis elegans exhibits that NOB has great antitumor activity and can enhance the lifespan by attenuation of heat shock and ultraviolet radiation [74]. We earlier mentioned that NOB is advantageous in suppressing iron overload-mediated oxidative stress. It is said that the high antioxidant activity of NOB results from improving the antioxidant defense system by targeting nuclear factor erythroid 2-related factor 2 (Nrf2) [75]. The activation of Nrf2 reduces ROS generation and oxidative stress via stimulation of superoxide dismutase, heme oxygenase-1, and NADPH quinone oxidoreductase 1 [75]. By induction of Nrf2, NOB decreases ROS levels to ameliorate hepatorenal toxicity [75]. Another pathological condition that can be alleviated by NOB is ischemic/reperfusion (I/R) injury, a condition involved in enhancing oxidative stress and inflammation [76]. Notably, NOB dually enhances the activity of antioxidant enzymes such as catalase and glutathione peroxidase (GSH-PX), whereas it diminishes the concentration of IL-6 and TNF-α, leading to amelioration of I/R-mediated inflammation and oxidative stress [77]. It has been demonstrated that antioxidant and anti-inflammatory activities of NOB during I/R is mediated by mitogen-activated protein kinase (MAPK) induction [78]. It is said that NOB is capable of the treatment of metabolic disorders and recovering cholesterol balance via the stimulation of bile acid synthesis [79]. In Table 1, we have summarized the therapeutic and biological activities of NOB. These studies highlight the potential of NOB in disease treatment and its protective effects. The newly published articles have shed some light on the capability of NOB in cancer therapy [80,81]. In the present review, we attempt to mechanistically examine the efficiency of NOB in cancer therapy by focusing on molecular pathways and mechanisms.

Table 1.
Therapeutic and biological activities of nobiletin (NOB).
5. Potential Role of NOB in Human Malignancies
5.1. Nobiletin and Chemotherapy
The estimates demonstrate that finding effective treatments for cancer is of importance due to the enhanced incidence rate of this life-threatening disorder. Chemotherapy is one of the most common ways in cancer therapy, and due to its minimally invasive nature, scientists have focused on cancer therapy using chemotherapeutic agents. However, a high number of patients with cancer are directed towards death due to chemotherapy failure caused by multidrug resistance (MDR) [91]. The transport-based classical and nonclassical MDR phenotypes are responsible for the cellular mechanisms of drug resistance [92]. The P-glycoprotein (P-gp) is a member of the ATP-binding cassette (ABC)-family efflux transporters encoded by the MDR1 gene. A variety of studies have evaluated the role of P-gp in different cancers. It is said that enhanced expression of P-gp elevates malignant behavior and the progression of cancer cells via the stimulation of epithelial-to-mesenchymal transition (EMT) [93]. The antitumor drugs exert their inhibitory effect on the proliferation and viability of MDR cancer cells via the inhibition of P-gp [94]. Consequently, scientists in the field of medicinal chemistry have attempted to develop novel drugs targeting and suppressing P-gp activity in cancer cells [95]. It has been demonstrated that NOB targets P-gp in cancer therapy. A newly published experiment developed a derivative of NOB to enhance its solubility and antitumor activity. This agent, known as compound 29d, can increase the accumulation of paclitaxel (PTX) in tumor cells (lung cancer, A549 cells) via reducing the P-gp activity [96]. On the other hand, increasing evidence demonstrates that the Nrf2/PI3K/Akt and the extracellular signal-regulated kinase (ERK) pathway pathways can stimulate chemoresistance [97]. The compound 29d administration is associated with the downregulation of ERK. Besides, NOB can inhibit the PI3K/Akt signaling pathway via Nrf2 downregulation [96]. This study highlights the fact that NOB and its derivatives target different pathways to sensitize cancer cells into chemotherapy. Similarly, another study evaluates the efficacy of NOB in enhancing the antitumor activity of PTX. The same molecular pathways were investigated. It is held that, by downregulation of Nrf2 and Akt and ERK phosphorylation, NOB sensitizes MDR lung cancer cells into PTX-mediated apoptosis [96]. Although these two studies showed similar findings of the Akt and Nrf2 pathways, the latter study exhibits that, in increasing PTX efficacy for the elimination of lung cancer cells, NOB does not affect P-gp activity. This difference is because compound 29d is a derivative of NOB with higher antitumor activity. However, more studies are required to examine this controversy. An experiment reveals that NOB can significantly decrease the viability and survival of cancer cells in a dose-dependent manner, but it does not affect the cell cycle. It is said that a combination of NOB and cisplatin has a more inhibitory effect on the viability of thyroid cancer cells compared to NOB or cisplatin alone. It is worth mentioning that, in reducing the viability of cancer cells, NOB does not negatively affect normal cells [98], making it a suitable option in chemotherapy. Sorafenib is a receptor tyrosine kinase (RTK) inhibitor approved by the Food and Drug Administration (FDA). The sorafenib is extensively applied in the treatment of different cancers with high efficacy [99,100]. The NOB can be co-administered with sorafenib to elevate its antitumor activity. A combination of NOB and sorafenib remarkably diminishes the proliferation and viability of prostate cancer cells by the stimulation of apoptosis and cell cycle arrest via enhancing the expression of Bax, Rb1, and CDKN1A [101]. The multidrug resistance-associated protein 1 (MRP1), known as ABCC1, was first recognized in lung cancer cells that had no expression of ABCB1 (MDR1 or P-gp) [102]. It has been reported that MRP1 stimulates chemoresistance via neuroblastoma-derived MYC (MYCN) [103,104,105]. The fibrous sheath-interacting protein 1 (FSIP1) is able to stimulate chemoresistance via MRP1 induction [106]. Notably, miR-7 functions as an onco-suppressor miR to sensitize breast cancer cells into chemotherapy via MRP1 inhibition [107]. The NOB follows the same route in sensitizing lung cancer cells into adriamycin chemotherapy. NOB enhances adriamycin accumulation in cancer cells via downregulation of MRP1, leading to the induction of apoptosis [108]. On the other hand, the Wnt/β-catenin signaling pathway plays a significant role in cancer development [109,110,111]. Antitumor drugs such as echinacoside diminish the malignancy and proliferation of cancer cells via Wnt inhibition [112]. Besides, miR-455-3p inhibits EMT and the invasion of cancer cells via downregulation of Wnt/β-catenin [112]. The Akt can reduce the activity of GSK-3β via its phosphorylation at serine9 to ensure the nuclear translocation of β-catenin and activation of the Wnt signaling pathway, whereas active GSK-3β inhibits the nuclear translocation of β-catenin via ubiquitination [113]. In enhancing the antitumor activity of adriamycin, NOB inhibits Akt to suppress the Wnt/β-catenin signaling pathway via elevating GSK-3β activity, leading to the reduced viability and proliferation of lung cancer cells [108]. One of the most well-known and studied signaling pathways is the PI3K/Akt/mTOR signaling pathway [114,115,116,117,118]. This axis participates in cell proliferation and metabolism. Consequently, tumor cells prefer to activate the PI3K/Akt/mTOR signaling pathway in enhancing their survival and growth [119]. The antitumor drugs negatively affect the PI3K/Akt/mTOR signaling pathway to suppress proliferation. For instance, sanggenol triggers apoptosis and cell cycle arrest in cancer cells via inhibition of the PI3K/Akt/mTOR axis [120]. Pitavastatin limits the migration and proliferation of cancer cells by the inhibition of angiogenesis via PI3K/Akt/mTOR downregulation [121]. In sensitizing cancer cells with oxaliplatin and reducing the viability and proliferation of colorectal cancer (CRC) cells, NOB inhibits the PI3K/Akt/mTOR signaling pathway, resulting in the induction of apoptosis via reducing the expression of Bcl-2 and enhancing the expression of Bax and caspase-3. The PI3K induces the mTOR signaling pathway via Akt phosphorylation. This axis results in cell proliferation and the growth of cancer cells. Targeting this pathway by NOB mediates its antitumor activity [122]. Overall, the studies exhibit that NOB not only can enhance chemosensitivity via the inhibition of P-gp, but also, it can suppress oncogene signaling pathways such as Nrf2 and Akt/ERK to inhibit cancer progression and sensitize them into chemotherapy [123]. It is worth mentioning that colonic metabolites of NOB such as 3/-DMN, 4/-DMN, and 3/,4/-DMN have chemo-preventive effects. In respect to the higher bioavailability of 3/-DMN, 4/-DMN, and 3/,4/-DMN compared to NOB, they can considerably suppress the invasion, proliferation, and survival of colon cancer cells via the stimulation of apoptosis and cell cycle arrest [41], making them suitable options in chemotherapy.
5.2. Relation between NOB and Metastasis
Metastasis is an increasing challenge in enhancing the overall survival rate of patients with cancer and associated with poor prognosis [124]. Earlier, we had described that EMT is a factor that enhances the metastasis and migration of cancer cells. MMPs are also able to provide the metastasis and invasion of tumor cells via the degradation of the extracellular matrix (ECM) [125,126]. Among MMPs, MMP-2 and MMP-9 are important due to their ability in the degradation of major components of the ECM, including gelatin, collagen, and laminin [127]. The overexpressions of MMP-2 and MMP-9 are related to the undesirable prognosis of patients with cancer [128]. Noteworthy, several molecular signaling pathways such as NF-κB, specificity protein-1 (SP-1), cAMP response element-binding protein (CREB), ERK, and JNK can regulate MMP expression [129,130,131]. The NOB suppresses the motility and invasion of cancer cells via the downregulation of MMP-2 and MMP-9. The investigation of molecular signaling pathways exhibits that NOB reduces MMP-2 and MMP-9 expressions through the inhibition of ERK and JNK pathways and downstream targets such as NF-κB, CREB, and SP-1. Overall, CREB and SP-1 interactions are necessary for MMP-2 expression, while NF-κB and SP-1 interactions are responsible for MMP-9 expression. In this way, JNK and ERK act as upstream mediators in the stimulation of CREB/SP-1/MMP-2 and NF-κB/SP-1/MMP-9 signaling pathways. In the inhibition of osteosarcoma migration, NOB negatively affects the aforementioned signaling pathways. NOB indirectly affects the target involved in the metastasis of osteosarcoma (MMP-2 and MMP-9) and, by downregulation of their upstream modulators, paves the way for the inhibition of metastasis and improving prognosis [132].
5.3. Head and Neck Cancers
The poly (ADP-ribose) polymerases (PARPs) are enzymes involved in catalyzing the poly (ADP-ribosylation (PARylation) [133]. Among the 18 members of PARPs, PARP-1/2 contribute to the repair of DNA injury [134]. The sirtuin 1 (SIRT1) is suggested to be a downstream target of PARP [135]. The implication of PARP/SIRT1 in cancer has been explored [134]. Inhibition of PARP2 by onco-suppressor miR-383 diminishes the progression of cancer cells and sensitizes them into cell death [136]. Interestingly, the administration of NOB is correlated with the downregulation of PARP2. As a downstream target of PARP2, SIRT1 undergoes upregulation that, in turn, induces the AMPK signaling pathway to stimulate apoptosis in nasopharyngeal carcinoma cells and to suppress their proliferation [137]. It is well-understood that EMT enhances the migration and metastasis of cancer cells. During this process, epithelial cells are transformed into mesenchymal ones that have high migratory and metastatic capabilities. In this way, E-cadherin as an epithelial protein undergoes downregulation, while an increase occurs in mesenchymal markers such as N-cadherin and vimentin [138,139,140,141]. Consequently, targeting this mechanism remarkably reduces the invasion of cancer cells. The administration of NOB is related to the downregulation of TGF-β and Slug, as upstream mediators involved in EMT induction, resulting in increasing E-cadherin and occluding levels and decreasing N-cadherin and fibronectin levels. The examination of molecular pathways demonstrates that TGF-β induces the nuclear translocation of β-catenin in EMT induction, and by the inhibition of TGF-β, NOB suppresses the EMT of glioma cells [142].
5.4. Thoracic Cancers
As we mentioned in the introduction section, NOB undergoes a transformation in the body and produces three common metabolites, including 3/-DMN, 4/-DMN, and 3/,4/-DMN. Interestingly, a newly published study has investigated the efficacy of NOB and its common metabolites in the treatment of lung cancer. This study displays that NOB and its metabolites have a great potential to suppress lung cancer tumorigenesis, but 4/-DMN and 3/,4/-DMN possess higher antitumor activity compared to NOB. The antitumor activity of NOB and its metabolites is mediated by their effect on the stimulation of apoptosis and cell cycle arrest via the overexpression of p21, CDK1, cyclin D1, CDK6, CDK4, Bax, and caspase, as well as PARP [143]. The accumulating data demonstrates that MMPs play a pivotal role in the migration and metastasis of cancer cells via degradation of the base membrane [144]. To suppress the migration and invasion of breast cancer cells, NOB downregulates the expression of MMP-2 and MMP-9 [145]. In respect to the role of MAPK in cell proliferation and apoptosis [146], targeting this pathway is of importance in cancer therapy. It seems that the stimulation of MAPK can inhibit both the migration and growth of cancer cells [147]. In breast cancer cells, NOB enhances p38 MAPK expression and its phosphorylation to inhibit breast cancer progression [145]. In the introduction section, we mentioned that the antioxidant activity of NOB relies on Nrf2 activation. However, the story is completely different in cancer cells. It is held that Nrf2 activation can ensure the proliferation of tumor cells and induces chemoresistance [148]. In breast cancer cells, NOB supplementation reduces the expression of Nrf2 and inhibits the nuclear translocation of Nrf2 to suppress breast cancer proliferation [145]. This study highlights the fact that NOB is able to simultaneously target different molecular pathways that make it an appropriate option in cancer therapy. It seems that CD36 participates in tumor metastasis via the regulation of lipid metabolism. The interaction between CD36 and TGF-β stimulates EMT mechanisms to enhance the migration and metastasis of cancer cells [149]. CD36 overexpression is associated with the poor prognosis and resistance of cancer cells into chemotherapy-mediated apoptosis [147]. The effect of CD36 on the migration and invasion of cancer cells is due to the downregulation of E-cadherin and β-catenin [150]. On the other hand, stimulation of the STAT3 and NF-κB signaling pathways mediates the angiogenesis of cancer cells, and the inhibition of STAT3 can suppress migration [151]. Normally, CD36 stimulates the nuclear translocation of STAT3 and NF-κB to induce angiogenesis. The activation of NF-κB occurs as a result of the nuclear translocation of STAT3. The administration of NOB restricts the angiogenesis, migration, and proliferation of breast cancer cells via inhibition of the CD36/STAT3/NF-κB axis [152]. Interestingly, studies have shown that CYP1 enzymes contribute to the bioactivation of flavonoids and mediating their antitumor activity [153]. This story is also true for NOB. The cytochrome P450 CYP1 plays a significant role in the bioactivation of NOB in breast cancer cells [154]. By bioactivation of NOB via CYP1, an increase occurs in its capability in the induction of apoptosis and cell cycle arrest at the G1 phase. Using a CYP1 inhibitor remarkably reduces antitumor activity against breast cancer cells [155], exhibiting that NOB metabolism by cytochrome P450 CYP1 can be targeted in further studies. Aromatase is another enzyme involved in estrogen biosynthesis [156]. The aromatase is a key member of cytochrome P450 CYP1 capable of converting androstenedione into estrone (E1) [157]. The expressions and activities of aromatase demonstrate an increase in patients with breast cancer [158,159]. So, aromatase is an oncogene factor in breast cancer, and its activity should be inhibited. Notably, the administration of NOB at high doses (10 μM) enhances the expression and activity of aromatase, while low doses (1 μM) inhibits the aromatase activity. This study highlights the fact that, in targeting the metabolism of breast cancer cells, low doses of NOB should be used [160]. In respect to the role of EMT in the migration and malignant behavior of cancer cells, much attention has been directed towards identifying molecular signaling pathways related to EMT induction [161,162,163,164,165]. The TGF-β1 is able to stimulate EMT by the phosphorylation of Smad2 and Smad3 and the subsequent formation of a complex with Smad4. Then, the Smad2/3/4 complex translocates into the nucleus to induce an EMT [166]. So, targeting Smads is of importance in suppressing metastasis. In lung cancer cells that have high metastatic ability and demonstrate migration into neighboring cells and tissues, reducing metastatic factors can alleviate poor prognosis. In this way, NOB disrupts TGF-β1 and Smad3 in EMT induction. As a consequence, the ability of cancer cells in migration undergoes downregulation [167]. The Notch1 is an oncogene factor that its role in different cancers has been evaluated. It is said that tumor-educated B (TEB) cells are able to enhance cancer progression via the activation of IL-1β/HIF-2α and subsequent induction of Notch1 [168]. As a histone methyltransferase, G9a increases the malignant behavior of cancer cells via Notch1 overexpression [169]. These studies show that Notch1 should be inhibited in cancer therapy. The NOB supplementation suppresses hypoxia-mediated EMT in lung tumor cells via the downregulation of Notch1. In this way, NOB inhibits downstream targets of Notch1 such as Hey1 and Hes1 and, also, Jagged1/2. It is worth mentioning that, by the downregulation of Notch1, EMT-related factors including Twist1, Snail1, and ZEB1/2 undergo a decrease [170]. On the other hand, miR-200b is an onco-suppressor factor that inhibits downstream targets such as laminin subunit alpha 4 (LAMA4) to reduce the invasion and proliferation of cancer cells [171]. During the metastasis of cancer cells, the expression of miR-200b undergoes downregulation [172]. So, antitumor drugs should elevate miR-200b expression. NOB enhances the expression of miR-200b in hypoxic conditions to suppress the EMT-mediated metastasis of lung cancer cells [170].
5.5. Gynecological Cancers
The programmed cell death (PCD) includes the apoptosis, pyroptosis, and autophagy that are made by caspases, lysosomal proteases, and endonucleases [173,174,175,176]. During recent decades, much attention has been directed towards targeting three major arms of PCD in cancer therapy. The pyroptosis participates in cell death via the induction of DNA fragmentation. GSDMD and GSDME are members of pyroptosis [177]. The NOB enhances ROS generation to stimulate mitochondrial dysfunction via decreasing the mitochondrial membrane potential, leading to autophagy activation. It seems that this pathway upregulates GSDMD/GSDME to trigger pyroptosis, resulting in a diminution in the viability of ovarian cancer cells [178]. The molecular biologists who work in the field of cancer believe that cancer cells can obtain resistance to chemotherapy and enhance their proliferation using autophagy induction [179,180]. Accumulating data has investigated the role of autophagy and its regulation in chemoresistance [181,182]. The TSPAN9 is a transmembrane protein that can stimulate the chemoresistance of cancer cells through autophagy induction [183]. It seems that there is a dual relationship between EMT and autophagy. By the inhibition of autophagy, the malignant behavior of cancer cells undergoes downregulation to sensitize cancer cells into chemotherapy [184]. Overall, studies are in agreement with the fact that autophagy activation may mediate chemoresistance [185]. In ovarian cancer cells, NOB targets autophagy to stimulate cell cycle arrest and apoptosis. Via upregulation of the Akt signaling pathway, NOB inhibits autophagy to sensitize cancer cells into apoptosis. Autophagy functions as a pro-survival mechanism, and its inhibition by NOB triggers the intrinsic pathway of apoptosis via the induction of caspase-9, caspase-3, and PARP [186].
5.6. Urological Cancers
The Toll-like receptors (TLRs) are expressed in a variety of immune cells, including macrophages, dendritic cells, and natural killer (NK) cells. There are 10 distinct types of TLRs (TLR1-10), and they undergo induction by endogenous or exogenous ligands carrying pathogen-associated molecular patterns (PAMPs) regions [187]. Although TLRs are involved in the immune response, TLR2, TLR4, and TLR9 contribute to cancer proliferation and progression [188]. So, scientists should consider the oncogene role of TLR4 and TLR9 in cancer cells. It seems that TLR4/MyD88/NF-κB and TLR4/TRIF/IRF3 are involved in the production of inflammatory cytokines after the identification of lipopolysaccharide (LPS) [189]. In prostate cancer cells, NOB exerts an inhibitory impact on their growth and proliferation. The investigation of molecular pathways shows that NOB is able to inhibit TLR9/IRF7 and TLR4/TRIF/IRF3 in suppressing the proliferation and growth of cancer cells [190]. In respect to the role of inflammation in cancer growth and the involvement of TLRs in the production of inflammatory factors such as interferon-γ (IFN-γ) and IFN-β, NOB inhibits prostate cancer growth by its anti-inflammatory activity. In the intrinsic pathway of apoptosis, the mitochondrion and endoplasmic reticulum (ER) play significant roles [191,192]. External stimuli such as ROS are able to disrupt the mitochondrial membrane integrity via the upregulation of Bax and downregulation of Bcl-2. Following cytochrome c release into the cytosol, the caspase-9 and caspase-3 are activated to induce apoptotic cell death [193,194]. The main function of ER is to modulate protein synthesis, protein folding, and calcium homeostasis [195]. The accumulation of unfolded proteins stimulates ER stress, leading to the activation of apoptosis, unfolded protein response (UPR), and ER-associated degradation (ERAD). The PKR-like ER-associated kinase (PERK), inositol requiring enzyme-1α (IRE1α), and activating transcription 6 (ATF6) are three major arms of the UPR that can either stimulate autophagy or apoptosis [196,197,198]. On the other hand, the PI3K/Akt/mTOR signaling pathway regulates apoptosis and cell proliferation [199]. The administration of NOB targets all of these pathways and mechanisms. By downregulation of the PI3K/Akt/mTOR axis, NOB inhibits the proliferation and growth of bladder cancer cells. NOB induces mitochondrial dysfunction to release cytochrome C, resulting in stimulation of proapoptotic factors caspase-3, caspase-9, Bad, and Bax. Besides, NOB triggers the PERK/elF2α/ATF4/CHOP axis through ER stress to activate apoptosis. These molecular pathways and mechanisms targeted by NOB reduce the invasion and proliferation of bladder cancer cells [200]. The signal transducer and activator of transcription 3 (STAT3) and YY1-associated protein 1 (YY1AP1) are two oncogene factors in cancer cells [201,202,203,204]. The YYAP1 upregulation is associated with the poor prognosis of patients with cancer [205]. The STAT3 signaling pathway accelerates the growth and proliferation of lung cancer cells via miR-33a-5p inhibition and the subsequent activation of karyopherin subunit alpha 4 (KDNA4) [206]. Besides, G-protein alpha-subunit (GNAS) as an upstream mediator can induce the STAT3 signaling pathway through IL-6 to enhance the malignancy and proliferation of cancer cells [207]. So, targeting these two signaling pathways is of importance in cancer therapy. The in vivo and in vitro experiments exhibit that NOB triggers apoptosis and cell cycle arrest. The examination of molecular signaling pathways demonstrates that NOB inhibits the phosphorylation of STAT3, YY1AP1, and SRC/Akt to exert its inhibitory impact on renal carcinoma cells [208]. The tumor microenvironment plays a significant role in the malignant behavior of cancer cells. Hypoxia is a feature of the tumor microenvironment that increases the metastasis of renal carcinoma cells and is associated with recurrence [209,210]. One of the molecular mechanisms involved in metastasis is the EMT [198]. Increasing evidence has shown that hypoxia can trigger the EMT to enhance the migration and invasion of cancer cells [211]. On the other hand, it has been demonstrated that hypoxia, in addition to other well-established inducers, can stimulate oncogenic NF-κB and Wnt/β-catenin signaling pathways [212,213]. These two pathways can function as upstream inducers of the EMT in cancer migration [214,215]. Noteworthy, NOB is capable of suppressing the invasion and migration of renal carcinoma cells. The examination of molecular pathways reveals that NOB downregulates the expression of Wnt and NF-κB to suppress the EMT in the hypoxic condition, leading to decreased the migration and metastasis of cancer cells (Figure 1) [216].
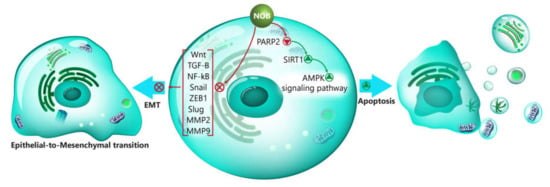
Figure 1.
The involvement of signaling pathways in the regulation of EMT by NOB. AMPK, AMP-activated protein kinase; SIRT1, sirtuin 1; PARP2, poly (ADP-ribose) polymerase 2; NOB, nobiletin; Wnt, Wingless-related integration site; TGF-β, transforming growth factor-β; NF-κB, nuclear factor-kappa B; ZEB1, zinc finger E-box binding homeobox 1; MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; and EMT, epithelial-to-mesenchymal transition.
5.7. Gastrointestinal Cancers
Colorectal cancer (CRC) is the most common cancer in males and females after lung and breast cancers [217]. A variety of factors contribute to CRC development, such as gender, age, genetic alterations, lifestyle, and inflammatory bowel disease (IBD). The incidence rate of CRC is higher in men compared to women [218,219]. It seems that plant-derived natural compounds are potential agents in CRC chemoprevention [220]. The efficacy of NOB in the treatment of colon cancer is related to its impact on the viability and survival of cancer cells. A metabolite of NOB, known as 4-DMN, and atorvastatin are able to suppress colon cancer malignancy via the stimulation of apoptosis and cell cycle arrest [221]. In this way, NOB enhances the expression of p21, while it reduces the levels of CDK2, CDK4, cyclin D, and cyclin E [222]. It is worth mentioning that inflammatory factors can lead to colon cancer development [218]. During IBD, a number of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β are secreted [223]. These factors are suggested to be involved in colon cancer carcinogenesis, since the downregulation of IL-6 reduced the colon cancer development [223]. The administration of NOB and atorvastatin decreases the levels of proinflammatory cytokines and downregulates the expression of COX-2 to inhibit inflammation-mediated colon cancer development [222]. In fact, in this case, the antitumor activity of NOB results from its anti-inflammatory activity. The epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein that begins several intracellular molecular signaling pathways, leading to cell proliferation and cell growth. β-elemene as an antitumor agent diminishes the migration and invasion of cancer cells by the inhibition of EGFR signaling [224]. The onco-suppressor upstream factors are able to inhibit cancer malignancy via the inhibition of EGFR [225]. These studies exhibit that EGFR signaling is a positive factor for the growth and proliferation of cancer cells, and its targeting is a potential strategy in cancer therapy. A combination of NOB and atorvastatin synergistically suppresses the proliferation and metastasis of colon cancer cells via EGFR downregulation [222]. The Ras homolog gene family member A (RhoA) is a key player of the Ras/Rho superfamily with involvement in different aspects of cells such as proliferation and migration. The abnormal expression of RhoA occurs in different cancers. It is said that the migration and survival of melanoma cells undergo inhibition via RhoA inhibition [226]. The great antitumor activity of lupeol depends on RhoA inhibition to suppress colon cancer capacity in proliferation and growth [227]. In the treatment of colon cancer, NOB negatively affects RhoA expression. The NOB supplementation along with atorvastatin suppresses the invasion and migration of colon cancer cells via RhoA downregulation [222]. In previous sections, we demonstrated that NOB was reported to induce apoptosis in cancer cells through both mitochondrial and ER pathways. The NOB enhances the expressions of ER stress-related proteins such as IRE-1α, ATF4, CHOP, and GRP78. This leads to the stimulation of apoptosis via caspase-4 activation. However, an interesting point is the induction of autophagy by NOB. It seems that the inhibition of autophagy in cancer cells may enhance the number of cells undergoing apoptosis [228]. In gastric cancer cells exposed to NOB, the inhibition of autophagy elevates the capability of NOB in the stimulation of apoptosis [229]. So, in order to enhance the antitumor activity of NOB, autophagy inhibitors such as rapamycin and chloroquine can be used to inhibit protective autophagy and maximize the efficacy of NOB in the elimination of cancer cells.
5.8. Hematological Cancers
The c-kit, known as CD117, encoded by the kit gene, is considered as an oncogene factor. The c-kit phosphorylates plasma membrane prohibitin (PHB) at tyrosine259 to ensure the invasion and migration of cancer cells and induces their resistance into chemotherapeutic agents [230,231]. The combination of irinotecan and tankyrase inhibitors diminishes the proliferation and growth of cancer cells via downregulation of the c-kit [232]. The microRNA (miR)-664, as an onco-suppressor, reduces the expression of the c-kit to suppress the proliferation and invasion of cancer cells [233]. In acute myeloid leukemia (AML) cells, NOB targets the c-kit. It appears that the viability and survival of AML cells undergo a decrease by NOB via reducing expression of the c-kit. Notably, a combination of NOB and cytarabine, a chemotherapeutic agent, remarkably decreases c-kit expression in AML therapy [234].
5.9. Anti-angiogenesis Effect
The process of sprouting new blood vessels from pre-existing ones is defined as angiogenesis [235]. This process is active during embryogenesis, and in adulthood, angiogenesis is transiently activated, for instance, during the reproductive cycle in females. Although angiogenesis seems to be vital for physiological conditions, its activation occurs in a variety of disorders, particularly cancer [236]. Molecularly, vascular endothelial growth factor (VEGF) plays a major role during angiogenesis, and in this way, it interacts with the epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) [237]. Upstream mediators target these molecular pathways to regulate angiogenesis. The steroid receptor coactivator (Src) and focal adhesion kinase (FAK) are tyrosine kinases capable of controlling angiogenesis. The EGFR effect on migration relies on FAK [238]. Src has been displayed to induce angiogenesis to elevate the growth and migration of cancer cells [239]. Src and EGFR regulate VEGF in angiogenesis by targeting STAT3 [240,241,242,243,244,245]. So, complicated signaling pathways are involved in the regulation of angiogenesis. The administration of NOB inhibits EGFR to downregulate the expression of its downstream targets, including Scr, FAK, and STAT3 (the Src/FAK/STAT3 signaling pathway). As a consequence, the nuclear translocation of STAT3 is inhibited, and its attachment into paxillin is suppressed, leading to the downregulation of angiogenesis and invasion and migration of breast cancer cells (Figure 2, Table 2) [246].
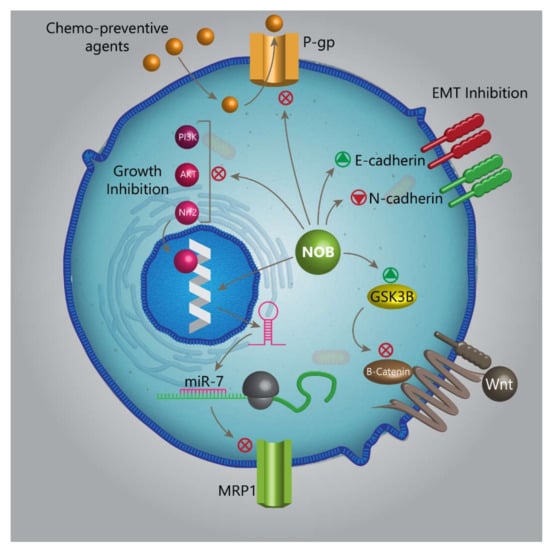
Figure 2.
The capability of NOB in targeting various molecular pathways and mechanisms, making it an appropriate option in cancer therapy. P-gp, P-glycoprotein; NOB, nobiletin; miR, microRNA; MRP1, multidrug-resistance-associated protein 1; GSK-3β, glycogen synthase kinase 3 beta; Nrf2, nuclear factor erythroid 2-related factor 2; Akt, protein kinase B; PI3K, phosphatidylinositide-3 kinase; and EMT, epithelial-to-mesenchymal transition.

Table 2.
The antitumor activity of nobiletin in different cancers.
6. Conclusion and Remarks
NOB is a naturally occurring compound with potential therapeutic effects that are shown in Table 1. However, we allotted this review to the antitumor activity of NOB. NOB can be used as a chemosensitizer. To date, studies have revealed that NOB can reduce the resistance of cancer cells into chemotherapeutic agents such as cisplatin, PTX, and OX. The chemoprevention impact of NOB is related to its effect on five distinct molecular pathways and mechanisms. The first molecular mechanism is P-gp, which, by inhibition of its activity, NOB paves the road into the penetration of chemo-preventive agents into cancer cells. Second, NOB inhibits the Nrf2/PI3K/Akt pathway to inhibit the growth of cancer cells. Third, NOB reduces the malignant behavior of cancer to sensitize them into chemotherapy via EMT inhibition. Fourth, NOB inhibits the Wnt/β-catenin signaling pathway via GSK-3β upregulation. Fifth, NOB enhances the expression of miR-7 to inhibit MRP1. In the induction of apoptosis, NOB affects various molecular pathways. One of them is the PARP2/SIRT1/AMPK axis. NOB inhibits PARP2 to stimulate the SIRT1/AMPK axis, leading to apoptotic cell death. One of the most important effects of NOB is its capability in suppressing the migration and invasion of cancer cells. In this way, NOB downregulates NF-κB, Wnt, TGF-β, Snail, Slug, and ZEB1 as upstream mediators of EMT, resulting in the reduced metastasis of cancer cells. In addition to EMT, NOB can inhibit MMP-2 and MMP-9 expressions to suppress the metastasis of cancer cells. It is worth mentioning that NOB can target the metabolism of cancer cells, so that it diminishes the aromatase activity, as a factor involved in the growth of breast cancer cells. NOB supplementation induces pro-survival autophagy. It seems that using autophagy inhibitors such as rapamycin and chloroquine enhances the efficacy of NOB in the stimulation of apoptosis. In the induction of apoptosis, NOB targets both the mitochondrion and ER. In inhibition of the migration of cancer cells during hypoxic conditions, NOB downregulates the expressions of NF-κB and Wnt signaling pathways. In respect to the role of inflammation in colon cancer carcinogenesis, NOB reduces the levels of inflammatory factors such as ILs and IFN to suppress cancer development and progression. The interesting point is that NOB exerts an anti-angiogenesis impact by the inhibition of STAT3 and VEGF. Several directions appear to be beneficial about NOB. Based on the minimal side-effects of NOB, it can be applied in clinical trials, and until now, there has been no research in this case. Besides, using different strategies such as nanocarriers seems to be advantageous in enhancing the antitumor activity of NOB via promoting its bioavailability.
Author Contributions
R.M. and M.A. provided the concept. M.N. and M.G. contributed to organization of manuscript. All the authors participated in writing and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
NOB, nobiletin; PMF, polymethoxyflavone; DMN, demethylnobiletin; CAGE, choline and geranic acid; DM, diabetes mellitus; TNF, tumor necrosis factor; ROS, reactive oxygen species; RA, rheumatoid arthritis; MMPs, matrix metalloproteinases; Nrf2, nuclear factor erythroid 2-related factor 2; SOD, superoxide dismutase; HO-1, heme ocygenase-1; NQO1, NADPH quinone reductase; I/R, ischemic/reperfusion; CAT, catalase; GSH-PX, glutathione-peroxidase; MAPK, mitogen activated-protein kinase; MDR, multidrug resistance; P-gp, P-glycoprotein; ABC, ATP-binding cassette; PTX, paclitaxel; RTK, receptor tyrosine kinase; FDA, Food and Drug Administration; MRP1, multidrug resistance-associated protein 1; MYCN, neuroblastoma-derived MYC; FSIP1, fibrous sheath-interacting protein 1; OX, oxaliplatin; PARP, poly (ADP-ribose) polymerase; SIRT1, sirtuin 1; EMT, epithelial-to-mesenchymal transition; E1, estrone; TEB, tumor-educated B; LAMA4, laminin subunit alpha 4; PCD, programmed cell death; TLRs, Toll-like receptors; NK, natural killer; PAMPs, pathogen-associated molecular patterns; LPS, lipopolysaccharide; IFN, interferon; ER, endoplasmic reticulum; UPR, unfolded protein response; ERAD, ER-associated degradation; PERK, PKR-like ER-associated kinase; IRE1α, inositol requiring enzyme-1α; ATF6, activating transcription factor 6; STAT3, signal transducer and activator of transcription 3; YY1AP1, YY1-associated protein 1; KDNA4, karyopherin subunit alpha 4; GNAS, G-protein alpha-subunit; CRC, colorectal cancer; IBD, inflammatory bowel disease; EGFR, epidermal growth factor receptor; RhoA, Ras homolog family, member A; PHB, prohibitin; miR, microRNA; AML, acute myeloid leukemia; ECM, extracellular matrix; SP-1, specificity protein-1; CREB, cAMP response element-binding protein; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; bFGF, basic fibroblast growth factor; Src, steroid receptor coactivator; FAK, focal adhesion kinase; and PXN, paxillin.
References
- Gupta, B.; Sadaria, D.; Warrier, V.U.; Kirtonia, A.; Kant, R.; Awasthi, A.; Baligar, P.; Pal, J.K.; Yuba, E.; Sethi, G.; et al. Plant lectins and their usage in preparing targeted nanovaccines for cancer immunotherapy. Semin. Cancer Biol. 2020. (In press) [CrossRef] [PubMed]
- Chang, H.-Y.; Wu, J.-R.; Gao, W.-Y.; Lin, H.-R.; Chen, P.-Y.; Chen, C.-I.; Wu, M.-J.; Yen, J.-H. The Cholesterol-Modulating Effect of Methanol Extract of Pigeon Pea (Cajanus cajan (L.) Millsp.) Leaves on Regulating LDLR and PCSK9 Expression in HepG2 Cells. Molecules 2019, 24, 493. [Google Scholar] [CrossRef] [PubMed]
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020, 153, 104635. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Weng, C.J.; Sethi, G.; Hu, D.N. Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid. Based Complement Altern. Med. 2013, 2013, 698190. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2019. (In press) [CrossRef] [PubMed]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40, 48–81. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40, 1–3. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Javanmardi, S.; Moradi-Ozarlou, M.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S.; Garg, M. Natural products and phytochemical nanoformulations targeting mitochondria in oncotherapy: An updated review on resveratrol. Biosci. Rep. 2020, 40, BSR20200257. [Google Scholar] [CrossRef]
- Mohan, C.D.; Rangappa, S.; Preetham, H.D.; Chandra Nayak, S.; Gupta, V.K.; Basappa, S.; Sethi, G.; Rangappa, K.S. Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature. Semin. Cancer Biol. 2020. (In press)
- Wang, H.; Ahn, K.S.; Alharbi, S.A.; Shair, O.H.M.; Arfuso, F.; Sethi, G.; Chinnathambi, A.; Tang, F.R. Celastrol Alleviates Gamma Irradiation-Induced Damage by Modulating Diverse Inflammatory Mediators. Int. J. Mol. Sci. 2020, 21, 1084. [Google Scholar] [CrossRef]
- Yang, M.H.; Jung, S.H.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. Attenuation of STAT3 Signaling Cascade by Daidzin Can Enhance the Apoptotic Potential of Bortezomib against Multiple Myeloma. Biomolecules 2019, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Kannaiyan, R.; Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: Role in the prevention and treatment of cancer. Nutr. Cancer 2011, 63, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Prasannan, R.; Kalesh, K.A.; Shanmugam, M.K.; Nachiyappan, A.; Ramachandran, L.; Nguyen, A.H.; Kumar, A.P.; Lakshmanan, M.; Ahn, K.S.; Sethi, G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharm. 2012, 84, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Verma, S.S.; Rai, V.; Awasthee, N.; Chava, S.; Hui, K.M.; Kumar, A.P.; Challagundla, K.B.; Sethi, G.; Gupta, S.C. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell Mol. Life Sci. 2019, 76, 1947–1966. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Sung, B.; Aggarwal, B.B. Pinitol targets nuclear factor-κB activation pathway leading to inhibition of gene products associated with proliferation, apoptosis, invasion, and angiogenesis. Mol. Cancer Ther. 2008, 7, 1604–1614. [Google Scholar] [CrossRef]
- Ramachandran, L.; Manu, K.A.; Shanmugam, M.K.; Li, F.; Siveen, K.S.; Vali, S.; Kapoor, S.; Abbasi, T.; Surana, R.; Smoot, D.T.; et al. Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor gamma activation pathway in gastric cancer. J. Biol. Chem. 2012, 287, 38028–38040. [Google Scholar] [CrossRef]
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-kappaB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634–648. [Google Scholar] [CrossRef]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef]
- Tan, S.M.; Li, F.; Rajendran, P.; Kumar, A.P.; Hui, K.M.; Sethi, G. Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J. Pharm. Exp. 2010, 334, 285–293. [Google Scholar] [CrossRef]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The multifunctional effects of nobiletin and its metabolites in vivo and in vitro. Evid. Based Complementary Altern. Med. 2016, 2016, 2918796. [Google Scholar]
- Chen, S.; Cai, D.; Pearce, K.; Sun, P.Y.; Roberts, A.C.; Glanzman, D.L. Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. Elife 2014, 3, e03896. [Google Scholar] [CrossRef] [PubMed]
- Uckoo, R.M.; Jayaprakasha, G.; Vikram, A.; Patil, B.S. Polymethoxyflavones isolated from the peel of Miaray Mandarin (Citrus miaray) have biofilm inhibitory activity in Vibrio harveyi. J. Agric. Food Chem. 2015, 63, 7180–7189. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Iwata, C.; Toda, H. Molecular cloning and characterization of a flavonoid-O-methyltransferase with broad substrate specificity and regioselectivity from Citrus depressa. BMC Plant Biol. 2016, 16, 180. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Charles, A.L.; Kung, H.-F.; Ho, C.-T.; Huang, T.-C. Extraction of nobiletin and tangeretin from Citrus depressa Hayata by supercritical carbon dioxide with ethanol as modifier. Ind. Crop. Prod. 2010, 31, 59–64. [Google Scholar] [CrossRef]
- Kohno, H.; Yoshitani, S.-i.; Tsukio, Y.; Murakami, A.; Koshimizu, K.; Yano, M.; Tokuda, H.; Nishino, H.; Ohigashi, H.; Tanaka, T. Dietary administration of citrus nobiletin inhibits azoxymethane-induced colonic aberrant crypt foci in rats. Life Sci. 2001, 69, 901–913. [Google Scholar] [CrossRef]
- Murakami, A.; Nakamura, Y.; Torikai, K.; Tanaka, T.; Koshiba, T.; Koshimizu, K.; Kuwahara, S.; Takahashi, Y.; Ogawa, K.; Yano, M. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion in mice. Cancer Res. 2000, 60, 5059–5066. [Google Scholar]
- Uckoo, R.M.; Jayaprakasha, G.K.; Patil, B.S. Rapid separation method of polymethoxyflavones from citrus using flash chromatography. Sep. Purif. Technol. 2011, 81, 151–158. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of flavonoid constituents in citrus fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [CrossRef]
- Tsukayama, M.; Ichikawa, R.; Yamamoto, K.; Sasaki, T.; Kawamura, Y. Microwave-assisted rapid extraction of polymethoxyflavones from dried peels of Citrus yuko Hort. ex Tanaka. Nippon Shokuhin Kagaku Kogaku Kaish J. Jpn. Soc. Food Sci. Technol. 2009, 56, 359–362. [Google Scholar] [CrossRef][Green Version]
- Silva, I.; Estrada, M.F.V.; Pereira, C.; da Silva, A.B.; Bronze, M.R.; Alves, P.M.; Duarte, C.M.; Brito, C.; Serra, A.T. Polymethoxylated flavones from orange peels inhibit cell proliferation in a 3D cell model of human colorectal cancer. Nutr. Cancer 2018, 70, 257–266. [Google Scholar] [CrossRef]
- Ko, H.-C.; Jang, M.-G.; Kang, C.-H.; Lee, N.-H.; Kang, S.-I.; Lee, S.-R.; Park, D.-B.; Kim, S.-J. Preparation of a polymethoxyflavone-rich fraction (PRF) of Citrus sunki Hort. ex Tanaka and its antiproliferative effects. Food Chem. 2010, 123, 484–488. [Google Scholar] [CrossRef]
- Asakawa, T.; Hiza, A.; Nakayama, M.; Inai, M.; Oyama, D.; Koide, H.; Shimizu, K.; Wakimoto, T.; Harada, N.; Tsukada, H. PET imaging of nobiletin based on a practical total synthesis. Chem. Commun. 2011, 47, 2868–2870. [Google Scholar] [CrossRef] [PubMed]
- Yoshigai, E.; Machida, T.; Okuyama, T.; Mori, M.; Murase, H.; Yamanishi, R.; Okumura, T.; Ikeya, Y.; Nishino, H.; Nishizawa, M. Citrus nobiletin suppresses inducible nitric oxide synthase gene expression in interleukin-1β-treated hepatocytes. Biochem. Biophys. Res. Commun. 2013, 439, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Atsumi, H.; Iwao, Y.; Kan, T.; Itai, S. Nobiletin: A citrus flavonoid displaying potent physiological activity. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72, 124–127. [Google Scholar] [CrossRef]
- Onoue, S.; Nakamura, T.; Uchida, A.; Ogawa, K.; Yuminoki, K.; Hashimoto, N.; Hiza, A.; Tsukaguchi, Y.; Asakawa, T.; Kan, T. Physicochemical and biopharmaceutical characterization of amorphous solid dispersion of nobiletin, a citrus polymethoxylated flavone, with improved hepatoprotective effects. Eur. J. Pharm. Sci. 2013, 49, 453–460. [Google Scholar] [CrossRef]
- Zheng, J.; Bi, J.; Johnson, D.; Sun, Y.; Song, M.; Qiu, P.; Dong, P.; Decker, E.; Xiao, H. Analysis of 10 metabolites of polymethoxyflavones with high sensitivity by electrochemical detection in high-performance liquid chromatography. J. Agric. Food Chem. 2015, 63, 509–516. [Google Scholar] [CrossRef]
- Zheng, J.; Song, M.; Dong, P.; Qiu, P.; Guo, S.; Zhong, Z.; Li, S.; Ho, C.T.; Xiao, H. Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Mol. Nutr. Food Res. 2013, 57, 1999–2007. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Sang, S.; Huang, M.T.; Ho, C.T. Identification of nobiletin metabolites in mouse urine. Mol. Nutr. Food Res. 2006, 50, 291–299. [Google Scholar] [CrossRef]
- Yasuda, T.; Yoshimura, Y.; Yabuki, H.; Nakazawa, T.; Ohsawa, K.; Mimaki, Y.; Sashida, Y. Urinary metabolites of nobiletin orally administered to rats. Chem. Pharm. Bull. 2003, 51, 1426–1428. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Zhang, G.; Xiao, H. Chemopreventive effects of nobiletin and its colonic metabolites on colon carcinogenesis. Mol. Nutr. Food Res. 2015, 59, 2383–2394. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Jonca, M.; Lambros, T.; Ferguson, S.; Goodnow, R.; Ho, C.T. Comparison of supercritical fluid chromatography and liquid chromatography for the separation of urinary metabolites of nobiletin with chiral and non-chiral stationary phases. Biomed. Chromatogr. 2006, 20, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Koga, N.; Ohta, C.; Kato, Y.; Haraguchi, K.; Endo, T.; Ogawa, K.; Ohta, H.; Yano, M. In vitro metabolism of nobiletin, a polymethoxy-flavonoid, by human liver microsomes and cytochrome P450. Xenobiotica 2011, 41, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, M. Biotransformation of Polymethoxyflavones and Its Implication on Biological Activities. Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 2017. [Google Scholar]
- Hattori, T.; Tagawa, H.; Inai, M.; Kan, T.; Kimura, S.I.; Itai, S.; Mitragotri, S.; Iwao, Y. Transdermal delivery of nobiletin using ionic liquids. Sci Rep 2019, 9, 20191. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liang, Y.; Pei, Y.; Li, B.; Liang, H. Plant exine capsules based encapsulation strategy: A high loading and long-term effective delivery system for nobiletin. Food Res. Int. 2020, 127, 108691. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Liu, Z.; Zhang, T.; Sun, S.; Ye, J.; Li, Z.; Mao, L.; Ren, J. Enhancement of Anti-Inflammatory Properties of Nobiletin in Macrophages by a Nano-Emulsion Preparation. J. Agric. Food Chem. 2018, 66, 91–98. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, J.; Ai, Z.; Su, J. Nobiletin-loaded micelles reduce ovariectomy-induced bone loss by suppressing osteoclastogenesis. Int. J. Nanomed. 2019, 14, 7839–7849. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Jabbarpour, Z.; Geramizadeh, B.; Motevaseli, E.; Nikeghbalian, S.; Shamsaeefar, A.; Motazedian, N.; Al-Abdullah, I.H.; Ghahremani, M.H.; et al. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci. Rep. 2019, 9, 11701. [Google Scholar] [CrossRef]
- Zhang, B.F.; Jiang, H.; Chen, J.; Guo, X.; Li, Y.; Hu, Q.; Yang, S. Nobiletin ameliorates myocardial ischemia and reperfusion injury by attenuating endoplasmic reticulum stress-associated apoptosis through regulation of the PI3K/AKT signal pathway. Int. Immunopharmacol. 2019, 73, 98–107. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, D.; Huang, L.; Jiang, C.; Pan, T.; Kang, X.; Pan, J. Nobiletin Inhibits IL-1beta-Induced Inflammation in Chondrocytes via Suppression of NF-kappaB Signaling and Attenuates Osteoarthritis in Mice. Front. Pharm. 2019, 10, 570. [Google Scholar] [CrossRef]
- Tsuboi, T.; Lu, R.; Yonezawa, T.; Watanabe, A.; Woo, J.T.; Abe-Dohmae, S.; Yokoyama, S. Molecular mechanism for nobiletin to enhance ABCA1/G1 expression in mouse macrophages. Atherosclerosis 2020, 297, 32–39. [Google Scholar] [CrossRef]
- Yuk, T.; Kim, Y.; Yang, J.; Sung, J.; Jeong, H.S.; Lee, J. Nobiletin Inhibits Hepatic Lipogenesis via Activation of AMP-Activated Protein Kinase. Evid. Based Complement. Altern. Med. 2018, 2018, 7420265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Du, Y.; Liu, P.; Zhang, J.; Li, Y.; Shen, H.; Xing, L.; Xue, X.; Chen, J.; et al. Long noncoding RNA TP53TG1 promotes pancreatic ductal adenocarcinoma development by acting as a molecular sponge of microRNA-96. Cancer Sci. 2019, 110, 2760–2772. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, H.; Otomo, R.; Sasaki, N.; Omi, T.; Sato, T.; Kaneda, T. Endothelium-independent vasodilator effects of nobiletin in rat aorta. J. Pharm. Sci. 2019, 140, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Lee, S.; Jun, M. Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of beta-Amyloid Peptide Toxicity. Nutrients 2019, 11, 2648. [Google Scholar] [CrossRef]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef]
- Zhang, P.A.; Sun, Q.; Li, Y.C.; Weng, R.X.; Wu, R.; Zhang, H.H.; Xu, G.Y. Overexpression of Purinergic P2X4 Receptors in Hippocampus Rescues Memory Impairment in Rats with Type 2 Diabetes. Neurosci. Bull. 2020. [Google Scholar] [CrossRef]
- Josefs, T.; Barrett, T.J.; Brown, E.J.; Quezada, A.; Wu, X.; Voisin, M.; Amengual, J.; Fisher, E.A. Neutrophil Extracellular Traps (NETs) promote macrophage inflammation and impair atherosclerosis resolution in mice with diabetes. Jci Insight 2020. (In press) [CrossRef]
- Nguyen-Ngo, C.; Salomon, C.; Quak, S.; Lai, A.; Willcox, J.C.; Lappas, M. Nobiletin exerts anti-diabetic and anti-inflammatory effects in an in vitro human model and in vivo murine model of gestational diabetes. Clin. Sci. 2020, 134, 571–592. [Google Scholar] [CrossRef]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loréal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Et Biophys. Acta Bba Gen. Subj. 2012, 1820, 403–410. [Google Scholar] [CrossRef]
- Gudjoncik, A.; Guenancia, C.; Zeller, M.; Cottin, Y.; Vergely, C.; Rochette, L. Iron, oxidative stress, and redox signaling in the cardiovascular system. Mol. Nutr. Food Res. 2014, 58, 1721–1738. [Google Scholar] [CrossRef]
- Finazzi, D.; Arosio, P. Biology of ferritin in mammals: An update on iron storage, oxidative damage and neurodegeneration. Arch. Toxicol. 2014, 88, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Gammella, E.; Recalcati, S.; Rybinska, I.; Buratti, P.; Cairo, G. Iron-induced damage in cardiomyopathy: Oxidative-dependent and independent mechanisms. Oxidative Med. Cell. Longev. 2015, 2015, 230182. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; He, H.; Zhang, Z.; Liao, Z.; Yin, D.; Liu, D.; Yi, B.; He, M. Long-term sodium ferulate supplementation scavenges oxygen radicals and reverses liver damage induced by iron overloading. Molecules 2016, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, X.; Zhang, L.; Cheng, L.; Li, X. LncRNA TUG1 promoted viability and associated with gemcitabine resistant in pancreatic ductal adenocarcinoma. J. Pharm. Sci. 2018, 137, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, H.; Yin, D.; Que, A.; Tang, L.; Liao, Z.; Huang, Q.; He, M. Mechanism of chronic dietary iron overload-induced liver damage in mice. Mol. Med. Rep. 2013, 7, 1173–1179. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Yi, B.; Liao, Z.; Tang, L.; Yin, D.; He, M. Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol. Med. Rep. 2014, 10, 2255–2262. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Chen, X.; Zhou, Q.; Li, H.; Chen, S.; Yin, D.; He, H.; He, M. Nobiletin Regulates ROS/ADMA/DDAHII/eNOS/NO Pathway and Alleviates Vascular Endothelium Injury by Iron Overload. Biol. Trace Elem. Res. 2020. (In press) [CrossRef]
- Lebre, M.C.; Vieira, P.L.; Tang, M.W.; Aarrass, S.; Helder, B.; Newsom-Davis, T.; Tak, P.P.; Screaton, G.R. Synovial IL-21/TNF-producing CD4+ T cells induce joint destruction in rheumatoid arthritis by inducing matrix metalloproteinase production by fibroblast-like synoviocytes. J. Leukoc. Biol. 2017, 101, 775–783. [Google Scholar] [CrossRef]
- Xing, R.; Jin, Y.; Sun, L.; Yang, L.; Li, C.; Li, Z.; Liu, X.; Zhao, J. Interleukin-21 induces migration and invasion of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Clin. Exp. Immunol. 2016, 184, 147–158. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, S.; Dong, Q. Nobiletin suppresses IL-21/IL-21 receptor-mediated inflammatory response in MH7A fibroblast-like synoviocytes (FLS): An implication in rheumatoid arthritis. Eur. J. Pharm. 2020, 875, 172939. [Google Scholar] [CrossRef]
- Morrow, N.M.; Burke, A.C.; Samsoondar, J.P.; Seigel, K.E.; Wang, A.; Telford, D.E.; Sutherland, B.G.; O’Dwyer, C.; Steinberg, G.R.; Fullerton, M.D.; et al. The citrus flavonoid nobiletin confers protection from metabolic dysregulation in high-fat-fed mice independent of AMPK. J. Lipid Res. 2020, 61, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Li, T.; Chen, L.; Zheng, B.; Liu, R.H. Nobiletin Delays Aging and Enhances Stress Resistance of Caenorhabditis elegans. Int. J. Mol. Sci. 2020, 21, 341. [Google Scholar] [CrossRef] [PubMed]
- Guvenc, M.; Cellat, M.; Gokcek, I.; Ozkan, H.; Arkali, G.; Yakan, A.; Yurdagul Ozsoy, S.; Aksakal, M. Nobiletin attenuates acetaminophen-induced hepatorenal toxicity in rats. J. Biochem. Mol. Toxicol. 2020, 34, e22427. [Google Scholar] [CrossRef] [PubMed]
- Ishiuchi, N.; Nakashima, A.; Doi, S.; Yoshida, K.; Maeda, S.; Kanai, R.; Yamada, Y.; Ike, T.; Doi, T.; Kato, Y.; et al. Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res. 2020, 11, 130. [Google Scholar]
- Guvenc, M.; Cellat, M.; Uyar, A.; Ozkan, H.; Gokcek, I.; Isler, C.T.; Yakan, A. Nobiletin Protects from Renal Ischemia-Reperfusion Injury in Rats by Suppressing Inflammatory Cytokines and Regulating iNOS-eNOS Expressions. Inflammation 2020, 43, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, F.; Yu, L.; Li, Z. Nobiletin alleviates cerebral ischemic-reperfusion injury via MAPK signaling pathway. Am. J. Transl. Res. 2019, 11, 5967–5977. [Google Scholar]
- Nohara, K.; Nemkov, T.; D’Alessandro, A.; Yoo, S.H.; Chen, Z. Coordinate Regulation of Cholesterol and Bile Acid Metabolism by the Clock Modifier Nobiletin in Metabolically Challenged Old Mice. Int. J. Mol. Sci. 2019, 20, 4281. [Google Scholar] [CrossRef]
- Yen, J.-H.; Lin, C.-Y.; Chuang, C.-H.; Chin, H.-K.; Wu, M.-J.; Chen, P.-Y. Nobiletin Promotes Megakaryocytic Differentiation through the MAPK/ERK-Dependent EGR1 Expression and Exerts Anti-Leukemic Effects in Human Chronic Myeloid Leukemia (CML) K562 Cells. Cells 2020, 9, 877. [Google Scholar] [CrossRef]
- Hermawan, A.; Putri, H. Bioinformatics Studies Provide Insight into Possible Target and Mechanisms of Action of Nobiletin against Cancer Stem Cells. Asian Pac. J. Cancer Prev. 2020, 21, 611–620. [Google Scholar] [CrossRef]
- Mao, Q.; Liang, X.; Wu, Y.; Lu, Y. Nobiletin protects against myocardial injury and myocardial apoptosis following coronary microembolization via activating PI3K/Akt pathway in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 392, 1121–1130. [Google Scholar] [CrossRef]
- Xu, K.; Huang, Y.; Zhou, T.; Wang, C.; Chi, Q.; Shi, J.; Zhu, P.; Dong, N. Nobiletin exhibits potent inhibition on tumor necrosis factor alpha-induced calcification of human aortic valve interstitial cells via targeting ABCG2 and AKR1B1. Phytother. Res. 2019, 33, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, H.; Li, Y.; Lu, X. Nobiletin suppresses oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Eur. J. Pharmacol. 2019, 854, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Liu, Z.; Liu, X. Nobiletin Protects against systemic inflammation-stimulated memory impairment via MAPK and NF-κB signaling pathways. J. Agric. Food Chem. 2019, 67, 5122–5134. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Xie, H.; Chen, C.; Tao, Z.; Zhang, C.; Cai, L. Inhibiting the PI3K/AKT/NF-κB signal pathway with nobiletin for attenuating the development of osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Potue, P.; Wunpathe, C.; Maneesai, P.; Kukongviriyapan, U.; Prachaney, P.; Pakdeechote, P. Nobiletin alleviates vascular alterations through modulation of Nrf-2/HO-1 and MMP pathways in l-NAME induced hypertensive rats. Food Funct. 2019, 10, 1880–1892. [Google Scholar] [CrossRef]
- Liu, Z.; Han, Y.; Zhao, F.; Zhao, Z.; Tian, J.; Jia, K. Nobiletin suppresses high-glucose–induced inflammation and ECM accumulation in human mesangial cells through STAT3/NF-κB pathway. J. Cell. Biochem. 2019, 120, 3467–3473. [Google Scholar] [CrossRef]
- He, P.P.; Shen, Q.Q.; Wen, M.; Zou, J.Q.; Wang, Y.; Yang, J.X.; Hu, L.Z.; Zheng, X.L.; Chen, Y.S.; Su, H.; et al. Nobiletin reduces LPL-mediated lipid accumulation and pro-in fl ammatory cytokine secretion through upregulation of miR-590 expression. Biochem Biophys Res Commun 2019, 508, 97–101. [Google Scholar] [CrossRef]
- Dusabimana, T.; Kim, S.R.; Kim, H.J.; Park, S.W.; Kim, H. Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Stock, C.-M.; Taylor, S.; Walsh, M.; Muddathir, A.K.; Verduzco, D.; Bashir, A.H.; Mohammed, O.Y.; Elhassan, G.O.; Harguindey, S. Resistance to cancer chemotherapy: Failure in drug response from ADME to P-gp. Cancer Cell Int. 2015, 15, 71. [Google Scholar] [CrossRef]
- Elshimali, Y.I.; Wu, Y.; Khaddour, H.; Wu, Y.; Gradinaru, D.; Sukhija, H.; Chung, S.S.; Vadgama, J.V. Optimization of cancer treatment through overcoming drug resistance. J. Cancer Res. Oncobiol. 2018, 1, 107. [Google Scholar] [CrossRef]
- Kamioka, H.; Tomono, T.; Fujita, A.; Onozato, R.; Iijima, M.; Tsuchida, S.; Arai, T.; Fujita, Y.; Zhang, X.; Yano, K.; et al. Moesin-Mediated P-Glycoprotein Activation during Snail-Induced Epithelial-Mesenchymal Transition in Lung Cancer Cells. J. Pharm. Sci. 2020. (In press) [CrossRef] [PubMed]
- Xu, S.W.; Law, B.Y.K.; Qu, S.L.Q.; Hamdoun, S.; Chen, J.; Zhang, W.; Guo, J.R.; Wu, A.G.; Mok, S.W.F.; Zhang, D.W.; et al. SERCA and P-glycoprotein inhibition and ATP depletion are necessary for celastrol-induced autophagic cell death and collateral sensitivity in multidrug-resistant tumor cells. Pharm. Res. 2020, 153, 104660. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Qin, Z.; Zhang, W.D.; Cheng, G.; Yehuda, A.G.; Ashby, C.R., Jr.; Chen, Z.S.; Cheng, X.D.; Qin, J.J. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: An update. Drug Resist Updat 2020, 49, 100681. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.-L.; Tian, Y.; Huo, S.; Qu, B.; Liu, R.-M.; Xu, P.; Li, Y.-Z.; Xie, Y. Nobiletin and its derivatives overcome multidrug resistance (MDR) in cancer: Total synthesis and discovery of potent MDR reversal agents. Acta Pharm. Sin. B 2020, 10, 327–343. [Google Scholar] [CrossRef]
- Wesołowska, O.; Wiśniewski, J.; Środa-Pomianek, K.; Bielawska-Pohl, A.; Paprocka, M.; Duś, D.; Duarte, N.l.; Ferreira, M.-J.U.; Michalak, K. Multidrug resistance reversal and apoptosis induction in human colon cancer cells by some flavonoids present in citrus plants. J. Nat. Prod. 2012, 75, 1896–1902. [Google Scholar] [CrossRef]
- Sousa, D.P.; Pojo, M.; Pinto, A.T.; Leite, V.; Serra, A.T.; Cavaco, B.M. Nobiletin Alone or in Combination with Cisplatin Decreases the Viability of Anaplastic Thyroid Cancer Cell Lines. Nutr. Cancer 2020, 72, 352–363. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kulkarni, N.S.; Farrales, P.; Kanabar, D.D.; Parvathaneni, V.; Kunda, N.K.; Muth, A.; Gupta, V. Sorafenib Loaded Inhalable Polymeric Nanocarriers against Non-Small Cell Lung Cancer. Pharm. Res. 2020, 37, 67. [Google Scholar] [CrossRef]
- Leineweber, C.G.; Pietzner, A.; Zhang, I.W.; Blessin, U.B.; Rothe, M.; Schott, E.; Schebb, N.H.; Weylandt, K.H. Assessment of the Effect of Sorafenib on Omega-6 and Omega-3 Epoxyeicosanoid Formation in Patients with Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 1875. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Deveci, A.O.; Bilir, C.; Kaleli, S. Synergistic effects of nobiletin and sorafenib combination on metastatic prostate cancer cells. Nutr. Cancer 2019, 71, 1299–1312. [Google Scholar] [CrossRef]
- Cole, S.P.; Bhardwaj, G.; Gerlach, J.H.; Mackie, J.E.; Grant, C.E.; Almquist, K.C.; Stewart, A.J.; Kurz, E.U.; Duncan, A.M.; Deeley, R.G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 1992, 258, 1650–1654. [Google Scholar] [CrossRef]
- Cole, S.P. Targeting multidrug resistance protein 1 (MRP1, ABCC1): Past, present, and future. Annu. Rev. Pharm. Toxicol. 2014, 54, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Manohar, C.F.; Bray, J.A.; Salwen, H.R.; Madafiglio, J.; Cheng, A.; Flemming, C.; Marshall, G.M.; Norris, M.D.; Haber, M.; Cohn, S.L. MYCN-mediated regulation of the MRP1 promoter in human neuroblastoma. Oncogene 2004, 23, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Scotto, K.W. Transcriptional regulation of ABC drug transporters. Oncogene 2003, 22, 7496–7511. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, J.; Ren, Y.; Li, L.; He, W.; Zhang, Y.; Liu, T.; Li, Z. Over-expression of FSIP1 promotes breast cancer progression and confers resistance to docetaxel via MRP1 stabilization. Cell Death Dis. 2019, 10, 204. [Google Scholar] [CrossRef]
- Hong, T.; Ding, J.; Li, W. miR-7 Reverses Breast Cancer Resistance to Chemotherapy by Targeting MRP1 and BCL2. Onco Targets 2019, 12, 11097–11105. [Google Scholar] [CrossRef]
- Moon, J.Y.; Hung, M.; Van, L.; Unno, T.; Cho, S.K. Nobiletin Enhances Chemosensitivity to Adriamycin through Modulation of the Akt/GSK3β/β–Catenin/MYCN/MRP1 Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. Nutrients 2018, 10, 1829. [Google Scholar] [CrossRef]
- Panda, P.K.; Naik, P.P.; Praharaj, P.P.; Meher, B.R.; Gupta, P.K.; Verma, R.S.; Maiti, T.K.; Shanmugam, M.K.; Chinnathambi, A.; Alharbi, S.A.; et al. Abrus agglutinin stimulates BMP-2-dependent differentiation through autophagic degradation of beta-catenin in colon cancer stem cells. Mol. Carcinog. 2018, 57, 664–677. [Google Scholar] [CrossRef]
- Bhuvanalakshmi, G.; Rangappa, K.S.; Dharmarajan, A.; Sethi, G.; Kumar, A.P.; Warrier, S. Breast Cancer Stem-Like Cells Are Inhibited by Diosgenin, a Steroidal Saponin, by the Attenuation of the Wnt beta-Catenin Signaling via the Wnt Antagonist Secreted Frizzled Related Protein-4. Front. Pharm. 2017, 8, 124. [Google Scholar] [CrossRef]
- Bhuvanalakshmi, G.; Gamit, N.; Patil, M.; Arfuso, F.; Sethi, G.; Dharmarajan, A.; Kumar, A.P.; Warrier, S. Stemness, Pluripotentiality, and Wnt Antagonism: sFRP4, a Wnt antagonist Mediates Pluripotency and Stemness in Glioblastoma. Cancers 2018, 11, 25. [Google Scholar] [CrossRef]
- Tang, C.; Gong, L.; Lvzi, X.; Qiu, K.; Zhang, Z.; Wan, L. Echinacoside inhibits breast cancer cells by suppressing the Wnt/beta-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2020. (In press) [CrossRef]
- Luo, J. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009, 273, 194–200. [Google Scholar] [CrossRef]
- Ong, P.S.; Wang, L.Z.; Dai, X.; Tseng, S.H.; Loo, S.J.; Sethi, G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharm. 2016, 7, 395. [Google Scholar] [CrossRef]
- Mohan, C.D.; Srinivasa, V.; Rangappa, S.; Mervin, L.; Mohan, S.; Paricharak, S.; Baday, S.; Li, F.; Shanmugam, M.K.; Chinnathambi, A.; et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE 2016, 11, e0153155. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-beta-Induced EMT of Lung Cancer Cells through PI3K/Akt/mTOR Inactivation. J. Cell Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.; Um, J.Y.; Sethi, G.; Ahn, K.S. Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers 2019, 11, 254. [Google Scholar] [CrossRef]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Guo, Q.; Liu, T.; Jiang, Y.; Zhao, M.; Zeng, K.; Tu, P. Cucurbitacin E Inhibits Huh7 Hepatoma Carcinoma Cell Proliferation and Metastasis via Suppressing MAPKs and JAK/STAT3 Pathways. Molecules 2020, 25, 560. [Google Scholar] [CrossRef]
- Won, Y.S.; Seo, K.I.; Sanggenol, L. Induces Apoptosis and Cell Cycle Arrest via Activation of p53 and Suppression of PI3K/Akt/mTOR Signaling in Human Prostate Cancer Cells. Nutrients 2020, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Shen, H.; Huang, H.; Yang, Z.; Zhou, Y.; Zhao, G. Cholesterol-lowering drug pitavastatin targets lung cancer and angiogenesis via suppressing prenylation-dependent Ras/Raf/MEK and PI3K/Akt/mTOR signaling. Anticancer Drugs 2020, 31, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Z.; Jiang, G.; Sun, H.; Yu, D. Nobiletin sensitizes colorectal cancer cells to oxaliplatin by PI3K/Akt/MTOR pathway. Front. Biosci. 2019, 24, 303–312. [Google Scholar]
- Ma, W.; Feng, S.; Yao, X.; Yuan, Z.; Liu, L.; Xie, Y. Nobiletin enhances the efficacy of chemotherapeutic agents in ABCB1 overexpression cancer cells. Sci. Rep. 2015, 5, 18789. [Google Scholar] [CrossRef] [PubMed]
- Ravn, S.; Heide-Jorgensen, U.; Christiansen, C.F.; Verwaal, V.J.; Hagemann-Madsen, R.H.; Iversen, L.H. Overall risk and risk factors for metachronous peritoneal metastasis after colorectal cancer surgery: A nationwide cohort study. Bjs Open 2020, 4, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Tang, M.L.; Ning, J.F.; Hao, Y.P.; Zhou, L.; Sun, X. Novel octapeptide-DTX prodrugs targeting MMP-7 as effective agents for the treatment of colorectal cancer with lower systemic toxicity. Eur. J. Med. Chem. 2020, 193, 112194. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.I.; Kil, J.H.; Yu, G.H.; Karadeniz, F.; Oh, J.H.; Seo, Y.; Kong, C.S. 3,5-Dicaffeoyl-epi-quinic acid inhibits the PMA-stimulated activation and expression of MMP-9 but not MMP-2 via downregulation of MAPK pathway. Z. Naturforsch. C. J. Biosci. 2020. (In press) [CrossRef]
- O’Reilly, M.S.; Wiederschain, D.; Stetler-Stevenson, W.G.; Folkman, J.; Moses, M.A. Regulation of angiostatin production by matrix metalloproteinase-2 in a model of concomitant resistance. J. Biol. Chem. 1999, 274, 29568–29571. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Ho, Y.C.; Lin, C.W.; Yang, W.E.; Yu, Y.L.; Tsai, M.C.; Yang, S.F.; Su, S.C. Salvianolic acid A suppresses MMP-2 expression and restrains cancer cell invasion through ERK signaling in human nasopharyngeal carcinoma. J. Ethnopharmacol. 2020, 252, 112601. [Google Scholar] [CrossRef]
- Lin, H.; Hao, Y.; Wan, X.; He, J.; Tong, Y. Baicalein inhibits cell development, metastasis and EMT and induces apoptosis by regulating ERK signaling pathway in osteosarcoma. J. Recept. Signal Transduct. Res. 2020, 40, 49–57. [Google Scholar] [CrossRef]
- Kanai, T.; Kondo, N.; Okada, M.; Sano, H.; Okumura, G.; Kijima, Y.; Ogose, A.; Kawashima, H.; Endo, N. The JNK pathway represents a novel target in the treatment of rheumatoid arthritis through the suppression of MMP-3. J. Orthop. Surg. Res. 2020, 15, 87. [Google Scholar] [CrossRef]
- Ha, S.H.; Kwon, K.M.; Park, J.Y.; Abekura, F.; Lee, Y.C.; Chung, T.W.; Ha, K.T.; Chang, H.W.; Cho, S.H.; Kim, J.S.; et al. Esculentoside H inhibits colon cancer cell migration and growth through suppression of MMP-9 gene expression via NF-kB signaling pathway. J. Cell Biochem. 2019, 120, 9810–9819. [Google Scholar] [CrossRef]
- Cheng, H.L.; Hsieh, M.J.; Yang, J.S.; Lin, C.W.; Lue, K.H.; Lu, K.H.; Yang, S.F. Nobiletin inhibits human osteosarcoma cells metastasis by blocking ERK and JNK-mediated MMPs expression. Oncotarget 2016, 7, 35208–35223. [Google Scholar] [CrossRef]
- Ali, S.O.; Khan, F.A.; Galindo-Campos, M.A.; Yelamos, J. Understanding specific functions of PARP-2: New lessons for cancer therapy. Am. J. Cancer Res. 2016, 6, 1842–1863. [Google Scholar] [PubMed]
- Megnin-Chanet, F.; Bollet, M.A.; Hall, J. Targeting poly(ADP-ribose) polymerase activity for cancer therapy. Cell Mol. Life Sci. 2010, 67, 3649–3662. [Google Scholar] [CrossRef] [PubMed]
- Vida, A.; Marton, J.; Miko, E.; Bai, P. Metabolic roles of poly(ADP-ribose) polymerases. Semin. Cell Dev. Biol. 2017, 63, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.; Jiao, Y.; Hao, M.; Tang, X. microRNA-383 suppresses the PI3K-AKT-MTOR signaling pathway to inhibit development of cervical cancer via down-regulating PARP2. J. Cell Biochem. 2018, 119, 5243–5252. [Google Scholar] [CrossRef]
- Zheng, G.D.; Hu, P.J.; Chao, Y.X.; Zhou, Y.; Yang, X.J.; Chen, B.Z.; Yu, X.Y.; Cai, Y. Nobiletin induces growth inhibition and apoptosis in human nasopharyngeal carcinoma C666-1 cells through regulating PARP-2/SIRT1/AMPK signaling pathway. Food Sci. Nutr. 2019, 7, 1104–1112. [Google Scholar] [CrossRef]
- Xu, Z.; Gu, C.; Yao, X.; Guo, W.; Wang, H.; Lin, T.; Li, F.; Chen, D.; Wu, J.; Ye, G.; et al. CD73 promotes tumor metastasis by modulating RICS/RhoA signaling and EMT in gastric cancer. Cell Death Dis. 2020, 11, 202. [Google Scholar] [CrossRef]
- Chatterjee, R.; Chatterjee, J. ROS and oncogenesis with special reference to EMT and stemness. Eur. J. Cell Biol. 2020, 99, 151073. [Google Scholar] [CrossRef]
- Stone, T.W. Dependence and Guidance Receptors-DCC and Neogenin-In Partial EMT and the Actions of Serine Proteases. Front. Oncol. 2020, 10, 94. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, X.; Zheng, X.; Zheng, Y.; Dong, X.; Zhao, N.; Liao, S.; Sun, B. The EMT transcription factor, Twist1, as a novel therapeutic target for pulmonary sarcomatoid carcinomas. Int. J. Oncol. 2020, 56, 750–760. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, K.; Li, C.; Zhao, Y.; Li, H.; Liu, X.; Long, Y.; Yao, J. Nobiletin inhibits invasion via inhibiting AKT/GSK3β/β-catenin signaling pathway in Slug-expressing glioma cells. Oncol. Rep. 2017, 37, 2847–2856. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Song, M.; Charoensinphon, N.; Zheng, J.; Qiu, P.; Wu, X.; Xiao, H. Inhibitory effects of nobiletin and its major metabolites on lung tumorigenesis. Food Funct. 2019, 10, 7444–7452. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, K.; Inoue, T.; Shimao, Y.; Sameshima, T. Matrix metalloproteinases in tumor invasion: Role for cell migration. Pathol. Int. 2002, 52, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.; Tian, S.; He, Y.; Lou, H.; Yang, Z.; Kong, Y.; Cao, X. Nobiletin inhibits breast cancer via p38 mitogen-activated protein kinase, nuclear transcription factor-kappaB, and nuclear factor erythroid 2-related factor 2 pathways in MCF-7 cells. Food Nutr. Res. 2018, 21, 62. [Google Scholar]
- Kang, N.; Wang, M.M.; Wang, Y.H.; Zhang, Z.N.; Cao, H.R.; Lv, Y.H.; Yang, Y.; Fan, P.H.; Qiu, F.; Gao, X.M. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem. Toxicol. 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Kubo, M.; Gotoh, K.; Eguchi, H.; Kobayashi, S.; Iwagami, Y.; Tomimaru, Y.; Akita, H.; Asaoka, T.; Noda, T.; Takeda, Y.; et al. Impact of CD36 on Chemoresistance in Pancreatic Ductal Adenocarcinoma. Ann. Surg. Oncol. 2020, 27, 610–619. [Google Scholar] [CrossRef]
- Yang, Q.; Li, K.; Huang, X.; Zhao, C.; Mei, Y.; Li, X.; Jiao, L.; Yang, H. lncRNA SLC7A11-AS1 Promotes Chemoresistance by Blocking SCF(beta-TRCP)-Mediated Degradation of NRF2 in Pancreatic Cancer. Mol. Nucleic Acids 2020, 19, 974–985. [Google Scholar] [CrossRef]
- Deng, M.; Cai, X.; Long, L.; Xie, L.; Ma, H.; Zhou, Y.; Liu, S.; Zeng, C. CD36 promotes the epithelial-mesenchymal transition and metastasis in cervical cancer by interacting with TGF-beta. J. Transl. Med. 2019, 17, 352. [Google Scholar] [CrossRef]
- Sakurai, K.; Tomihara, K.; Yamazaki, M.; Heshiki, W.; Moniruzzaman, R.; Sekido, K.; Tachinami, H.; Ikeda, A.; Imaue, S.; Fujiwara, K.; et al. CD36 expression on oral squamous cell carcinoma cells correlates with enhanced proliferation and migratory activity. Oral Dis. 2019, 26, 745–755. [Google Scholar] [CrossRef]
- Masoumi-Dehghi, S.; Babashah, S.; Sadeghizadeh, M. microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-kappaB signaling pathways. J. Cell Commun. Signal 2020. (In press) [CrossRef]
- Sp, N.; Kang, D.Y.; Kim, D.H.; Park, J.H.; Lee, H.G.; Kim, H.J.; Darvin, P.; Park, Y.-M.; Yang, Y.M. Nobiletin inhibits CD36-dependent tumor angiogenesis, migration, invasion, and sphere formation through the Cd36/Stat3/Nf-Κb signaling axis. Nutrients 2018, 10, 772. [Google Scholar] [CrossRef]
- Androutsopoulos, V.P.; Tsatsakis, A.M. Benzo [a] pyrene sensitizes MCF7 breast cancer cells to induction of G1 arrest by the natural flavonoid eupatorin-5-methyl ether, via activation of cell signaling proteins and CYP1-mediated metabolism. Toxicol. Lett. 2014, 230, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Surichan, S.; Androutsopoulos, V.P.; Sifakis, S.; Koutala, E.; Tsatsakis, A.; Arroo, R.R.; Boarder, M.R. Bioactivation of the citrus flavonoid nobiletin by CYP1 enzymes in MCF7 breast adenocarcinoma cells. Food Chem. Toxicol. 2012, 50, 3320–3328. [Google Scholar] [CrossRef] [PubMed]
- Surichan, S.; Arroo, R.R.; Ruparelia, K.; Tsatsakis, A.M.; Androutsopoulos, V.P. Nobiletin bioactivation in MDA-MB-468 breast cancer cells by cytochrome P450 CYP1 enzymes. Food Chem. Toxicol. 2018, 113, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.D.; Thompson, L.U. Mammalian lignans and genistein decrease the activities of aromatase and 17β-hydroxysteroid dehydrogenase in MCF-7 cells. J. Steroid Biochem. Mol. Biol. 2005, 94, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.H.; Doerge, D.R.; Woodling, K.A.; Hartman, J.A.; Kwak, J.; Helferich, W.G. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis 2008, 29, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chan, F.L.; Chen, S.; Leung, L.K. The citrus flavonone hesperetin inhibits growth of aromatase-expressing MCF-7 tumor in ovariectomized athymic mice. J. Nutr. Biochem. 2012, 23, 1230–1237. [Google Scholar] [CrossRef]
- Ye, L.; Gho, W.M.; Chan, F.L.; Chen, S.; Leung, L.K. Dietary administration of the licorice flavonoid isoliquiritigenin deters the growth of MCF-7 cells overexpressing aromatase. Int. J. Cancer 2009, 124, 1028–1036. [Google Scholar] [CrossRef]
- Rahideh, S.T.; Keramatipour, M.; Nourbakhsh, M.; Koohdani, F.; Hoseini, M.; Talebi, S.; Shidfar, F. Comparison of the effects of nobiletin and letrozole on the activity and expression of aromatase in the MCF-7 breast cancer cell line. Biochem. Cell Biol. 2017, 95, 468–473. [Google Scholar] [CrossRef]
- Yang, M.H.; Lee, J.H.; Ko, J.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules 2019, 24, 1584. [Google Scholar] [CrossRef]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Wang, L.; Wang, H.; Tang, F.R.; Cai, W.Q.; Sethi, G.; Xin, H.W.; Ma, Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells 2019, 8, 1178. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Ahn, K.S.; Wang, L.Z.; Kim, C.; Deivasigamni, A.; Arfuso, F.; Um, J.-Y.; Kumar, A.P.; Chang, Y.-C.; Kumar, D. Ascochlorin enhances the sensitivity of doxorubicin leading to the reversal of epithelial-to-mesenchymal transition in hepatocellular carcinoma. Mol. Cancer Ther. 2016, 15, 2966–2976. [Google Scholar] [CrossRef] [PubMed]
- Ko, H. Geraniin inhibits TGF-beta1-induced epithelial-mesenchymal transition and suppresses A549 lung cancer migration, invasion and anoikis resistance. Bioorg. Med. Chem. Lett. 2015, 25, 3529–3534. [Google Scholar] [CrossRef]
- Da, C.; Liu, Y.; Zhan, Y.; Liu, K.; Wang, R. Nobiletin inhibits epithelial-mesenchymal transition of human non-small cell lung cancer cells by antagonizing the TGF-beta1/Smad3 signaling pathway. Oncol. Rep. 2016, 35, 2767–2774. [Google Scholar] [CrossRef]
- Li, S.; Huang, C.; Hu, G.; Ma, J.; Chen, Y.; Zhang, J.; Huang, Y.; Zheng, J.; Xue, W.; Xu, Y.; et al. Tumor-educated B cells promote renal cancer metastasis via inducing the IL-1beta/HIF-2alpha/Notch1 signals. Cell Death Dis. 2020, 11, 163. [Google Scholar] [CrossRef]
- Dang, N.N.; Jiao, J.; Meng, X.; An, Y.; Han, C.; Huang, S. Abnormal overexpression of G9a in melanoma cells promotes cancer progression via upregulation of the Notch1 signaling pathway. Aging 2020, 12, 2393–2407. [Google Scholar] [CrossRef]
- Gao, X.J.; Liu, J.W.; Zhang, Q.G.; Zhang, J.J.; Xu, H.T.; Liu, H.J. Nobiletin inhibited hypoxia-induced epithelial-mesenchymal transition of lung cancer cells by inactivating of Notch-1 signaling and switching on miR-200b. Pharmazie 2015, 70, 256–262. [Google Scholar]
- Li, Y.; Guan, B.; Liu, J.; Zhang, Z.; He, S.; Zhan, Y.; Su, B.; Han, H.; Zhang, X.; Wang, B.; et al. MicroRNA-200b is downregulated and suppresses metastasis by targeting LAMA4 in renal cell carcinoma. EBioMedicine 2019, 44, 439–451. [Google Scholar] [CrossRef]
- Liu, P.; Chen, S.; Huang, Y.; Xu, S.; Song, H.; Zhang, W.; Sun, N. LINC00667 promotes Wilms’ tumor metastasis and stemness by sponging miR-200b/c/429 family to regulate IKK-beta. Cell Biol. Int. 2020. (In press) [CrossRef]
- Chung, S.D.; Lai, T.Y.; Chien, C.T.; Yu, H.J. Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS ONE 2012, 7, 47299. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.J.F.; Marchi, S.; Petroni, G.; Kroemer, G.; Galluzzi, L.; Pervaiz, S. Noncanonical Cell Fate Regulation by Bcl-2 Proteins. Trends Cell Biol. 2020. (In press) [CrossRef] [PubMed]
- Hazari, Y.; Bravo-San Pedro, J.M.; Hetz, C.; Galluzzi, L.; Kroemer, G. Autophagy in hepatic adaptation to stress. J. Hepatol. 2020, 72, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Buqué, A.; Rodriguez-Ruiz, M.E.; Fucikova, J.; Galluzzi, L. Apoptotic caspases cut down the immunogenicity of radiation. OncoImmunology 2019, 8, e1655364. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, B.; Li, D.; Wang, G.; Han, X.; Sun, X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem. Biophys. Res. Commun. 2018, 495, 1418–1425. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Mao, L.; Guo, Y.; Hao, Y.; Deng, Y.; Han, X.; Li, Q.; Liao, W.; Yuan, M. Nobiletin Triggers Reactive Oxygen Species-Mediated Pyroptosis through Regulating Autophagy in Ovarian Cancer Cells. J. Agric. Food Chem. 2020, 68, 1326–1336. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wang, X.; Zhang, C.Y. LncRNA SNHG7 enhances chemoresistance in neuroblastoma through cisplatin-induced autophagy by regulating miR-329-3p/MYO10 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3805–3817. [Google Scholar]
- Guo, H.; Ren, H.; Li, J.; Hao, M.; Hao, J.; Ren, H.; Guo, L.; Liu, R. TIPE2 suppressed cisplatin resistance by inducing autophagy via mTOR signalling pathway. Exp. Mol. Pathol. 2020, 113, 104367. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, P.; Xu, X.; Wang, J.; Wang, D.; Peng, P.; Zheng, C.; Meng, Q.J.; Yang, L.; Luo, Z. Knockdown of cytokeratin 8 overcomes chemoresistance of chordoma cells by aggravating endoplasmic reticulum stress through PERK/eIF2alpha arm of unfolded protein response and blocking autophagy. Cell Death Dis. 2019, 10, 887. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, E.; Tang, Y.; Mao, J.; Shen, J.; Zheng, X.; Xie, S.; Zhang, S.; Wu, Y.; Liu, H.; et al. miR-223 overexpression inhibits doxorubicin-induced autophagy by targeting FOXO3a and reverses chemoresistance in hepatocellular carcinoma cells. Cell Death Dis. 2019, 10, 843. [Google Scholar] [CrossRef]
- Qi, Y.; Qi, W.; Liu, S.; Sun, L.; Ding, A.; Yu, G.; Li, H.; Wang, Y.; Qiu, W.; Lv, J. TSPAN9 suppresses the chemosensitivity of gastric cancer to 5-fluorouracil by promoting autophagy. Cancer Cell Int. 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jing, F.J.; Xu, W.; Li, X.; Li, X.; Sun, J.L.; Xing, X.M.; Zhou, C.K.; Jing, F.B. Ubenimex induces autophagy inhibition and EMT suppression to overcome cisplatin resistance in GC cells by perturbing the CD13/EMP3/PI3K/AKT/NF-kappaB axis. Aging 2019, 12, 80–105. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Li, T.; Qiu, W.; Zhu, Z. Claudin-1 silencing increases sensitivity of liver cancer HepG2 cells to 5-fluorouracil by inhibiting autophagy. Oncol. Lett. 2019, 18, 5709–5716. [Google Scholar] [CrossRef]
- Jiang, Y.-P.; Guo, H.; Wang, X.-B. Nobiletin (NOB) suppresses autophagic degradation via over-expressing AKT pathway and enhances apoptosis in multidrug-resistant SKOV3/TAX ovarian cancer cells. Biomed. Pharmacother. 2018, 103, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kaisho, T.; Akira, S. Toll-like receptors. Annu. Rev. Immunol. 2003, 21, 335–376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Y.; Zhang, Q.; Wang, F.; Zhang, D. Toll-like receptors and prostate cancer. Front. Immunol. 2014, 5, 352. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Deveci Ozkan, A. Anti-inflammatory effects of nobiletin on TLR4/TRIF/IRF3 and TLR9/IRF7 signaling pathways in prostate cancer cells. Immunopharmacol. Immunotoxicol. 2020, 42, 93–100. [Google Scholar] [CrossRef]
- Denicourt, C.; Dowdy, S.F. Targeting apoptotic pathways in cancer cells. Science 2004, 305, 1411–1413. [Google Scholar] [CrossRef]
- Liao, C.-T.; Chang, J.T.-C.; Wang, H.-M.; Ng, S.-H.; Hsueh, C.; Lee, L.-Y.; Lin, C.-H.; Chen, I.-H.; Huang, S.-F.; Cheng, A.-J. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann. Surg. Oncol. 2008, 15, 915–922. [Google Scholar] [CrossRef]
- Amarante-Mendes, G.P.; Naekyung Kim, C.; Liu, L.; Huang, Y.; Perkins, C.L.; Green, D.R.; Bhalla, K. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood J. Am. Soc. Hematol. 1998, 91, 1700–1705. [Google Scholar]
- Nicholson, D.W.; Thornberry, N.A. Life and death decisions. Science 2003, 299, 214–215. [Google Scholar] [CrossRef] [PubMed]
- RV, E.H.; Bredesen, D. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004, 11, 372380Rodrgue. [Google Scholar]
- Zhang, X.; Huang, J.; Yu, C.; Xiang, L.; Li, L.; Shi, D.; Lin, F. Quercetin Enhanced Paclitaxel Therapeutic Effects Towards PC-3 Prostate Cancer Through ER Stress Induction and ROS Production. Onco. Targets 2020, 13, 513–523. [Google Scholar] [CrossRef]
- Maeyashiki, C.; Melhem, H.; Hering, L.; Baebler, K.; Cosin-Roger, J.; Schefer, F.; Weder, B.; Hausmann, M.; Scharl, M.; Rogler, G.; et al. Activation of pH-Sensing Receptor OGR1 (GPR68) Induces ER Stress Via the IRE1alpha/JNK Pathway in an Intestinal Epithelial Cell Model. Sci. Rep. 2020, 10, 1438. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.; Zhang, J.; Tian, J. FNDC3B is associated with ER stress and poor prognosis in cervical cancer. Oncol. Lett. 2020, 19, 406–414. [Google Scholar] [CrossRef]
- Fu, G.; Xu, Z.; Chen, X.; Pan, H.; Wang, Y.; Jin, B. CDCA5 functions as a tumor promoter in bladder cancer by dysregulating mitochondria-mediated apoptosis, cell cycle regulation and PI3k/AKT/mTOR pathway activation. J. Cancer 2020, 11, 2408–2420. [Google Scholar] [CrossRef]
- Goan, Y.G.; Wu, W.T.; Liu, C.I.; Neoh, C.A.; Wu, Y.J. Involvement of Mitochondrial Dysfunction, Endoplasmic Reticulum Stress, and the PI3K/AKT/mTOR Pathway in Nobiletin-Induced Apoptosis of Human Bladder Cancer Cells. Molecules 2019, 24, 2881. [Google Scholar] [CrossRef]
- Kim, C.; Cho, S.K.; Kapoor, S.; Kumar, A.; Vali, S.; Abbasi, T.; Kim, S.H.; Sethi, G.; Ahn, K.S. beta-Caryophyllene oxide inhibits constitutive and inducible STAT3 signaling pathway through induction of the SHP-1 protein tyrosine phosphatase. Mol. Carcinog. 2014, 53, 793–806. [Google Scholar] [CrossRef]
- Mohan, C.D.; Bharathkumar, H.; Bulusu, K.C.; Pandey, V.; Rangappa, S.; Fuchs, J.E.; Shanmugam, M.K.; Dai, X.; Li, F.; Deivasigamani, A.; et al. Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J. Biol. Chem. 2014, 289, 34296–34307. [Google Scholar] [CrossRef]
- Wong, A.L.A.; Hirpara, J.L.; Pervaiz, S.; Eu, J.Q.; Sethi, G.; Goh, B.C. Do STAT3 inhibitors have potential in the future for cancer therapy? Expert Opin. Investig. Drugs 2017, 26, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hirpara, J.L.; Eu, J.Q.; Sethi, G.; Wang, L.; Goh, B.C.; Wong, A.L. Targeting STAT3 and oxidative phosphorylation in oncogene-addicted tumors. Redox Biol. 2019, 25, 101073. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.J.; Lin, Y.M.; Huang, Y.C.; Shi, B.; Yeh, K.T.; Gong, Z.; Lu, J.W. Prognostic significance of high YY1AP1 and PCNA expression in colon adenocarcinoma. Biochem. Biophys. Res. Commun. 2017, 494, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, W.; Zhang, S. STAT3-induced upregulation of circCCDC66 facilitates the progression of non-small cell lung cancer by targeting miR-33a-5p/KPNA4 axis. Biomed. Pharm. 2020, 126, 110019. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, X.; Su, Y.; Jia, C.; Dai, C. GNAS promotes inflammation-related hepatocellular carcinoma progression by promoting STAT3 activation. Cell Mol. Biol. Lett. 2020, 25, 8. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, G.; Zhu, Z.; Zheng, Y.; Yan, F.; Pan, C.; Wang, Z.; Li, X.; Wang, F.; Meng, P.; et al. Nobiletin Inhibits Cell Viability via the SRC/AKT/STAT3/YY1AP1 Pathway in Human Renal Carcinoma Cells. Front. Pharm. 2019, 10, 690. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, F.; Roche, O.; Sanchez-Prieto, R.; Aragones, J. Hypoxia-Inducible Factor 2-Dependent Pathways Driving Von Hippel-Lindau-Deficient Renal Cancer. Front. Oncol. 2018, 8, 214. [Google Scholar] [CrossRef]
- Robinson, C.M.; Poon, B.P.K.; Kano, Y.; Pluthero, F.G.; Kahr, W.H.A.; Ohh, M. A Hypoxia-Inducible HIF1-GAL3ST1-Sulfatide Axis Enhances ccRCC Immune Evasion via Increased Tumor Cell-Platelet Binding. Mol. Cancer Res. 2019, 17, 2306–2317. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Tanaka, F.; Morita, H.; Hiraki, A.; Hashimoto, S. Hypoxia-induced HIF-1alpha and ZEB1 are critical for the malignant transformation of ameloblastoma via TGF-beta-dependent EMT. Cancer Med. 2019, 8, 7822–7832. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y.; Xiong, W.; Zhou, R.; Song, N.; Liu, L.; Qian, J. Dexmedetomidine protects H9C2 against hypoxia/reoxygenation injury through miR-208b-3p/Med13/Wnt signaling pathway axis. Biomed. Pharm. 2020, 125, 110001. [Google Scholar] [CrossRef]
- Al-Anazi, A.; Parhar, R.; Saleh, S.; Al-Hijailan, R.; Inglis, A.; Al-Jufan, M.; Bazzi, M.; Hashmi, S.; Conca, W.; Collison, K.; et al. Data on hypoxia-induced VEGF, leptin and NF-kB p65 expression. Data Brief 2018, 21, 2395–2397. [Google Scholar] [CrossRef] [PubMed]
- Asgarova, A.; Asgarov, K.; Godet, Y.; Peixoto, P.; Nadaradjane, A.; Boyer-Guittaut, M.; Galaine, J.; Guenat, D.; Mougey, V.; Perrard, J.; et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology 2018, 7, e1423170. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhai, R.; Chen, T.; Gao, C.; Xue, R.; Wang, N.; Wang, J.; Xu, Y.; Gui, D. Panax Notoginseng Ameliorates Podocyte EMT by Targeting the Wnt/beta-Catenin Signaling Pathway in STZ-Induced Diabetic Rats. Drug Des. Devel. 2020, 14, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, S.; Yin, M.; Guo, L.; Xu, M.; Wang, Y. Nobiletin inhibits hypoxia-induced epithelial-mesenchymal transition in renal cell carcinoma cells. J. Cell. Biochem. 2019, 120, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics 2017. Ca Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.N.; Blanchard, J.F.; Kliewer, E.; Wajda, A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer 2001, 91, 854–862. [Google Scholar] [CrossRef]
- Rubin, D.C.; Shaker, A.; Levin, M.S. Chronic intestinal inflammation: Inflammatory bowel disease and colitis-associated colon cancer. Front. Immunol. 2012, 3, 107. [Google Scholar] [CrossRef]
- Goh, J.X.H.; Tan, L.T.; Goh, J.K.; Chan, K.G.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers 2019, 11, 867. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Qiu, P.; Li, F.; Wang, M.; Zheng, J.; Wang, Q.; Xu, F.; Xiao, H. A metabolite of nobiletin, 4′-demethylnobiletin and atorvastatin synergistically inhibits human colon cancer cell growth by inducing G0/G1 cell cycle arrest and apoptosis. Food Funct. 2018, 9, 87–95. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Qiu, P.; Rakariyatham, K.; Li, F.; Gao, Z.; Cai, X.; Wang, M.; Xu, F.; Zheng, J.; et al. Synergistic chemopreventive effects of nobiletin and atorvastatin on colon carcinogenesis. Carcinogenesis 2017, 38, 455–464. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Liu, B.; Song, H.; Yu, R.; Zou, D.; Chen, Y.; Ma, Y.; Lv, F.; Xu, L.; Zhang, Z.; et al. beta-Elemene inhibits the metastasis of multidrug-resistant gastric cancer cells through miR-1323/Cbl-b/EGFR pathway. Phytomedicine 2020, 69, 153184. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Guo, H.; Zhu, L.; Xu, L.; Pei, Q.; Cao, Y. LINC00152 Knock-down Suppresses Esophageal Cancer by EGFR Signaling Pathway. Open Med. 2020, 15, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Peng, X.; Zhang, Y. Cytotoxicity of amide-linked local anesthetics on melanoma cells via inhibition of Ras and RhoA signaling independent of sodium channel blockade. BMC Anesth. 2020, 20, 43. [Google Scholar] [CrossRef]
- Jiang, Y.; Hong, D.; Lou, Z.; Tu, X.; Jin, L. Lupeol inhibits migration and invasion of colorectal cancer cells by suppressing RhoA-ROCK1 signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2020. (In press) [CrossRef]
- Shan, C.; Hui, W.; Li, H.; Wang, Z.; Guo, C.; Peng, R.; Gu, J.; Chen, Y.; Ouyang, Q. Discovery of Novel Autophagy Inhibitors and Their Sensitization Abilities for Vincristine-Resistant Esophageal Cancer Cell Line Eca109/VCR. ChemMedChem 2020. (In press) [CrossRef]
- Moon, J.Y.; Cho, S.K. Nobiletin Induces Protective Autophagy Accompanied by ER-Stress Mediated Apoptosis in Human Gastric Cancer SNU-16 Cells. Molecules 2016, 21, 914. [Google Scholar] [CrossRef]
- Fang, C.H.; Lin, Y.T.; Liang, C.M.; Liang, S.M. A novel c-Kit/phospho-prohibitin axis enhances ovarian cancer stemness and chemoresistance via Notch3-PBX1 and beta-catenin-ABCG2 signaling. J. Biomed. Sci. 2020, 27, 42. [Google Scholar] [CrossRef]
- Garg, M.; Nagata, Y.; Kanojia, D.; Mayakonda, A.; Yoshida, K.; Haridas Keloth, S.; Zang, Z.J.; Okuno, Y.; Shiraishi, Y.; Chiba, K.; et al. Profiling of somatic mutations in acute myeloid leukemia with FLT3-ITD at diagnosis and relapse. Blood 2015, 126, 2491–2501. [Google Scholar] [CrossRef]
- Jang, M.K.; Mashima, T.; Seimiya, H. Tankyrase Inhibitors Target Colorectal Cancer Stem Cells via AXIN-Dependent Downregulation of c-KIT Tyrosine Kinase. Mol. Cancer 2020, 19, 765–776. [Google Scholar] [CrossRef]
- Lv, M.; Ou, R.; Zhang, Q.; Lin, F.; Li, X.; Wang, K.; Xu, Y. MicroRNA-664 suppresses the growth of cervical cancer cells via targeting c-Kit. Drug Des. Devel. 2019, 13, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Chen, Y.-T.; Gao, W.-Y.; Wu, M.-J.; Yen, J.-H. Nobiletin Down-Regulates c-KIT Gene Expression and Exerts Antileukemic Effects on Human Acute Myeloid Leukemia Cells. J. Agric. Food Chem. 2018, 66, 13423–13434. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Buonavoglia, A.; Fasano, R.; Solimando, A.G.; De Re, V.; Cicco, S.; Vacca, A.; Racanelli, V. Insights into the Regulation of Tumor Angiogenesis by Micro-RNAs. J. Clin. Med. 2019, 8, 2030. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Cui, J.; Si, T. Endostar blocks the metastasis, invasion and angiogenesis of ovarian cancer cells. Neoplasma 2020. (In press) [CrossRef] [PubMed]
- Danielsen, T.; Rofstad, E.K. VEGF, bFGF and EGF in the angiogenesis of human melanoma xenografts. Int. J. Cancer 1998, 76, 836–841. [Google Scholar] [CrossRef]
- Sieg, D.J.; Hauck, C.R.; Ilic, D.; Klingbeil, C.K.; Schaefer, E.; Damsky, C.H.; Schlaepfer, D.D. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000, 2, 249–256. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Src protein-tyrosine kinase structure and regulation. Biochem. Biophys. Res. Commun. 2004, 324, 1155–1164. [Google Scholar] [CrossRef]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar] [CrossRef]
- Chen, Z.; Han, Z.C. STAT3: A critical transcription activator in angiogenesis. Med. Res. Rev. 2008, 28, 185–200. [Google Scholar] [CrossRef]
- Zhang, J.; Ahn, K.S.; Kim, C.; Shanmugam, M.K.; Siveen, K.S.; Arfuso, F.; Samym, R.P.; Deivasigamanim, A.; Lim, L.H.; Wang, L.; et al. Nimbolide-Induced Oxidative Stress Abrogates STAT3 Signaling Cascade and Inhibits Tumor Growth in Transgenic Adenocarcinoma of Mouse Prostate Model. Antioxid. Redox. Signal 2016, 24, 575–589. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Sethi, G.; Ahn, K.S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 2015, 6, 6386–6405. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chiang, S.Y.; Nam, D.; Chung, W.S.; Lee, J.; Na, Y.S.; Sethi, G.; Ahn, K.S. Capillarisin inhibits constitutive and inducible STAT3 activation through induction of SHP-1 and SHP-2 tyrosine phosphatases. Cancer Lett. 2014, 345, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shanmugam, M.K.; Chen, L.; Chatterjee, S.; Basha, J.; Kumar, A.P.; Kundu, T.K.; Sethi, G. Garcinol, a polyisoprenylated benzophenone modulates multiple proinflammatory signaling cascades leading to the suppression of growth and survival of head and neck carcinoma. Cancer Prev. Res. 2013, 6, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Sp, N.; Kang, D.Y.; Joung, Y.H.; Park, J.H.; Kim, W.S.; Lee, H.K.; Song, K.D.; Park, Y.M.; Yang, Y.M. Nobiletin Inhibits Angiogenesis by Regulating Src/FAK/STAT3-Mediated Signaling through PXN in ER(+) Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 935. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, S.M.; Nam, D.; Lee, J.H.; Ahn, K.S.; Choi, S.H.; Kim, S.H.; Shim, B.S.; Chang, I.M.; Ahn, K.S. Antimetastatic effect of nobiletin through the down-regulation of CXC chemokine receptor type 4 and matrix metallopeptidase-9. Pharm. Biol. 2012, 50, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ono, M.; Takeshima, M.; Nakano, S. Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Res. 2014, 34, 1785–1792. [Google Scholar]
- Ma, X.; Jin, S.; Zhang, Y.; Wan, L.; Zhao, Y.; Zhou, L. Inhibitory effects of nobiletin on hepatocellular carcinoma in vitro and in vivo. Phytother. Res. PTR 2014, 28, 560–567. [Google Scholar] [CrossRef]
- Shi, M.D.; Liao, Y.C.; Shih, Y.W.; Tsai, L.Y. Nobiletin attenuates metastasis via both ERK and PI3K/Akt pathways in HGF-treated liver cancer HepG2 cells. Phytomedicine 2013, 20, 743–752. [Google Scholar] [CrossRef]
- Nemoto, K.; Ikeda, A.; Yoshida, C.; Kimura, J.; Mori, J.; Fujiwara, H.; Yokosuka, A.; Mimaki, Y.; Ohizumi, Y.; Degawa, M. Characteristics of nobiletin-mediated alteration of gene expression in cultured cell lines. Biochem. Biophys. Res. Commun. 2013, 431, 530–534. [Google Scholar] [CrossRef]
- Yoshimizu, N.; Otani, Y.; Saikawa, Y.; Kubota, T.; Yoshida, M.; Furukawa, T.; Kumai, K.; Kameyama, K.; Fujii, M.; Yano, M.; et al. Anti-tumour effects of nobiletin, a citrus flavonoid, on gastric cancer include: Antiproliferative effects, induction of apoptosis and cell cycle deregulation. Aliment. Pharmacol. Ther. 2004, 20, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Cho, M.; Ahn, K.S.; Cho, S.K. Nobiletin induces apoptosis and potentiates the effects of the anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric cancer cells. Nutr. Cancer 2013, 65, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Cheng, T.H.; Lee, J.S.; Chen, J.H.; Liao, Y.C.; Fong, Y.; Wu, C.H.; Shih, Y.W. Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/Akt and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol. Cell. Biochem. 2011, 347, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Lien, L.M.; Wang, M.J.; Chen, R.J.; Chiu, H.C.; Wu, J.L.; Shen, M.Y.; Chou, D.S.; Sheu, J.R.; Lin, K.H.; Lu, W.J. Nobiletin, a polymethoxylated flavone, inhibits glioma cell growth and migration via arresting cell cycle and suppressing MAPK and Akt pathways. Phytother. Res. 2016, 30, 214–221. [Google Scholar] [CrossRef]
- Uesato, S.; Yamashita, H.; Maeda, R.; Hirata, Y.; Yamamoto, M.; Matsue, S.; Nagaoka, Y.; Shibano, M.; Taniguchi, M.; Baba, K.; et al. Synergistic antitumor effect of a combination of paclitaxel and carboplatin with nobiletin from Citrus depressa on non-small-cell lung cancer cell lines. Planta Med. 2014, 80, 452–457. [Google Scholar] [CrossRef]
- Chien, S.Y.; Hsieh, M.J.; Chen, C.J.; Yang, S.F.; Chen, M.K. Nobiletin inhibits invasion and migration of human nasopharyngeal carcinoma cell lines by involving ERK1/2 and transcriptional inhibition of MMP-2. Expert Opin. Ther. Targets 2015, 19, 307–320. [Google Scholar] [CrossRef]
- Chen, J.; Chen, A.Y.; Huang, H.; Ye, X.; Rollyson, W.D.; Perry, H.E.; Brown, K.C.; Rojanasakul, Y.; Rankin, G.O.; Dasgupta, P.; et al. The flavonoid nobiletin inhibits tumor growth and angiogenesis of ovarian cancers via the Akt pathway. Int. J. Oncol. 2015, 46, 2629–2638. [Google Scholar] [CrossRef]
- Chen, J.; Creed, A.; Chen, A.Y.; Huang, H.; Li, Z.; Rankin, G.O.; Ye, X.; Xu, G.; Chen, Y.C. Nobiletin suppresses cell viability through AKT pathways in PC-3 and DU-145 prostate cancer cells. BMC Pharmacol. Toxicol. 2014, 15, 59. [Google Scholar] [CrossRef]
- Chien, S.Y.; Hsieh, M.J.; Chen, C.J.; Yang, S.F.; Chen, M.K. A citrus polymethoxyflavonoid, nobiletin, is a novel MEK inhibitor that exhibits antitumor metastasis in human fibrosarcoma HT-1080 cells. Biochem. Biophys. Res. Commun. 2008, 366, 168–173. [Google Scholar]
- Miyata, Y.; Sato, T.; Yano, M.; Ito, A. Activation of protein kinase C βII/ε-c-Jun NH2-terminal kinase pathway and inhibition of mitogen-activated protein/extracellular signal-regulated kinase 1/2 phosphorylation in antitumor invasive activity induced by the polymethoxy flavonoid, nobiletin. Mol. Cancer Ther. 2004, 3, 839–847. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).