New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders
Abstract
1. Introduction
2. Atopic Dermatitis
2.1. St. John’s Wort (Hypericum perforatum (L.))
2.2. Licorice (Glycyrrhiza glabra (L.))
2.3. Tormentil (Potentilla erecta (L.))
2.4. Bitter Substances
2.5. Evening Primrose (Oenothera biennis (L.))
3. Psoriasis Vulgaris
3.1. Araroba Tree (Vataireopsis araroba (Aguiar) Ducke)
3.2. Lace Flower (Ammi majus(L.) and Ammi visnaga (L.))
3.3. Barberry Bark (Mahonia aquifolium (Pursh) Nutt.)
3.4. Indigo (Baphicacanthus cusia, Brem.)
3.5. Turmeric (Curcuma longa (L.))
3.6. Olibanum (Boswellia Serrata, Triana & Planch.)
3.7. St. John’s Wort (Hypericum perforatum (L.))
4. Herpes Simplex
4.1. Lemon Balm (Melissa officinalis (L.))
4.2. Sage (Salvia officinalis (L.)) and rhubarb (Rheum palmatum (L.))
5. Actinic Keratosis
5.1. Birch Bark (Betula spp.)
5.2. Petty Spurge (Euphorbia peplus (L.))
6. Photoprotection and Esthetic Dermatology
6.1. Green Tea (Non-Fermented Camellia sinensis (L.))
6.2. Dyer’s Weed (Reseda luteola (L.))
6.3. Cocoa Tree (Theobroma cacao (L.))
6.4. Carotinoids
6.5. Citrus Fruits (Citrus spp.)
6.6. Coffee Plants (Coffea spec.)
6.7. Licorice (Glycyrrhiza glabra (L.))
6.8. Pine Bark (Pinus pinaster, Ailton)
6.9. Gotu Kola (Centella asiatica (L.) Urban)
7. Wound Healing
7.1. Birch Bark (Betula spp.)
7.2. Onion (Allium cepa (L.))
8. Rosacea
8.1. Green Tea (Non-Fermented Camellia sinensis (L.))
8.2. Licorice Root (Glycyrrhiza inflata, Batalin)
8.3. Bitter Wood (Simarouba amara Aubl.)
8.4. Tormentil (Potentilla erecta (L.))
9. Acne Vulgaris
9.1. Tea Tree (Melaleuca alternifolia (Maiden & Betche) Cheel)
9.2. Green Tea (non-fermented Camellia sinensis (L.))
9.3. Hop (Humulus lupulus (L.))
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reuter, J.; Wölfle, U.; Weckesser, S.; Schempp, C. Which plant for which skin disease? Part 1: Atopic dermatitis, psoriasis, acne, condyloma and herpes simplex. J. Dtsch. Dermatol. Ges. 2010, 8, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Reuter, J.; Wölfle, U.; Korting, H.C.; Schempp, C. Which plant for which skin disease? Part 2: Dermatophytes, chronic venous insufficiency, photoprotection, actinic keratoses, vitiligo, hair loss, cosmetic indications. J. Dtsch. Dermatol. Ges. 2010, 8, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Oxford Centre for Evidence-Based Medicine. Levels of Evidence and Grades of Recommendation, 2009. Available online: http://www.cebm.net/index.aspx?o=1025#levels (accessed on 26 March 2010).
- Vieira, B.L.; Lim, N.R.; Lohman, M.E.; Lio, P.A. Complementary and Alternative Medicine for Atopic Dermatitis: An Evidence-Based Review. Am. J. Clin. Dermatol. 2016, 17, 557–581. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Seelinger, G.; Schempp, C.M. Topical application of St. John’s wort (Hypericum perforatum). Planta Med. 2014, 80, 109–120. [Google Scholar]
- Schempp, C.M.; Windeck, T.; Hezel, S.; Simon, J.C. Topical treatment of atopic dermatitis with St. John’s wort cream--a randomized, placebo controlled, double blind half-side comparison. Phytomedicine Int. J. Phytother. Phytopharm. 2003, 10 (Suppl. S4), 31–37. [Google Scholar] [CrossRef]
- Yang, R.; Wang, L.; Yuan, B.; Liu, Y. The Pharmacological Activities of Licorice. Planta Med. 2015, 81, 1654–1669. [Google Scholar] [CrossRef]
- Lee, Y.M.; Hirota, S.; Jippo-Kanemoto, T.; Kim, H.R.; Shin, T.Y.; Yeom, Y.; Lee, K.K.; Kitamura, Y.; Nomura, S.; Kim, H.M. Inhibition of histamine synthesis by glycyrrhetinic acid in mast cells cocultured with Swiss 3T3 fibroblasts. Int. Arch. Allergy Immunol. 1996, 110, 272–277. [Google Scholar] [CrossRef]
- Afnan, Q.; Adil, M.D.; Nissar-Ul, A.; Rafiq, A.R.; Amir, H.F.; Kaiser, P.; Gupta, V.K.; Vishwakarma, R.; Tasduq, S.A. Glycyrrhizic acid (GA), a triterpenoid saponin glycoside alleviates ultraviolet-B irradiation-induced photoaging in human dermal fibroblasts. Phytomedicine Int. J. Phytother. Phytopharm. 2012, 19, 658–664. [Google Scholar] [CrossRef]
- Farrukh, M.R.; Nissar, U.-A.; Kaiser, P.J.; Afnan, Q.; Sharma, P.R.; Bhushan, S.; Tasduq, S.A. Glycyrrhizic acid (GA) inhibits reactive oxygen Species mediated photodamage by blocking ER stress and MAPK pathway in UV-B irradiated human skin fibroblasts. J. Photochem. Photobiol. B 2015, 148, 351–357. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.; Li, Y.; Li, M.; Chen, G. Effect of isoliquiritigenin for the treatment of atopic dermatitis-like skin lesions in mice. Arch. Dermatol. Res. 2017, 309, 805–813. [Google Scholar] [CrossRef]
- Kühnl, J.; Roggenkamp, D.; Gehrke, S.A.; Stäb, F.; Wenck, H.; Kolbe, L.; Neufang, G. Licochalcone A activates Nrf2 in vitro and contributes to licorice extract-induced lowered cutaneous oxidative stress in vivo. Exp. Dermatol. 2015, 24, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Song, N.R.; Kim, J.-E.; Park, J.S.; Kim, J.R.; Kang, H.; Lee, E.; Kang, Y.-G.; Son, J.E.; Seo, S.G.; Heo, Y.S.; et al. Licochalcone A, a polyphenol present in licorice, suppresses UV-induced COX-2 expression by targeting PI3K, MEK1, and B-Raf. Int. J. Mol. Sci. 2015, 16, 4453–4470. [Google Scholar] [CrossRef] [PubMed]
- Sulzberger, M.; Worthmann, A.-C.; Holtzmann, U.; Buck, B.; Jung, K.A.; Schoelermann, A.M.; Rippke, F.; Stäb, F.; Wenck, H.; Neufang, G.; et al. Effective treatment for sensitive skin: 4-t-butylcyclohexanol and licochalcone A. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. S1), 9–17. [Google Scholar] [CrossRef] [PubMed]
- Abramovits, W.; Boguniewicz, M.; Adult Atopiclair Study Group. A multicenter, randomized, vehicle-controlled clinical study to examine the efficacy and safety of MAS063DP (Atopiclair) in the management of mild to moderate atopic dermatitis in adults. J. Drugs Dermatol. 2006, 5, 236–244. [Google Scholar] [PubMed]
- Angelova-Fischer, I.; Rippke, F.; Richter, D.; Filbry, A.; Arrowitz, C.; Weber, T.; Fischer, T.W.; Zillikens, D. Stand-alone Emollient Treatment Reduces Flares After Discontinuation of Topical Steroid Treatment in Atopic Dermatitis: A Double-blind, Randomized, Vehicle-controlled, Left-right Comparison Study. Acta Derm. Venereol. 2018, 98, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, J.; Tasiopoulou, G.; Hoffmann, J.; Wölfle, U.; Schwabe, K.; Quirin, K.-W.; Schempp, C.M. Anti-Inflammatory Effect of a Novel Topical Herbal Composition (VEL-091604) Consisting of Gentian Root, Licorice Root and Willow Bark Extract. Planta Med. 2019, 85, 608–614. [Google Scholar] [CrossRef]
- Wölfle, U.; Hoffmann, J.; Haarhaus, B.; Rao Mittapalli, V.; Schempp, C.M. Anti-inflammatory and vasoconstrictive properties of Potentilla erecta—A traditional medicinal plant from the northern hemisphere. J. Ethnopharmacol. 2017, 204, 86–94. [Google Scholar] [CrossRef]
- Hoffmann, J.; Wölfle, U.; Schempp, C.M.; Casetti, F. Tannins from Potentilla officinalis display antiinflammatory effects in the UV erythema test and on atopic skin. J. Dtsch. Dermatol. Ges. 2016, 14, 917–922. [Google Scholar]
- Wölfle, U.; Elsholz, F.A.; Kersten, A.; Haarhaus, B.; Müller, W.E.; Schempp, C.M. Expression and functional activity of the bitter taste receptors TAS2R1 and TAS2R38 in human keratinocytes. Skin Pharmacol. Physiol. 2015, 28, 137–146. [Google Scholar] [CrossRef]
- Wölfle, U.; Haarhaus, B.; Seiwerth, J.; Cawelius, A.; Schwabe, K.; Quirin, K.-W.; Schempp, C.M. The Herbal Bitter Drug Gentiana lutea Modulates Lipid Synthesis in Human Keratinocytes In Vitro and In Vivo. Int. J. Mol. Sci. 2017, 18, 1814. [Google Scholar] [CrossRef]
- Morse, N.L.; Clough, P.M. A meta-analysis of randomized, placebo-controlled clinical trials of Efamol evening primrose oil in atopic eczema. Where do we go from here in light of more recent discoveries? Curr. Pharm. Biotechnol. 2006, 7, 503–524. [Google Scholar] [CrossRef] [PubMed]
- Farahnik, B.; Sharma, D.; Alban, J.; Sivamani, R.K. Topical Botanical Agents for the Treatment of Psoriasis: A Systematic Review. Am. J. Clin. Dermatol. 2017, 18, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Gamret, A.C.; Price, A.; Fertig, R.M.; Lev-Tov, H.; Nichols, A.J. Complementary and Alternative Medicine Therapies for Psoriasis: A Systematic Review. JAMA Dermatol. 2018, 154, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Topically Used Herbal Products for the Treatment of Psoriasis—Mechanism of Action, Drug Delivery, Clinical Studies. Planta Med. 2016, 82, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Van de Kerkhof, P.C.M.; van der Valk, P.G.M.; Swinkels, O.Q.J.; Kucharekova, M.; de Rie, M.A.; de Vries, H.J.C.; Damstra, R.; Oranje, A.P.; de Waard-van der Spek, F.B.; van Neer, P.; et al. A comparison of twice-daily calcipotriol ointment with once-daily short-contact dithranol cream therapy: A randomized controlled trial of supervised treatment of psoriasis vulgaris in a day-care setting. Br. J. Dermatol. 2006, 155, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Markham, T.; Rogers, S.; Collins, P. Narrowband UV-B (TL-01) phototherapy vs oral 8-methoxypsoralen psoralen-UV-A for the treatment of chronic plaque psoriasis. Arch. Dermatol. 2003, 139, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Vongthongsri, R.; Konschitzky, R.; Seeber, A.; Treitl, C.; Hönigsmann, H.; Tanew, A. Randomized, double-blind comparison of 1 mg/L versus 5 mg/L methoxsalen bath-PUVA therapy for chronic plaque-type psoriasis. J. Am. Acad. Dermatol. 2006, 55, 627–631. [Google Scholar] [CrossRef]
- Amornpinyokeit, N.; Asawanonda, P. 8-Methoxypsoralen cream plus targeted narrowband ultraviolet B for psoriasis. Photodermatol. Photoimmunol. Photomed. 2006, 22, 285–289. [Google Scholar] [CrossRef]
- Bernstein, S.; Donsky, H.; Gulliver, W.; Hamilton, D.; Nobel, S.; Norman, R. Treatment of mild to moderate psoriasis with Reliéva, a Mahonia aquifolium extract--a double-blind, placebo-controlled study. Am. J. Ther. 2006, 13, 121–126. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Chang, C.-J.; Chang, Y.-C.; Wong, W.-R.; Chang, S.-C.; Pang, J.-H.S. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch. Dermatol. 2008, 144, 1457–1464. [Google Scholar] [CrossRef]
- Lin, Y.-K.; See, L.-C.; Huang, Y.-H.; Chang, Y.-C.; Tsou, T.-C.; Lin, T.-Y.; Lin, N.-L. Efficacy and safety of Indigo naturalis extract in oil (Lindioil) in treating nail psoriasis: A randomized, observer-blind, vehicle-controlled trial. Phytomedicine Int. J. Phytother. Phytopharm. 2014, 21, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-K.; See, L.-C.; Huang, Y.-H.; Chi, C.-C.; Hui, R.C.-Y. Comparison of indirubin concentrations in indigo naturalis ointment for psoriasis treatment: A randomized, double-blind, dosage-controlled trial. Br. J. Dermatol. 2018, 178, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Antiga, E.; Bonciolini, V.; Volpi, W.; Del Bianco, E.; Caproni, M. Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Heng, M.C.; Song, M.K.; Harker, J.; Heng, M.K. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br. J. Dermatol. 2000, 143, 937–949. [Google Scholar] [CrossRef]
- Reddy, S.; Aggarwal, B.B. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Lett. 1994, 341, 19–22. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Mishra, A.; Prabhu, S.; Rafiq, M.; Rangesh, P. Imiquimod-induced psoriasis-like inflammation in differentiated Human keratinocytes: Its evaluation using curcumin. Eur. J. Pharmacol. 2017, 813, 33–41. [Google Scholar] [CrossRef]
- Nardo, V.D.; Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Barygina, V.; Lotti, J.; Daaboul, F.; Lotti, T. Use of Curcumin in Psoriasis. Open Access Maced. J. Med. Sci. 2018, 6, 218–220. [Google Scholar] [CrossRef]
- Muhammed, M.; Nagabhushanam, K.; Sankarab, N.; Sood, R.; Kumar Karri, S. Clinical evaluation of AKBBA in the management of psoriasis. Clin. Dermatol. 2014, 2, 17–24. [Google Scholar] [CrossRef]
- Leuner, K.; Kraus, M.; Woelfle, U.; Beschmann, H.; Harteneck, C.; Boehncke, W.-H.; Schempp, C.M.; Müller, W.E. Reduced TRPC channel expression in psoriatic keratinocytes is associated with impaired differentiation and enhanced proliferation. PLoS ONE 2011, 6, e14716. [Google Scholar] [CrossRef]
- Najafizadeh, P.; Hashemian, F.; Mansouri, P.; Farshi, S.; Surmaghi, M.S.; Chalangari, R. The evaluation of the clinical effect of topical St Johns wort (Hypericum perforatum L.) in plaque type psoriasis vulgaris: A pilot study. Australas. J. Dermatol. 2012, 53, 131–135. [Google Scholar] [CrossRef]
- Mansouri, P.; Mirafzal, S.; Najafizadeh, P.; Safaei-Naraghi, Z.; Salehi-Surmaghi, M.H.; Hashemian, F. The impact of topical Saint John’s Wort (Hypericum perforatum) treatment on tissue tumor necrosis factor-alpha levels in plaque-type psoriasis: A pilot study. J. Postgrad. Med. 2017, 63, 215–220. [Google Scholar] [PubMed]
- Hassan, S.T.S.; Masarčíková, R.; Berchová, K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015, 67, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, K.; Okudaira, N.; Adachi, K.; Odai-Ide, R.; Watanabe, S.; Ohno, H.; Yamamoto, M.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; et al. Antiviral and Antitumor Activity of Licorice Root Extracts. Vivo Athens Greece 2016, 30, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Goswami, D.; Mahapatra, A.D.; Banerjee, S.; Kar, A.; Ojha, D.; Mukherjee, P.K.; Chattopadhyay, D. Boswellia serrata oleo-gum-resin and β-boswellic acid inhibits HSV-1 infection in vitro through modulation of NF-κB and p38 MAP kinase signaling. Phytomedicine Int. J. Phytother. Phytopharm. 2018, 51, 94–103. [Google Scholar] [CrossRef]
- Koytchev, R.; Alken, R.G.; Dundarov, S. Balm mint extract (Lo-701) for topical treatment of recurring herpes labialis. Phytomedicine Int. J. Phytother. Phytopharm. 1999, 6, 225–230. [Google Scholar] [CrossRef]
- Saller, R.; Büechi, S.; Meyrat, R.; Schmidhauser, C. Combined herbal preparation for topical treatment of Herpes labialis. Res. Complement. Nat. Class Med. 2001, 8, 373–382. [Google Scholar]
- Kacerovská, D.; Pizinger, K.; Majer, F.; Smíd, F. Photodynamic therapy of nonmelanoma skin cancer with topical hypericum perforatum extract--a pilot study. Photochem. Photobiol. 2008, 84, 779–785. [Google Scholar] [CrossRef]
- Pflugfelder, A.; Andonov, E.; Weide, B.; Dirschka, T.; Schempp, C.; Stockfleth, E.; Stratigos, A.; Krüger-Krasagakis, S.; Bauer, J.; Garbe, C.; et al. Lack of activity of betulin-based Oleogel-S10 in the treatment of actinic keratoses: A randomized, multicentre, placebo-controlled double-blind phase II trial. Br. J. Dermatol. 2015, 172, 926–932. [Google Scholar] [CrossRef]
- Huyke, C.; Reuter, J.; Rodig, M.; Kersten, A.; Laszczyk, M.; Scheffler, A.; Nashan, D.; Schempp, C. Treatment of actinic keratoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J. Dtsch. Dermatol. Ges. 2009, 7, 128–133. [Google Scholar]
- Huyke, C.; Laszczyk, M.; Scheffler, A.; Ernst, R.; Schempp, C.M. Treatment of actinic keratoses with birch bark extract: A pilot study. J. Dtsch. Dermatol. Ges. 2006, 4, 132–136. [Google Scholar] [CrossRef]
- Woelfle, U.; Laszczyk, M.N.; Kraus, M.; Leuner, K.; Kersten, A.; Simon-Haarhaus, B.; Scheffler, A.; Martin, S.F.; Müller, W.E.; Nashan, D.; et al. Triterpenes promote keratinocyte differentiation in vitro, ex vivo and in vivo: A role for the transient receptor potential canonical (subtype) 6. J. Investig. Dermatol. 2010, 130, 113–123. [Google Scholar] [CrossRef]
- Anderson, L.; Schmieder, G.J.; Werschler, W.P.; Tschen, E.H.; Ling, M.R.; Stough, D.B.; Katsamas, J. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol mebutate gel 0.025% and 0.05% for actinic keratosis. J. Am. Acad. Dermatol. 2009, 60, 934–943. [Google Scholar] [CrossRef]
- Siller, G.; Gebauer, K.; Welburn, P.; Katsamas, J.; Ogbourne, S.M. PEP005 (ingenol mebutate) gel, a novel agent for the treatment of actinic keratosis: Results of a randomized, double-blind, vehicle-controlled, multicentre, phase IIa study. Australas. J. Dermatol. 2009, 50, 16–22. [Google Scholar] [CrossRef]
- Chen, A.C.; Damian, D.L.; Halliday, G.M. Oral and systemic photoprotection. Photodermatol. Photoimmunol. Photomed. 2014, 30, 102–111. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (-)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Ahmad, N.; Mukhtar, H. Green tea and skin. Arch. Dermatol. 2000, 136, 989–994. [Google Scholar] [CrossRef]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef]
- Wölfle, U.; Esser, P.R.; Simon-Haarhaus, B.; Martin, S.F.; Lademann, J.; Schempp, C.M. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic. Biol. Med. 2011, 50, 1081–1093. [Google Scholar] [CrossRef]
- Wölfle, U.; Heinemann, A.; Esser, P.R.; Haarhaus, B.; Martin, S.F.; Schempp, C.M. Luteolin prevents solar radiation-induced matrix metalloproteinase-1 activation in human fibroblasts: A role for p38 mitogen-activated protein kinase and interleukin-20 released from keratinocytes. Rejuvenation Res. 2012, 15, 466–475. [Google Scholar] [CrossRef]
- Casetti, F.; Jung, W.; Wölfle, U.; Reuter, J.; Neumann, K.; Gilb, B.; Wähling, A.; Wagner, S.; Merfort, I.; Schempp, C.M. Topical application of solubilized Reseda luteola extract reduces ultraviolet B-induced inflammation in vivo. J. Photochem. Photobiol. B 2009, 96, 260–265. [Google Scholar] [CrossRef]
- Martin, S.F.; Esser, P.R.; Weber, F.C.; Jakob, T.; Freudenberg, M.A.; Schmidt, M.; Goebeler, M. Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy 2011, 66, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Schempp, C.M.; Meinke, M.C.; Lademann, J.; Ferrari, Y.; Brecht, T.; Gehring, W. Topical antioxidants protect the skin from chemical-induced irritation in the repetitive washing test: A placebo-controlled, double-blind study. Contact Dermat. 2012, 67, 234–237. [Google Scholar] [CrossRef]
- Heinrich, U.; Neukam, K.; Tronnier, H.; Sies, H.; Stahl, W. Long-term ingestion of high flavanol cocoa provides photoprotection against UV-induced erythema and improves skin condition in women. J. Nutr. 2006, 136, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Gärtner, C.; Wiebusch, M.; Eichler, O.; Sies, H.; Tronnier, H.; Stahl, W. Supplementation with beta-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J. Nutr. 2003, 133, 98–101. [Google Scholar] [CrossRef]
- Aust, O.; Stahl, W.; Sies, H.; Tronnier, H.; Heinrich, U. Supplementation with tomato-based products increases lycopene, phytofluene, and phytoene levels in human serum and protects against UV-light-induced erythema. Int. J. Vitam. Nutr. Res. 2005, 75, 54–60. [Google Scholar] [CrossRef]
- Rizwan, M.; Rodriguez-Blanco, I.; Harbottle, A.; Birch-Machin, M.A.; Watson, R.E.B.; Rhodes, L.E. Tomato paste rich in lycopene protects against cutaneous photodamage in humans in vivo: A randomized controlled trial. Br. J. Dermatol. 2011, 164, 154–162. [Google Scholar] [CrossRef]
- Hakim, I.A.; Harris, R.B.; Ritenbaugh, C. Citrus peel use is associated with reduced risk of squamous cell carcinoma of the skin. Nutr. Cancer 2000, 37, 161–168. [Google Scholar] [CrossRef]
- Song, F.; Qureshi, A.A.; Han, J. Increased caffeine intake is associated with reduced risk of basal cell carcinoma of the skin. Cancer Res. 2012, 72, 3282–3289. [Google Scholar] [CrossRef]
- Corona, R.; Dogliotti, E.; D’Errico, M.; Sera, F.; Iavarone, I.; Baliva, G.; Chinni, L.; Gobello, T.; Mazzanti, C.; Puddu, P.; et al. Risk factors for basal cell carcinoma in a Mediterranean population. Arch. Dermatol. 2001, 137, 1162–1168. [Google Scholar] [CrossRef]
- Armanini, D.; Nacamulli, D.; Francini-Pesenti, F.; Battagin, G.; Ragazzi, E.; Fiore, C. Glycyrrhetinic acid, the active principle of licorice, can reduce the thickness of subcutaneous thigh fat through topical application. Steroids 2005, 70, 538–542. [Google Scholar] [CrossRef]
- Yokota, T.; Nishio, H.; Kubota, Y.; Mizoguchi, M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment. Cell Res. 1998, 11, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. French Maritime Pine Bark Extract (Pycnogenol®) Effects on Human Skin: Clinical and Molecular Evidence. Skin Pharmacol. Physiol. 2016, 29, 13–17. [Google Scholar] [CrossRef]
- Ni, Z.; Mu, Y.; Gulati, O. Treatment of melasma with Pycnogenol. Phytother. Res. PTR 2002, 16, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Mallol, J.; Belda, M.A.; Costa, D.; Noval, A.; Sola, M. Prophylaxis of Striae gravidarum with a topical formulation. A double blind trial. Int. J. Cosmet. Sci. 1991, 13, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Lee, H.; Seo, Y.; Koh, J.; Park, D. Evaluation of the effects of a preparation containing asiaticoside on periocular wrinkles of human volunteers. Int. J. Cosmet. Sci. 2008, 30, 167–173. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Rafiee, E.; Mehrabian, A.; Feily, A. Skin wound healing and phytomedicine: A review. Skin Pharmacol. Physiol. 2014, 27, 303–310. [Google Scholar] [CrossRef]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, chemical and pharmacological characterization of a new oleogel-forming triterpene extract from the outer bark of birch (betulae cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef]
- Ebeling, S.; Naumann, K.; Pollok, S.; Wardecki, T.; Vidal-Y-Sy, S.; Nascimento, J.M.; Boerries, M.; Schmidt, G.; Brandner, J.M.; Merfort, I. From a traditional medicinal plant to a rational drug: Understanding the clinically proven wound healing efficacy of birch bark extract. PLoS ONE 2014, 9, e86147. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Brandner, J.M.; Schumann, H.; Bross, F.; Fimmers, R.; Böttger, K.; Scheffler, A.; Podmelle, F. Accelerated reepithelialization by triterpenes: Proof of concept in the healing of surgical skin lesions. Skin Pharmacol. Physiol. 2015, 28, 1–11. [Google Scholar] [CrossRef]
- Barret, J.P.; Podmelle, F.; Lipový, B.; Rennekampff, H.-O.; Schumann, H.; Schwieger-Briel, A.; Zahn, T.R.; Metelmann, H.-R.; BSH-12 and BSG-12 Study Groups. Accelerated re-epithelialization of partial-thickness skin wounds by a topical betulin gel: Results of a randomized phase III clinical trials program. Burns 2017, 43, 1284–1294. [Google Scholar] [CrossRef]
- Frew, Q.; Rennekampff, H.-O.; Dziewulski, P.; Moiemen, N.; BBW-11 Study Group; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, A. The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside. Planta Med. 2019, 85, 524–527. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Assessment Report of the Committee for Medicinal Products for Human Use (CHMP) on Episalvan, International Non-Proprietary Name: Birch Bark Extract. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/episalvan (accessed on 20 November 2019).
- Fang, Q.-Q.; Chen, C.-Y.; Zhang, M.-X.; Huang, C.-L.; Wang, X.-W.; Xu, J.-H.; Wu, L.-H.; Zhang, L.-Y.; Tan, W.-Q. The Effectiveness of Topical Anti-scarring Agents and a Novel Combined Process on Cutaneous Scar Management. Curr. Pharm. Des. 2017, 23, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. The ability of onion extract gel to improve the cosmetic appearance of postsurgical scars. J. Cosmet. Dermatol. 2008, 7, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Fisk, W.A.; Lev-Tov, H.A.; Clark, A.K.; Sivamani, R.K. Phytochemical and Botanical Therapies for Rosacea: A Systematic Review. Phytother. Res. PTR 2015, 29, 1439–1451. [Google Scholar] [CrossRef]
- Domingo, D.S.; Camouse, M.M.; Hsia, A.H.; Matsui, M.; Maes, D.; Ward, N.L.; Cooper, K.D.; Baron, E.D. Anti-angiogenic effects of epigallocatechin-3-gallate in human skin. Int. J. Clin. Exp. Pathol. 2010, 3, 705–709. [Google Scholar]
- Weber, T.M.; Ceilley, R.I.; Buerger, A.; Kolbe, L.; Trookman, N.S.; Rizer, R.L.; Schoelermann, A. Skin tolerance, efficacy, and quality of life of patients with red facial skin using a skin care regimen containing Licochalcone A. J. Cosmet. Dermatol. 2006, 5, 227–232. [Google Scholar] [CrossRef]
- Jovanovic, Z.; Angabini, N.; Ehlen, S.; Mokos, Z.B.; Subotic, M.; Neufang, G. Efficacy and Tolerability of a Cosmetic Skin Care Product with Trans-4-t-butylcyclohexanol and Licochalcone A in Subjects with Sensitive Skin Prone to Redness and Rosacea. J. Drugs Dermatol. 2017, 16, 605–610. [Google Scholar]
- Ferrari, A.; Diehl, C. Evaluation of the efficacy and tolerance of a topical gel with 4% quassia extract in the treatment of rosacea. J. Clin. Pharmacol. 2012, 52, 84–88. [Google Scholar] [CrossRef]
- Sudhapriyadhars, G. Topical glucocorticoid—A review. J. Pharm. Sci. Res. 2014, 6, 244–246. [Google Scholar]
- Sauermann, G.; Jaspers, S.; Hoppe, U.; Salzer, B.; Steinkraus, V. Influence of NO-synthase antagonists in rosacea patients. J. Investig. Dermatol. 1997, 4, 657. [Google Scholar]
- Fisk, W.A.; Lev-Tov, H.A.; Sivamani, R.K. Botanical and phytochemical therapy of acne: A systematic review. Phytother. Res. PTR 2014, 28, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Bassett, I.B.; Pannowitz, D.L.; Barnetson, R.S. A comparative study of tea-tree oil versus benzoylperoxide in the treatment of acne. Med. J. Aust. 1990, 153, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Enshaieh, S.; Jooya, A.; Siadat, A.H.; Iraji, F. The efficacy of 5% topical tea tree oil gel in mild to moderate acne vulgaris: A randomized, double-blind placebo-controlled study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 22–25. [Google Scholar] [PubMed]
- Yoon, J.Y.; Kwon, H.H.; Min, S.U.; Thiboutot, D.M.; Suh, D.H. Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P. acnes. J. Investig. Dermatol. 2013, 133, 429–440. [Google Scholar] [CrossRef]
- Elsaie, M.L.; Abdelhamid, M.F.; Elsaaiee, L.T.; Emam, H.M. The efficacy of topical 2% green tea lotion in mild-to-moderate acne vulgaris. J. Drugs Dermatol. 2009, 8, 358–364. [Google Scholar]
- Weber, N.; Biehler, K.; Schwabe, K.; Haarhaus, B.; Quirin, K.-W.; Frank, U.; Schempp, C.M.; Wölfle, U. Hop Extract Acts as an Antioxidant with Antimicrobial Effects against PropionibacteriumAcnes and Staphylococcus Aureus. Molecules 2019, 24, 223. [Google Scholar] [CrossRef]
| Disease | Plant | Compound | Treatment | L O E | Ref |
|---|---|---|---|---|---|
| Atopic dermatitis | St. John’s wort (Hypericum perforatum (L.)) | Hyperforin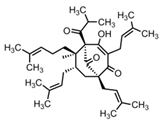 | - Ointment with 1.5% hyperforin twice daily for 4 weeks | A | [6] |
| Licorice (Glycyrrhiza glabra (L.)) | Glycyrrhetinic acid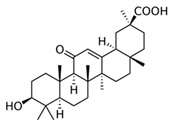 | - Hydrophilic ointment with 2% glycyrrhetinic acid three times daily for 5 weeks | A | [15] | |
| - Cream with 0.6% glycyrrhizinic acid and licorice extract (0.1% Glycyrrhiza uralensis root extract) twice daily for 2 weeks | B | [17] | |||
Licochalcone A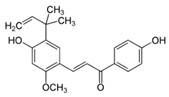 | - Cream with < 1% licochalcone A | A | [16] | ||
| Tormentil (Potentilla erecta (L.)) | Tannins | - Cream with 2% tannins from the rhizome of tormentil for 48 h in an UV-erythema test | B | [18] | |
| - Cream with 2% tannins from the rhizome of tormentil twice daily for 2 weeks | B | [19] | |||
| Bitter substances | Gentian extract | - Cream with 5% gentain extract twice daily for 4 weeks increases in volunteers epidermal lipids that are reduced in atopic dermatitis | B | [21] | |
| Atopic dermatitis | Evening primrose (Oenothera biennis (L.)) | Primrose oil | - Meta-analysis for the use of primrose oil | A | [22] |
| Psoriasis vulgaris | Araroba tree (Vataireopsis araroba (Aguiar) Ducke) | Dithranol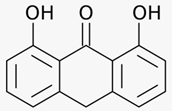 | - Daily short treatments (15 - 45 min) with dithranol cream of stepwise rising concentrations up to 5% for 12 weeks | A | [26] |
| Lace flower (Ammi majus (L.) and Ammi visnaga (L.)) | 8-methoxypsoralen (8-MOP)  | - Oral 8-MOP PUVA (0.6 mg/kg) twice per week | A | [27] | |
| - Bath PUVA with 5 mg/l 8-MOP 4 times per week | A | [28] | |||
| - Cream PUVA with 0.1% 8-MOP 12 treatments | A | [29] | |||
| 5-methoxypsoralen (5-MOP) 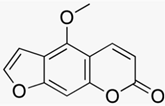 | - Oral 5-MOP PUVA (1.2 mg/kg) twice per week | A | [27] | ||
| Barberry bark (Mahonia aquifolium (Pursh) Nutt.) | Mahonia ointment | - 10% Mahonia ointment twice daily for 12 weeks | A | [30] | |
| Indigo (Baphicacanthus cusia Brem.) | Indigo naturalis containing indigo and indirubin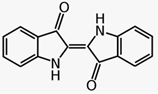 | - 10% indigo ointment (1.4% indigo and 0.16% indirubin) daily for 12 weeks | A | [31] | |
| Psoriasis vulgaris | Indigo extract | - Indigo ointment (50 μg/g and 200 μg/g indigo extract) applied twice daily over 8 weeks | A | [33] | |
| Turmeric (Curcuma longa (L.)) | Curcumin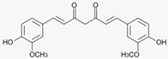 | - No randomized placebo-controlled studies available, only in vitro studies or in vivo studies using mice | |||
| Olibanum (Boswellia serrata, Triana & Planch) | 3-O-Acetyl-11-keto- β-boswellic acid 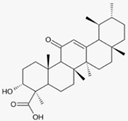 | - Olibanum ointment containing 5% 3-O-Acetyl-11-keto-β-boswellic acid three times daily for 12 weeks | B | [39] | |
| St. John’s wort (Hypericum perforatum (L.)) | St. John’s wort extract | - 5% St. John’s wort extract twice daily for 4 weeks | B | [41] | |
| - 5% St. John’s wort extract once daily for 4 weeks | B | [42] | |||
| Herpes simplex | Lemon balm (Melissa officinalis (L.)) | Lemon balm extract | - Cream containing 1% dried Melissa officialis extract applied 4 times daily for 5 days | A | [46] |
| Sage (Salvia officinalis (L.)) and Rhubarb (Rheum palmatum (L.)) | Sage and rhubarb extract | - Treatment with cream containing sage extract (23 mg/g) or combination of sage and rhubarb extract (23 mg/g each) until healing (7.6 days with sage and 6.7 with combination) | A | [47] | |
| Actinic keratoses | Birch bark (Betula spp.) | Betulin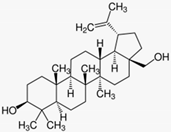 | - Treatment with betulin oleogel for 3 months without effect in high grade actinic keratoses | A | [49] |
| - Birch bark ointment (betulin oleogel) for 2 months | B | [50] | |||
| - Betulin oleogel for 3 months | B | [51] | |||
| Petty spurge (Euphorbia peplus (L.)) | Ingenol mebutate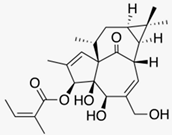 | - Gel with 0.025% or 0.05% ingenol mebutate daily for 3 days | A | [53] | |
| - Gel with 0.0025%, 0.01% or 0.05% ingenol mebutate on two consecutive days or on day 1 and day 8 | A | [54] | |||
| Photo-protection and esthetic dermatology | Green tea (Camellia sinensis (L.)) | Epigallocatechin-3-gallate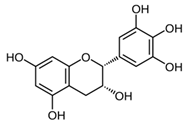 | - 1 mg/cm2 skin of epigallocatechin-3-gallate before UV exposure | D | [56] |
| Dyer’s weed (Reseda luteola (L.)) | Luteolin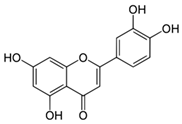 | - Dyer’s weed extract containing 2.5% luteolin | A | [61] | |
| Dyer’s weed extract | - Cream with 0.1% Dyer’s weed extract, 0.1% tocopherol and 0.05% ubiquinone three times daily for 7 days | A | [63] | ||
| Cocoa tree (Theobroma cacao (L.)) | Flavanol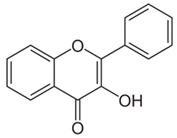 | - Ingestion of cocoa with high (326 mg/d) or low (27 mg/d) flavanol content in water over 12 weeks | A | [64] | |
| Photo-protection and esthetic dermatology | Carotinoids | β-carotene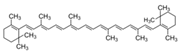 | - 24 mg β-carotene orally daily for up to 8 weeks | A | [65] |
Lycopene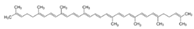 | - 10 mg lycopene daily for 12 weeks | B | [66] | ||
| - Tomato paste containing 16 mg lycopene daily for 12 weeks | B | [67] | |||
| Citrus fruits (Citrus spp.) | Limonene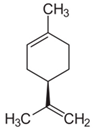 | - Regular citrus fruit consumption with or without peel | C | [68] | |
| Coffee plants (Coffea spec.) | Caffeine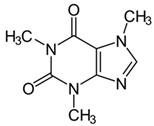 | - Observatory studies on caffeine intake | B B | [69] [70] | |
| Licorice (Glycyrrhiza glabra (L.)) | β-glycyrrhetinic acid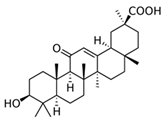 | - Ointment with 2.5% β-glycyrrhetinic acid twice daily over 4 weeks | A | [71] | |
Glabridin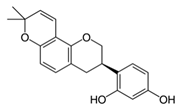 | - Ointment with 0.5% glabridin on guinea pig skin | D | [72] | ||
| Photo-protection and esthetic dermatology | Licochalcone A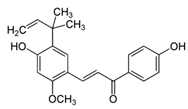 | - 9 μM Licochalcone A in in vitro experiments | D | [12] | |
| - Ointment containing licochalcone A twice daily for 2 weeks | A | [12] | |||
| Pine bark (Pinus pinaster Ailton) | Pine bark extract | - Oral ingestion of 25 mg pine bark extract three times daily for 30 days | B | [74] | |
| Gotu kola (Centella asiatica (L.) Urban) | Gotu kola extract | - Cream with Centella asiatica extract applied daily from the end of the 12th week of pregnancy until delivery | A | [75] | |
Asiaticoside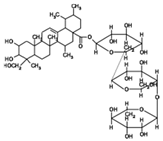 | - Cream containing 0.1% asiaticoside twice daily for 12 weeks | B | [76] | ||
| Wound healing | Birch bark (Betula spp.) | Betulin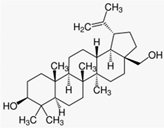 | - Wounds including second degree burns and split-thickness skin graft wounds were treated with betulin oleogel (containing 10% betulin) till wound closure or up to 28 days | A | [81] |
| Onion (Allium cepa (L.)) | Onion extract | - Onion extract twice daily for 10 weeks after initial primary wound healing | A | [86] | |
| Rosacea | Green tea(Camellia sinensis (L.)) | Epigallocatechin-3-gallate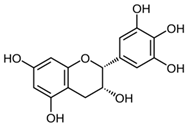 | - Cream containing 2.5% epigallocatechin gallate twice daily for 6 weeks | D | [88] |
| Licorice root (Glycyrrhiza inflata Batalin) | Licochalcone A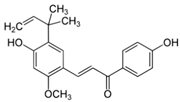 | - Licochalcone A in different vehicles applied daily for 8 weeks | B | [89] | |
| - Treatment with licochalcone A and trans-4-t-butylcyclohexanol (TRPV1 inhibitor) twice a day for 4 weeks | B | [90] | |||
| Bitter-wood (Simarouba amara Aubl.) | Bitter-wood extract | - Gel containing 4% Simarouba amara extract twice daily for 6 weeks | B | [91] | |
| Tormentil (Potentilla erecta (L.)) | Tormentil extract | - Tormentil extract (5%) applied topically once for 48 h to volunteers in a patch test | C | [18] | |
| Acne vulgaris | Tea tree (Melaleuca alternifolia (Maiden & Betche) Cheel) | Tea tree oil | - Gel containing 5% Melaleuca alternifolia oil applied topically for 3 months | B | [95] |
| - Gel containing 5% Melaleuca alternifolia oil applied twice daily for 45 days | A | [96] | |||
| Green tea (Camellia sinensis (L.)) | Epigallocatechin-3-gallate | - Treatment with 1% or 5% epigallocatechin-3-gallate solution twice daily for 8 weeks | A | [97] | |
| Acne vulgaris | Green tea extract | - Lotion with 2% Camellia sinensis extract twice daily for 6 weeks | B | [98] | |
| Hop (Humulus lupulus (L.)) | Hop extract | - 3.1 μg/mL and 9.4 μg/mL of Humulus lupulus extract in vitro | C | [99] | |
| - Gel containing 0.3% Humulus lupulus extract in vitro |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, J.; Gendrisch, F.; Schempp, C.M.; Wölfle, U. New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines 2020, 8, 27. https://doi.org/10.3390/biomedicines8020027
Hoffmann J, Gendrisch F, Schempp CM, Wölfle U. New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines. 2020; 8(2):27. https://doi.org/10.3390/biomedicines8020027
Chicago/Turabian StyleHoffmann, Julia, Fabian Gendrisch, Christoph Mathis Schempp, and Ute Wölfle. 2020. "New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders" Biomedicines 8, no. 2: 27. https://doi.org/10.3390/biomedicines8020027
APA StyleHoffmann, J., Gendrisch, F., Schempp, C. M., & Wölfle, U. (2020). New Herbal Biomedicines for the Topical Treatment of Dermatological Disorders. Biomedicines, 8(2), 27. https://doi.org/10.3390/biomedicines8020027





