Luteolin Induces Selective Cell Death of Human Pluripotent Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Cardiomyocyte Differentiation
2.3. Cell Death Analysis
2.4. Fluorescence-Based Competition Assay
2.5. Immunofluorescence and Immunoblotting
2.6. RNA Extraction and Quantitative Real-Time PCR Assay
2.7. Flow-Cytometry
2.8. Measurement of Intracellular Ca2+ Influx
2.9. Statistical Analysis
3. Results
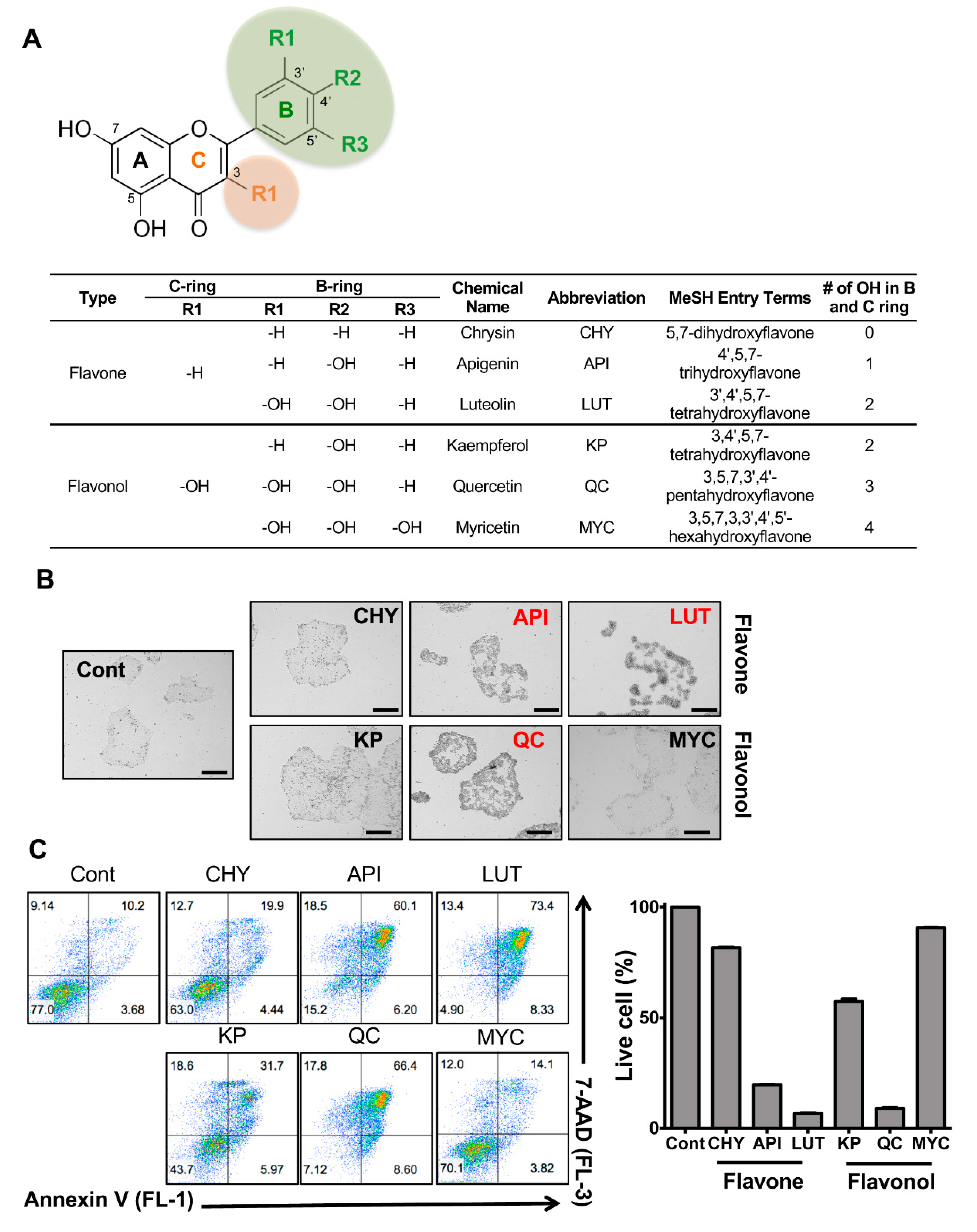
3.1. Stemotoxic Screening of Flavonoids
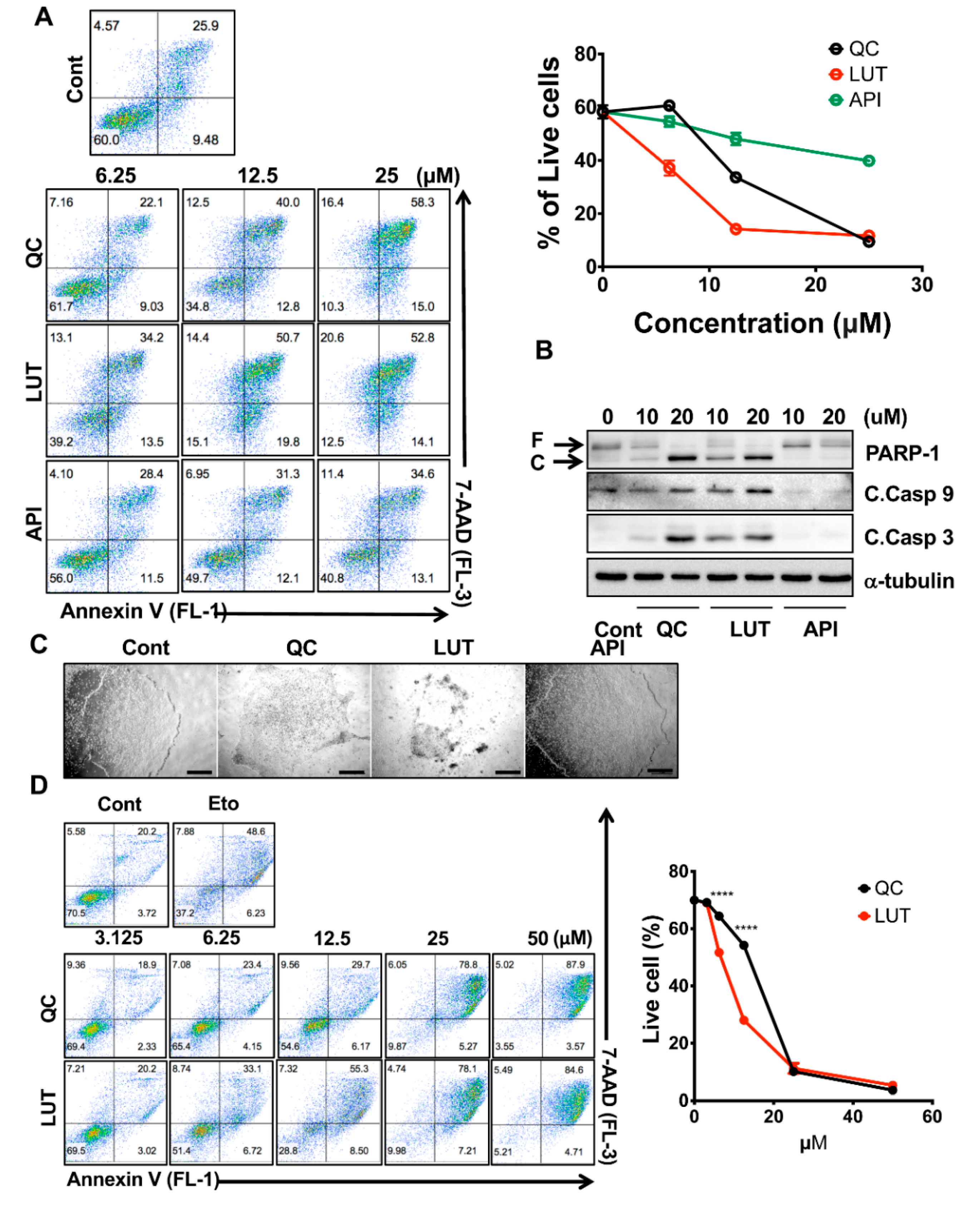
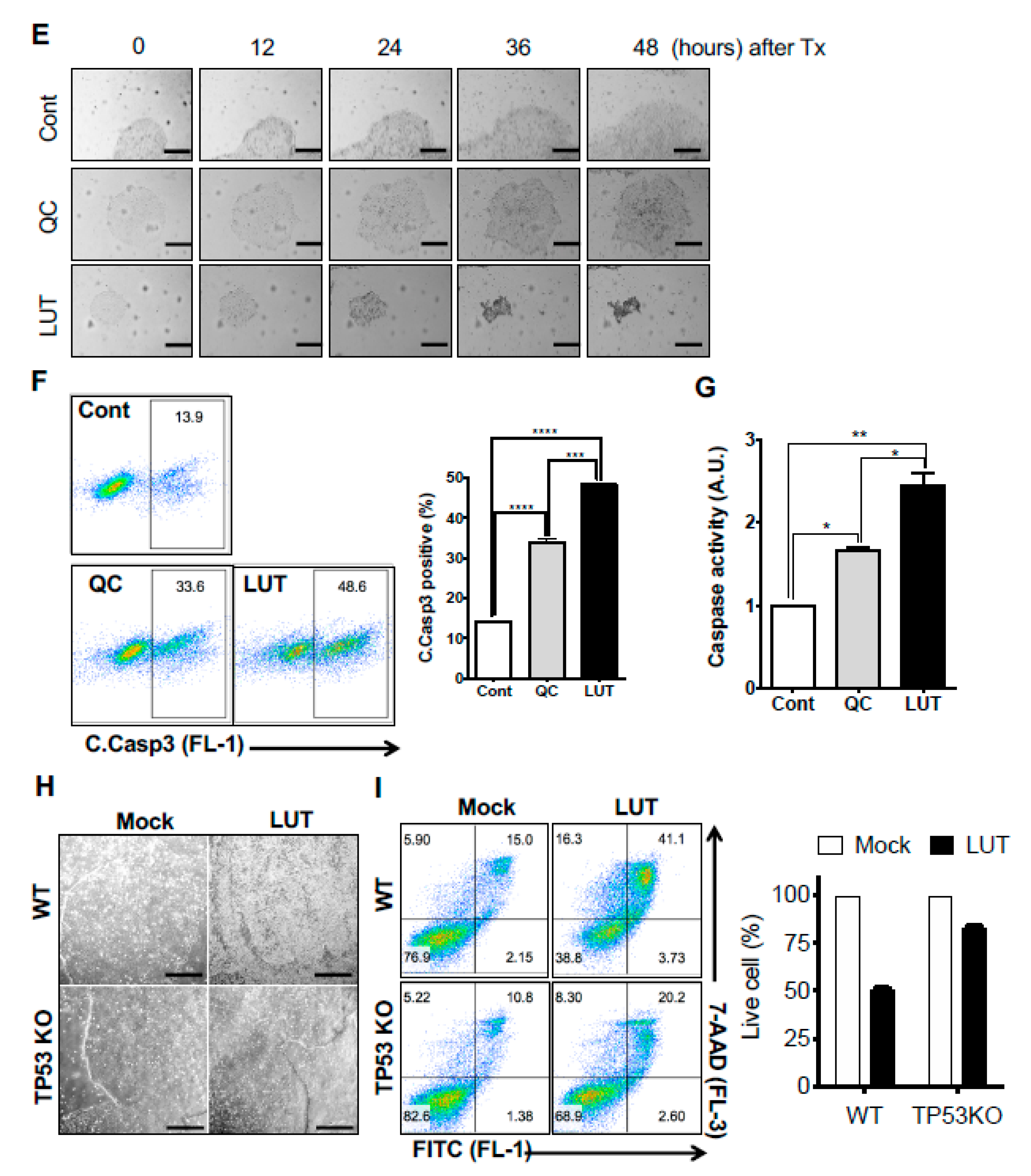
3.2. Potent Stemotoxic Effects of Luteolin
3.3. Selectivity of Luteolin toward hPSCs
3.4. Normal Functioning of hESC-Derived Cardiomyocytes after Luteolin Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Data Accessibility
References
- Trounson, A.; DeWitt, N.D. Pluripotent stem cells progressing to the clinic. Nat. Rev. Mol. Cell Biol. 2016, 17, 194–200. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Goldring, C.E.; Duffy, P.A.; Benvenisty, N.; Andrews, P.W.; Ben-David, U.; Eakins, R.; French, N.; Hanley, N.A.; Kelly, L.; Kitteringham, N.R.; et al. Assessing the safety of stem cell therapeutics. Cell Stem Cell 2011, 8, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, U.; Benvenisty, N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 2011, 11, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013, 19, 998–1004. [Google Scholar] [CrossRef]
- Jeong, H.C.; Cho, S.J.; Lee, M.O.; Cha, H.J. Technical approaches to induce selective cell death of pluripotent stem cells. Cell. Mol. Life Sci. 2017. [Google Scholar] [CrossRef]
- Knoepfler, P.S. Deconstructing stem cell tumorigenicity: A roadmap to safe regenerative medicine. Stem Cells 2009, 27, 1050–1056. [Google Scholar] [CrossRef]
- Lee, M.O.; Moon, S.H.; Jeong, H.C.; Yi, J.Y.; Lee, T.H.; Shim, S.H.; Rhee, Y.H.; Lee, S.H.; Oh, S.J.; Lee, M.Y.; et al. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, E3281–E3290. [Google Scholar] [CrossRef]
- Cho, S.J.; Kim, S.Y.; Park, S.J.; Song, N.; Kwon, H.Y.; Kang, N.Y.; Moon, S.H.; Chang, Y.T.; Cha, H.J. Photodynamic Approach for Teratoma-Free Pluripotent Stem Cell Therapy Using CDy1 and Visible Light. ACS Cent. Sci. 2016, 2, 604–607. [Google Scholar] [CrossRef]
- Ben-David, U.; Gan, Q.-F.; Golan-Lev, T.; Arora, P.; Yanuka, O.; Oren Yifat, S.; Leikin-Frenkel, A.; Graf, M.; Garippa, R.; Boehringer, M.; et al. Selective Elimination of Human Pluripotent Stem Cells by an Oleate Synthesis Inhibitor Discovered in a High-Throughput Screen. Cell Stem Cell 2013, 12, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.F.; Mao, D.; Hirata, N.; Khambu, B.; Kimura, Y.; Kawase, E.; Shimogawa, H.; Ojika, M.; Nakatsuji, N.; Ueda, K.; et al. Selective elimination of human pluripotent stem cells by a marine natural product derivative. J. Am. Chem. Soc. 2014, 136, 9798–9801. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kita, A.; Yamanaka, K.; Mori, M.; Amino, N.; Takeuchi, M.; Tominaga, F.; Hatakeyama, S.; Kinoyama, I.; Matsuhisa, A.; et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007, 67, 8014–8021. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Jeong, H.C.; Kim, C.Y.; Kim, E.Y.; Heo, S.H.; Cho, S.J.; Hong, K.S.; Cha, H.J. Intact wound repair activity of human mesenchymal stem cells after YM155 mediated selective ablation of undifferentiated human embryonic stem cells. J. Dermatol. Sci. 2017, 86, 123–131. [Google Scholar] [CrossRef][Green Version]
- Kim, K.T.; Park, J.C.; Jang, H.K.; Lee, H.; Park, S.; Kim, J.; Kwon, O.S.; Go, Y.H.; Jin, Y.; Kim, W.; et al. Safe scarless cassette-free selection of genome-edited human pluripotent stem cells using temporary drug resistance. Biomaterials 2020, 262, 120295. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, H.C.; Hong, S.K.; Lee, M.O.; Cho, S.J.; Cha, H.J. Quercetin induced ROS production triggers mitochondrial cell death of human embryonic stem cells. Oncotarget 2017, 8, 64964–64973. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Heinz, S.A.; Henson, D.A.; Austin, M.D.; Jin, F.; Nieman, D.C. Quercetin supplementation and upper respiratory tract infection: A randomized community clinical trial. Pharm. Res. 2010, 62, 237–242. [Google Scholar] [CrossRef]
- Go, Y.-H.; Lim, C.; Jeong, H.-C.; Kwon, O.-S.; Chung, S.; Lee, H.; Kim, W.; Suh, Y.-G.; Son, W.S.; Lee, M.-O.; et al. Structure-Activity Relationship Analysis of YM155 for Inducing Selective Cell Death of Human Pluripotent Stem Cells. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Go, Y.-H.; Lee, H.-J.; Kong, H.-J.; Jeong, H.-C.; Lee, D.Y.; Hong, S.-K.; Sung, S.H.; Kwon, O.-S.; Cha, H.-J. Screening of cytotoxic or cytostatic flavonoids with quantitative Fluorescent Ubiquitination-based Cell Cycle Indicator-based cell cycle assay. R. Soc. Open Sci. 2018, 5, 181303. [Google Scholar] [CrossRef]
- Lee, T.H.; Song, S.H.; Kim, K.L.; Yi, J.Y.; Shin, G.H.; Kim, J.Y.; Kim, J.; Han, Y.M.; Lee, S.H.; Lee, S.H.; et al. Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells. Circ. Res. 2010, 106, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, J.H.; Jeong, S.; Kim, B.Y.; Kang, Y.K.; Xu, Y.; Chung, S.K. K120R mutation inactivates p53 by creating an aberrant splice site leading to nonsense-mediated mRNA decay. Oncogene 2019, 38, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Hong, K.S.; Song, W.K.; Bae, D.; Hwang, I.K.; Kim, J.S.; Chung, H.M. Perivascular Progenitor Cells Derived From Human Embryonic Stem Cells Exhibit Functional Characteristics of Pericytes and Improve the Retinal Vasculature in a Rodent Model of Diabetic Retinopathy. Stem Cells Transl. Med. 2016, 5, 1268–1276. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, R.Y.; Park, B.W.; Lee, S.; Choi, S.W.; Park, J.H.; Choi, J.J.; Kim, S.W.; Jang, J.; Cho, D.W.; et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat. Commun. 2019, 10, 3123. [Google Scholar] [CrossRef]
- Cho, S.J.; Kim, K.T.; Jeong, H.C.; Park, J.C.; Kwon, O.S.; Song, Y.H.; Shin, J.G.; Kang, S.; Kim, W.; Shin, H.D.; et al. Selective Elimination of Culture-Adapted Human Embryonic Stem Cells with BH3 Mimetics. Stem Cell Rep. 2018. [Google Scholar] [CrossRef]
- Kwon, O.S.; Lee, H.; Kong, H.J.; Kwon, E.J.; Park, J.E.; Lee, W.; Kang, S.; Kim, M.; Kim, W.; Cha, H.J. Connectivity map-based drug repositioning of bortezomib to reverse the metastatic effect of GALNT14 in lung cancer. Oncogene 2020. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Han, M.J.; Cha, H.J.; Zoldan, J.; Burkart, A.; Jung, J.H.; Jang, Y.; Kim, C.H.; Jeong, H.C.; Kim, B.G.; et al. Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis. Nat. Cell Biol. 2017, 19, 445–456. [Google Scholar] [CrossRef]
- Fernandez, C.; Nieto, O.; Fontenla, J.A.; Rivas, E.; de Ceballos, M.L.; Fernandez-Mayoralas, A. Synthesis of glycosyl derivatives as dopamine prodrugs: Interaction with glucose carrier GLUT-1. Org. Biomol. Chem. 2003, 1, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Gynther, M.; Ropponen, J.; Laine, K.; Leppanen, J.; Haapakoski, P.; Peura, L.; Jarvinen, T.; Rautio, J. Glucose promoiety enables glucose transporter mediated brain uptake of ketoprofen and indomethacin prodrugs in rats. J. Med. Chem. 2009, 52, 3348–3353. [Google Scholar] [CrossRef]
- Patra, M.; Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. A Potent Glucose-Platinum Conjugate Exploits Glucose Transporters and Preferentially Accumulates in Cancer Cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 2550–2554. [Google Scholar] [CrossRef]
- Calvaresi, E.C.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319–2333. [Google Scholar] [CrossRef] [PubMed]
- Sequiera, G.L.; Mehta, A.; Shim, W. A Simple Protocol for the Generation of Cardiomyocytes from Human Pluripotent Stem Cells. Methods Mol. Biol. 2016, 1307, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Kang, S.W.; Park, S.J.; Bae, D.; Kim, S.J.; Lee, H.A.; Kim, K.S.; Hong, K.S.; Kim, J.S.; Do, J.T.; et al. The use of aggregates of purified cardiomyocytes derived from human ESCs for functional engraftment after myocardial infarction. Biomaterials 2013, 34, 4013–4026. [Google Scholar] [CrossRef]
- Dubois, N.C.; Craft, A.M.; Sharma, P.; Elliott, D.A.; Stanley, E.G.; Elefanty, A.G.; Gramolini, A.; Keller, G. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat. Biotechnol. 2011, 29, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Uosaki, H.; Fukushima, H.; Takeuchi, A.; Matsuoka, S.; Nakatsuji, N.; Yamanaka, S.; Yamashita, J.K. Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS ONE 2011, 6, e23657. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, S.; Hattori, F.; Sano, M.; Hishiki, T.; Nagahata, Y.; Matsuura, T.; Hashimoto, H.; Suzuki, T.; Yamashita, H.; Satoh, Y.; et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 2013, 12, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Viatchenko-Karpinski, S.; Fleischmann, B.K.; Liu, Q.; Sauer, H.; Gryshchenko, O.; Ji, G.J.; Hescheler, J. Intracellular Ca2+ oscillations drive spontaneous contractions in cardiomyocytes during early development. Proc. Natl. Acad. Sci. USA 1999, 96, 8259–8264. [Google Scholar] [CrossRef]
- Sirenko, O.; Grimm, F.A.; Ryan, K.R.; Iwata, Y.; Chiu, W.A.; Parham, F.; Wignall, J.A.; Anson, B.; Cromwell, E.F.; Behl, M.; et al. In vitro cardiotoxicity assessment of environmental chemicals using an organotypic human induced pluripotent stem cell-derived model. Toxicol. Appl. Pharm. 2017, 322, 60–74. [Google Scholar] [CrossRef]
- Sirenko, O.; Cromwell, E.F.; Crittenden, C.; Wignall, J.A.; Wright, F.A.; Rusyn, I. Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicol. Appl. Pharm. 2013, 273, 500–507. [Google Scholar] [CrossRef]
- Kang, S.J.; Park, Y.I.; Hwang, S.R.; Yi, H.; Tham, N.; Ku, H.O.; Song, J.Y.; Kang, H.G. Hepatic population derived from human pluripotent stem cells is effectively increased by selective removal of undifferentiated stem cells using YM155. Stem Cell Res. Ther. 2017, 8, 78. [Google Scholar] [CrossRef]
- Bedel, A.; Beliveau, F.; Lamrissi-Garcia, I.; Rousseau, B.; Moranvillier, I.; Rucheton, B.; Guyonnet-Duperat, V.; Cardinaud, B.; de Verneuil, H.; Moreau-Gaudry, F.; et al. Preventing Pluripotent Cell Teratoma in Regenerative Medicine Applied to Hematology Disorders. Stem Cells Transl. Med. 2017, 6, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Cyranoski, D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 2018, 557, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Mallapaty, S. Revealed: Two men in China were first to receive pioneering stem-cell treatment for heart disease. Nature 2020, 581, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.A.; Braam, S.R.; Koutsis, K.; Ng, E.S.; Jenny, R.; Lagerqvist, E.L.; Biben, C.; Hatzistavrou, T.; Hirst, C.E.; Yu, Q.C.; et al. NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nat. Methods 2011, 8, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A Flavonoid that Has Multiple Cardio-Protective Effects and Its Molecular Mechanisms. Front. Pharm. 2017, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xu, T.; Wu, P.; Pan, D.; Chen, J.; Chen, J.; Zhang, B.; Zhu, H.; Li, D. Luteolin improves cardiac dysfunction in heart failure rats by regulating sarcoplasmic reticulum Ca(2+)-ATPase 2a. Sci. Rep. 2017, 7, 41017. [Google Scholar] [CrossRef]
- Rodriguez, J.; Yanez, J.; Vicente, V.; Alcaraz, M.; Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Lozano, J.A. Effects of several flavonoids on the growth of B16F10 and SK-MEL-1 melanoma cell lines: Relationship between structure and activity. Melanoma Res. 2002, 12, 99–107. [Google Scholar] [CrossRef]




| Gene | Forward Sequence (5′ to 3′) | Reverse Sequence (5′ to 3′) |
|---|---|---|
| POU5F1 | GTGGAGGAAGCTGACAACAA | ATTCTCCAGGTTGCCTCTCA |
| SOX2 | TTCACATGTCCCAGCACTACCAGA | TCACATGTGTGAGAGGGGCAGTGTGC |
| NANOG | AAATTGGTGATGAAGATGTATTCG | GCAAAACAGAGCCAAAAACG |
| TNNT2 | ATGAGCGGGAGAAGGAGCGGCAGAAC | TCAATGGCCAGCACCTTCCTCCTCTC |
| MYH7 | CACCAACAACCCCTACGATT | ACTCATTGCCCACTTTCACC |
| NKX2-5 | GTTCCAGAACCGGCGCTACAAGTG | GCTTGCCATCGCGCACCAGCACTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, Y.-H.; Kim, J.; Jeong, H.-C.; Kim, S.-M.; Kim, Y.-J.; Park, S.-J.; Moon, S.-H.; Cha, H.-J. Luteolin Induces Selective Cell Death of Human Pluripotent Stem Cells. Biomedicines 2020, 8, 453. https://doi.org/10.3390/biomedicines8110453
Go Y-H, Kim J, Jeong H-C, Kim S-M, Kim Y-J, Park S-J, Moon S-H, Cha H-J. Luteolin Induces Selective Cell Death of Human Pluripotent Stem Cells. Biomedicines. 2020; 8(11):453. https://doi.org/10.3390/biomedicines8110453
Chicago/Turabian StyleGo, Young-Hyun, Jumee Kim, Ho-Chang Jeong, Seong-Min Kim, Yun-Jeong Kim, Soon-Jung Park, Sung-Hwan Moon, and Hyuk-Jin Cha. 2020. "Luteolin Induces Selective Cell Death of Human Pluripotent Stem Cells" Biomedicines 8, no. 11: 453. https://doi.org/10.3390/biomedicines8110453
APA StyleGo, Y.-H., Kim, J., Jeong, H.-C., Kim, S.-M., Kim, Y.-J., Park, S.-J., Moon, S.-H., & Cha, H.-J. (2020). Luteolin Induces Selective Cell Death of Human Pluripotent Stem Cells. Biomedicines, 8(11), 453. https://doi.org/10.3390/biomedicines8110453






