Galactomannan Pentasaccharide Produced from Copra Meal Enhances Tight Junction Integration of Epithelial Tissue through Activation of AMPK
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of MOS5
2.3. Determination of MOS5 Effects on TEER and Calcium Switch Assay
2.4. Western Blot Analysis
2.5. Purification of α-Galactosidase from Achantina Fulica
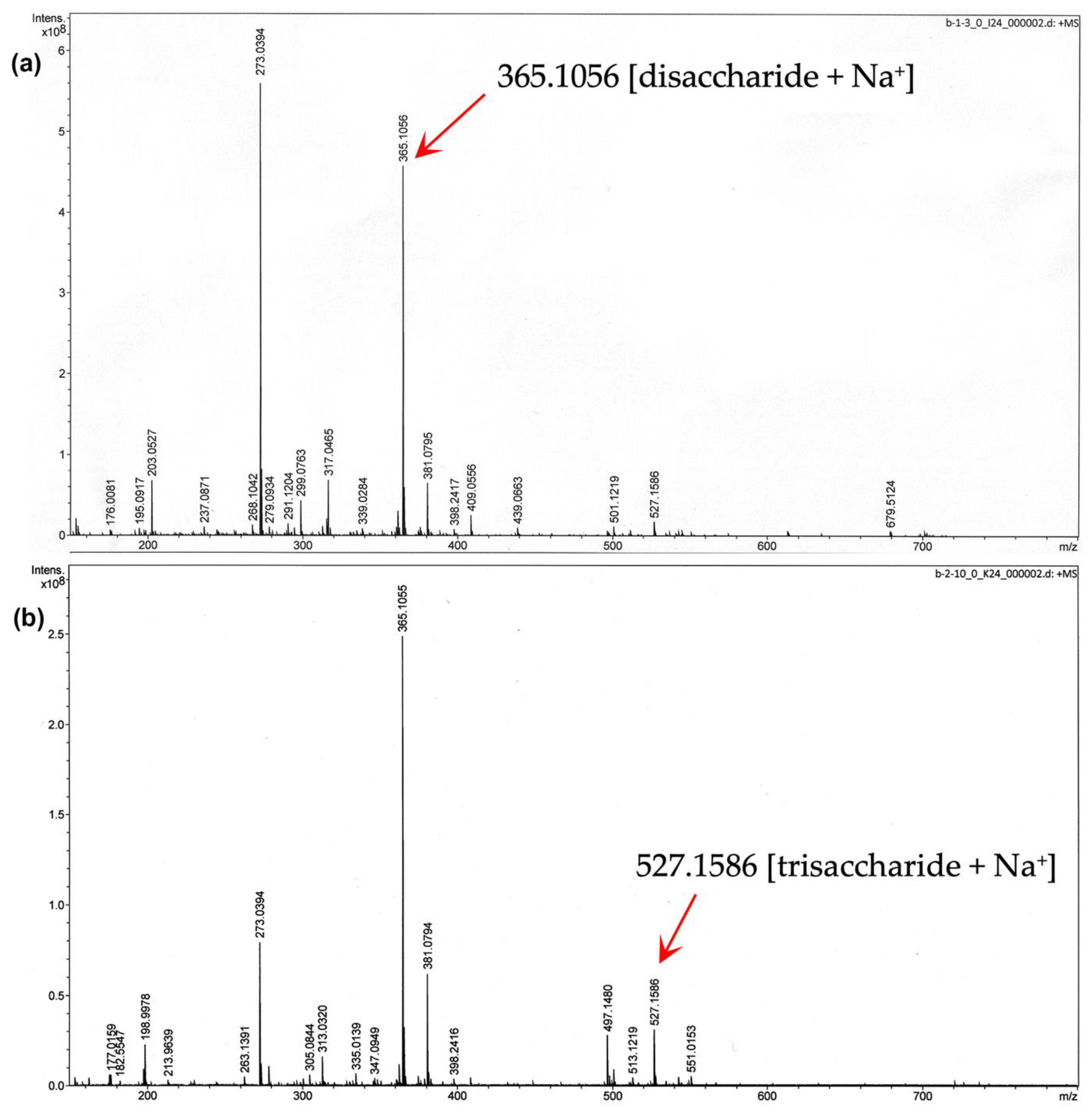
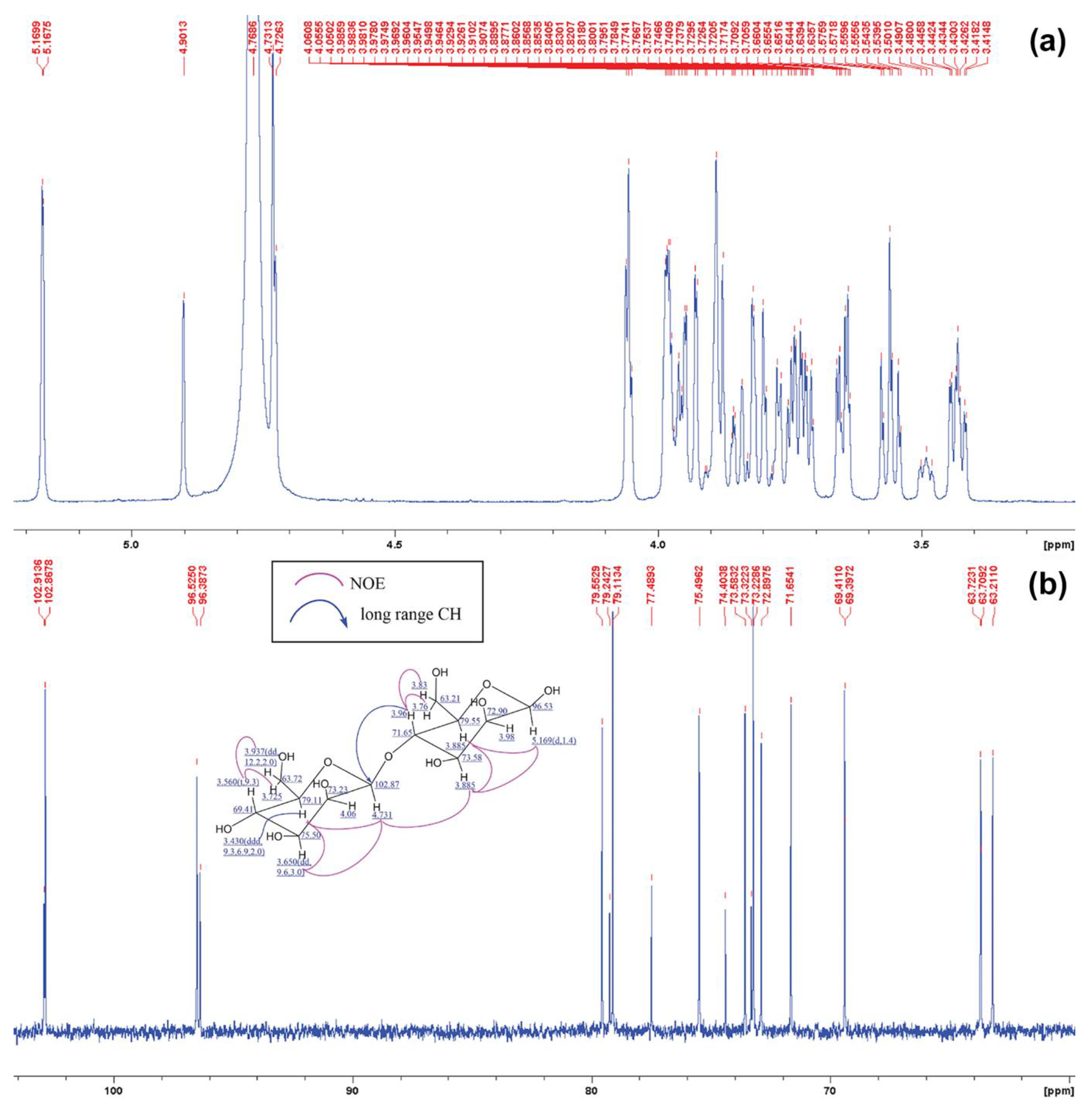
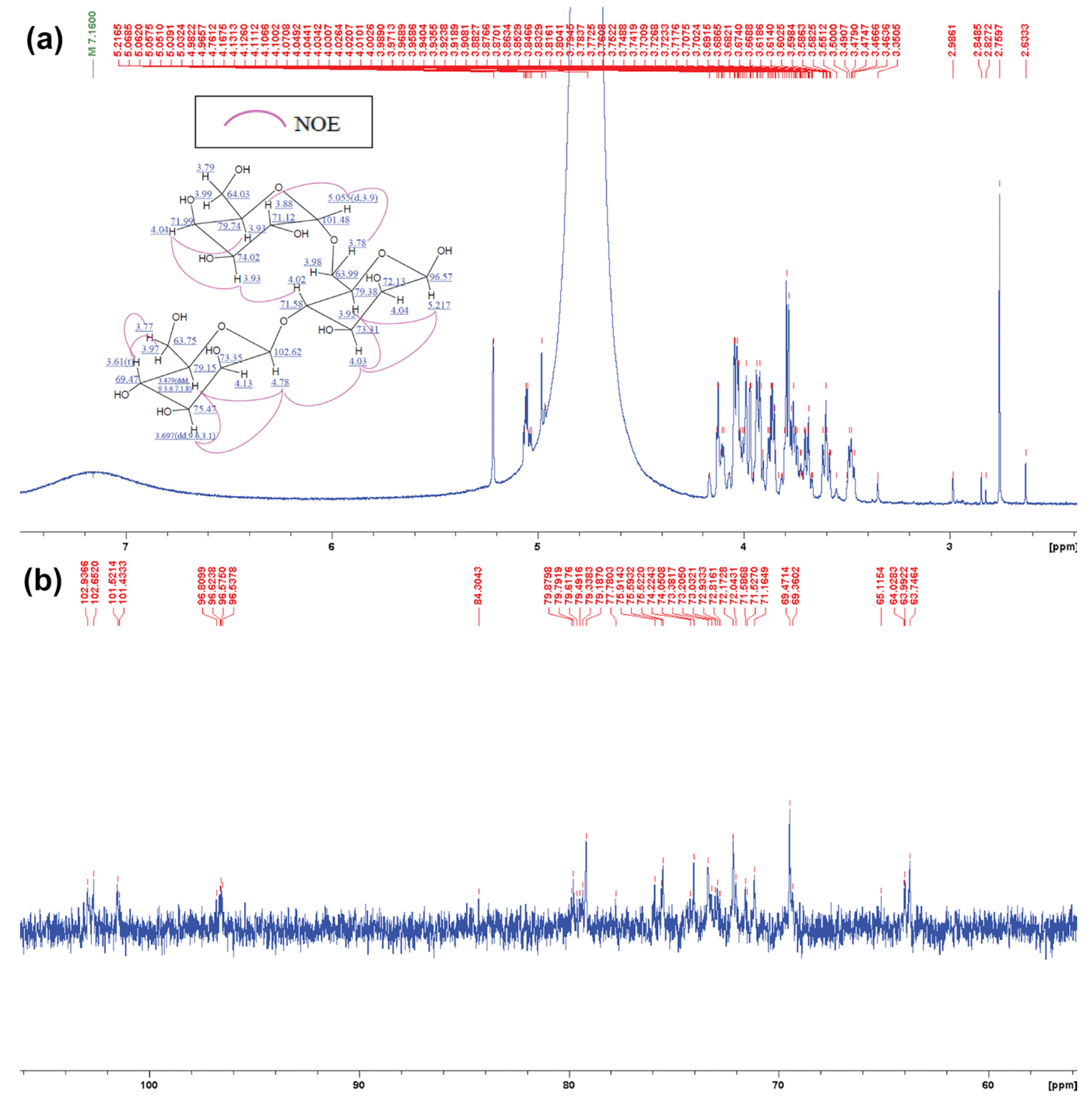
2.6. Structural Analysis of MOS5 Products
2.7. Statistical Analysis
3. Results
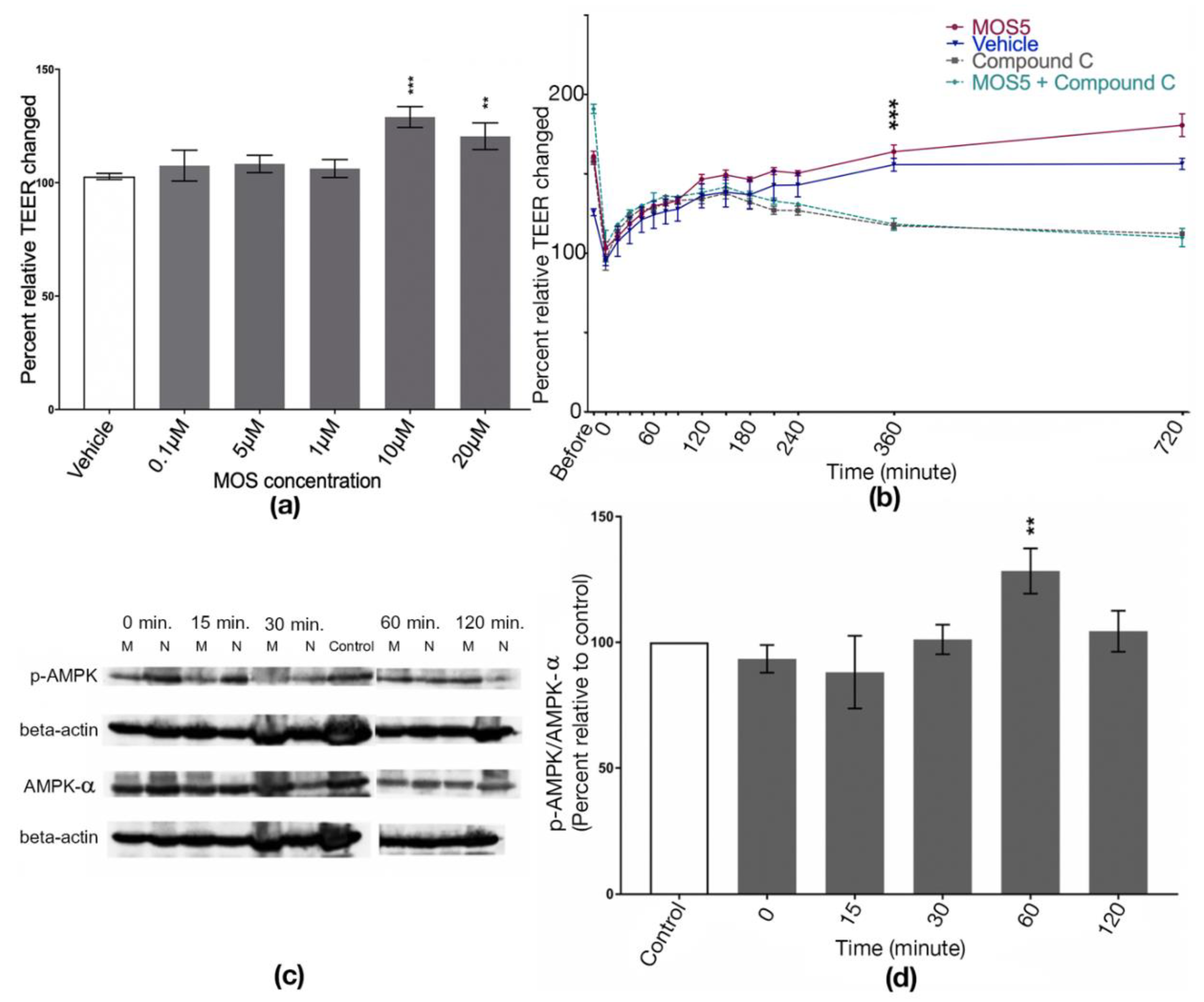
3.1. Effects of MOS5 on Tight Junction Assembly of MOS5 via AMPK Pathway
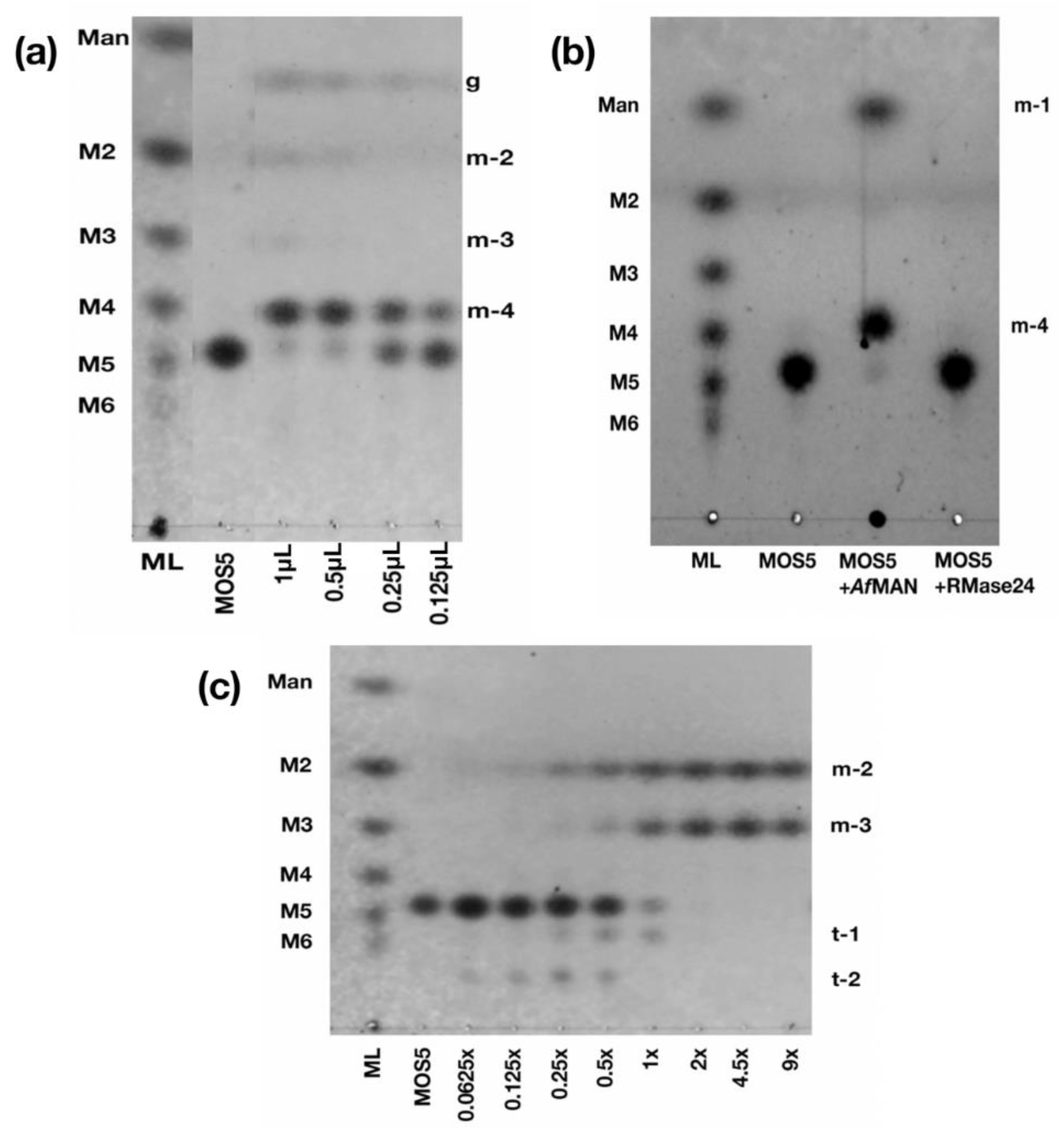
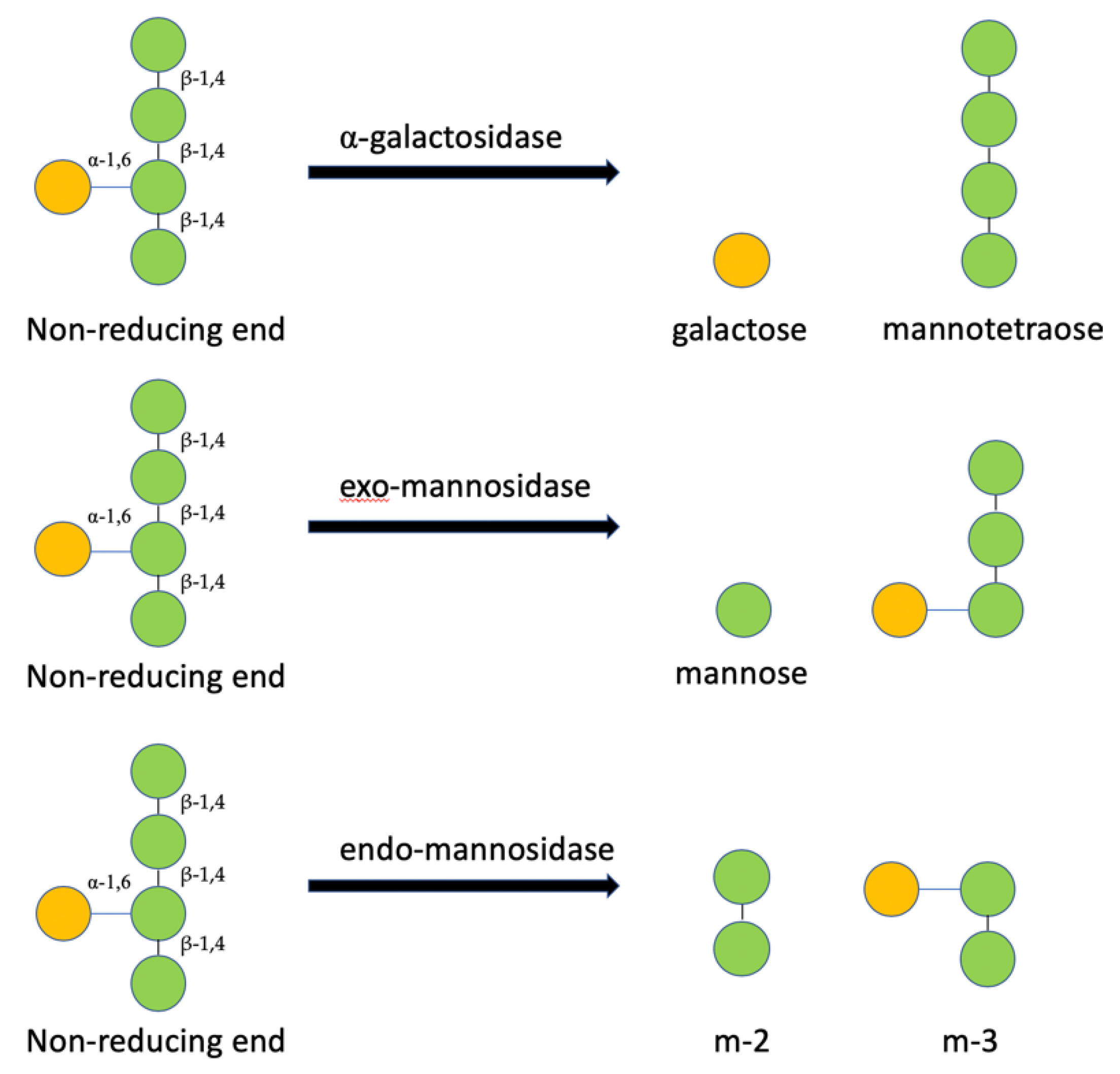
3.2. Determination of MOS5 Structure via Enzymatic Hydrolysis Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edelblum, K.L.; Turner, J.R. The tight junction in inflammatory disease: Communication breakdown. Curr. Opin. Pharmacol. 2009, 9, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Gu, Y.; Gao, Z.; Guo, L.; Guo, W.; Wu, X.; Shen, Y.; Sun, Y.; Wu, X.; Xu, Q. EGFR inhibitor-driven endoplasmic reticulum stress-mediated injury on intestinal epithelial cells. Life Sci. 2014, 119, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.S.; Levin, F.; Chu, D.S. Persistent corneal epithelial defect associated with erlotinib treatment. Cornea 2009, 28, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Clayburgh, D.R.; Shen, L.; Turner, J.R. A porous defense: The leaky epithelial barrier in intestinal disease. Lab. Investig. 2004, 84, 282. [Google Scholar] [CrossRef] [PubMed]
- Shen, L. Tight junctions on the move: Molecular mechanisms for epithelial barrier regulation. Ann. New York Acad. Sci. 2012, 1258, 9. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Su, L.; Turner, J.R. Mechanisms and functional implications of intestinal barrier defects. Dig. Dis. 2009, 27, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Shen, L.; Clayburgh, D.R.; Nalle, S.C.; Sullivan, E.A.; Meddings, J.B.; Abraham, C.; Turner, J.R. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 2009, 136, 551–563. [Google Scholar] [CrossRef]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- Sawada, N.; Murata, M.; Kikuchi, K.; Osanai, M.; Tobioka, H.; Kojima, T.; Chiba, H. Tight junctions and human diseases. Med. Electron. Microsc. 2003, 36, 147–156. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799. [Google Scholar] [CrossRef]
- Mitic, L.L.; Van Itallie, C.M.; Anderson, J.M. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: Lessons from mutant animals and proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G250–G254. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem. Pharm. 2015, 96, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Mattaveewong, T.; Wongkrasant, P.; Chanchai, S.; Pichyangkura, R.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide suppresses tumor progression in a mouse model of colitis-associated colorectal cancer through AMPK activation and suppression of NF-κB and mTOR signaling. Carbohydr. Polym. 2016, 145, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Hu, Y.; Zhu, J.; Woodruff, T.K.; Jardetzky, T.S. Structure of betaglycan zona pellucida (ZP)-C domain provides insights into ZP-mediated protein polymerization and TGF-β binding. Proc. Natl. Acad. Sci. USA 2011, 108, 5232–5236. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Spring, P.; Sweetman, J.; Moate, R.; Davies, S.J. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture 2010, 300, 182–188. [Google Scholar] [CrossRef]
- Cheled-Shoval, S.; Gamage, N.W.; Amit-Romach, E.; Forder, R.; Marshal, J.; Van Kessel, A.; Uni, Z. Differences in intestinal mucin dynamics between germ-free and conventionally reared chickens after mannan-oligosaccharide supplementation. Poult. Sci. 2014, 93, 636–644. [Google Scholar] [CrossRef]
- Corrigan, A.; Horgan, K.; Clipson, N.; Murphy, R. Effect of dietary prebiotic (mannan oligosaccharide) supplementation on the caecal bacterial community structure of turkeys. Microb. Ecol. 2012, 64, 826–836. [Google Scholar] [CrossRef]
- Mansour, M.R.; Akrami, R.; Ghobadi, S.; Denji, K.A.; Ezatrahimi, N.; Gharaei, A. Effect of dietary mannan oligosaccharide (MOS) on growth performance, survival, body composition, and some hematological parameters in giant sturgeon juvenile (Huso huso Linnaeus, 1754). Fish. Physiol. Biochem. 2012, 38, 829–835. [Google Scholar] [CrossRef]
- Ai, Q.; Xu, H.; Mai, K.; Xu, W.; Wang, J.; Zhang, W. Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture 2011, 317, 155–161. [Google Scholar] [CrossRef]
- Mourão, J.L.; Pinheiro, V.; Alves, A.; Guedes, C.; Pinto, L.; Saavedra, M.J.; Spring, P.; Kocher, A. Effect of mannan oligosaccharides on the performance, intestinal morphology and cecal fermentation of fattening rabbits. Anim. Feed Sci. Technol. 2006, 126, 107–120. [Google Scholar] [CrossRef]
- Grisdale-Helland, B.; Helland, S.J.; Gatlin III, D.M. The effects of dietary supplementation with mannanoligosaccharide, fructooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar). Aquaculture 2008, 283, 163–167. [Google Scholar] [CrossRef]
- Staykov, Y.; Spring, P.; Denev, S.; Sweetman, J. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2007, 15, 153–161. [Google Scholar] [CrossRef]
- Genc, M.; Aktas, M.; Genc, E.; Yilmaz, E. Effects of dietary mannan oligosaccharide on growth, body composition and hepatopancreas histology of Penaeus semisulcatus (de Haan 1844). Aquac. Nutr. 2007, 13, 156–161. [Google Scholar] [CrossRef]
- Nopvichai, C.; Charoenwongpaiboon, T.; Luengluepunya, N.; Ito, K.; Muanprasat, C.; Pichyangkura, R. Production and purification of mannan oligosaccharide with epithelial tight junction enhancing activity. PeerJ 2019, 7, e7206. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharm. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharm. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef]
- Peshev, D.; Van den Ende, W. Fructans: Prebiotics and immunomodulators. J. Funct. Foods 2014, 8, 348–357. [Google Scholar] [CrossRef]
- Shi, L.; Fang, B.; Yong, Y.; Li, X.; Gong, D.; Li, J.; Yu, T.; Gooneratne, R.; Gao, Z.; Li, S. Chitosan oligosaccharide-mediated attenuation of LPS-induced inflammation in IPEC-J2 cells is related to the TLR4/NF-κB signaling pathway. Carbohydr. Polym. 2019, 219, 269–279. [Google Scholar] [CrossRef]
- Ghosh, A.; Verma, A.K.; Tingirikari, J.R.; Shukla, R.; Goyal, A. Recovery and purification of oligosaccharides from copra meal by recombinant endo-β-mannanase and deciphering molecular mechanism involved and its role as potent therapeutic agent. Mol. Biotechnol. 2015, 57, 111–127. [Google Scholar] [CrossRef]
- Saittagaroon, S.; Kawakishi, S.; Namiki, M. Characterisation of polysaccharides of copra meal. J. Sci. Food Agric. 1983, 34, 855–860. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Watsanit, K.; Adachi, S. Carbohydrate content and composition of product from subcritical water treatment of coconut meal. J. Ind. Eng. Chem. 2012, 18, 225–229. [Google Scholar] [CrossRef]
- Abe, J.-i.; Hossain, M.Z.; Huzukuri, S. Isolation of β-mannanase-producing microorganism. J. Ferment. Bioeng. 1994, 78, 259–261. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Abe, J.-i.; Hizukuri, S. Multiple forms of β-mannanase from Bacillus sp. KK01. Enzym. Microb. Technol. 1996, 18, 95–98. [Google Scholar] [CrossRef]
- McCleary, B.; Matheson, N.; Small, D.M. Galactomannans and a galactoglucomannan in legume seed endosperms: Structural requirements for β-mannanase hydrolysis. Phytochemistry 1976, 15, 1111–1117. [Google Scholar] [CrossRef]
- Ademark, P.; Varga, A.; Medve, J.; Harjunpää, V.; Drakenberg, T.; Tjerneld, F.; Stålbrand, H. Softwood hemicellulose-degrading enzymes from Aspergillus niger: Purification and properties of a β-mannanase. J. Biotechnol. 1998, 63, 199–210. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nopvichai, C.; Pongkorpsakol, P.; Wongkrasant, P.; Wangpaiboon, K.; Charoenwongpaiboon, T.; Ito, K.; Muanprasat, C.; Pichyangkura, R. Galactomannan Pentasaccharide Produced from Copra Meal Enhances Tight Junction Integration of Epithelial Tissue through Activation of AMPK. Biomedicines 2019, 7, 81. https://doi.org/10.3390/biomedicines7040081
Nopvichai C, Pongkorpsakol P, Wongkrasant P, Wangpaiboon K, Charoenwongpaiboon T, Ito K, Muanprasat C, Pichyangkura R. Galactomannan Pentasaccharide Produced from Copra Meal Enhances Tight Junction Integration of Epithelial Tissue through Activation of AMPK. Biomedicines. 2019; 7(4):81. https://doi.org/10.3390/biomedicines7040081
Chicago/Turabian StyleNopvichai, Chatchai, Pawin Pongkorpsakol, Preedajit Wongkrasant, Karan Wangpaiboon, Thanapon Charoenwongpaiboon, Kazuo Ito, Chatchai Muanprasat, and Rath Pichyangkura. 2019. "Galactomannan Pentasaccharide Produced from Copra Meal Enhances Tight Junction Integration of Epithelial Tissue through Activation of AMPK" Biomedicines 7, no. 4: 81. https://doi.org/10.3390/biomedicines7040081
APA StyleNopvichai, C., Pongkorpsakol, P., Wongkrasant, P., Wangpaiboon, K., Charoenwongpaiboon, T., Ito, K., Muanprasat, C., & Pichyangkura, R. (2019). Galactomannan Pentasaccharide Produced from Copra Meal Enhances Tight Junction Integration of Epithelial Tissue through Activation of AMPK. Biomedicines, 7(4), 81. https://doi.org/10.3390/biomedicines7040081



