Linitis Plastica-like Metastases to the Gastrointestinal Tract on Cross-Sectional Imaging
Abstract
1. Introduction
2. Primary Gastrointestinal LP
2.1. Primary Gastric LP
Primary LP GI Tumours: Cross-Sectional Imaging
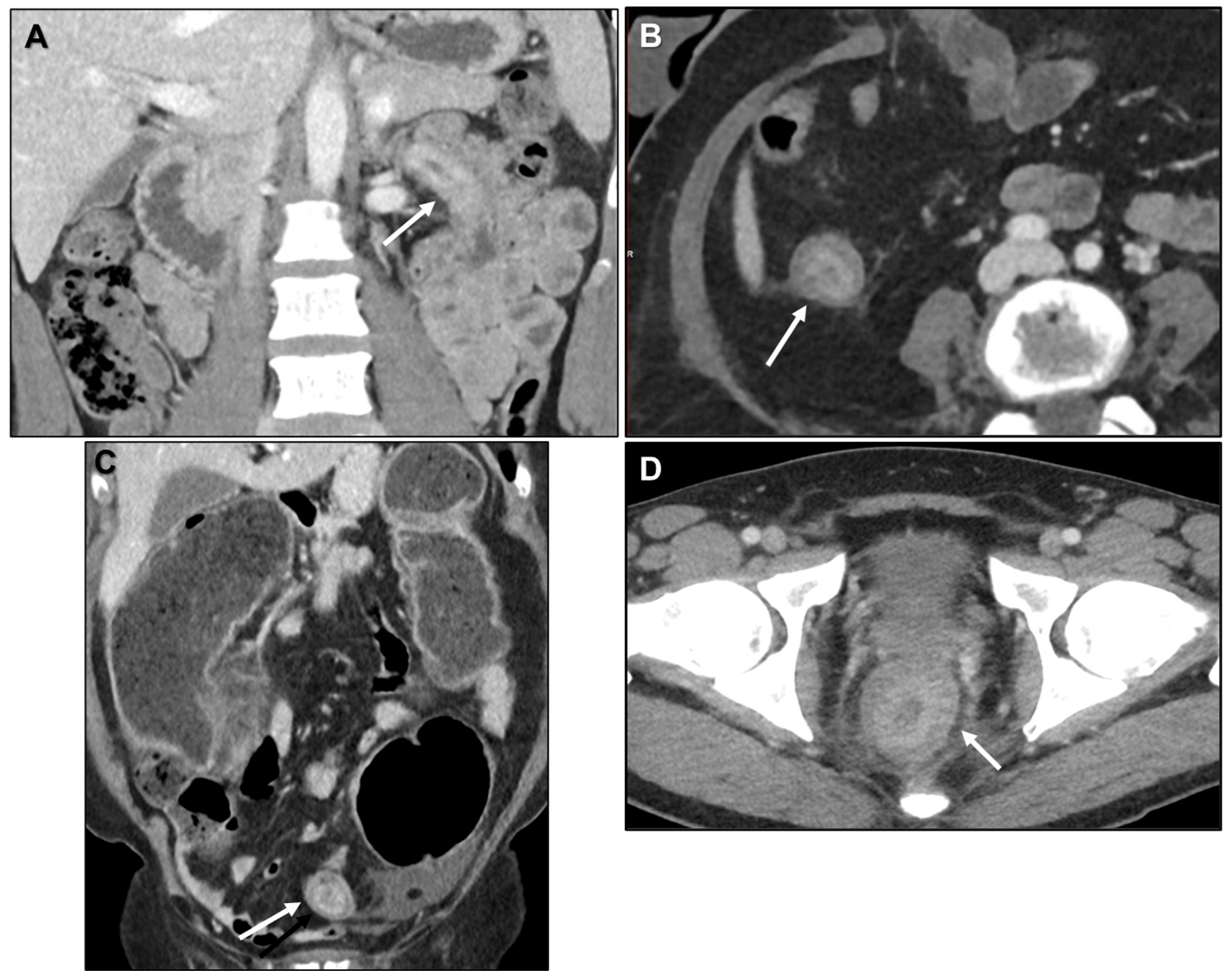
2.2. LP-like Metastases to the GI Tract: Primary Tumours
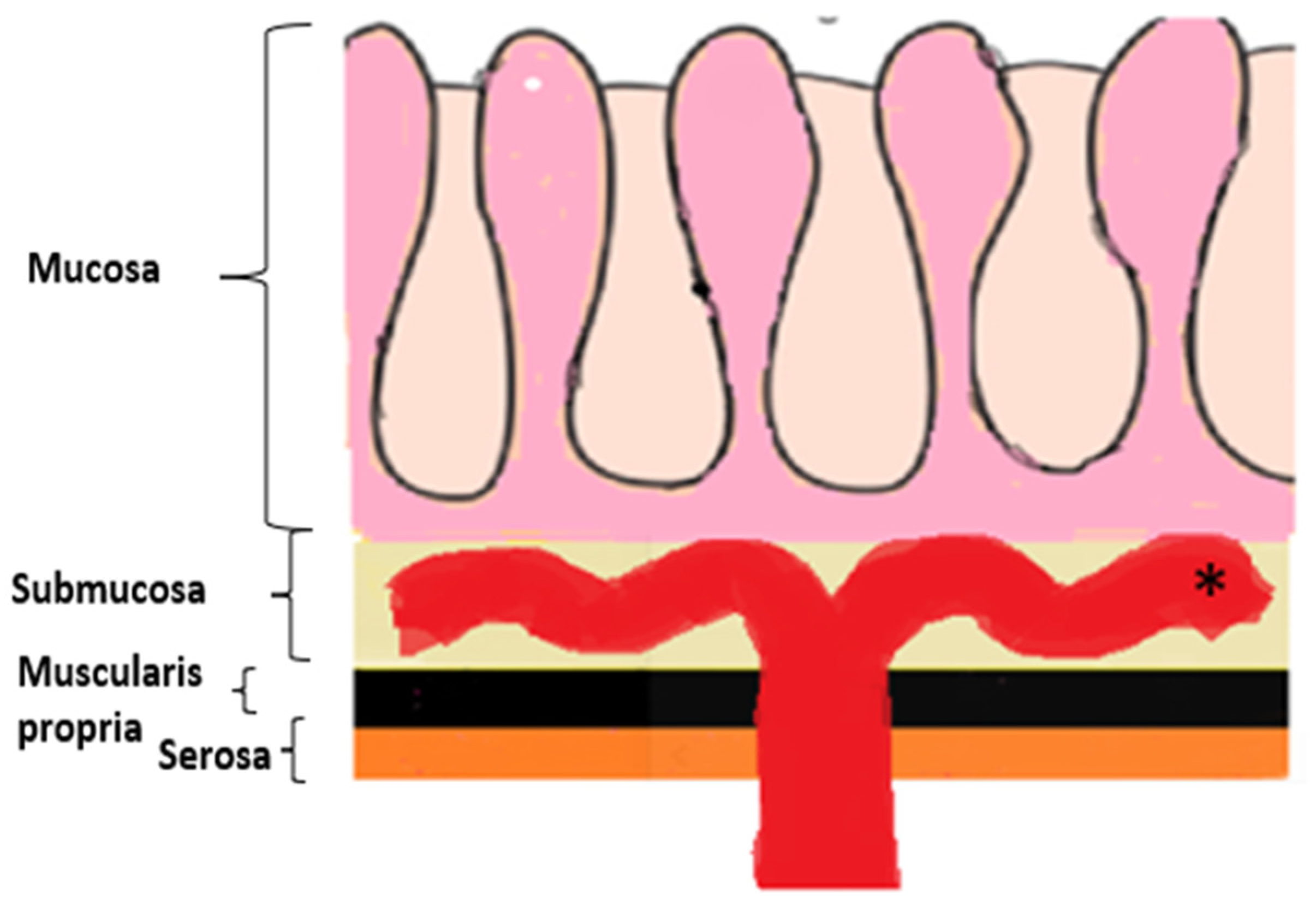
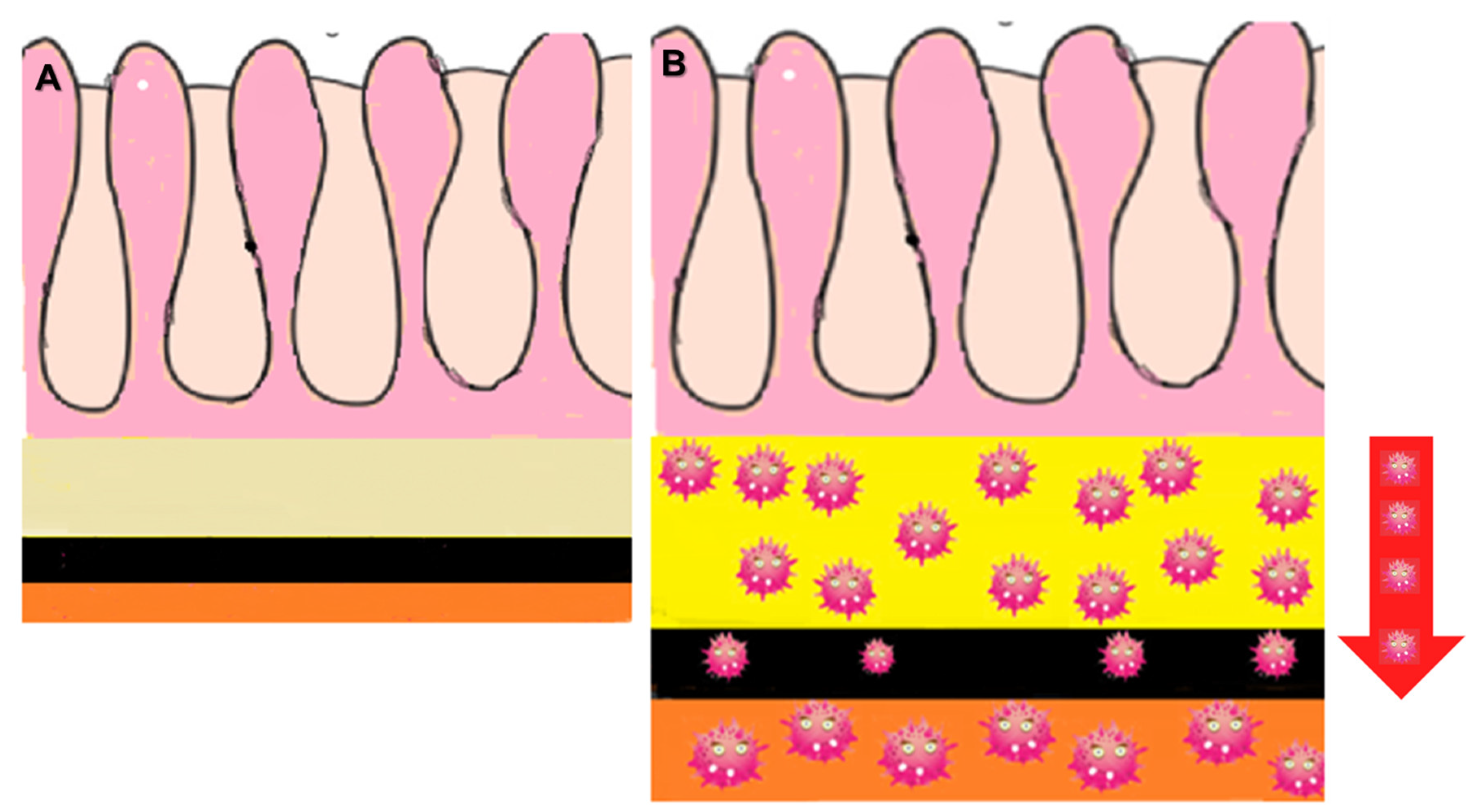
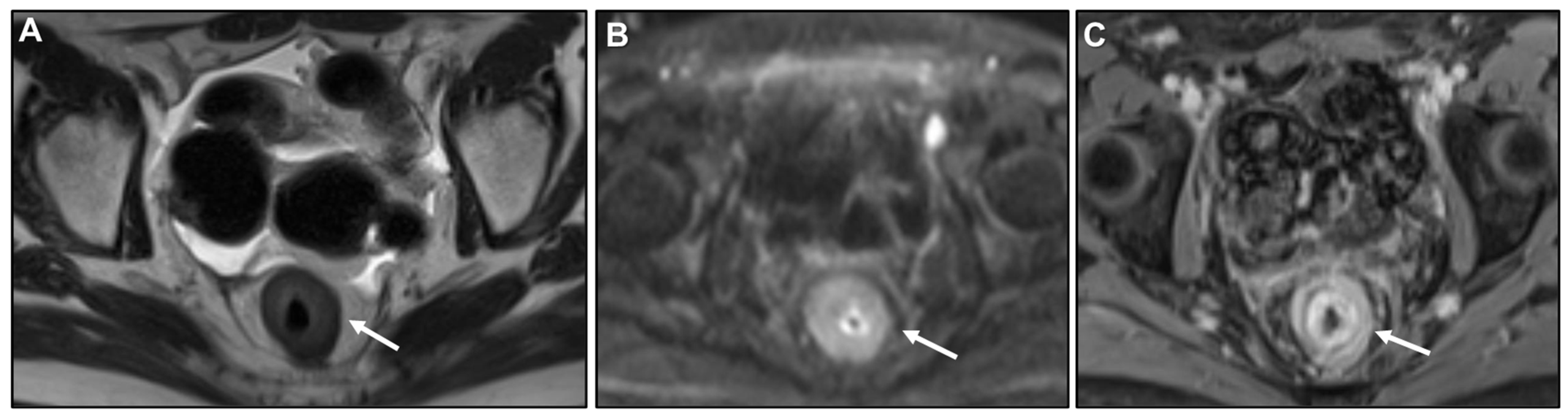
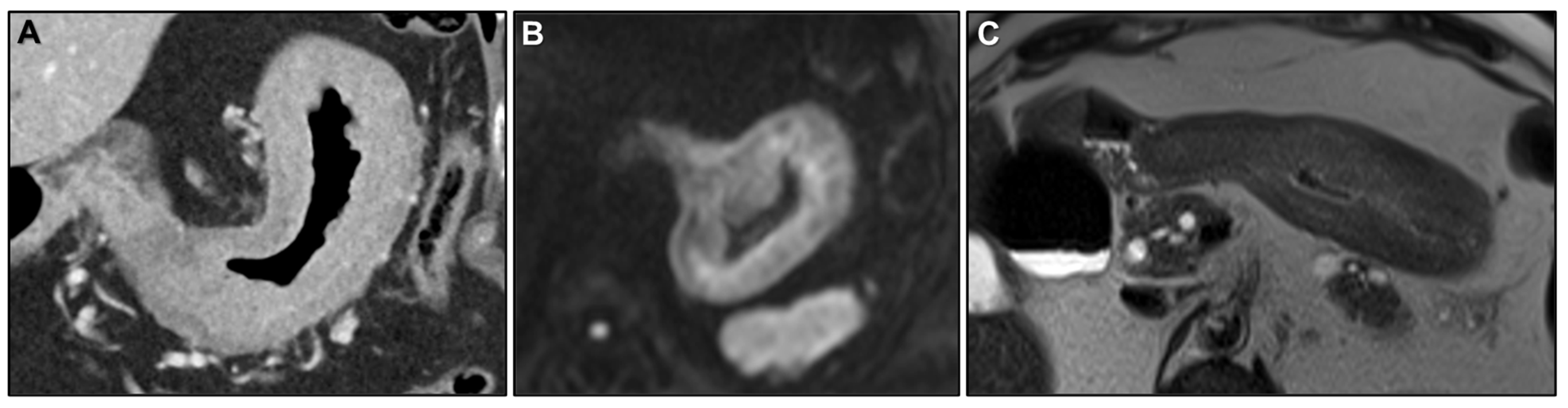
2.2.1. LP-like Metastases to the GI Tract: Imaging Features
Malignant Target Sign
Homogeneous Delayed Enhancement
Concentric Ring Pattern
Length
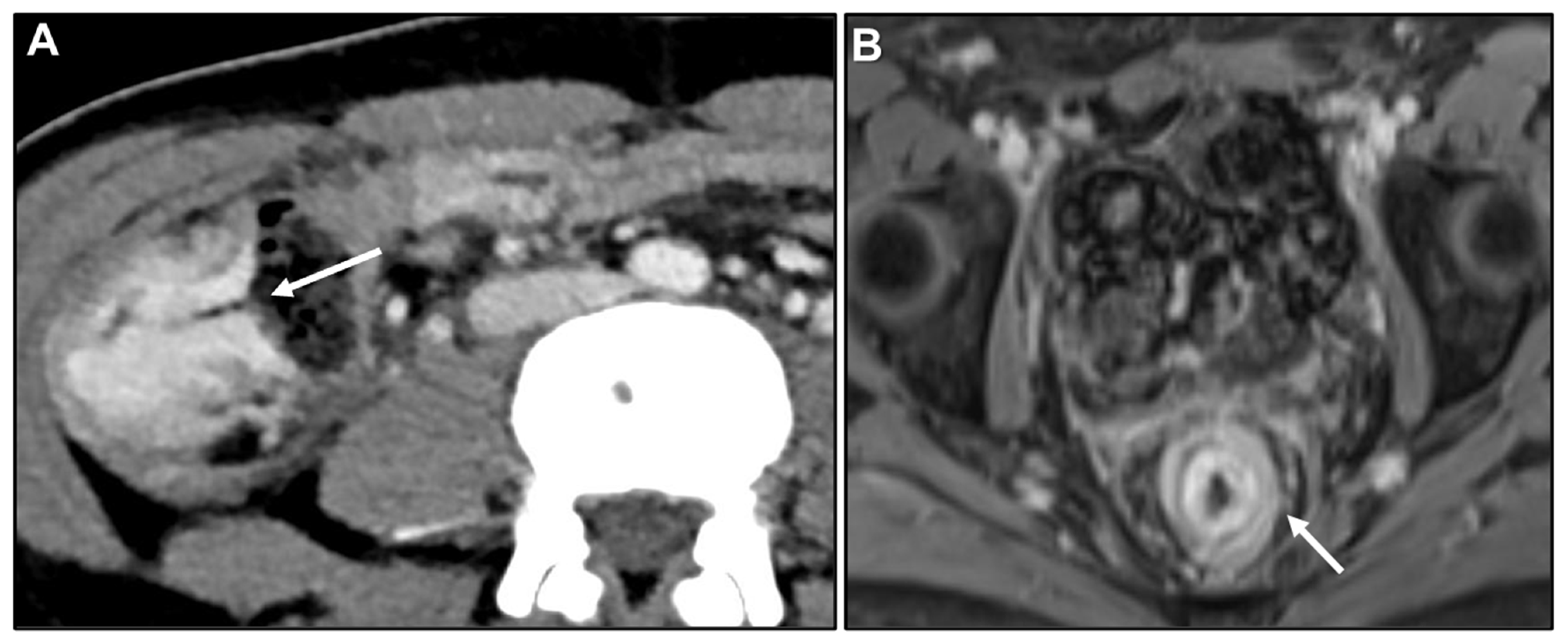
2.2.2. Associated Imaging Features
Peritoneal Carcinomatosis
Presacral Fat Tissue and Rectovaginal Septum Infiltration and T2-Weighted Imaging (T2WI) Hypointense Extramural Tumour Component
Intestinal Obstruction
2.3. Diagnostic Challenges
2.3.1. Anamnesis
2.3.2. Clinical Presentation
2.3.3. Imaging Findings
2.3.4. Pathology
2.4. Differential Diagnosis
2.4.1. Malignant Causes
2.4.2. Benign Causes
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | apparent diffusion coefficient |
| CE | contrast enhanced |
| CT | computer tomography |
| CK7 | cytokeratin 7 |
| DWI | Diffusion-weighted imaging |
| GI | gastrointestinal |
| GRE-T1WI | gradient recalled echo T1-weighted images |
| H&E | hematoxylin and eosin |
| IHC | immunohistochemistry |
| ILC | invasive lobular carcinoma |
| LP | linitis plastica |
| MP | muscularis propria |
| MPR | multiplanar reconstruction |
| MR | magnetic resonance |
| SB | small bowel |
| PC | peritoneal carcinomatosis |
| T2WI | T2-weighted imaging |
References
- Feczko, P.J.; Collins, D.D.; Mezwa, D.G. Metastatic disease involving the gastrointestinal tract. Radiol. Clin. N. Am. 1993, 31, 1359–1373. [Google Scholar] [CrossRef]
- Cormier, W.J.; Gaffey, T.A.; Welch, J.M.; Welch, J.S.; Edmonson, J.H. Linitis plastica caused by metastatic lobular carcinoma of the breast. Mayo Clin. Proc. 1980, 55, 747–753. [Google Scholar]
- Mastoraki, A.; Papanikolaou, I.S.; Sakorafas, G.; Safioleas, M. Facing the challenge of managing linitis plastica-review of the literature. Hepatogastroenterology 2009, 56, 1773–1778. [Google Scholar] [PubMed]
- Agnes, A.; Estrella, J.S.; Badgwell, B. The significance of a nineteenth century definition in the era of genomics: Linitis plastica. World J. Surg. Oncol. 2017, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Lyle, H.H., VIII. Linitis Plastica (Cirrhosis of Stomach): With a Report of a Case Cured by Gastro-Jejunostomy. Ann. Surg. 1911, 54, 625–668. [Google Scholar] [CrossRef] [PubMed]
- Stout, A.P. Tumors of the Stomach; Armed Forces Institute of Pathology: Washington DC, USA, 1953; pp. 1–134. [Google Scholar]
- Consul, N.; DiSantis, D.J.; Dyer, R.B. The “leather bottle” stomach. Abdom. Radiol. 2018, 43, 2210–2211. [Google Scholar] [CrossRef]
- Agarwal, N.; Ulahannan, M.J.; Mandile, M.A.; Cayten, C.G.; Pitchumoni, C.S. Increased risk of colorectal cancer following breast cancer. Ann. Surg. 1986, 203, 307–310. [Google Scholar] [CrossRef]
- Christodoulidis, G.; Koumarelas, K.E.; Kouliou, M.N.; Samara, M.; Thodou, E.; Zacharoulis, D. The Genomic Signatures of Linitis Plastica Signal the Entrance into a New Era: Novel Approaches for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 14680. [Google Scholar] [CrossRef]
- Balthazar, E.J.; Rosenberg, H.D.; Davidian, M.M. Primary and metastatic scirrrhous carcinoma of the rectum. AJR Am. J. Roentgenol. 1979, 132, 711–715. [Google Scholar] [CrossRef][Green Version]
- Mariette, C.; Carneiro, F.; Grabsch, H.I.; van der Post, R.S.; Allum, W.; de Manzoni, G. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer 2019, 22, 1–9. [Google Scholar] [CrossRef]
- El-Nakeep, S.; Kasi, A. Linitis Plastica; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Piessen, G.; Messager, M.; Leteurtre, E.; Jean-Pierre, T.; Mariette, C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann. Surg. 2009, 250, 878–887. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours of the Digestive System, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2010.
- Ikoma, N.; Agnes, A.; Chen, H.C.; Wang, X.; Blum, M.M.; Das, P.; Minsky, B.; Estrella, J.S.; Mansfield, P.; Ajani, J.A.; et al. Linitis Plastica: A Distinct Type of Gastric Cancer. J. Gastrointest. Surg. 2020, 24, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.P. Linitis plastica: A study of ten cases. QJM 1933, 2, 59–78. [Google Scholar] [CrossRef][Green Version]
- Fornasarig, M.; Capuano, A.; Maiero, S.; Pivetta, E.; Canzonieri, V.; Belluco, C.; Mongiat, M.; Cannizzaro, R.; Spessotto, P. pCLE detects mucosal neoplastic vascular pattern in gastric linitis plastica. Clin. Exp. Med. 2023, 23, 547–551. [Google Scholar] [CrossRef]
- Muraoka, S.; Tsuchida, K.; Iwasaki, M.; Izawa, N.; Jinnai, H.; Komatsubara, T.; Tsunemi, M.; Sakuma, F.; Kashima, K.; Fukushi, K.; et al. A case report of gastric linitis plastica diagnosed by endoscopic ultrasound-guided fine needle aspiration. Medicine 2017, 96, e8937. [Google Scholar] [CrossRef]
- Meyers, M.A.; Oliphant, M.; Teixidor, H.; Weiser, P. Metastatic carcinoma simulating inflammatory colitis. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1975, 123, 74–83. [Google Scholar] [CrossRef][Green Version]
- Gollub, M.J.; Schwartz, M.B.; Shia, J. Scirrhous metastases to the gastrointestinal tract at CT: The malignant target sign. AJR Am. J. Roentgenol. 2009, 192, 936–940. [Google Scholar] [CrossRef][Green Version]
- Balthazar, E.J. CT of the gastrointestinal tract: Principles and interpretation. AJR Am. J. Roentgenol. 1991, 156, 23–32. [Google Scholar] [CrossRef]
- Balthazar, E.J.; Siegel, S.E.; Megibow, A.J.; Scholes, J.; Gordon, R. CT in patients with scirrhous carcinoma of the GI tract: Imaging findings and value for tumor detection and staging. AJR Am. J. Roentgenol. 1995, 165, 839–845. [Google Scholar] [CrossRef]
- Laufman, H.; Saphir, O. Primary linitis plastica type of carcinoma of the colon. AMA Arch. Surg. 1951, 62, 79–91. [Google Scholar] [CrossRef]
- Kim, H.J.; Ha, H.K.; Cho, K.S.; Yu, E.; Kim, J.C.; Yoo, C.S.; Sik, C.; Kim, H.C.; Chul, H.; Yang, S.K.; et al. CT features of primary colorectal signet-ring cell carcinoma. J. Comput. Assist. Tomogr. 2001, 25, 225–230. [Google Scholar] [CrossRef]
- Rao, T.R.; Hambrick, E.; Abcarian, H.; Salgia, K.; Recant, W.M. Colorectal linitis plastica. Dis. Colon. Rectum 1982, 25, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, C.; Hannan, E.; Duggan, W.; Mullen, D.; Buckley, M.; Stafford, A.T. Primary colorectal linitis plastica presenting as rapid acute deterioration: A diagnostic dilemma. Ann. R. Coll. Surg. Engl. 2020, 102, e187–e189. [Google Scholar] [CrossRef]
- Shirouzu, K.; Isomoto, H.; Morodomi, T.; Ogata, Y.; Akagi, Y.; Kakegawa, T. Primary linitis plastica carcinoma of the colon and rectum. Cancer 1994, 74, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.H.; Chen, Y.Y.; Soon, M.S. Primary linitis plastica of the jejunum. Gastrointest. Endosc. 2006, 63, 503–504. [Google Scholar] [CrossRef]
- Raskin, M.M.; Viamonte, M.; Viamonte, M., Jr. Primary linitis plastica carcinoma of the colon. Radiology 1974, 113, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Papp, J.P., Jr.; Levine, E.J.; Thomas, F.B. Primary linitis plastica carcinoma of the colon and rectum. Am. J. Gastroenterol. 1995, 90, 141–145. [Google Scholar]
- Jang, H.J.; Lim, H.K.; Kim, H.S.; Cho, E.Y.; Lee, S.J.; Kim, K.A.; Choi, D. Intestinal metastases from gastric adenocarcinoma: Helical CT findings. J. Comput. Assist. Tomogr. 2001, 25, 61–67. [Google Scholar] [CrossRef]
- Fisher, E.R.; Brown, C.H. Linitis plastica carcinoma of the stomach with extensive metastases simulating a colonic lesion. Gastroenterology 1952, 20, 503–508. [Google Scholar] [CrossRef]
- Kondo, K.; Usui, Y.; Matsukawa, M.; Yamada, S.; Negoro, T.; Kan, T.; Yoshida, K.; Sasaki, J.; Ishioka, T.; Shirakabe, H. An autopsy case of gastric metastasis simulating linitis plastica carcinoma from primary linitis plastica carcinoma of the rectum. Gan No Rinsho 1988, 34, 1996–2001. [Google Scholar]
- Eljabu, W.; Finch, G.; Nottingham, J.; Vaingankar, N. Metastatic deposits of breast lobular carcinoma to small bowel and rectum. Int. J. Breast Cancer 2011, 2011, 413949. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.M.; Anderson, T.J.; Page, D.L.; Lee, D.; Duffy, S.W. Infiltrating lobular carcinoma of the breast. Histopathology 1982, 6, 149–161. [Google Scholar] [CrossRef]
- Lamovec, J.; Bracko, M. Metastatic pattern of infiltrating lobular carcinoma of the breast: An autopsy study. J. Surg. Oncol. 1991, 48, 28–33. [Google Scholar] [CrossRef]
- Winston, C.B.; Hadar, O.; Teitcher, J.B.; Caravelli, J.F.; Sklarin, N.T.; Panicek, D.M.; Liberman, L. Metastatic lobular carcinoma of the breast: Patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am. J. Roentgenol. 2000, 175, 795–800. [Google Scholar] [CrossRef]
- El-Hage, A.; Ruel, C.; Afif, W.; Wissanji, H.; Hogue, J.C.; Desbiens, C.; Leblanc, B.; Poirier, E. Metastatic pattern of invasive lobular carcinoma of the breast-Emphasis on gastric metastases. J. Surg. Oncol. 2017, 114, 543–547. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Kutasovic, J.R.; Lakhani, S.R.; Simpson, P.T. Invasive lobular carcinoma of the breast: Morphology, biomarkers and ’omics. Breast Cancer Res. 2015, 17, 12. [Google Scholar] [CrossRef]
- McLemore, E.C.; Pockaj, B.A.; Reynolds, C.; Gray, R.J.; Hernandez, J.L.; Grant, C.S.; Donohue, J.H. Breast cancer: Presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann. Surg. Oncol. 2005, 12, 886–894. [Google Scholar] [CrossRef]
- Ambroggi, M.; Stroppa, E.M.; Mordenti, P.; Biasini, C.; Zangrandi, A.; Michieletti, E.; Belloni, E.; Cavanna, L. Metastatic breast cancer to the gastrointestinal tract: Report of five cases and review of the literature. Int. J. Breast Cancer 2012, 4, 439023. [Google Scholar] [CrossRef]
- Janjic, O.; Labgaa, I.; Hübner, M.; Demartines, N.; Joliat, G.R. Metastasis to the rectum: A systematic review of the literature. Eur. J. Surg. Oncol. 2022, 48, 822–833. [Google Scholar] [CrossRef]
- Lior, T.; Chin Ng, S.; Ng, M.K. A rare case of linitis plastica of the colon from ovarian carcinoma. J. Surg. Case Rep. 2019, 2019, rjz089. [Google Scholar] [CrossRef]
- Ha, H.K.; Jee, K.R.; Yu, E.; Yu, C.S.; Rha, S.E.; Lee, I.J.; Hee, J.Y.; Jin, C.K.; Kun, C.P.; Ho, Y. CT features of metastatic linitis plastica to the rectum in patients with peritoneal carcinomatosis. AJR Am. J. Roentgenol. 2000, 174, 463–466. [Google Scholar] [CrossRef]
- Fernet, P.; Azar, H.A.; Stout, A.P. Intramural (tubal) spread of linitis plastica along the alimentary tract. Gastroenterology 1965, 48, 419–424. [Google Scholar] [CrossRef]
- Burgain, C.; Germain, A.; Bastien, C.; Orry, X.; Choné, L.; Claudon, M.; Laurent, V. Computed tomography features of gastrointestinal linitis plastica: Spectrum of findings in early and delayed phase imaging. Abdom. Radiol. 2016, 41, 1370–1377. [Google Scholar] [CrossRef]
- Lau, L.C.; Wee, B.; Wang, S.; Thian, Y.L. Metastatic breast cancer to the rectum: A case report with emphasis on MRI features. Medicine 2017, 96, e6739. [Google Scholar] [CrossRef] [PubMed]
- Rudralingam, V.; Dobson, M.J.; Pitt, M.; Stewart, D.J.; Hearn, A.; Susnerwala, S. MR imaging of linitis plastica of the rectum. AJR Am. J. Roentgenol. 2003, 181, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Mazza, S.; Laurenza, C.; Elvo, B.; Tanzi, G.; Ungari, M.; Soro, S.; Verga, M.C.; Drago, A.; Grassia, R. Rectal linitis plastica as the first presentation of metastatic lobular breast cancer: An endoscopic ultrasound diagnosis. Clin. J. Gastroenterol. 2022, 15, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.C.; Kies, D.A.; van der Laan, J.; Wonders, J. Linitis plastica of the rectum secondary to prostate carcinoma. BMJ Case Rep. 2022, 15, e248462. [Google Scholar] [CrossRef]
- Dresen, R.C.; Beets, G.L.; Rutten, H.J.T.; Engelen, S.M.E.; Lahaye, M.J.; Vliegen, R.F.A.; de Bruïne, A.P.; Kessels, A.G.H.; Lammering, G.; Beets-Tan, R.G.H. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part I. Are we able to predict tumor confined to the rectal wall? Radiology 2009, 252, 71–80. [Google Scholar] [CrossRef]
- Franceschini, G.; Manno, A.; Mulè, A.; Verbo, A.; Rizzo, G.; Sermoneta, D.; Petito, L.; D’ALba, P.; Maggiore, C.; Terribile, D.; et al. Gastro-intestinal symptoms as clinical manifestation of peritoneal and retroperitoneal spread of an invasive lobular breast cancer: Report of a case and review of the literature. BMC Cancer 2006, 6, 193. [Google Scholar] [CrossRef]
- Ruymbeke, H.; Harlet, L.; Stragier, B.; Steenkiste, E.; Ryckx, M.; Marolleau, F. Anorectal metastasis from breast carcinoma: A case report and review of the literature. BMC Res. Notes 2018, 11, 268. [Google Scholar] [CrossRef]
- Taal, B.G.; den Hartog Jager, F.C.; Steinmetz, R.; Peterse, H. The spectrum of gastrointestinal metastases of breast carcinoma: II. The colon and rectum. Gastrointest. Endosc. 1992, 38, 136–141. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J. Gastric cancer with large bowel obstruction as the first presentation: A case report. Oncol. Lett. 2013, 6, 1377–1379. [Google Scholar] [CrossRef]
- Tariq, T.; Turk, A.; Reaume, M.; Muddasani, A.; Parmar, M. Blocked by a Ring: A Case of Gastric Linitis Plastica Presenting as Large Bowel Obstruction Secondary to Rectal Stenosis. ACG Case Rep. J. 2019, 6, e00007. [Google Scholar] [CrossRef] [PubMed]
- Benfiguig, A.; Anciaux, M.L.; Eugène, C.I.; Benkémoun, G.; Etienne, J.C. Gastric metastasis of breast cancer occurring after a cancer-free interval of 30 years. Ann. Gastroenterol. Hepatol. 1992, 28, 175–177. [Google Scholar]
- Rydén, L.; Chebil, G.; Jönsson, P.-E. Small bowel obstruction caused by intestinal metastases from undiagnosed breast cancer: Report of two cases. Eur. J. Surg. 2002, 168, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Greco, F.A.; Pavlidis, N.; Daugaard, G.; Oien, K.; Pentheroudakis, G.; ESMO Guidelines Committee. Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v133–v138. [Google Scholar] [CrossRef]
- Fayemi, A.O.; Ali, M.; Braun, E.V. Metastatic carcinoma simulating linitis plastica of the colon. A case report. Am. J. Gastroenterol. 1979, 71, 311–314. [Google Scholar]
- Meyers, M.A. Intraperitoneal spread of malignancies and its effect on the bowel. Clin. Radiol. 1981, 32, 129–146. [Google Scholar] [CrossRef]
- Haberstich, R.; Tuech, J.J.; Wilt, M.; Rodier, J.F. Anal localization as first manifestation of metastatic ductal breast carcinoma. Tech. Coloproctol. 2005, 9, 237–238. [Google Scholar] [CrossRef]
- Harris, M.; Howell, A.; Chrissohou, M.; Swindell, R.I.; Hudson, M.; Sellwood, R.A. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating duct carcinoma of the breast. Br. J. Cancer 1984, 5, 23–30. [Google Scholar] [CrossRef]
- Ferri, L.E.; Onerheim, R.; Emond, C. Linitis plastica as the first indication of metastatic lobular carcinoma of the breast: Case report and literature review. Can. J. Surg. 1999, 42, 466–469. [Google Scholar]
- Hsieh, P.S.; Yeh, C.Y.; Chen, J.R.; Changchien, C.R. Ileocecal breast carcinoma metastasis. Int. J. Color. Dis. 2004, 19, 607–608. [Google Scholar] [CrossRef] [PubMed]
- Tot, T. The role of cytokeratins 20 and 7 and estrogen receptor analysis in separation of metastatic lobular carcinoma of the breast and metastatic signet ring cell carcinoma of the gastrointestinal tract. APMIS 2000, 108, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.S.; Pantongrag-Brown, L.; Aguilera, N.S.; Buck, J.L.; Buetow, P.C. Non-Hodgkin lymphoma of the stomach: A cause of linitis plastica. Radiology 1996, 201, 375–378. [Google Scholar] [CrossRef]
- Ozyilkan, O.; Ozyilkan, E. Gastric Hodgkin’s disease presenting with radiological appearance of linitis plastica. Am. J. Gastroenterol. 1999, 94, 3661–3662. [Google Scholar] [CrossRef]
- Lo Re, G.; Federica, V.; Midiri, F.; Picone, D.; La Tona, G.; Galia, M.; Casto, A.L.; Lagalla, R.; Midiri, M. Radiological Features of Gastrointestinal Lymphoma. Gastroenterol. Res. Pract. 2016, 2016, 2498143. [Google Scholar] [CrossRef]
- Cervia, J.S. Gastric Kaposi’s sarcoma without skin lesions presenting as linitis plastica. Gastrointest. Endosc. 1992, 38, 96. [Google Scholar] [CrossRef]
- Hadjiyane, C.; Lee, Y.H.; Stein, L.; Jayagopal, S.; Shih, H.; Pellecchia, C. Kaposi’s sarcoma presenting as linitis plastica. Am. J. Gastroenterol. 1991, 86, 1823–1825. [Google Scholar]
- Murray, J.G.; Evans, S.J.; Jeffrey, P.B.; Halvorsen, R.A., Jr. Cytomegalovirus colitis in AIDS: CT features. AJR Am. J. Roentgenol. 1995, 165, 67–71. [Google Scholar] [CrossRef]
- Abdu, R.A.; Carter, K.; Pomidor, W.J. Gastric syphilis mimicking linitis plastica. Arch. Surg. 1993, 128, 103–104. [Google Scholar] [CrossRef]
- Rubesin, S.E.; Levine, M.S.; Laufer, I. Double-contrast upper gastrointestinal radiography: A pattern approach for diseases of the stomach. Radiology 2008, 246, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Calafat, P.; de Diller, A.B.; Sanchez, C. Breast carcinoma metastasis in ileum-colon and gallbladder simulating inflammatory diseases. Rev. Fac. Cien Med. Univ. Nac. Cordoba 1999, 56, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Macari, M.; Balthazar, E.J. CT of bowel wall thickening: Significance and pitfalls of interpretation. AJR Am. J. Roentgenol. 2001, 176, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Macari, M.; Megibow, A.J.; Balthazar, E.J. A pattern approach to the abnormal small bowel: Observations at MDCT and CT enterography. AJR Am. J. Roentgenol. 2007, 188, 1344–1355. [Google Scholar] [CrossRef]
- DiPiro, P.J.; Tirumani, S.H.; Cruz, G.P.; Ramaiya, N.H.; Lester, S.C.; Shinagare, A.B. Lobular breast cancer: Patterns of intraabdominal metastatic spread on imaging and prognostic significance. Abdom. Radiol. 2019, 44, 362–369. [Google Scholar] [CrossRef]
- Hristova, L.; Soyer, P.; Hoeffel, C.; Marteau, P.; Oussalah, A.; Lavergne-Slove, A.; Boudiaf, M.; Dohan, A.; Laurent, V. Colorectal cancer in inflammatory bowel diseases: CT features with pathological correlation. Abdom. Imaging 2013, 38, 421–435. [Google Scholar] [CrossRef]
- Dória, M.T.; Maesaka, J.Y.; Filho, S.N.; Silveira, T.P.; Boufelli, G.; Siqueira, S.A.C.; Baracat, E.C.; Filassi, J.R. Gastric metastasis as the first manifestation of an invasive lobular carcinoma of the breast. Autops. Case Rep. 2015, 5, 49–53. [Google Scholar] [CrossRef]
- Manohar, P.M.; Davidson, N.E. Updates in endocrine therapy for metastatic breast cancer. Cancer Biol. Med. 2021, 19, 202–212. [Google Scholar] [CrossRef]
- Ethier, S.P. Using Functional Genomics and Artificial Intelligence to Reverse Engineer Human Cancer Cells; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2023; p. 161. [Google Scholar]

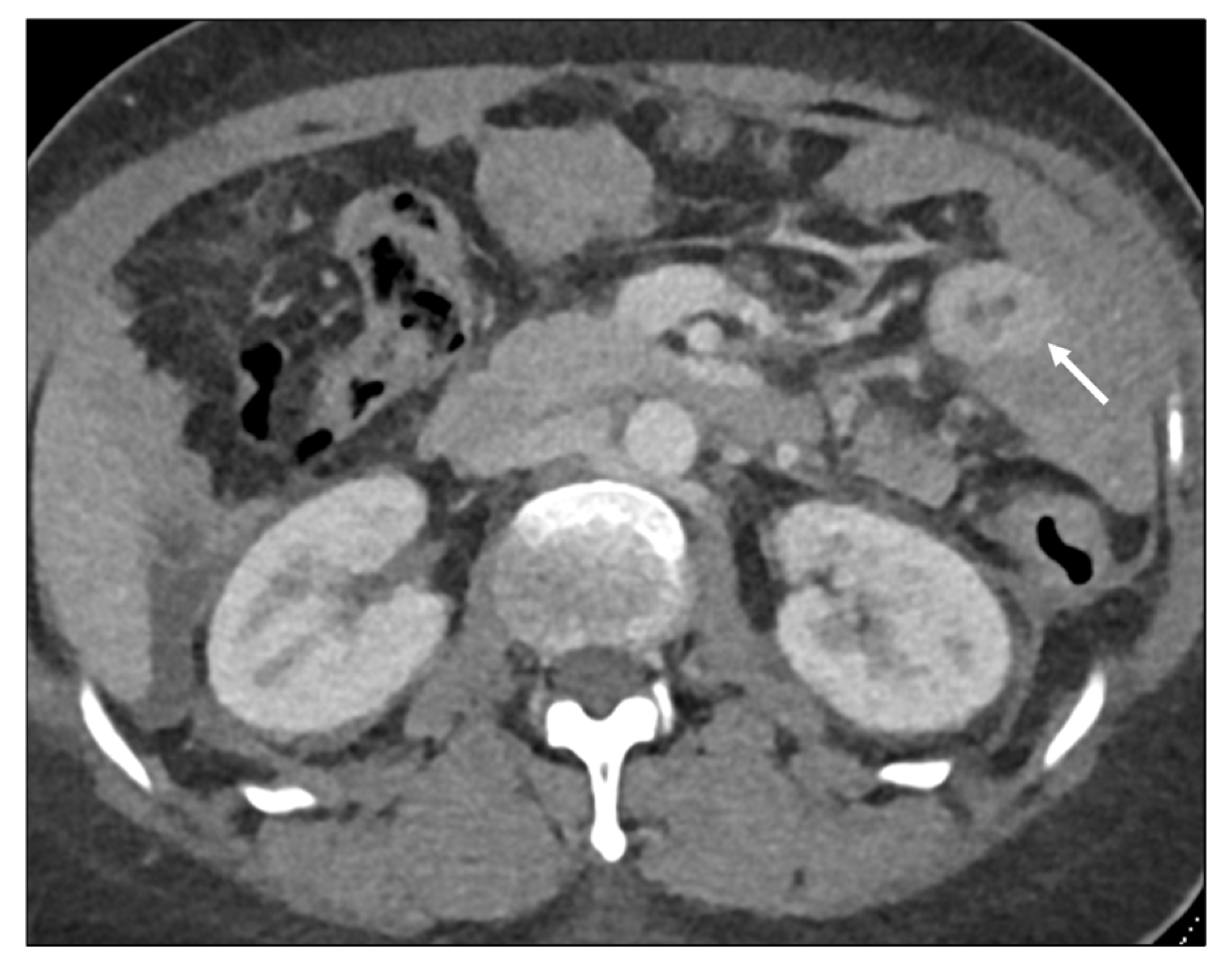
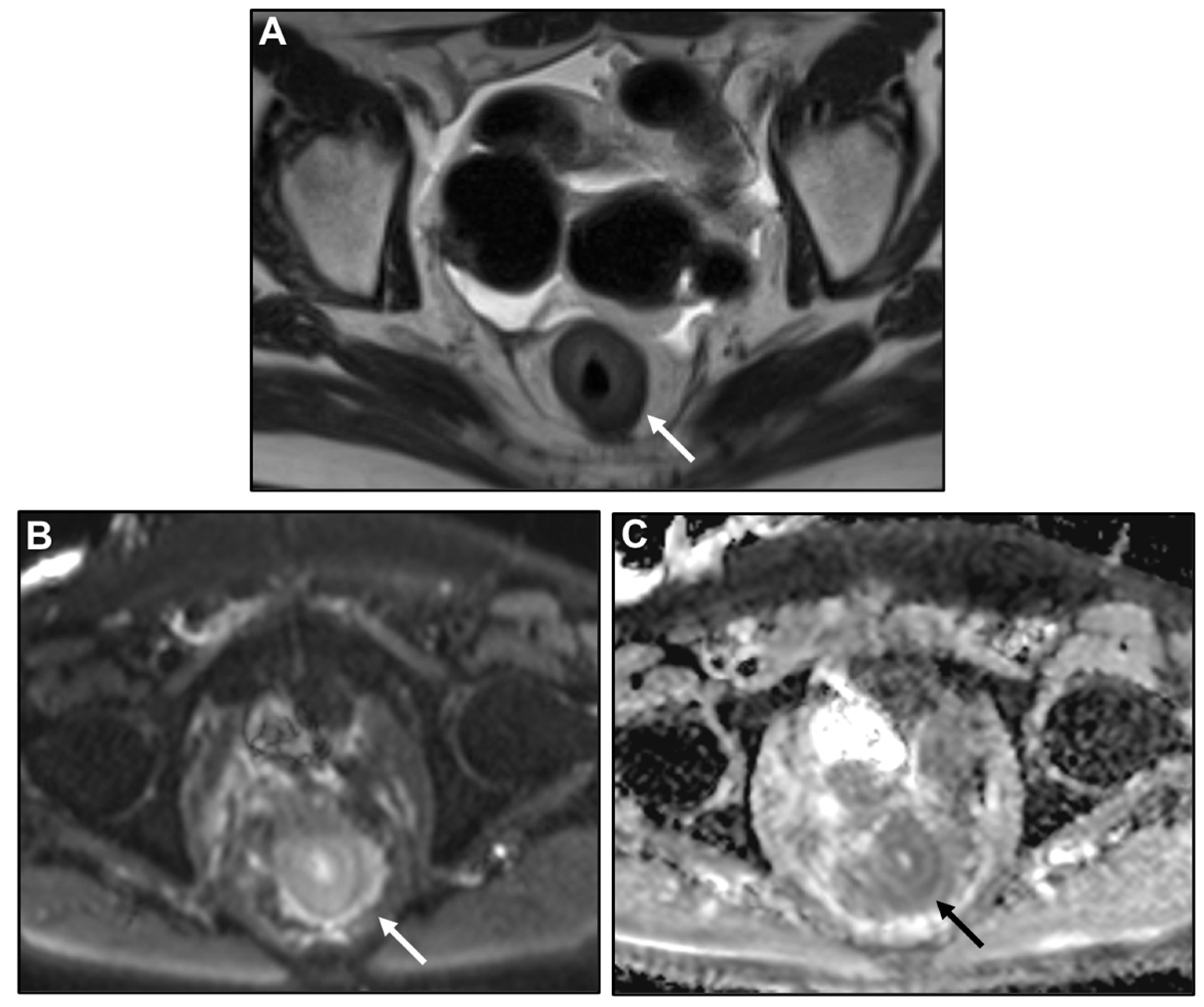
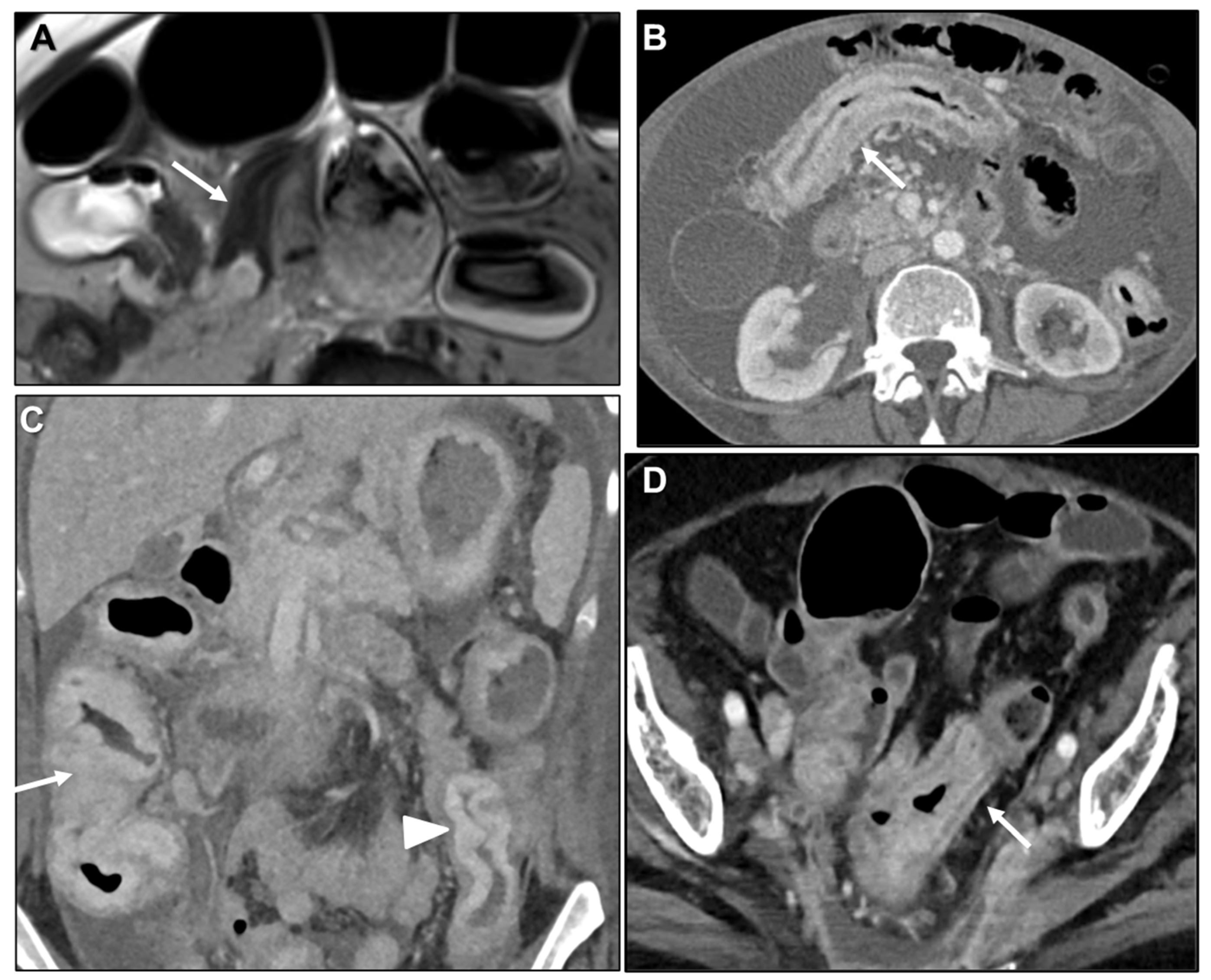
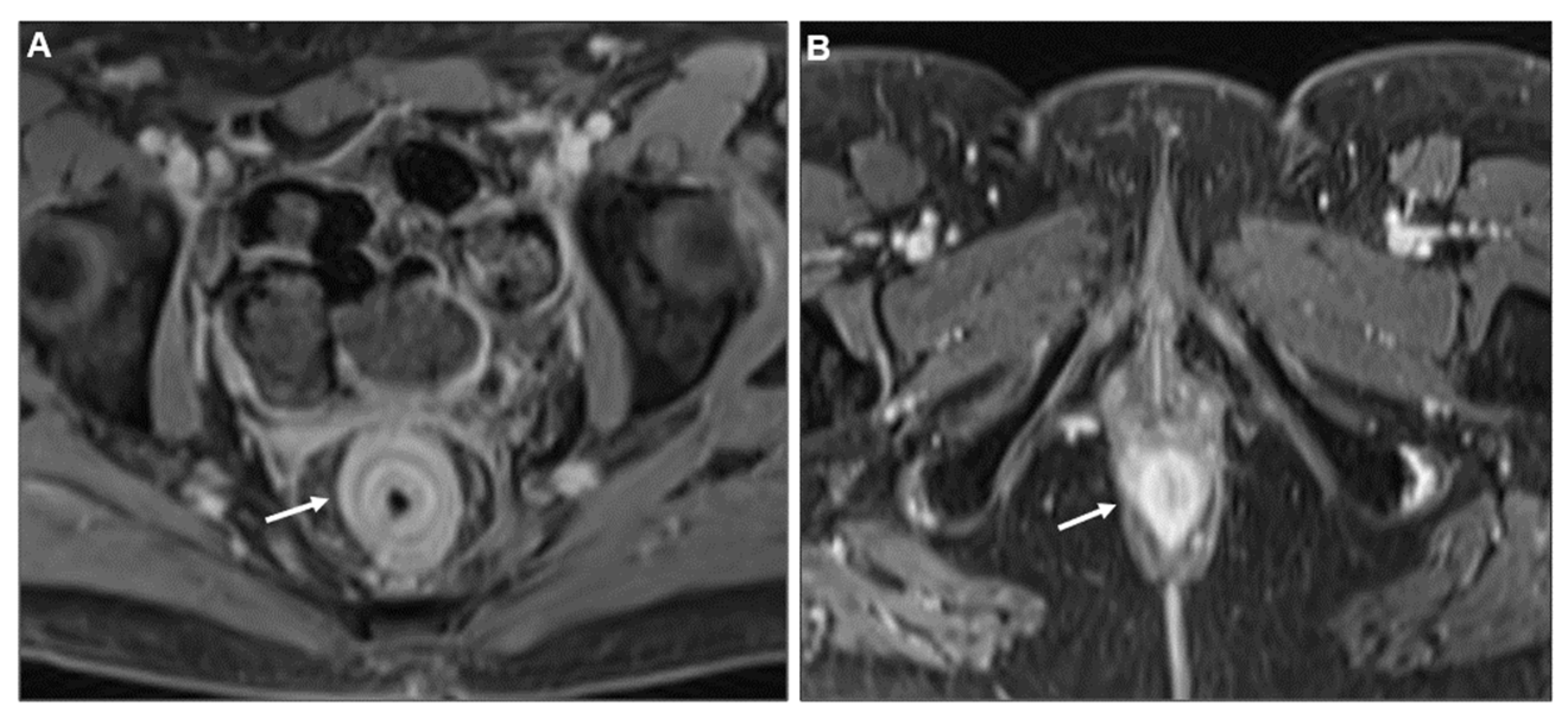
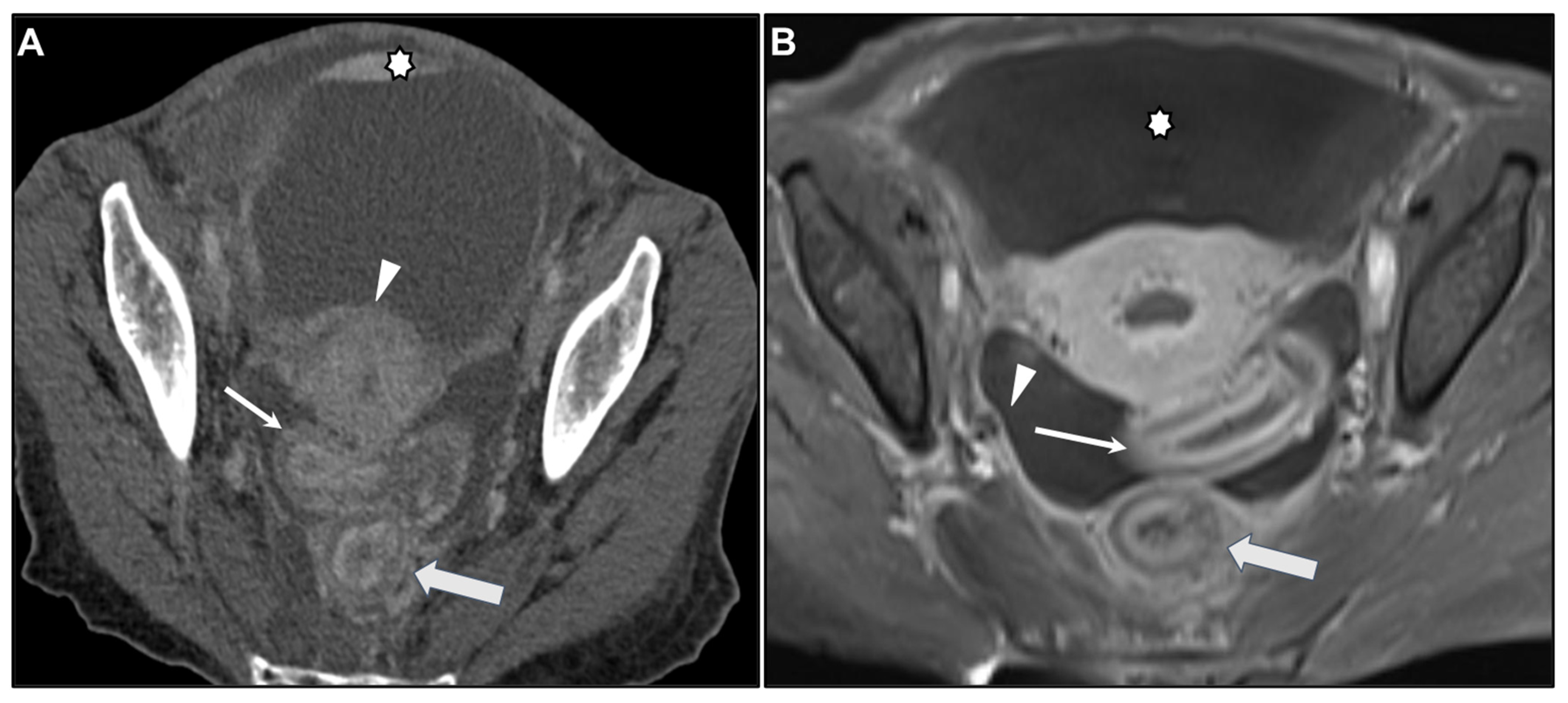
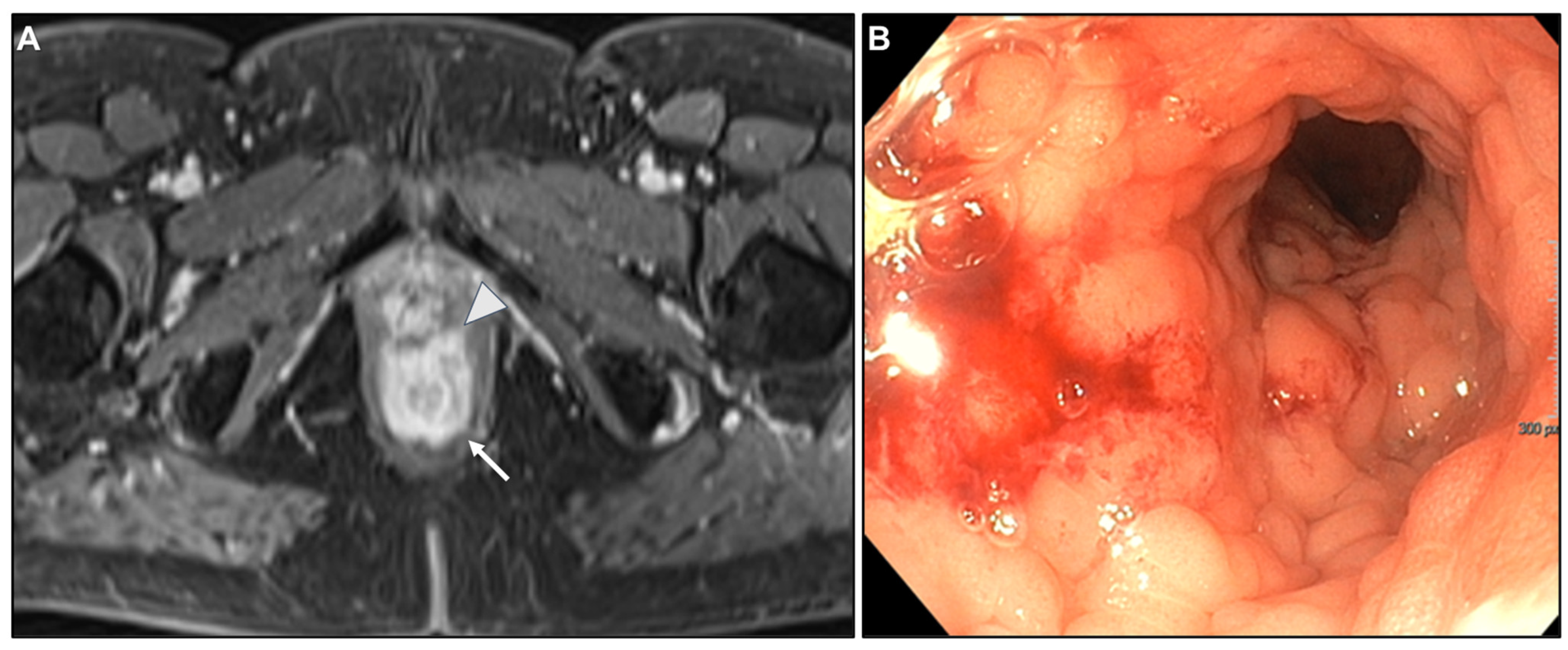

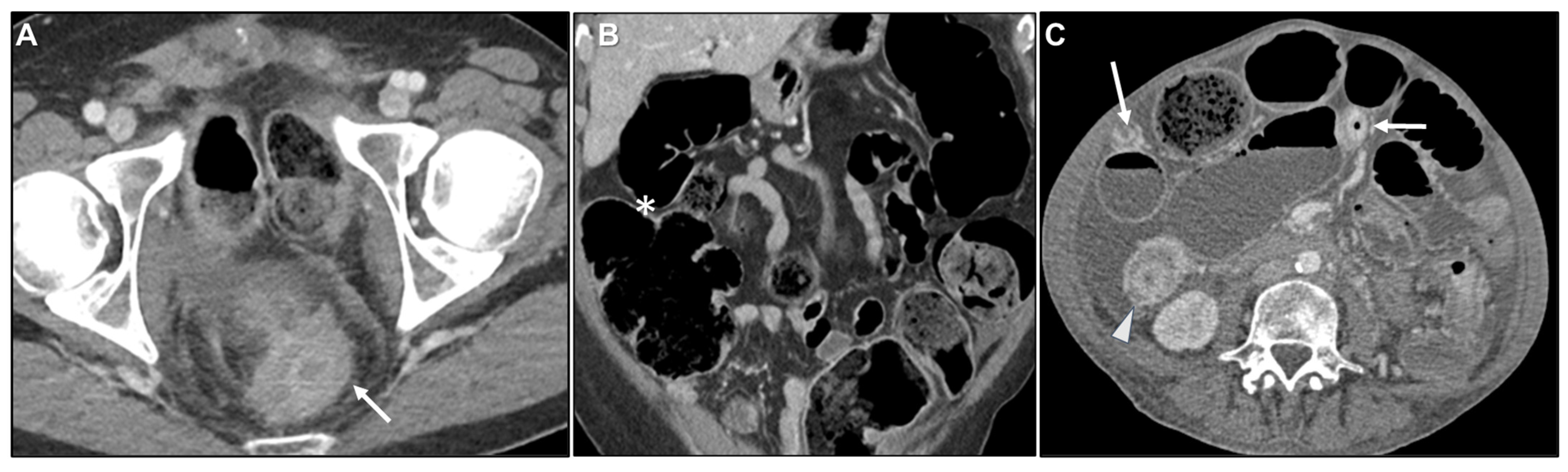
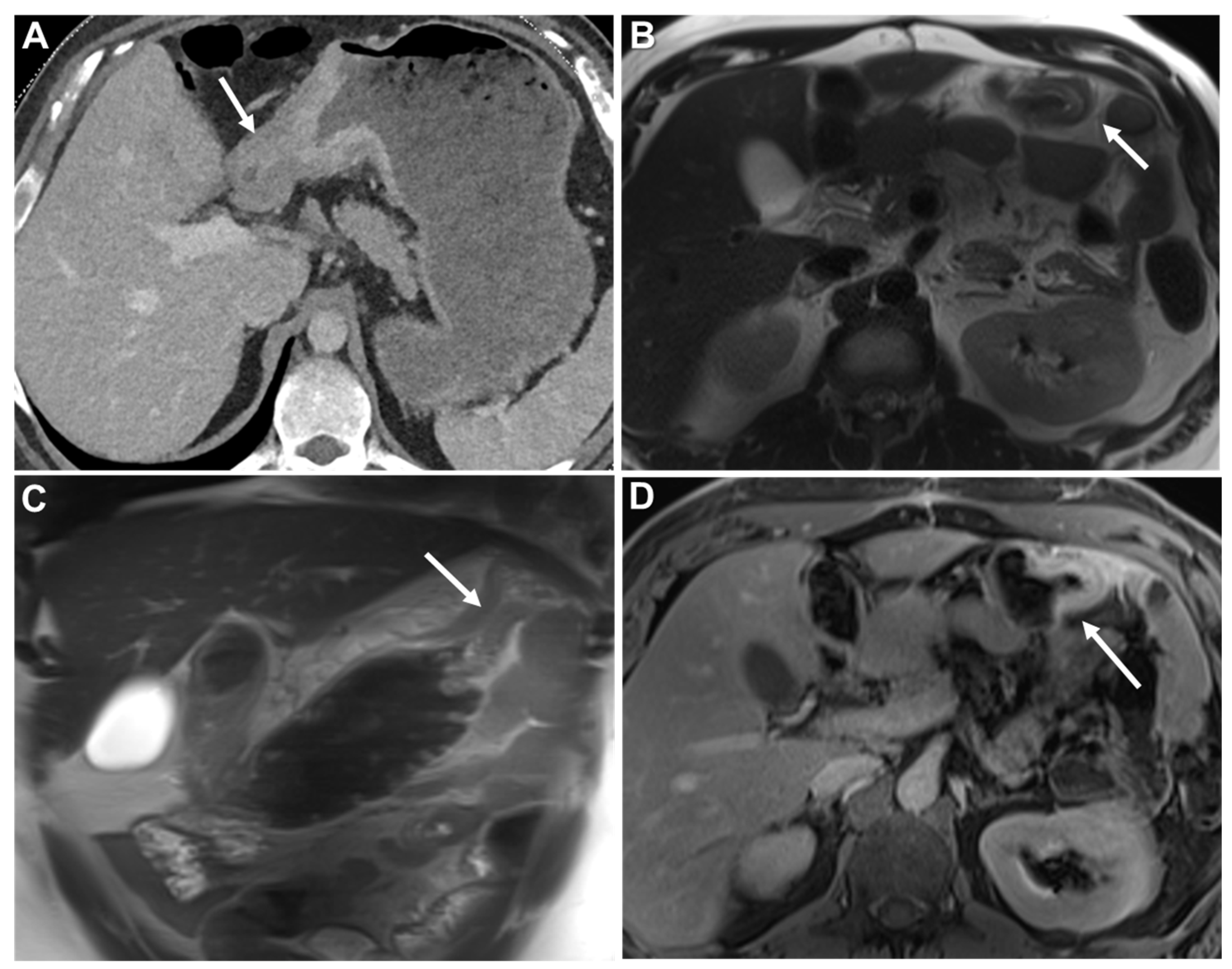


| Key Imaging Features |
|---|
| Malignant target sign |
| Delayed homogeneous enhancement |
| Concentric ring pattern |
| Associated Imaging Features |
|---|
| Peritoneal carcinomatosis |
| Presacral fat tissue and rectovaginal septum infiltration |
| Extramural tumour component markedly hypointense on T2WI |
| Intestinal obstruction |
| Malignant | Benign |
|---|---|
| Primary adenocarcinoma | Infectious (CMV, syphilis, tuberculosis) |
| Hodgkin and non-Hodgkin gastric lymphoma | Inflammatory diseases (sarcoidosis, acute Crohn’s disease, radiation induced changes) |
| Kaposi’s sarcoma | Chronic inflammatory bowel disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veron Sanchez, A.; Canales Lachen, E.; Gomez Galdon, M.; Moretti, L.; Maris, C.; Bucalau, A.M.; Khaled, C.; Bali, M.A. Linitis Plastica-like Metastases to the Gastrointestinal Tract on Cross-Sectional Imaging. Biomedicines 2025, 13, 2197. https://doi.org/10.3390/biomedicines13092197
Veron Sanchez A, Canales Lachen E, Gomez Galdon M, Moretti L, Maris C, Bucalau AM, Khaled C, Bali MA. Linitis Plastica-like Metastases to the Gastrointestinal Tract on Cross-Sectional Imaging. Biomedicines. 2025; 13(9):2197. https://doi.org/10.3390/biomedicines13092197
Chicago/Turabian StyleVeron Sanchez, Ana, Elena Canales Lachen, Maria Gomez Galdon, Luigi Moretti, Calliope Maris, Ana Maria Bucalau, Charif Khaled, and Maria Antonietta Bali. 2025. "Linitis Plastica-like Metastases to the Gastrointestinal Tract on Cross-Sectional Imaging" Biomedicines 13, no. 9: 2197. https://doi.org/10.3390/biomedicines13092197
APA StyleVeron Sanchez, A., Canales Lachen, E., Gomez Galdon, M., Moretti, L., Maris, C., Bucalau, A. M., Khaled, C., & Bali, M. A. (2025). Linitis Plastica-like Metastases to the Gastrointestinal Tract on Cross-Sectional Imaging. Biomedicines, 13(9), 2197. https://doi.org/10.3390/biomedicines13092197






