Inhibiting UNC13B Suppresses Cell Proliferation by Upregulating the Apoptotic Pathway in Multiple Myeloma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Transfection and Selection
2.3. Quantitative Real-Time PCR (qPCR) for UNC13B Expression
2.4. Cell Proliferation Assay (CCK-8)
2.5. Soft Agar Colony Formation Assay
2.6. Cell Cycle Analysis
2.7. Apoptosis Assay
2.8. Western Blotting
2.9. Data Analysis
3. Results
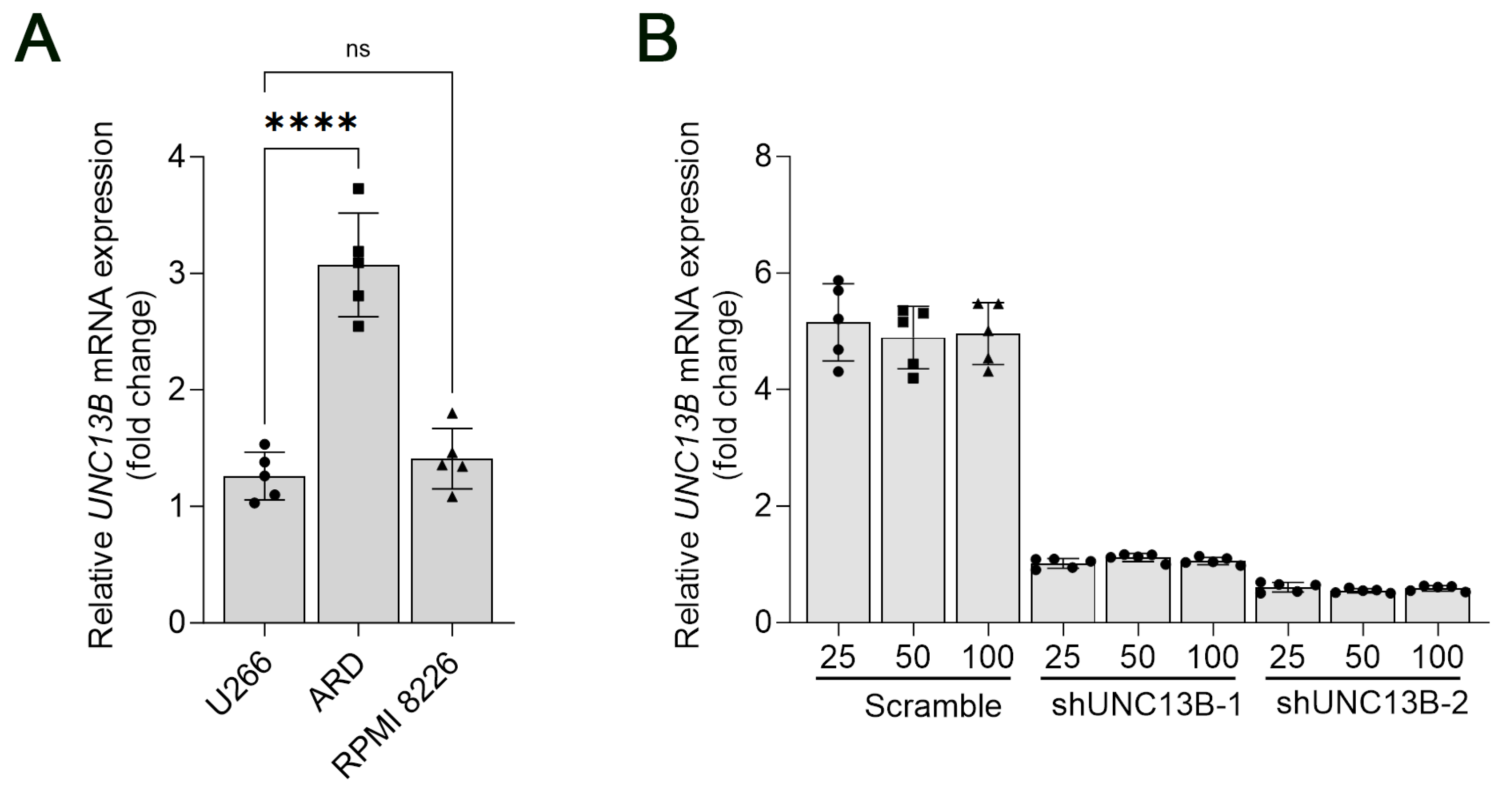
3.1. Upregulation of UNC13B Gene Expression in Multiple Myeloma Cell Lines
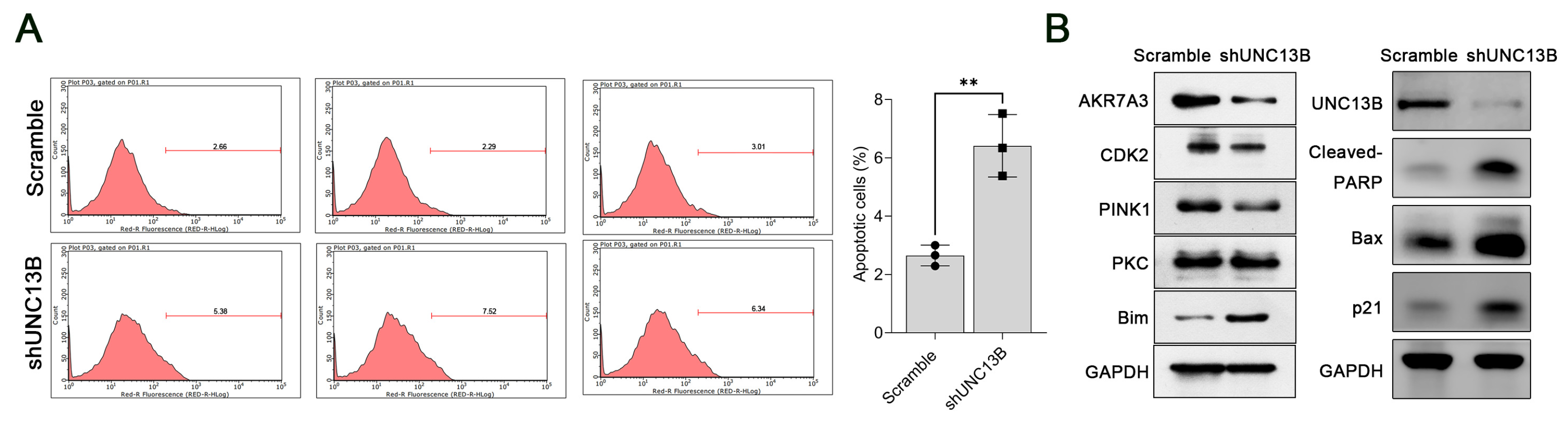
3.2. Downregulation of UNC13B Inhibits Cell Proliferation and Induces Apoptosis in ARD Cells
3.3. UNC13B Modulates the Expression of PINK1, CDK2, AKR7A3, and Bim
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MM | Multiple myeloma |
| MGUS | Monoclonal gammopathy of undetermined significance |
| SMM | Smoldering multiple myeloma |
| FBS | Fetal bovine serum |
| qPCR | Quantitative polymerase chain reaction |
| shRNA | Short hairpin RNA |
| PI | Propidium iodide |
| CCK-8 | Cell Counting Kit-8 |
| PVDF | Polyvinylidene difluoride |
| BSA | Bovine serum albumin |
| PKC | Protein kinase C |
| PINK1 | PTEN-induced kinase 1 |
| CDK2 | Cyclin-dependent kinase 2 |
| AKR7A3 | Aldo-keto reductase family 7 member A3 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| ANOVA | Analysis of variance |
| SD | Standard deviation |
References
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and management of multiple myeloma: A review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Rajkumar, V.; Kyle, R.A.; van Duin, M.; Sonneveld, P.; Mateos, M.V.; Gay, F.; Anderson, K.C. Multiple myeloma. Nat. Rev. Dis. Primers 2017, 3, 17046. [Google Scholar] [CrossRef]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global burden of multiple myeloma: A systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, staging, and management of multiple myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef]
- Minnie, S.A.; Hill, G.R. Immunotherapy of multiple myeloma. J. Clin. Investig. 2020, 130, 1565–1575. [Google Scholar] [CrossRef]
- Bazarbachi, A.H.; Al Hamed, R.; Malard, F.; Harousseau, J.L.; Mohty, M. Relapsed refractory multiple myeloma: A comprehensive overview. Leukemia 2019, 33, 2343–2357. [Google Scholar] [CrossRef]
- Manier, S.; Salem, K.Z.; Park, J.; Landau, D.A.; Getz, G.; Ghobrial, I.M. Genomic complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 100–113. [Google Scholar] [CrossRef]
- Mateos, M.V.; Landgren, O. Mgus and smoldering multiple myeloma: Diagnosis and epidemiology. Cancer Treat. Res. 2016, 169, 3–12. [Google Scholar] [PubMed]
- Kyle, R.A.; Remstein, E.D.; Therneau, T.M.; Dispenzieri, A.; Kurtin, P.J.; Hodnefield, J.M.; Larson, D.R.; Plevak, M.F.; Jelinek, D.F.; Fonseca, R.; et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N. Engl. J. Med. 2007, 356, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fulciniti, M.; Samur, M.K.; Ho, M.; Deng, S.; Liu, L.; Wen, K.; Yu, T.; Chyra, Z.; Dereibal, S.; et al. Ywhae/14-3-3epsilon expression impacts the protein load, contributing to proteasome inhibitor sensitivity in multiple myeloma. Blood 2020, 136, 468–479. [Google Scholar] [CrossRef]
- Franqui-Machin, R.; Hao, M.; Bai, H.; Gu, Z.; Zhan, X.; Habelhah, H.; Jethava, Y.; Qiu, L.; Frech, I.; Tricot, G.; et al. Destabilizing nek2 overcomes resistance to proteasome inhibition in multiple myeloma. J. Clin. Investig. 2018, 128, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz-Krzeminska, I.; Krzeminski, P.; Corchete, L.A.; Quwaider, D.; Rojas, E.A.; Herrero, A.B.; Gutierrez, N.C. Factors regulating microrna expression and function in multiple myeloma. Non-Coding RNA 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Pawlyn, C.; Morgan, G.J. Evolutionary biology of high-risk multiple myeloma. Nat. Rev. Cancer 2017, 17, 543–556. [Google Scholar] [CrossRef]
- Swamydas, M.; Murphy, E.V.; Ignatz-Hoover, J.J.; Malek, E.; Driscoll, J.J. Deciphering mechanisms of immune escape to inform immunotherapeutic strategies in multiple myeloma. J. Hematol. Oncol. 2022, 15, 17. [Google Scholar] [CrossRef]
- Russell, B.M.; Avigan, D.E. Immune dysregulation in multiple myeloma: The current and future role of cell-based immunotherapy. Int. J. Hematol. 2023, 117, 652–659. [Google Scholar] [CrossRef]
- Dittman, J.S. Unc13: A multifunctional synaptic marvel. Curr. Opin. Neurobiol. 2019, 57, 17–25. [Google Scholar] [CrossRef]
- Michelassi, F.; Liu, H.; Hu, Z.; Dittman, J.S. A c1-c2 module in munc13 inhibits calcium-dependent neurotransmitter release. Neuron 2017, 95, 577–590.e5. [Google Scholar] [CrossRef]
- Nakamura, T.; Jimbo, K.; Nakajima, K.; Tsuboi, T.; Kato, T. De novo unc13b mutation identified in a bipolar disorder patient increases a rare exon-skipping variant. Neuropsychopharmacol. Rep. 2018, 38, 210–213. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, J.D.; Liu, X.R.; Liu, D.T.; Chen, Y.H.; Wu, Y.; Sun, Y.; Yu, J.; Ren, R.N.; Mei, Z.; et al. Unc13b variants associated with partial epilepsy with favourable outcome. Brain A J. Neurol. 2021, 144, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Green, T.E.; Scheffer, I.E.; Berkovic, S.F.; Hildebrand, M.S. Unc13b and focal epilepsy. Brain A J. Neurol. 2022, 145, e10–e12. [Google Scholar] [CrossRef]
- Banushi, B.; Joseph, S.R.; Lum, B.; Lee, J.J.; Simpson, F. Endocytosis in cancer and cancer therapy. Nat. Rev. Cancer 2023, 23, 450–473. [Google Scholar] [CrossRef]
- Mellman, I.; Yarden, Y. Endocytosis and cancer. Cold Spring Harb. Perspect. Biol. 2013, 5, a016949. [Google Scholar] [CrossRef]
- Goh, L.K.; Sorkin, A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a017459. [Google Scholar] [CrossRef]
- Hendrix, A.; Westbroek, W.; Bracke, M.; De Wever, O. An ex(o)citing machinery for invasive tumor growth. Cancer Res. 2010, 70, 9533–9537. [Google Scholar] [CrossRef]
- Wang, X.B.; Yuan, L.H.; Yan, L.P.; Ye, Y.B.; Lu, B.; Xu, X. Unc13b promote arsenic trioxide resistance in chronic lymphoid leukemia through mitochondria quality control. Front. Oncol. 2022, 12, 920999. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Begum, N.; Tryphena, K.P.; Singh, S.B.; Srivastava, S.; Rai, S.N.; Vamanu, E.; Khatri, D.K. Inter and intracellular mitochondrial transfer: Future of mitochondrial transplant therapy in parkinson’s disease. Biomed. Pharmacother. Biomed. Pharmacother. 2023, 159, 114268. [Google Scholar] [CrossRef]
- Han, H.; Tan, J.; Wang, R.; Wan, H.; He, Y.; Yan, X.; Guo, J.; Gao, Q.; Li, J.; Shang, S.; et al. Pink1 phosphorylates drp1(s616) to regulate mitophagy-independent mitochondrial dynamics. EMBO Rep. 2020, 21, e48686. [Google Scholar] [CrossRef]
- Yu, S.; Du, M.; Yin, A.; Mai, Z.; Wang, Y.; Zhao, M.; Wang, X.; Chen, T. Bcl-xl inhibits pink1/parkin-dependent mitophagy by preventing mitochondrial parkin accumulation. Int. J. Biochem. Cell Biol. 2020, 122, 105720. [Google Scholar] [CrossRef] [PubMed]
- Burchell, V.S.; Nelson, D.E.; Sanchez-Martinez, A.; Delgado-Camprubi, M.; Ivatt, R.M.; Pogson, J.H.; Randle, S.J.; Wray, S.; Lewis, P.A.; Houlden, H.; et al. The parkinson’s disease-linked proteins fbxo7 and parkin interact to mediate mitophagy. Nat. Neurosci. 2013, 16, 1257–1265. [Google Scholar] [CrossRef]
- Jin, Y.; Penning, T.M. Aldo-keto reductases and bioactivation/detoxication. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M. Human aldo-keto reductases and the metabolic activation of polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2014, 27, 1901–1917. [Google Scholar] [CrossRef]
- Hlavac, V.; Brynychova, V.; Vaclavikova, R.; Ehrlichova, M.; Vrana, D.; Pecha, V.; Trnkova, M.; Kodet, R.; Mrhalova, M.; Kubackova, K.; et al. The role of cytochromes p450 and aldo-keto reductases in prognosis of breast carcinoma patients. Medicine 2014, 93, e255. [Google Scholar] [CrossRef]
- Chow, R.K.K.; Tsz-Kwan Sin, S.; Liu, M.; Li, Y.; Man Chan, T.H.; Song, Y.; Chen, L.; Lai-Wan Kwong, D.; Guan, X.Y. Akr7a3 suppresses tumorigenicity and chemoresistance in hepatocellular carcinoma through attenuation of erk, c-jun and nf-kappab signaling pathways. Oncotarget 2017, 8, 83469–83479. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Song, Y.; Min, J.; Wang, R.; Li, H.; Zhu, L.; Guo, Y.; Gan, D.; Li, S.; Ma, P.; et al. Akr7a3 modulates the metastasis of pancreatic ductal adenocarcinoma through regulating phgdh-suppressed autophagy. Cancer Sci. 2023, 114, 3101–3113. [Google Scholar] [CrossRef]
- Ansari, U.; Chen, V.; Sedighi, R.; Syed, B.; Muttalib, Z.; Ansari, K.; Ansari, F.; Nadora, D.; Razick, D.; Lui, F. Role of the unc13 family in human diseases: A literature review. AIMS Neurosci. 2023, 10, 388–400. [Google Scholar] [CrossRef]
- Man, K.N.; Imig, C.; Walter, A.M.; Pinheiro, P.S.; Stevens, D.R.; Rettig, J.; Sorensen, J.B.; Cooper, B.H.; Brose, N.; Wojcik, S.M. Identification of a munc13-sensitive step in chromaffin cell large dense-core vesicle exocytosis. eLife 2015, 4, 1–28. [Google Scholar] [CrossRef]
- da Costa, V.R.; Araldi, R.P.; Vigerelli, H.; D’Amelio, F.; Mendes, T.B.; Gonzaga, V.; Policiquio, B.; Colozza-Gama, G.A.; Valverde, C.W.; Kerkis, I. Exosomes in the tumor microenvironment: From biology to clinical applications. Cells 2021, 10, 2617. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Octaviani, S.; Tian, Z.; Wang, S.R.; Huang, C.; Huang, J. Mitochondrial quality control in hematopoietic stem cells: Mechanisms, implications, and therapeutic opportunities. Stem Cell Res. Ther. 2025, 16, 180. [Google Scholar] [CrossRef]
- Chen, X.; Bao, Y.; Sun, G.; Wang, X.; Zhu, J. Unc13b regulates the sensitivity of wilms’ tumor cells to doxorubicin by modulating lysosomes. Oncol. Lett. 2024, 28, 446. [Google Scholar] [CrossRef]
- Tadesse, S.; Caldon, E.C.; Tilley, W.; Wang, S. Cyclin-dependent kinase 2 inhibitors in cancer therapy: An update. J. Med. Chem. 2019, 62, 4233–4251. [Google Scholar] [CrossRef]
- Chauhan, S.; Diril, M.K.; Lee, J.H.; Bisteau, X.; Manoharan, V.; Adhikari, D.; Ratnacaram, C.K.; Janela, B.; Noffke, J.; Ginhoux, F.; et al. Cdk2 catalytic activity is essential for meiotic cell division in vivo. Biochem. J. 2016, 473, 2783–2798. [Google Scholar] [CrossRef]
- Aleem, E.; Kiyokawa, H.; Kaldis, P. Cdc2-cyclin e complexes regulate the g1/s phase transition. Nat. Cell Biol. 2005, 7, 831–836. [Google Scholar] [CrossRef]
- Fagundes, R.; Teixeira, L.K. Cyclin e/cdk2: DNA replication, replication stress and genomic instability. Front. Cell Dev. Biol. 2021, 9, 774845. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.; Anshabo, A.T.; Portman, N.; Lim, E.; Tilley, W.; Caldon, C.E.; Wang, S. Targeting cdk2 in cancer: Challenges and opportunities for therapy. Drug Discov. Today 2020, 25, 406–413. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.; Strasser, A.; O’Reilly, L.A.; Hausmann, G.; Adams, J.M.; Cory, S.; Huang, D.C. Bim: A novel member of the bcl-2 family that promotes apoptosis. EMBO J. 1998, 17, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Saxena, S.; Singh, B.K.; Kakkar, P. Bh3-only protein bim: An emerging target in chemotherapy. Eur. J. Cell Biol. 2017, 96, 728–738. [Google Scholar] [CrossRef]
- Aira, L.E.; Villa, E.; Colosetti, P.; Gamas, P.; Signetti, L.; Obba, S.; Proics, E.; Gautier, F.; Bailly-Maitre, B.; Jacquel, A.; et al. The oncogenic tyrosine kinase lyn impairs the pro-apoptotic function of bim. Oncogene 2018, 37, 2122–2136. [Google Scholar] [CrossRef]
- Merino, D.; Best, S.A.; Asselin-Labat, M.L.; Vaillant, F.; Pal, B.; Dickins, R.A.; Anderson, R.L.; Strasser, A.; Bouillet, P.; Lindeman, G.J.; et al. Pro-apoptotic bim suppresses breast tumor cell metastasis and is a target gene of snai2. Oncogene 2015, 34, 3926–3934. [Google Scholar] [CrossRef]
- Newton, A.C. Protein kinase c: Perfectly balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 208–230. [Google Scholar] [CrossRef]
- Redig, A.J.; Platanias, L.C. The protein kinase c (pkc) family of proteins in cytokine signaling in hematopoiesis. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2007, 27, 623–636. [Google Scholar] [CrossRef]
- Koivunen, J.; Aaltonen, V.; Peltonen, J. Protein kinase c (pkc) family in cancer progression. Cancer Lett. 2006, 235, 1–10. [Google Scholar] [CrossRef]
- Kannaiyan, R.; Mahadevan, D. A comprehensive review of protein kinase inhibitors for cancer therapy. Expert Rev. Anticancer Ther. 2018, 18, 1249–1270. [Google Scholar] [CrossRef]
- Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.H. Activators and inhibitors of protein kinase c (pkc): Their applications in clinical trials. Pharmaceutics 2021, 13, 1748. [Google Scholar] [CrossRef]
- Isakov, N. Protein kinase c (pkc) isoforms in cancer, tumor promotion and tumor suppression. Semin. Cancer Biol. 2018, 48, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, S.; James, D.J.; Quintana Serrano, M.; Esquibel, J.; Woo, S.S.; Kielar-Grevstad, E.; Crummy, E.; Qurashi, R.; Kowalchyk, J.A.; Martin, T.F.J. Small molecules that inhibit the late stage of munc13-4-dependent secretory granule exocytosis in mast cells. J. Biol. Chem. 2018, 293, 8217–8229. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.A.; Czikora, A.; Kedei, N.; You, Y.; Mitchell, G.A.; Pany, S.; Ghosh, A.; Blumberg, P.M.; Das, J. Munc13 is a molecular target of bryostatin 1. Biochemistry 2019, 58, 3016–3030. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Yuan, L.; Ding, Y.; Xie, R.; Liu, F.; Zhang, Z.; Xu, X.; Wang, X. Inhibiting UNC13B Suppresses Cell Proliferation by Upregulating the Apoptotic Pathway in Multiple Myeloma. Biomedicines 2025, 13, 2086. https://doi.org/10.3390/biomedicines13092086
Tao Y, Yuan L, Ding Y, Xie R, Liu F, Zhang Z, Xu X, Wang X. Inhibiting UNC13B Suppresses Cell Proliferation by Upregulating the Apoptotic Pathway in Multiple Myeloma. Biomedicines. 2025; 13(9):2086. https://doi.org/10.3390/biomedicines13092086
Chicago/Turabian StyleTao, Yuan, Lihua Yuan, Yuntian Ding, Rongli Xie, Fangjie Liu, Zhongming Zhang, Xiaojun Xu, and Xiaobo Wang. 2025. "Inhibiting UNC13B Suppresses Cell Proliferation by Upregulating the Apoptotic Pathway in Multiple Myeloma" Biomedicines 13, no. 9: 2086. https://doi.org/10.3390/biomedicines13092086
APA StyleTao, Y., Yuan, L., Ding, Y., Xie, R., Liu, F., Zhang, Z., Xu, X., & Wang, X. (2025). Inhibiting UNC13B Suppresses Cell Proliferation by Upregulating the Apoptotic Pathway in Multiple Myeloma. Biomedicines, 13(9), 2086. https://doi.org/10.3390/biomedicines13092086





