Prognostic Risk Model of Megakaryocyte–Erythroid Progenitor (MEP) Signature Based on AHSP and MYB in Acute Myeloid Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Data
Information Processing for ScRNA-Seq
2.2. Cell-to-Cell Communication
2.3. Differential Expression Assessment Using ScRNA-Seq
2.4. GO and KEGG Analyses
2.5. Formation and Validation of a Prognostic Model Based on DEGs
2.6. Developing and Validating a Nomogram
2.7. Single-Cell Trajectory Analysis
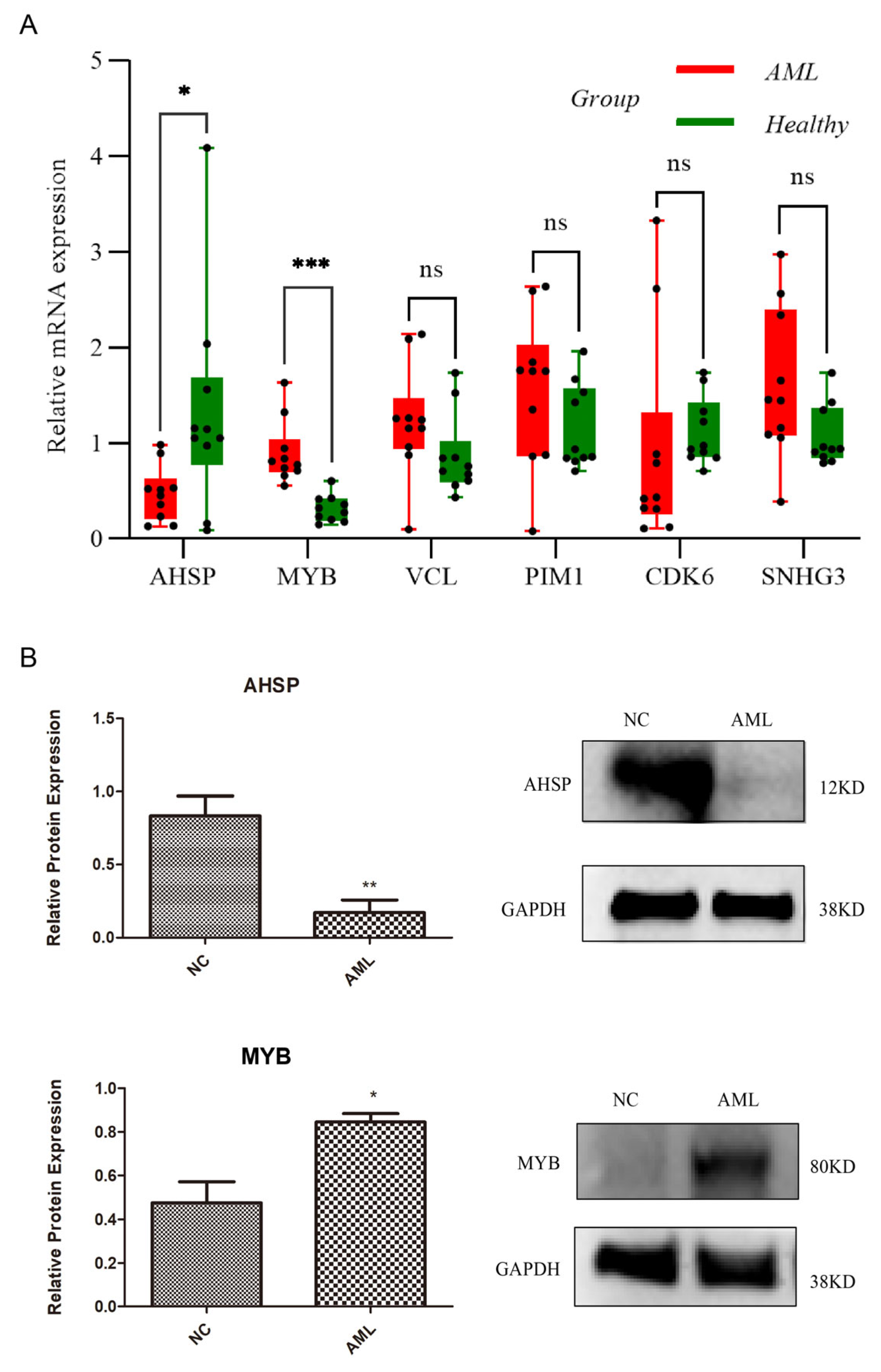
2.8. Using Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) for Validation
2.9. Western Blotting for the Verification of Post-Transcriptional Expression
3. Results
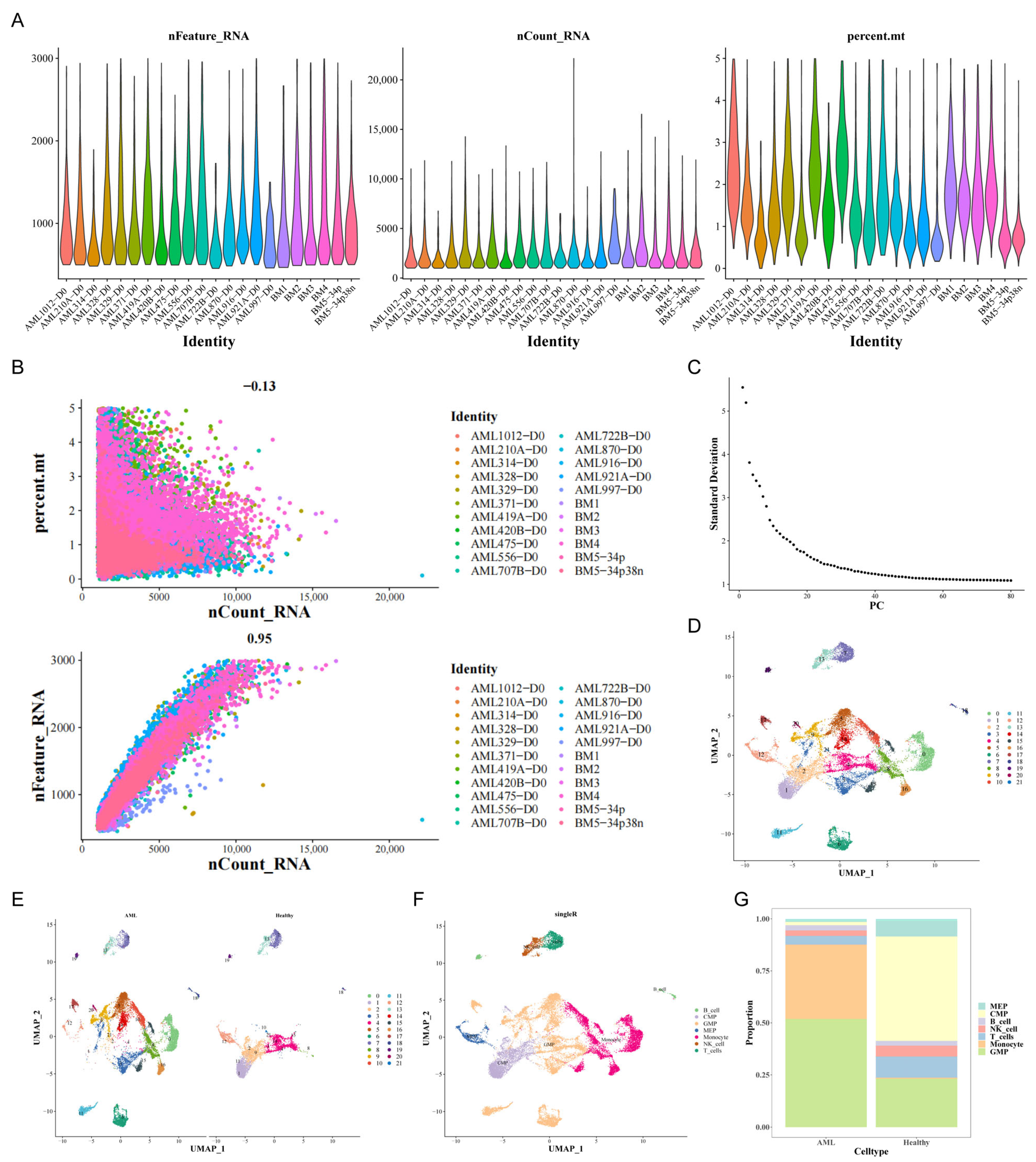
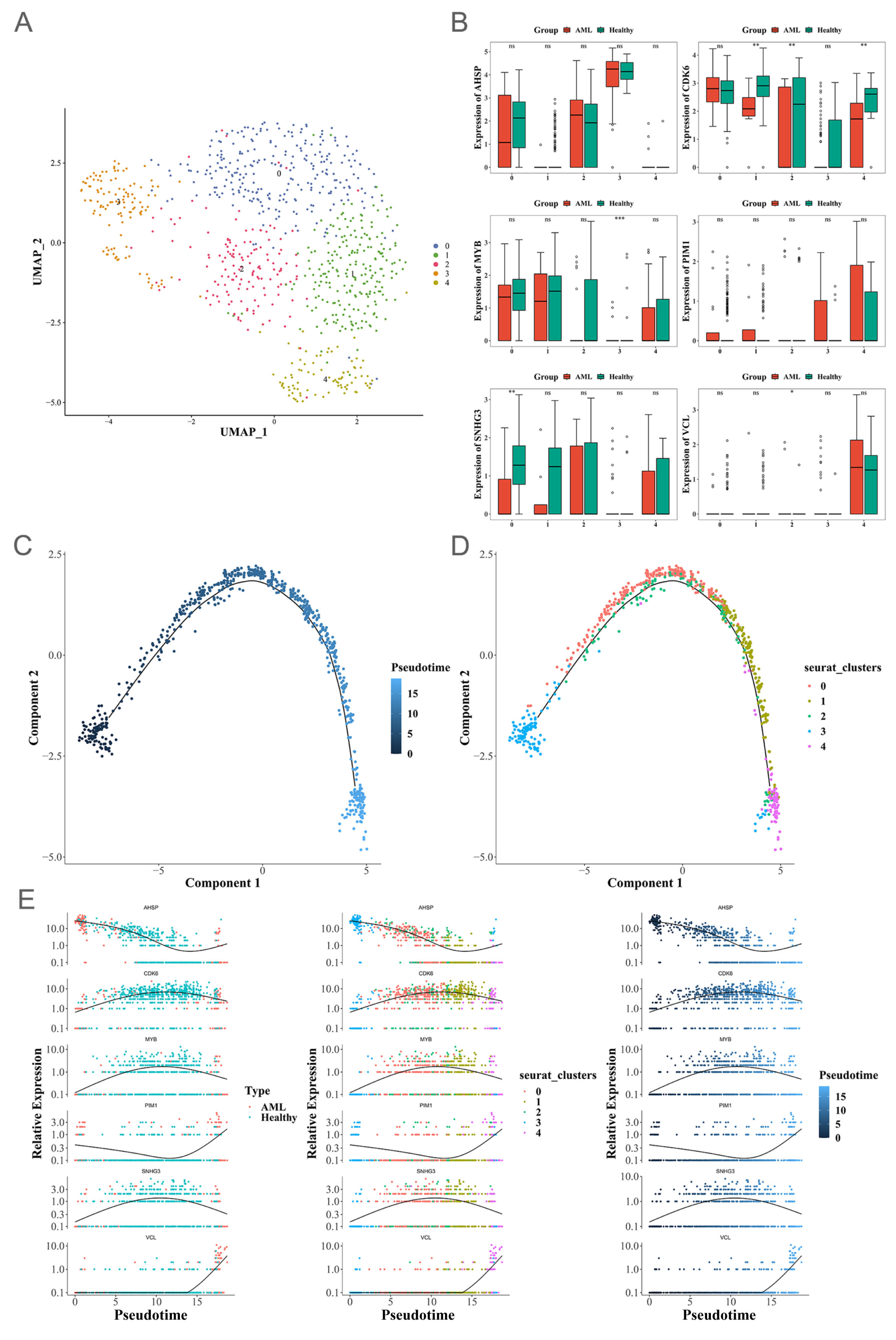
3.1. Assessment of ScRNA-Seq Data and Identification of Genes Associated with MEP
3.2. An Assessment of the Interaction Between Cells in Patients with AML
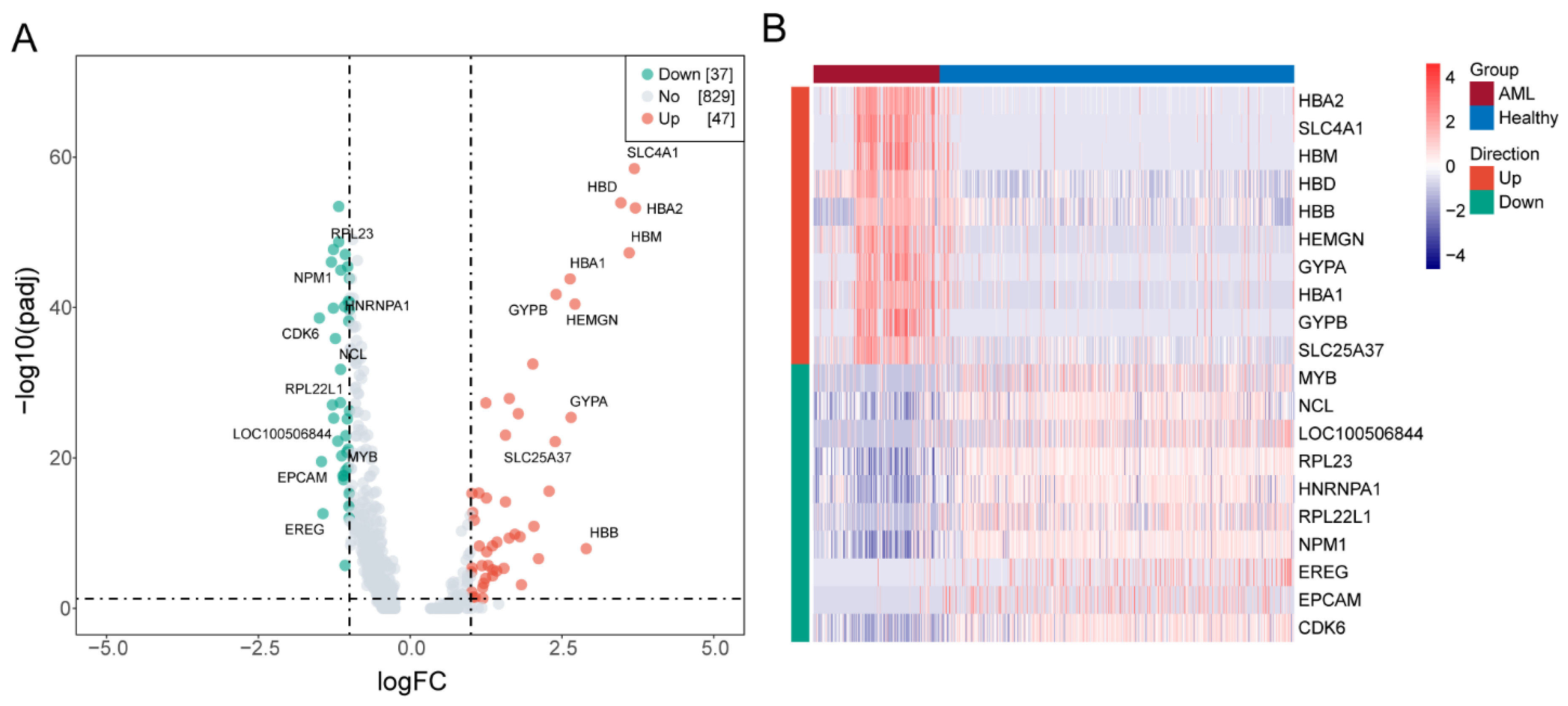
3.3. Identifying DEGs
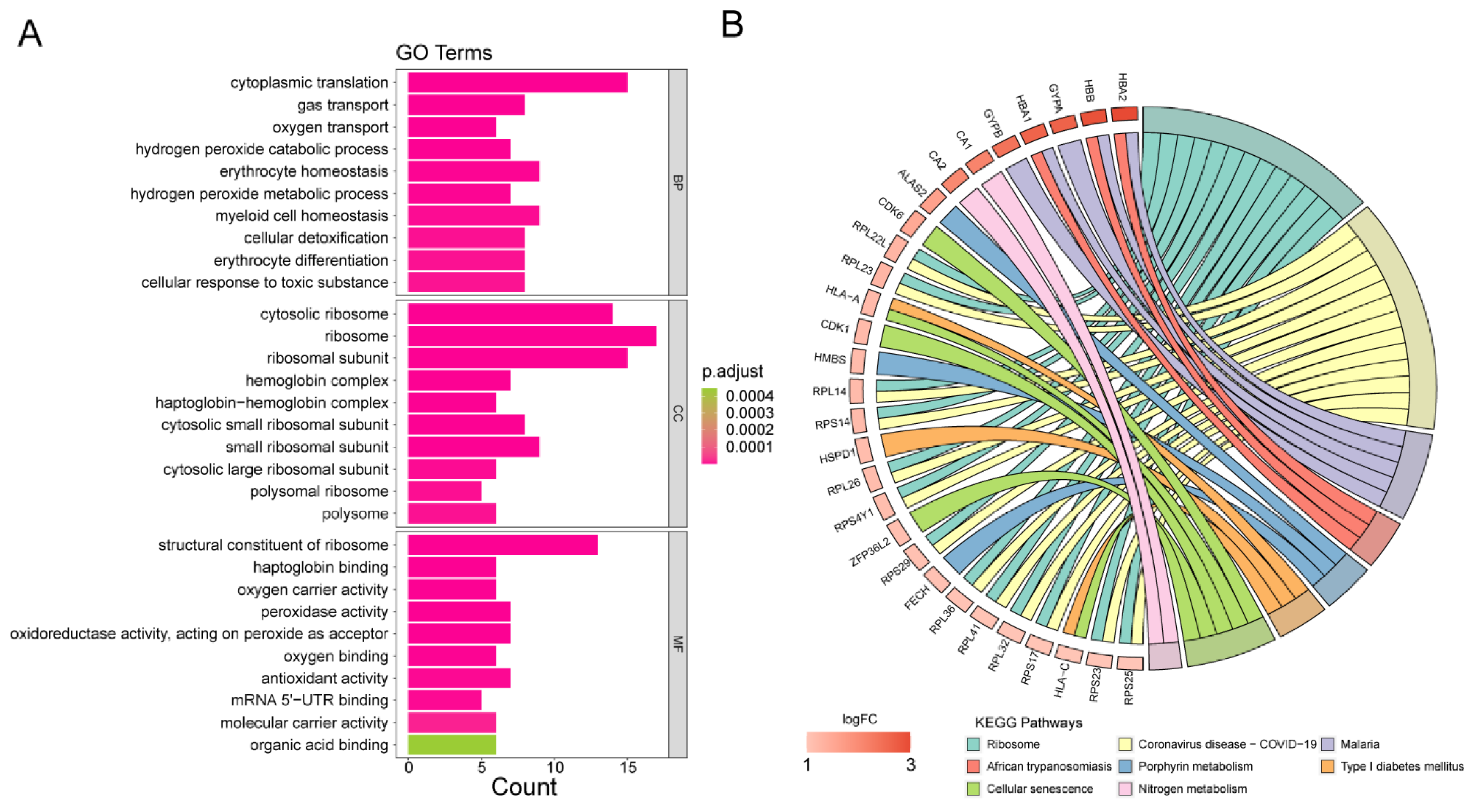
3.4. Analyses of Functional Enrichment
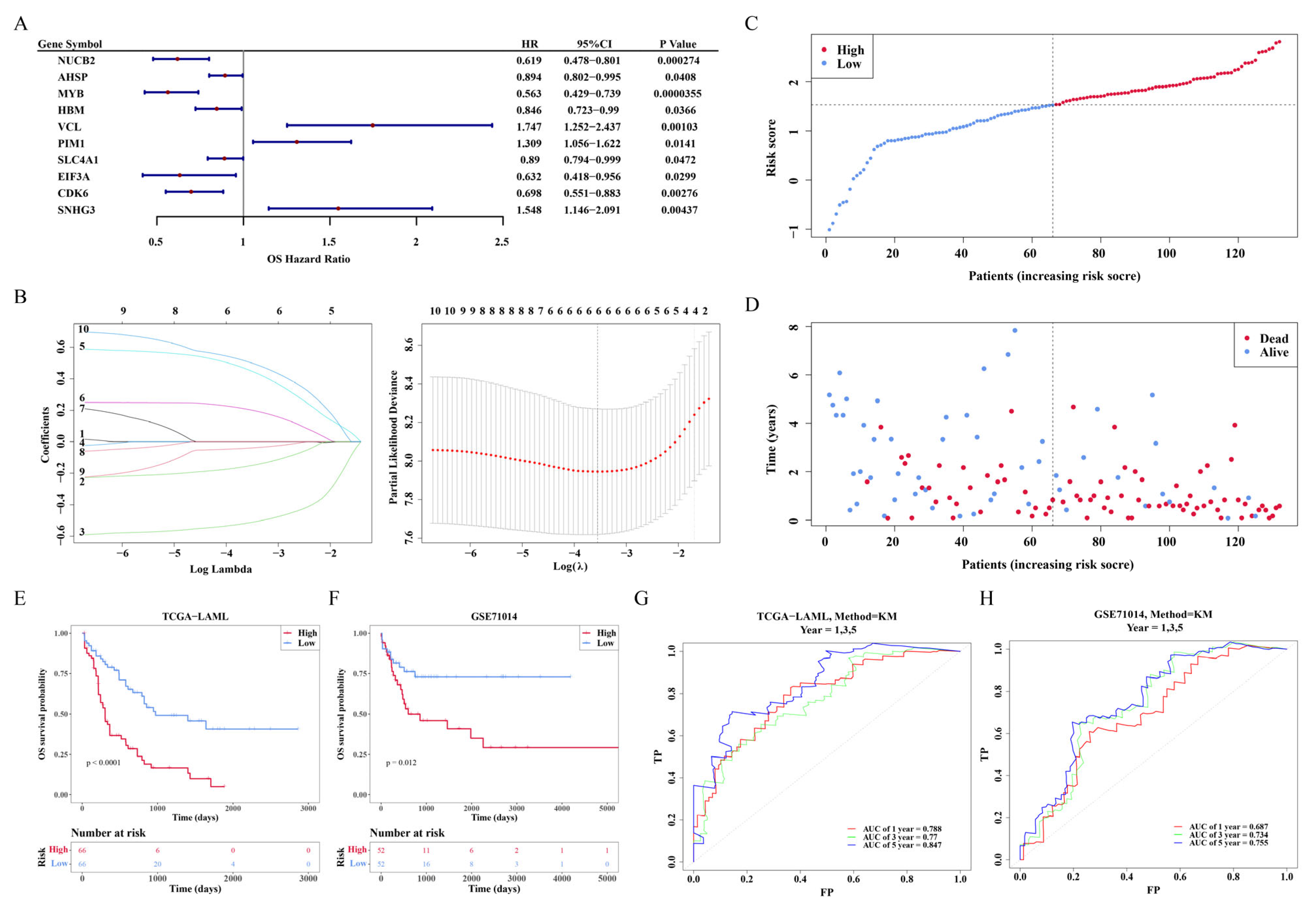
3.5. The Development of a Risk Model
3.6. Nomogram Construction
3.7. The Connection Involving the Risk Score as Well as Clinicopathological Attributes
3.8. Pseudotime Analysis
3.9. Two Signature Genes That Are Validated Using Expressions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chen, X.; Pan, J.; Wang, S.; Hong, S.; Hong, S.; He, S. The Epidemiological Trend of Acute Myeloid Leukemia in Childhood: A Population-Based Analysis. J. Cancer 2019, 10, 4824–4835. [Google Scholar] [CrossRef]
- De Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Short, N.J.; Rytting, M.E.; Cortes, J.E. Acute myeloid leukaemia. Lancet 2018, 392, 593–606. [Google Scholar] [CrossRef]
- Tabata, R.; Chi, S.; Yuda, J.; Minami, Y. Emerging Immunotherapy for Acute Myeloid Leukemia. Int. J. Mol. Sci. 2021, 22, 1944. [Google Scholar] [CrossRef]
- Kaspers, G.J.; Zimmermann, M.; Reinhardt, D.; Gibson, B.E.; Tamminga, R.Y.; Aleinikova, O.; Armendariz, H.; Dworzak, M.; Ha, S.Y.; Hasle, H.; et al. Improved outcome in pediatric relapsed acute myeloid leukemia: Results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J. Clin. Oncol. 2013, 31, 599–607. [Google Scholar] [CrossRef]
- Klimchenko, O.; Mori, M.; Distefano, A.; Langlois, T.; Larbret, F.; Lecluse, Y.; Feraud, O.; Vainchenker, W.; Norol, F.; Debili, N. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood 2009, 114, 1506–1517. [Google Scholar] [CrossRef]
- Cai, Q.; Jeannet, R.; Hua, W.K.; Cook, G.J.; Zhang, B.; Qi, J.; Liu, H.; Li, L.; Chen, C.C.; Marcucci, G.; et al. CBFβ-SMMHC creates aberrant megakaryocyte-erythroid progenitors prone to leukemia initiation in mice. Blood 2016, 128, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Morita, K.; Hill, M.C.; Jiang, Y.; Kitano, A.; Saito, Y.; Wang, F.; Mao, X.; Hoegenauer, K.A.; Morishita, K.; et al. PRDM16s transforms megakaryocyte-erythroid progenitors into myeloid leukemia-initiating cells. Blood 2019, 134, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kong, G.; Rajagopalan, A.; Lu, L.; Song, J.; Hussaini, M.; Zhang, X.; Ranheim, E.A.; Liu, Y.; Wang, J.; et al. p53-/- synergizes with enhanced NrasG12D signaling to transform megakaryocyte-erythroid progenitors in acute myeloid leukemia. Blood 2017, 129, 358–370. [Google Scholar] [CrossRef]
- Nilsson, T.; Waraky, A.; Östlund, A.; Li, S.; Staffas, A.; Asp, J.; Fogelstrand, L.; Abrahamsson, J.; Palmqvist, L. An induced pluripotent stem cell t(7;12)(q36;p13) acute myeloid leukemia model shows high expression of MNX1 and a block in differentiation of the erythroid and megakaryocytic lineages. Int. J. Cancer 2022, 151, 770–782. [Google Scholar] [CrossRef] [PubMed]
- van Galen, P.; Hovestadt, V.; Wadsworth Ii, M.H.; Hughes, T.K.; Griffin, G.K.; Battaglia, S.; Verga, J.A.; Stephansky, J.; Pastika, T.J.; Lombardi Story, J.; et al. Single-Cell RNA-Seq Reveals AML Hierarchies Relevant to Disease Progression and Immunity. Cell 2019, 176, 1265–1281.e1224. [Google Scholar] [CrossRef]
- Lee, S.H.; Chiu, Y.C.; Li, Y.H.; Lin, C.C.; Hou, H.A.; Chou, W.C.; Tien, H.F. High expression of dedicator of cytokinesis 1 (DOCK1) confers poor prognosis in acute myeloid leukemia. Oncotarget 2017, 8, 72250–72259. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Aran, D.; Looney, A.P.; Liu, L.; Wu, E.; Fong, V.; Hsu, A.; Chak, S.; Naikawadi, R.P.; Wolters, P.J.; Abate, A.R.; et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019, 20, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhou, X.; Yang, L.; Zhou, H.; Meng, M.; Zhang, L.; Li, J. A Cuproptosis Activation Scoring model predicts neoplasm-immunity interactions and personalized treatments in glioma. Comput. Biol. Med. 2022, 148, 105924. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Salehzadeh, A.; Talesh Sasani, S.; Tarang, A. The miR526b-5p-Related Single Nucleotide Polymorphisms, rs72618599, Located in 3′-UTR of TCF3 Gene, is Associated with the Risk of Breast and Gastric Cancers. Iran. Biomed. J. 2022, 26, 53–63. [Google Scholar]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Qiu, X.; Mao, Q.; Tang, Y.; Wang, L.; Chawla, R.; Pliner, H.A.; Trapnell, C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 2017, 14, 979–982. [Google Scholar] [CrossRef]
- Chen, N.; Fan, B.; He, Z.; Yu, X.; Wang, J. Identification of HBEGF+ fibroblasts in the remission of rheumatoid arthritis by integrating single-cell RNA sequencing datasets and bulk RNA sequencing datasets. Arthritis Res. Ther. 2022, 24, 215. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Bao, L.; Wu, F.; Wang, S.; Hao, S. Intercellular Communication Reveals Therapeutic Potential of Epithelial-Mesenchymal Transition in Triple-Negative Breast Cancer. Biomolecules 2022, 12, 1478. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, S.; Wang, D.; Edwards, H.; Thibodeau, J.; Kim, S.; Stemmer, P.; Wang, G.; Jin, J.; Savasan, S.; et al. Panobinostat sensitizes AraC-resistant AML cells to the combination of azacitidine and venetoclax. Biochem. Pharmacol. 2024, 228, 116065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Liang, Y.C.; Wang, J.X.; Zhang, J.; La, T.; Li, Q.Z. Unlocking the potential: A novel prognostic index signature for acute myeloid leukemia. Comput. Biol. Med. 2024, 173, 108396. [Google Scholar] [CrossRef]
- Luo, C.J.; Abudukeremu, Y.; Rao, M.L.; Zhou, D.H.; Fang, J.P.; Li, Y.; Xu, L.H. A Novel Prognostic Risk-Scoring Model Based on RAS Gene-Associated Cluster in Pediatric Acute Myeloid Leukemia. Cancer Med. 2025, 14, e70716. [Google Scholar] [CrossRef]
- Zhu, G.; Cai, J.; Fu, W.; Sun, Y.; Wang, T.; Zhong, H. Elucidating the immune landscape and potential prognostic model in acute myeloid leukemia with TP53 mutation. Hematology 2024, 29, 2400620. [Google Scholar] [CrossRef]
- Raess, P.W.; Paessler, M.E.; Bagg, A.; Weiss, M.J.; Choi, J.K. α-Hemoglobin-stabilizing protein is a sensitive and specific marker of erythroid precursors. Am. J. Surg. Pathol. 2012, 36, 1538–1547. [Google Scholar] [CrossRef]
- Yu, H.; Pinkus, J.L.; Pinkus, G.S. α-Hemoglobin-stabilizing Protein: An Effective Marker for Erythroid Precursors in Bone Marrow Biopsy Specimens. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 51–56. [Google Scholar] [CrossRef]
- Wang, D.; Shen, B.; Li, Y. A novel erythroid differentiation related gene EDRF1 upreguated globin gene expression in HEL cells. Zhonghua Yi Xue Za Zhi 2001, 81, 1512–1515. [Google Scholar] [PubMed]
- Zhu, G.Z.; Yang, Y.L.; Zhang, Y.J.; Liu, W.; Li, M.P.; Zeng, W.J.; Zhao, X.L.; Chen, X.P. High Expression of AHSP, EPB42, GYPC and HEMGN Predicts Favorable Prognosis in FLT3-ITD-Negative Acute Myeloid Leukemia. Cell Physiol. Biochem. 2017, 42, 1973–1984. [Google Scholar] [CrossRef]
- Klempnauer, K.H. C/EBPβ cooperates with MYB to maintain the oncogenic program of AML cells. Oncotarget 2023, 14, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.L.; Gabrielsen, O.S.; Frampton, J. MYB as a Critical Transcription Factor and Potential Therapeutic Target in AML. Adv. Exp. Med. Biol. 2024, 1459, 341–358. [Google Scholar]
- Nicosia, L.; Spencer, G.J.; Brooks, N.; Amaral, F.M.R.; Basma, N.J.; Chadwick, J.A.; Revell, B.; Wingelhofer, B.; Maiques-Diaz, A.; Sinclair, O.; et al. Therapeutic targeting of EP300/CBP by bromodomain inhibition in hematologic malignancies. Cancer Cell 2023, 41, 2136–2153.e2113. [Google Scholar] [CrossRef]
- Negri, A.; Ward, C.; Bucci, A.; D’Angelo, G.; Cauchy, P.; Radesco, A.; Ventura, A.B.; Walton, D.S.; Clarke, M.; Mandriani, B.; et al. Reversal of MYB-dependent suppression of MAFB expression overrides leukaemia phenotype in MLL-rearranged AML. Cell Death Dis. 2023, 14, 763. [Google Scholar] [CrossRef]
- Murray, H.C.; Miller, K.; Brzozowski, J.S.; Kahl, R.G.S.; Smith, N.D.; Humphrey, S.J.; Dun, M.D.; Verrills, N.M. Synergistic Targeting of DNA-PK and KIT Signaling Pathways in KIT Mutant Acute Myeloid Leukemia. Mol. Cell Proteom. 2023, 22, 100503. [Google Scholar] [CrossRef]
- Fiskus, W.; Mill, C.P.; Bose, P.; Masarova, L.; Pemmaraju, N.; Dunbar, A.; Birdwell, C.E.; Davis, J.A.; Das, K.; Hou, H.; et al. Preclinical efficacy of CDK7 inhibitor-based combinations against myeloproliferative neoplasms transformed to AML. Blood 2025, 145, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Aroua, N.; Liu, Y.; Rohde, C.; Cheng, J.; Wirth, A.K.; Fijalkowska, D.; Göllner, S.; Lotze, M.; Yun, H.; et al. A Dynamic rRNA Ribomethylome Drives Stemness in Acute Myeloid Leukemia. Cancer Discov. 2023, 13, 332–347. [Google Scholar] [CrossRef]
- Xu, J.; Zhong, A.; Zhang, S.; Chen, M.; Zhang, L.; Hang, X.; Zheng, J.; Wu, B.; Deng, X.; Pan, X.; et al. KMT2D Deficiency Promotes Myeloid Leukemias which Is Vulnerable to Ribosome Biogenesis Inhibition. Adv. Sci. 2023, 10, e2206098. [Google Scholar] [CrossRef] [PubMed]
- Colin Sieff, M. FRCPath. Diamond-Blackfan Anemia. In GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 2009. Available online: https://www.ncbi.nlm.nih.gov/books/NBK7047/ (accessed on 10 July 2022).
- Sun, L.; Wang, W.; Han, C.; Huang, W.; Sun, Y.; Fang, K.; Zeng, Z.; Yang, Q.; Pan, Q.; Chen, T.; et al. The oncomicropeptide APPLE promotes hematopoietic malignancy by enhancing translation initiation. Mol. Cell 2021, 81, 4493–4508.e4499. [Google Scholar] [CrossRef]
- Mill, C.P.; Fiskus, W.; DiNardo, C.D.; Birdwell, C.; Davis, J.A.; Kadia, T.M.; Takahashi, K.; Short, N.; Daver, N.; Ohanian, M.; et al. Effective therapy for AML with RUNX1 mutation by cotreatment with inhibitors of protein translation and BCL2. Blood 2022, 139, 907–921. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Y.; Sun, J.; Sun, H.; Chen, H.; Zhu, Y.; Fu, H.; Yu, C.; E., W.; Lai, S.; et al. A single-cell survey of cellular hierarchy in acute myeloid leukemia. J. Hematol. Oncol. 2020, 13, 128. [Google Scholar] [CrossRef]
- Ramaswamy, K.; Forbes, L.; Minuesa, G.; Gindin, T.; Brown, F.; Kharas, M.G.; Krivtsov, A.V.; Armstrong, S.A.; Still, E.; de Stanchina, E.; et al. Peptidomimetic blockade of MYB in acute myeloid leukemia. Nat. Commun. 2018, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Uttarkar, S.; Dassé, E.; Coulibaly, A.; Steinmann, S.; Jakobs, A.; Schomburg, C.; Trentmann, A.; Jose, J.; Schlenke, P.; Berdel, W.E.; et al. Targeting acute myeloid leukemia with a small molecule inhibitor of the Myb/p300 interaction. Blood 2016, 127, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Li, Y. Research progress of epigenetic regulation and therapy in acute myeloid leukemia. J. Clin. Pathol. Res. 2021, 41, 1411–1419. [Google Scholar][Green Version]
- Zhang, S.; Huang, F.; Wang, Y.; Long, Y.; Li, Y.; Kang, Y.; Gao, W.; Zhang, X.; Wen, Y.; Wang, Y.; et al. NAT10-mediated mRNA N(4)-acetylcytidine reprograms serine metabolism to drive leukaemogenesis and stemness in acute myeloid leukaemia. Nat. Cell Biol. 2024, 26, 2168–2182. [Google Scholar] [CrossRef] [PubMed]

| Primer | Primer Sequence | |
|---|---|---|
| Forward Primer | Reverse Primer | |
| AHSP | TTACAGGCAGCAGGTGACAGG | AGGTGTCAGGGTAGAGTGGCAG |
| MYB | TCTGCTCACACCACTGGGAAG | ATCTGCCACAGGCGAGGTC |
| PIM1 | CAGGACAGTGCTTGATACAGGAAC | AGAAGAGAGTATCTATGGGAGGAGTT |
| CDK6 | AGTGGTCGTCACGCTGTGGTA | AGGTCCTGGAAGTATGGGTGA |
| SNHG3 | TGCTCAGGAAGAAGCCAGATG | GTAGTTACAGCAGGACACGGATG |
| VCL | AAGGCCGGGGAGGTGATT | ATGATGTCATTGCCCTTGCTGG |
| Actin | TGGCACCCAGCACAATGAA | AGGGTGTAACGCAACTAAGTCATAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin, T.; Wang, Y.; Tang, J.; Xu, X.-J.; Lin, C.; Lu, B. Prognostic Risk Model of Megakaryocyte–Erythroid Progenitor (MEP) Signature Based on AHSP and MYB in Acute Myeloid Leukemia. Biomedicines 2025, 13, 1845. https://doi.org/10.3390/biomedicines13081845
Bin T, Wang Y, Tang J, Xu X-J, Lin C, Lu B. Prognostic Risk Model of Megakaryocyte–Erythroid Progenitor (MEP) Signature Based on AHSP and MYB in Acute Myeloid Leukemia. Biomedicines. 2025; 13(8):1845. https://doi.org/10.3390/biomedicines13081845
Chicago/Turabian StyleBin, Ting, Ying Wang, Jing Tang, Xiao-Jun Xu, Chao Lin, and Bo Lu. 2025. "Prognostic Risk Model of Megakaryocyte–Erythroid Progenitor (MEP) Signature Based on AHSP and MYB in Acute Myeloid Leukemia" Biomedicines 13, no. 8: 1845. https://doi.org/10.3390/biomedicines13081845
APA StyleBin, T., Wang, Y., Tang, J., Xu, X.-J., Lin, C., & Lu, B. (2025). Prognostic Risk Model of Megakaryocyte–Erythroid Progenitor (MEP) Signature Based on AHSP and MYB in Acute Myeloid Leukemia. Biomedicines, 13(8), 1845. https://doi.org/10.3390/biomedicines13081845






