1. Introduction
Early on in the COVID-19 pandemic, researchers started to investigate whether patients with inflammatory bowel diseases (IBDs)—including Crohn’s disease (CD) and ulcerative colitis (UC)—represent a risk group for COVID-19 and whether immunosuppressive therapies for these patients worsen the outcome of SARS-CoV-2 infection [
1]. This concern originates from the fact that IBD patients often have compromised immune systems, either as a result of the underlying disease or due to immunomodulatory treatment, potentially predisposing them to the risk of more frequent and more severe infections [
1]. Previous studies have reported conflicting findings [
1,
2,
3]. These range from no difference compared to the healthy general population to the influence of different immunosuppressive therapies on the outcome of the disease. Initially, systemic steroids in particular, but also tumour necrosis factor (TNF) inhibitors, stood out as potentially having a negative influence on the outcome of COVID-19 [
4,
5,
6].
With the introduction of vaccines against SARS-CoV-2 infection, the field of research expanded to explore which side effects the vaccinations may trigger in IBD patients and to what extent the serological response to vaccination may be impaired by IBD and related therapies. With regard to the first question, the incidence of side effects to vaccination in IBD patients is comparable to that of the general population. Flares of IBD or severe side effects are very rare [
7,
8,
9]. The answer to the second question cannot definitely be given in the literature. Some studies [
10,
11,
12,
13,
14] indicate that TNF inhibitors, in particular when used in combination with thiopurines, as well as Janus kinase (JAK) inhibitors, affect the serological response. However, a study by Macaluso et al. (2023) did report an attenuating effect of TNF inhibitors compared to other therapies but concluded that the overall finding of lower antibody titres in IBD patients compared to healthy controls appeared to be largely independent of immunosuppressive treatment [
15]. More recent studies continued to observe titre development in relation to the third COVID-19 vaccination. For example, IBD patients showed an increase in serological response after the third vaccination [
16,
17]. In addition, studies [
18,
19] show that this increase was weakened by certain immunosuppressive therapies. Here again, TNF inhibitors used in combination with thiopurines and JAK inhibitors are particularly noteworthy. As expected, the titres decrease over time [
20]. Infliximab appears to accelerate the decrease in titres [
11,
21,
22]. However, an infection prior to vaccination can also lead to higher serological responses [
23].
What has been missing in many of these studies so far is a long-term perspective on COVID-19 infections and vaccinations in IBD patients. The present study addresses this topic by observing IBD patients at Heidelberg University Hospital from July 2021 to August 2022 and recording the prevalence of infections, vaccinations, and side effects of its IBD cohort, and, in a second step, investigating the development of serological responses, both after several vaccinations and in combination with COVID-19 disease (mostly in the context of the Omicron wave after the third vaccination). The study also explored whether IBD therapies had an influence on the development of serological responses.
2. Materials and Methods
All study participants were recruited at the IBD outpatient clinic of the University Hospital Heidelberg, which is a tertiary referral centre for the treatment of IBD patients. The study period was from July 2021 to August 2022. Data were retrieved from paper-based standardised questionnaires and electronic records from the clinic information system.
The standardised questionnaires were completed manually by the patients as part of their routine outpatient visits. Information on the history of COVID-19 was requested, including the time point of infection and the need for inpatient or intensive care treatment. Patients were also interviewed on their COVID-19 vaccination course, including the number, dates, and types of vaccinations, as well as side effects and associated IBD relapse symptoms. Completing the questionnaire was expected to take about five minutes on average. Some patients visited the IBD outpatient clinic more than once so that they could complete further questionnaires in case new information on COVID-19 and/or vaccinations was available.
A large part of the data was obtained from the electronic patient records at the clinic information system of Heidelberg University Hospital. Thus various sample characteristics could be extracted in addition to demographic data. At that, it was possible to track which IBD therapies the patients received at the time of vaccinations and infections using the electronic follow-up forms of the IBD outpatient clinic. To minimise errors, the data was checked twice.
Antibody titres against COVID-19 were determined as part of the routine laboratory tests for the IBD patients at the outpatient clinic. Spike-specific IgG antibodies (S1-RBD) were measured using the ADVIA Centaur SARS-CoV-2 IgG (sCOVG) assay (Siemens Healthineers, Munich, Germany), a CLIA targeting IgG to the SARS-CoV-2 spike S1 receptor-binding domain. Results ≥ 1.0 index were considered positive and converted to BAU/mL using the WHO factor of 21.8 BAU/mL per index. Nucleocapsid antibodies were determined using the Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics, Rotkreuz, Switzerland) to differentiate infection-induced from vaccine-induced seroconversion. All assays were performed according to the manufacturer’s instructions; serum samples were processed promptly or stored at 2–8 °C (≤8 h) or −20 °C (long-term). Titres of patients who visited the outpatient clinic more than once within the indicated time frame were measured repeatedly.
All IBD patients who visited the IBD outpatient clinic for a regular appointment within the indicated time frame were offered to participate in the survey voluntarily. They were free to complete the above-mentioned questionnaire in the waiting room and therein agreed to the evaluation of their data for the purpose of this study.
This prospective, monocentric, uncontrolled observational study has two main objectives: first, to document the occurrence of COVID-19 and vaccinations of IBD patients and note any possible side effects of the vaccinations; second, to describe the development of antibody titres on a descriptive level. The main focus was on S-IgG antibodies, which were measured in BAU/mL and were therefore ratio-scaled variables. The test for S-IgG antibodies turns positive both as a result of infection and of vaccination. Therefore, the available S-IgG antibody titres were analysed inferentially. This included mean comparisons of the antibody titres in relation to the number of vaccinations and COVID-19 infections, as well as in relation to the different IBD medications of the patients. Linear models with mixed effects were applied to analyse potential influences on the S-IgG antibody titres of the vaccinated patients. The JASP (version 0.17.1, University of Amsterdam, Amsterdam, The Netherlands) and RStudio (version 2023.03.0+386, Posit PBC, Boston, MA, USA) programs were used for these analyses, with the former employed primarily for the first part of the analysis plan and the description of the sample.
In the descriptive part of the study, results were indicated either in frequencies and percentages or in mean values, standard deviations, medians and ranges, depending on the type of variable. In the inferential statistical part, various non-parametric test procedures were used depending on the type of mean comparison. The non-parametric methods were chosen because no normal distribution could be assumed for the antibody titres as the target variable. As an additional challenge for this assumption, Kolmogorov–Smirnov tests (RStudio, version 2023.03.0+386) were performed on antibody titres. These differed significantly from the null hypothesis (p < 0.05), meaning that a normal distribution could not be assumed. The methods used included the Friedman test (RStudio, version 2023.03.0+386) for comparing several dependent samples, the Wilcoxon signed-rank test (RStudio, version 2023.03.0+386) for comparing two dependent samples, and the Kruskal–Wallis (RStudio, version 2023.03.0+386) test for comparing several independent samples.
In the final step, linear models with mixed effects were used for the calculation in order to better identify the variables influencing the vaccination titres. In the model, a random intercept was assumed for each individual so that respective differences in titre development and discrepancies between the numbers of measurements of individual patients were taken into account. The number of vaccinations was set as a fixed effect, meaning that the analysis factored in whether the measured values were taken under the conditions of a basic immunisation or a booster vaccination. The time interval and the type of immunosuppressive therapy were also assumed to be fixed effects.
3. Results
3.1. Study Population and Overall Characteristics of the Patients
A total of 537 patients completed the questionnaire at least once. Among these, 14 patients were excluded because the diagnosis of IBD could not be confirmed. Three further patients died during the survey period, so their data were excluded (the cause of death was in no case COVID-19). Thus, 520 IBD patients whose clinical data was recorded as part of their evaluation via the clinic information system of Heidelberg University Hospital had completed at least one questionnaire. Among these 520 patients,
n = 107 completed the questionnaire on COVID-19 and vaccinations twice, and
n = 76 at least three times during the survey period. Four hundred and fifty-five patients (85.6%) completed the questionnaires in full. In 420 of the included patients, quantitative determination of S-IgG-AK titres was performed at least once during the study period. Among the 520 included IBD patients, 269 were female, the mean age was 45.3 years (SD = ±15.5), 60.6% suffered from CD, 35.4% from UC, and 4.0% from IBD unclassified (IBD-U). The demographic and clinical characteristics of the study population are shown in
Table 1 and
Table 2.
3.2. Prevalence of Infections, Vaccinations, and Side Effects
Four hundred eighty (92.3%) were vaccinated against COVID-19 at least once, and 154 (29.6%) patients knowingly or unknowingly experienced SARS-CoV-2 infection (23.6% without, 4.9% after the first, 16.0% after the second, 52.1% after the third, and 3.5% after the fourth vaccination). Unknown cases of prior infection were identified based on discrepancies between patients’ self-reported history (denial of prior infection) and the presence of nucleocapsid antibodies. None of the patients reported the need for inpatient treatment. The vaccinated and infected patients showed a seroconversion rate of 94.4% (cut-off: 21.8 BAU/mL). The frequency of side effects and IBD flare symptoms may be viewed in
Table 3. The most common side effects mentioned were fatigue (35.2%), discomfort in the vaccination arm (32.4%), headaches (32.4%), and elevated temperature (24.1%). Diarrhoea/increased stool frequency (60.0%), abdominal pain/cramps (42.4%), blood in the stool (15.2%), and worsening of extraintestinal symptoms (15.2%) were mentioned as the most common flare effects. Details on vaccine types, dosing intervals, and homologous versus heterologous regimens are now provided in an additional
Table S3.
3.3. Serological Responses to Vaccination with or Without Infection
The individual mean values of the serological responses of the three event groups (second vaccination, third vaccination and third vaccination with additional COVID-19 disease) are displayed in
Table 4. In addition, the last column of the table shows the time interval in days between the last event (vaccination or infection) and the titre determination. In this context, ‘three vaccinations’ denotes a booster dose following a two-dose primary vaccination series. Regarding hybrid immunity, all analyses were performed for infections occurring after three vaccinations, as this subgroup contained the largest number of cases, most of which were recorded during the Omicron wave.
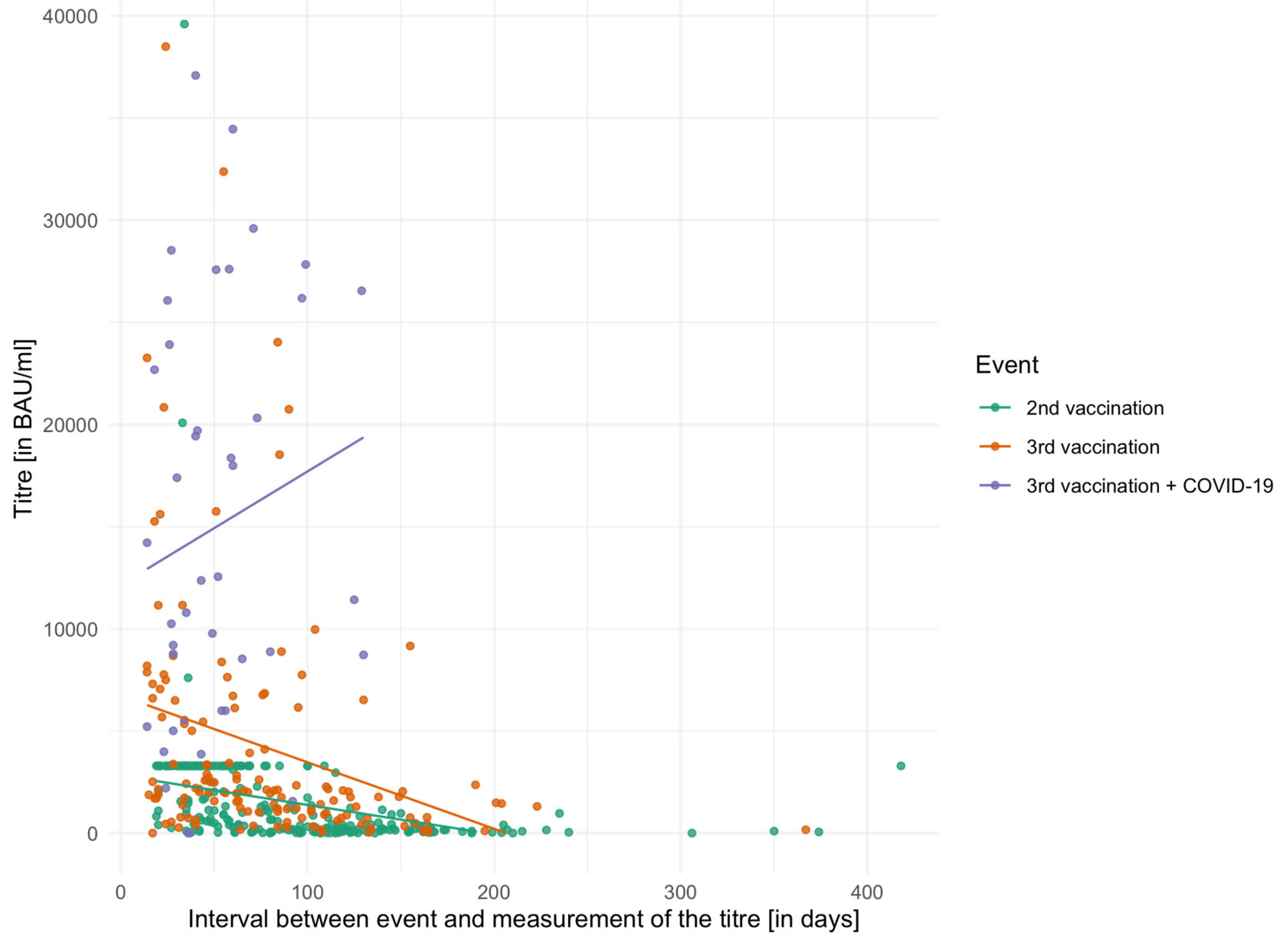
Figure 1 shows the vaccination titres of the individual event groups in relation to the time interval between titre measurements. The figure also shows that there was a cap on the titre measurement during the titre survey for the second vaccination and that, therefore, in many patients only a maximum value of 3291.80 BAU/mL could be measured.
The mean titres of the three event groups were examined using the Friedman test and differed significantly from one another (χ
2 = 16.51,
p < 0.001, df = 3). In the pairwise comparison of the three groups using the Bonferroni-corrected Wilcoxon signed-rank test, all differed significantly: 2nd vaccination vs. 3rd vaccination (
p = 0.010), 2nd vaccination vs. 3rd vaccination + COVID-19 (
p = 0.006), and 3rd vaccination vs. 3rd vaccination plus COVID-19 (
p = 0.013). The
Supplementary Materials include two tables (S1 and S2) presenting serological titres stratified by Crohn’s disease and ulcerative colitis. No statistically significant differences between the two disease groups were detected using the Kruskal–Wallis test, either after the second vaccination (χ
2 = 0.82, df = 2,
p = 0.66), the third vaccination (χ
2 = 0.22, df = 2,
p = 0.89), or after three vaccinations plus SARS-CoV2 infection (χ
2 = 0.65, df = 1,
p = 0.42).
3.4. Antibody Titre Decay over Time
For 29 of the included patients, two titre measurements were performed at different time points following the 2nd vaccination. The average interval between these two titre measurements was 78.6 days. In the Wilcoxon signed-rank test, the mean values of the two titres differed significantly from each other (V = 370, p < 0.001). Comparable results were found for the patients (n = 56) in whom two titres were determined after the third vaccination (V = 1573, p < 0.001). The mean interval between the two measurements was 59.6 days.
3.5. Influence of IBD Therapies on Serological Responses
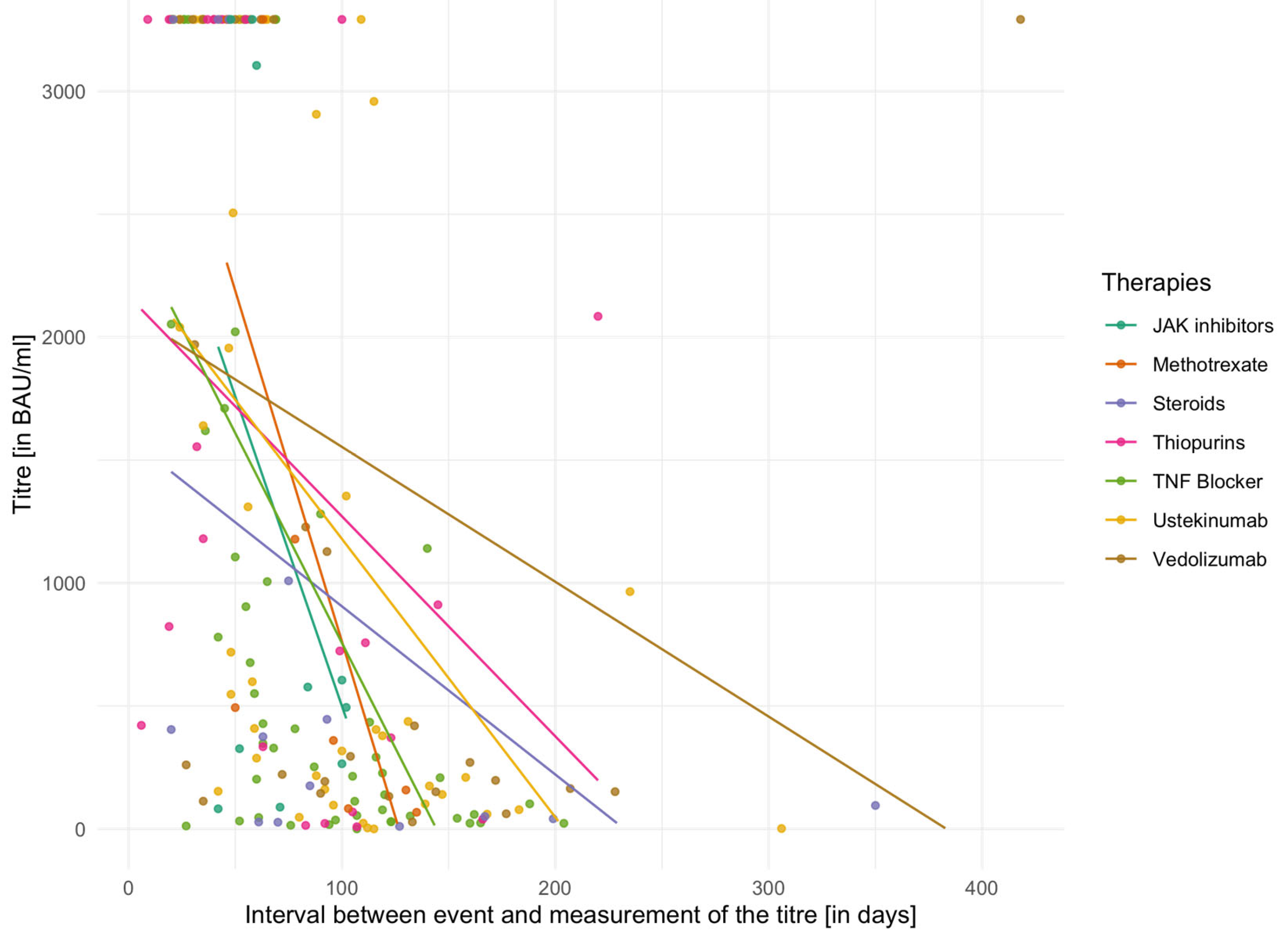
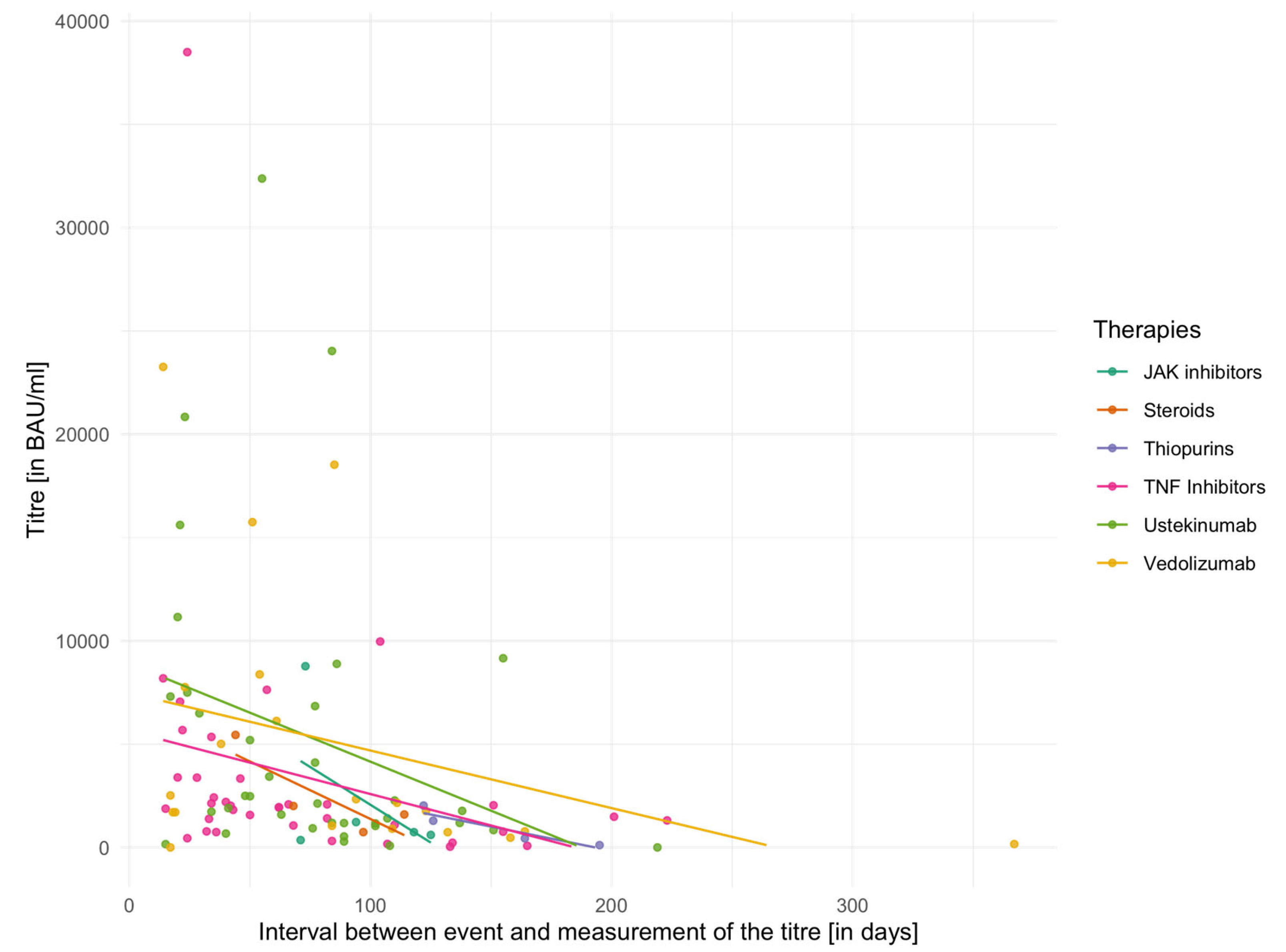
The individual mean values of vaccination titres for different IBD medications are shown in
Table 5 for the second vaccination and in
Table 6 for the third vaccination. In addition,
Figure 2 and
Figure 3 demonstrate vaccination titres for individual therapies after the second or third vaccination in relation to the interval to titre determination. Here, too, the capping of the titres after the second vaccination has to be considered. The Kruskal–Wallis test did not reveal any significant differences between groups either in relation to the second vaccination (χ
2 = 6.60,
p = 0.36, df = 6) or in relation to the third vaccination (χ
2 = 4.97,
p = 0.42, df = 5).
A linear model with mixed effects was used to analyse whether the type of immunosuppressive therapy (TNF-inhibitor, ustekinumab, or vedolizumab) or other predictors (time interval between vaccination and titre determination, number of vaccinations) influenced vaccination titres. In this model, the predictors (therapies, time intervals and number of vaccinations) represented the fixed effects, with the assumption of additional random effects for every individual. For the estimation of the model, 197 observations from 157 patients were used. The model showed a significant effect both for the time interval between titre measurement and vaccination (ß = −20.42, SE = ±5.59, p < 0.001) and for the number of vaccinations (ß = 2859.38, SE = ±2626.91, p < 0.001). The type of therapy was not significant as a predictor (ß = −307.59, SE = ±2290.21, p = 0.29). A random intercept was added for individual subjects. Here, the variance was σ2 = 5,822,408 (SD = ±2413). Based on the parameters described above, the following model was derived: titrei j = 5258.949 − 318.12 * therapy − 20.65 * time interval + (1∣number of vaccinationsj) +/− (1∣personi) +εij. It represented the most parsimonious specification, as additional factors such as sex, age, or vaccine type did not demonstrate any significant effects in alternative model configurations.
4. Discussion
The aim of this study was to obtain an overview of SARS-CoV-2 infections, vaccinations and antibody titres in the IBD cohort at Heidelberg University Hospital, which represents a mainly difficult-to-treat patient group at a tertiary referral centre. One clear benefit of this study is the establishment of a basis for recommendations regarding vaccinations to be passed on to the patients, most of whom are treated with immunosuppressive medications. In addition, the long-term perspective of the study (data collection period from July 2021 through August 2022) may contribute to the existing research landscape. This large time window made it possible to collect several titres after an event (2nd or 3rd vaccination), allowing sufficient time for titre development. The long observation period also made it possible to record the combination of vaccinations and COVID-19 itself and their influence on antibody titre development in IBD patients.
In the first step of the analyses, it was revealed that 29.6% of the included IBD patients had been infected with SARS-CoV-2 at least once by the end of the survey in August 2022. In comparison, by then, approximately 32.2 million cumulative infections had been registered in the German general population, which represents an approximate share of 38.6% [
24]. In addition, a large proportion of patients (92.3%) opted for at least one vaccination and thus stood out from the German general population, with a proportion of 76.4% of people with basic immunisation [
25]. It may be interpreted that patients with a serious underlying disease are more health-conscious and therefore more likely to be vaccinated and less likely to enter risky situations with the potential to expose them to severe infection [
26]. With regard to possible vaccine side effects, the vaccinated patients in our study reported fewer side effects as compared to the ones described in some other studies [
9,
27]. A potential explanation may be that our patients had to actively recall their side effects using an open question format, whereas other investigators asked the patients to fill in a predefined list of symptoms. Open-ended questioning is inherently more susceptible to recall bias and underreporting, particularly of mild or transient symptoms, which may not be spontaneously recalled or considered relevant by respondents. The prevalence of IBD flares associated with vaccinations was low and consistent with previous studies [
7,
8].
In line with results of previous studies [
16,
17], serological immune responses differed significantly after two and three doses of vaccine. Patients who received three doses of vaccine achieved higher S-IgG antibody titres than those with two doses. Furthermore, patients with additional infection after the third vaccination had an additional significant benefit in terms of antibody development. Similar results have been observed in a healthy population [
28]. However, the inclusion of a control group comprising healthy individuals would have enhanced the generalisability of the findings. For IBD patients, Doherty et al. (2023) found a similar effect of previous infection on titre development [
23]. Our study supports these data, even though the present study dealt with subsequent infection after the vaccinations, while the other studies dealt with initial infection and subsequent vaccinations.
In the subgroup of our patients with several titres available after every event (second or third vaccination), antibody concentrations decreased significantly over time. This is in line with the findings of various other studies [
29,
30]. In those studies, however, the examined cohorts consisted of healthy subjects. An implication that arises from this finding is the need for booster vaccinations. Importantly, it must be noted that the immune system does not only consist of IgG antibodies circulating in the blood, but that other components, especially cellular immunity, also contribute to protection against severe SARS-CoV-2 infection after vaccination [
31].
Many IBD patients feel uncomfortable about potential detrimental effects of their immunosuppressive therapy on the development of their immune response to vaccination or COVID-19. In response to this need for guidance, several other studies have been published. In particular, TNF inhibitors and their combination with thiopurines and JAK inhibitors have emerged as potential negative influencing factors on titre development [
20,
21,
22]. The present study failed to confirm this observation, as none of the analysed IBD therapies had a particularly detrimental effect on titre development. However, such therapy-specific attenuating effects have been demonstrated in larger multicentre cohorts, including the ICARUS-IBD study, which reported that immunosuppressive treatments can negatively influence serological responses in IBD patients [
32]. One possible explanation for this discrepancy is the limited number of patients in certain therapy subgroups, especially JAK inhibitors and thiopurines, which may have reduced the statistical power to detect small effect sizes. Importantly, our analyses regarding therapy subgroups were restricted to patients without prior SARS-CoV-2 infection, and antibody responses were evaluated only after the second or third vaccination. This approach was chosen to avoid confounding by hybrid immunity and to allow for a more homogeneous assessment of vaccine-induced humoral responses. The results of our study provide a further heterogeneous picture regarding the effects of IBD therapies on titre development.
4.1. Limitations
In addition to the limitations already discussed in the previous section, the most prominent limitations of this study are of a methodological nature. This is a purely observational study without an experimental approach. As a result, certain parameters were not controllable. For example, the intervals between vaccinations and antibody determination were not standardised, so that only mean values of the time intervals could be specified. One of the major methodological limitations of the study is that changes were made by the laboratory concerning titre measurements after the second vaccination, and titres had to be assumed to reach a certain maximum value of 3291.80 BAU/mL in this study. We continued to be able to detect when titre development was reduced, but not up to what maximum titre development would actually have been possible. This drawback is limited to the period following the second vaccination; the maximum titre increase can also be observed on the basis of the third vaccination or additional SARS-CoV-2 infections, from which realistic values can be inferred. Another limitation is that data on the total clinic census and systematic comparisons between participants and non-participants were not collected, which may limit the assessment of representativeness.
Some of the patients in the different therapy groups may have treated their disease with 5-aminosalicylic acid (5-ASA) preparations. As these are widely used and frequently combined with other therapeutic agents, it was not possible to establish a separate group for this class of medication. Importantly, 5-ASA preparations have immunomodulatory effects, which may also have an influence on titre development. Possibly due to the high proportion of 5-ASA preparations in the treatment of IBD, there are not many studies investigating the individual effect of these preparations on titre development. For example, Casas Deza et al. (2024) found no influence of mesalazine on titre development [
33]. However, since 5-ASA preparations were represented in each of the individual therapy groups of the study, we accepted their presence.
There are also limitations concerning the interpretation of data. Our patient cohort is characterised by particularly severe, complex and refractory IBD courses. A more health-conscious behaviour in this special patient group might have resulted in a bias, as it may have influenced infection and vaccination rates as compared to healthy individuals and individuals with less complex disease courses [
26]. When interpreting the results, it must also be taken into account that although antibody titres correlate with the responsiveness of the immune system, they say nothing about the risk of infection.
As a final point, it should be mentioned that due to the length of the study period and the rapid further evolution of the virus, several COVID-19 variants emerged during the observation period of the study. Due to the individual characteristics of these variants, the results cannot be automatically extrapolated from one infection to another.
4.2. Strengths
By conducting the study at a tertiary treatment centre for IBD, it was possible to observe a large number of patients with moderate-to-severe disease and a high percentage of treatment-refractory courses. It should be acknowledged, however, that a single clinic cannot represent the full spectrum of IBD patients. Above all, this close observation included a long follow-up period during which the titres of individual patients could be recorded several times. Tight monitoring also made it possible to record the combination of vaccinations and COVID-19 itself and to analyse resulting antibody titres. Previously, the combination of triple vaccination and subsequent infection had only been analysed in healthy populations [
34]. Beyond that, a large treatment centre such as ours made it possible to observe different immunosuppressive therapies and compare them with one another.
Finally, a central aspect and relevant advantage of this study is that in the context of the SARS-CoV-2 pandemic and the introduction of newly developed vaccines, the opportunity to observe the widespread vaccination coverage of an entire patient group is well utilised. The data illustrates a convenient example of vaccination responses in immunocompromised people, which may also provide lessons to be kept in mind regarding other vaccinations unrelated to COVID-19.
4.3. Implications
Several important implications can be drawn from this study. Most notably, vaccination is beneficial. Patients with IBD develop detectable antibody titres and are protected against severe courses of COVID-19. While patients with hybrid immunity exhibit the highest titres, our findings also underscore the importance of booster vaccinations. Nevertheless, serological titres do not fully reflect the overall immune defence, highlighting the need for future research to investigate cellular immunity in IBD patients following vaccination.
5. Conclusions
Due to the nature of IBD itself and aggravated by immunosuppressive medications, patients with IBD may be at increased risk of severe SARS-CoV-2 infections and impaired development of serological responses after vaccination against COVID-19. This comprehensive study investigated the prevalence and side effects of COVID-19 and vaccinations as well as spike-IgG-antibody titres in vaccinated and infected patients over the period of a whole year. Our data suggests that IBD patients are not exposed to increased risks (relapse symptoms, side effects, severe courses) in relation to COVID-19 or related vaccinations. The highest serological responses were found in IBD patients with three vaccinations in addition to SARS-CoV-2 infection when compared to double- and triple-vaccinated patients without infection. Further, a significant decay of antibody titres over time was observed. Surprisingly, the study revealed that IBD therapies did not influence antibody concentrations.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/biomedicines13092072/s1, Table S1: Average vaccination titres (in BAU/mL) after the respective events (two, three, three + additional infections) for Crohn’s disease; Table S2: Average vaccination titres (in BAU/mL) after the respective events (two, three, three + additional infections) for ulcerative colitis; Table S3. Vaccination regimens.
Author Contributions
Conceptualisation, L.K. and A.G.; methodology, L.K.; software, L.K.; validation, L.K., M.K. and A.G.; formal analysis, L.K.; resources, M.K. and A.G.; data curation, L.K. and M.K.; writing—original draft preparation, L.K.; writing—review and editing, M.K. and A.G.; visualisation, L.K.; supervision, A.G.; project administration, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of The University of Heidelberg (protocol number: S-820/2021, approval date: 8 November 2021).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Pseudonymised data is available on reasonable request.
Acknowledgments
C.W. Gauss helped with the English proofreading. K. Sadus helped regarding statistical questions.
Conflicts of Interest
A.G. received travel fees from AbbVie, Johnson&Johnson, and Tillotts; lecture fees from AbbVie, Johnson&Johnson, Takeda, GSK, Pfizer, Amgen, Sandoz, Falk Foundation; consulting fees from Abbvie, Johnson&Johnson, Takeda, Amgen, GSK; M.K. received travel fees from Johnson&Johnson; L.K. has no conflicts of interest to declare.
Abbreviations
| BMI | body mass index |
| CD | Crohn’s disease |
| 5-ASA | 5-aminosalicyc acid |
| IBD | inflammatory bowel disease |
| JAK | Janus kinase |
| SD | standard deviation |
| S-IgG antibody | Spike-IgG-antibody |
| TNF | tumour necrosis factor |
| UC | ulcerative colitis |
References
- Papa, A.; Gasbarrini, A.; Tursi, A. Epidemiology and the Impact of Therapies on the Outcome of COVID-19 in Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2020, 15, 1722–1724. [Google Scholar] [CrossRef]
- Bezzio, C.; Saibeni, S.; Variola, A.; Allocca, M.; Massari, A.; Gerardi, V.; Casini, V.; Ricci, C.; Zingone, F.; Amato, A.; et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: An IG-IBD study. Gut 2020, 69, 1213–1217. [Google Scholar] [CrossRef]
- Burke, K.E.; Kochar, B.; Allegretti, J.R.; Winter, R.W.; Lochhead, P.; Khalili, H.; Colizzo, F.P.; Hamilton, M.J.; Chan, W.W.; Ananthakrishnan, A.N. Immunosuppressive Therapy and Risk of COVID-19 Infection in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 155–161. [Google Scholar] [CrossRef]
- Ungaro, R.C.; Brenner, E.J.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.F.; Reinisch, W.; Steinwurz, F.; et al. Effect of IBD medications on COVID-19 outcomes: Results from an international registry. Gut 2021, 70, 725–732. [Google Scholar] [CrossRef]
- Tripathi, K.; Brewer, G.G.; Thu Nguyen, M.; Singh, Y.; Ismail, M.S.; Sauk, J.S.; Parian, A.M.; Limketkai, B.N. COVID-19 and Outcomes in Patients with Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2022, 28, 1265–1279. [Google Scholar] [CrossRef]
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, But Not TNF Antagonists, Are Associated with Adverse COVID-19 Outcomes in Patients with Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology 2020, 159, 481–491. [Google Scholar] [CrossRef]
- James, D.; Jena, A.; Bharath, P.N.; Choudhury, A.; Singh, A.K.; Sebastian, S.; Sharma, V. Safety of SARS-CoV-2 vaccination in patients with inflammatory bowel disease: A systematic review and meta-analysis. Dig. Liver Dis. 2022, 54, 713–721. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Focht, G.; Lujan, R.; Mendelovici, A.; Friss, C.; Greenfeld, S.; Kariv, R.; Ben-Tov, A.; Matz, E.; Nevo, D.; et al. COVID-19 Vaccine Is Effective in Inflammatory Bowel Disease Patients and Is Not Associated with Disease Exacerbation. Clin. Gastroenterol. Hepatol. 2022, 20, e1263–e1282. [Google Scholar] [CrossRef]
- Tabesh, E.; Soheilipour, M.; Rezaeisadrabadi, M.; Zare-Farashbandi, E.; Mousavi-Roknabadi, R.S. Comparison the effects and side effects of COVID-19 vaccination in patients with inflammatory bowel disease (IBD): A systematic scoping review. BMC Gastroenterol. 2022, 22, 393. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Contributors to the CLARITY IBD study. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef]
- Charilaou, P.; Tricarico, C.; Battat, R.; Scherl, E.J.; Longman, R.S.; Lukin, D.J. Impact of Inflammatory Bowel Disease Therapies on Durability of Humoral Response to SARS-CoV-2 Vaccination. Clin. Gastroenterol. Hepatol. 2022, 20, e1493–e1499. [Google Scholar] [CrossRef]
- Tepasse, P.R.; Vollenberg, R.; Nowacki, T.M. Vaccination against SARS-CoV-2 in Patients with Inflammatory Bowel Diseases: Where Do We Stand? Life 2021, 11, 1220. [Google Scholar] [CrossRef]
- Magro, F.; Estevinho, M.M. COVID-19 Vaccines in IBD Patients: Particularities and Future Perspectives. J. Crohn’s Colitis 2022, 16, 1343–1344. [Google Scholar] [CrossRef]
- Alexander, J.L.; Kennedy, N.A.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Seoane, R.C.; Liu, Z.; Nice, R.; Bewshea, C.; D’Mello, A.; et al. VIP study investigators. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 342–352. [Google Scholar] [CrossRef]
- Macaluso, F.S.; Principi, M.; Facciotti, F.; Contaldo, A.; Todeschini, A.; Saibeni, S.; Bezzio, C.; Castiglione, F.; Nardone, O.M.; Spagnuolo, R.; et al. Reduced humoral response to two doses of COVID-19 vaccine in patients with inflammatory bowel disease: Data from ESCAPE-IBD, an IG-IBD study. Dig. Liver Dis. 2023, 55, 154–159. [Google Scholar] [CrossRef]
- Shehab, M.; Alrashed, F.; Alfadhli, A.; Alsayegh, A.; Aldallal, U.; Alsayegh, M.; Cherian, P.; Alkhair, I.; Thanaraj, T.A.; Channanath, A.; et al. Immunogenicity of BNT162b2 Vaccine Booster Dose in Patients with Inflammatory Bowel Disease Receiving Infliximab Combination Therapy: A Prospective Observational Study. Front. Med. 2022, 9, 933996. [Google Scholar] [CrossRef] [PubMed]
- Schell, T.L.; Knutson, K.L.; Saha, S.; Wald, A.; Phan, H.S.; Almasry, M.; Chun, K.; Grimes, I.; Lutz, M.; Hayney, M.S.; et al. Humoral Immunogenicity of 3 COVID-19 Messenger RNA Vaccine Doses in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2022, 28, 1781–1786. [Google Scholar] [CrossRef]
- Lutz, M.; Lazarus, S.; Caldera, F. COVID-19 vaccination in adults with inflammatory bowel disease. Ther. Adv. Gastroenterol. 2023, 16, 17562848231173130. [Google Scholar] [CrossRef]
- Ferreira, F.B.; Rafael, M.A.; Coimbra, L.; Boavida, N.; Arrobas, F.; Correia, F.P.; Figueiredo, L.M.; Carvalho, E.; Branco, J.; Lourenço, L.C.; et al. Anti-tumor necrosis factor therapy is associated with attenuated humoral response to SARS-CoV-2 vaccines in patients with inflammatory bowel disease. Vaccine 2023, 41, 3862–3871. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Liu, Z.; Sandoval, D.M.; Reynolds, C.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Seoane, R.C.; Anand, N.; Nice, R.; et al. VIP study investigators. COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; James, D.; Singh, A.K.; Dutta, U.; Sebastian, S.; Sharma, V. Effectiveness and Durability of COVID-19 Vaccination in 9447 Patients with IBD: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1456–1479.e18. [Google Scholar] [CrossRef]
- Edelman-Klapper, H.; Rabinowitz, K.M.; Zittan, E.; Shitrit, A.B.-G.; Goren, I.; Avni-Biron, I.; Ollech, J.E.; Lichtenstein, L.; Banai-Eran, H.; Yanai, H.; et al. Serologic Response and Safety after a Third Dose of the COVID-19 BNT162b2 Vaccine in Patients with Inflammatory Bowel Diseases. Vaccines 2023, 11, 1263. [Google Scholar] [CrossRef]
- Doherty, J.; O’Morain, N.; Stack, R.; Tosetto, M.; Inzitiari, R.; O’Reilly, S.; Gu, L.; Sheridan, J.; Cullen, G.; Mc Dermott, E.; et al. Reduced Serological Response to COVID-19 Booster Vaccine is Associated with Reduced B Cell Memory in Patients with Inflammatory Bowel Disease; VARIATION [VAriability in Response in IBD AgainsT SARS-CoV-2 ImmunisatiON]. J. Crohn’s Colitis 2023, 17, 1445–1456. [Google Scholar] [CrossRef]
- Corona in Zahlen. COVID-19 Fallzahlen und Statistiken. Available online: https://www.corona-in-zahlen.de (accessed on 4 March 2025).
- Impfdashboard. COVID-19 Impfzahlen in Deutschland. Available online: https://impfdashboard.de (accessed on 4 March 2025).
- Weidner, G.; Sieverding, M.; Chesney, M.A. The role of self-regulation in health and illness. Psychol. Health Med. 2016, 21, 135–137. [Google Scholar] [CrossRef]
- Markovinović, A.; Quan, J.; Herauf, M.; Hracs, L.; Windsor, J.W.; Sharifi, N.; Coward, S.; Caplan, L.; Gorospe, J.; Ernest-Suarez, K.; et al. Adverse Events and Serological Responses After SARS-CoV-2 Vaccination in Individuals with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2023, 118, 1693–1697. [Google Scholar] [CrossRef]
- Ogrič, M.; Žigon, P.; Podovšovnik, E.; Lakota, K.; Sodin-Semrl, S.; Rotar, Ž.; Čučnik, S. Differences in SARS-CoV-2-Specific Antibody Responses After the First, Second, and Third Doses of BNT162b2 in Naïve and Previously Infected Individuals: A 1-Year Observational Study in Healthcare Professionals. Front. Immunol. 2022, 13, 876533. [Google Scholar] [CrossRef]
- Asahi, N.; Sakamaki, I.; Hida, Y.; Torii, K.; Hashimoto, N.; Iwasaki, H.; Iwano, M.; Kimura, H. Antibody level dynamics until after the third dose of COVID-19 vaccination. Heliyon 2023, 9, e17477. [Google Scholar] [CrossRef]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; und Magen, E. Large-Scale Study of Antibody Titer Decay Following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef]
- Pulendran, B.; Davis, M.M. The science and medicine of human immunology. Science 2020, 369, eaay4014. [Google Scholar] [CrossRef]
- Wong, S.Y.; Wellens, J.; Helmus, D.; Marlow, L.; Brann, S.; Martinez Pazos, V.; Satsangi, J. Geography Influences Susceptibility to SARS-CoV-2 Serological Response in Patients With Inflammatory Bowel Disease: Multinational Analysis From the ICARUS-IBD Consortium. Inflamm. Bowel Dis. 2023, 29, 1693–1705. [Google Scholar] [CrossRef]
- Deza, D.C.; Gomara, A.B.J.; Biota, E.C.; Beltrán, B.; Domènech, E.; Casbas, A.G.; Mañosa, M.; Zabana, Y.; Alfaro, L.R.; Romero, E.V.; et al. Impact of mesalazine on the response to COVID-19 vaccination in patients with inflammatory bowel disease: Results of a prospective multicentre study of GETECCU (VACOVEII study). Gastroenterol. Hepatol. 2024, 47, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, K.; Carreño, J.M.; Gleason, C.; Monahan, B.; Singh, G.; Abbad, A.; Tcheou, J.; Raskin, A.; Kleiner, G.; van Bakel, H.; et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 2024, 57, 587–599.e584. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).