Targeting Dermal Fibroblast Senescence: From Cellular Plasticity to Anti-Aging Therapies
Abstract
1. Introduction
2. Molecular Mechanisms of Dermal Fibroblast Plasticity
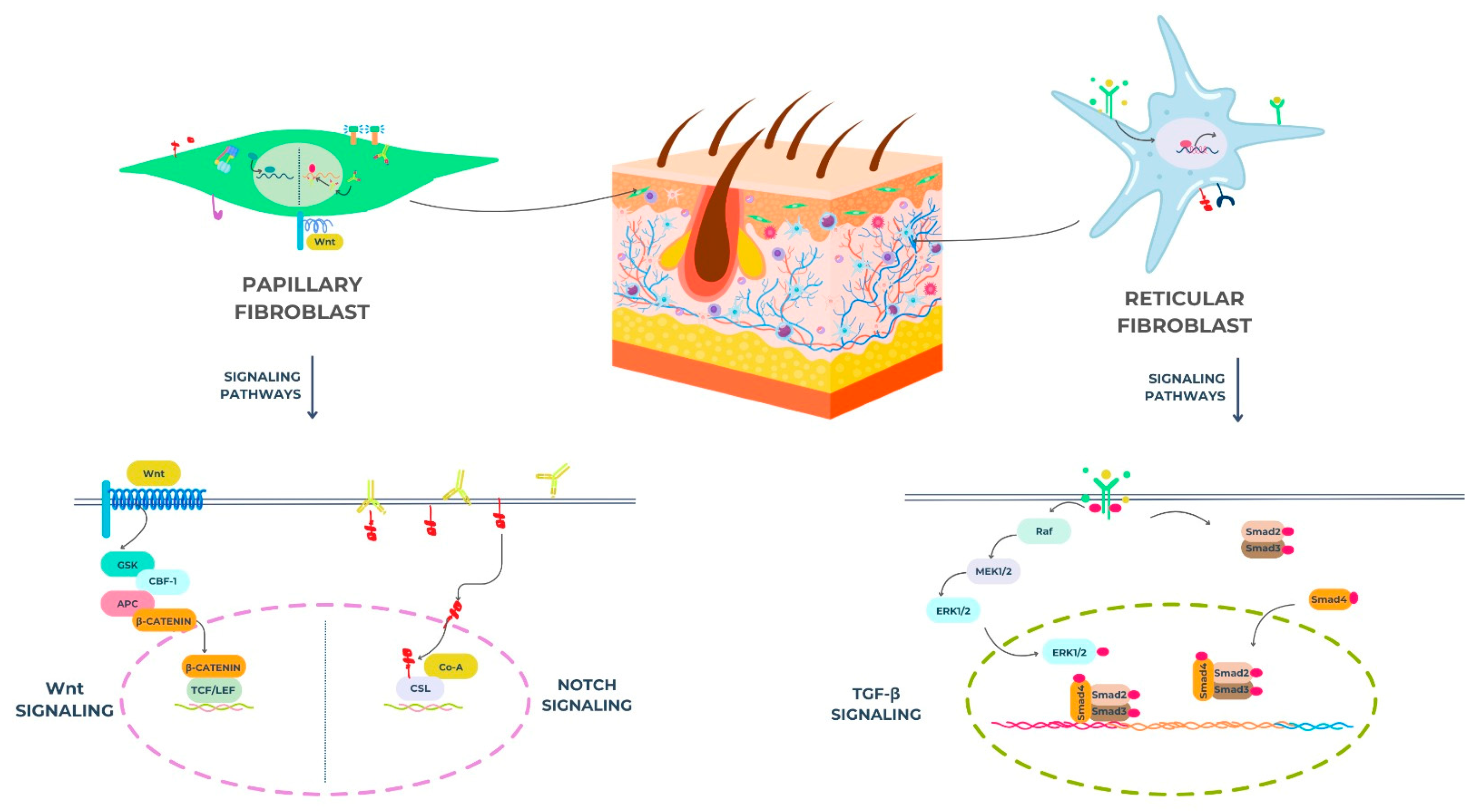
2.1. Key Signaling Pathways
- Transforming Growth Factor-beta (TGF-β) Signaling
- Fibroblast Growth Factor (FGF) Signaling
- Platelet-Derived Growth Factor (PDGF) Signaling
- Wnt Signaling
2.2. Epigenetic Regulation
- DNA Methylation
- Histone Modifications
2.3. MicroRNAs (miRNAs)
2.4. Signaling Pathways and Dermal Fibroblast Subpopulations
- Wnt: wingless/integrase-1 (refers to a family of signaling pathways);
- GSK: glycogen synthase Kkinase;
- CBF-1: CBF1 transactivator/also known as RBP-Jκ (recombination signal binding protein for immunoglobulin kappa J region);
- APC: adenomatous polyposis coli;
- β-catenin: beta-catenin protein;
- TCF: T-cell factor;
- LEF: lymphoid enhancer-binding factor;
- CSL: CBF1/suppressor of hairless/LAG-1 (a DNA-binding protein involved in Notch signaling).
- Raf: rapidly accelerated fibrosarcoma kinase;
- MEK1/2: mitogen-activated protein kinase kinase 1/2;
- ERK1/2: extracellular signal-regulated kinase 1/2;
- RBP-J: recombination signal binding protein for immunoglobulin kappa J region (also known as CBF1);
- Dll: delta-like ligand (e.g., Dll1, Dll3, Dll4);
- Notch: refers to the Notch receptor signaling pathway;
- Smad2, Smad3, Smad4: mothers against decapentaplegic homologs 2, 3, and 4 (intracellular signaling mediators for TGF-β).
3. Extracellular Matrix (ECM) and the Role of Fibroblasts
3.1. Extracellular Matrix
- Collagen
- Elastin
- Proteoglycans
- Glycosaminoglycans (GAGs)
- Adhesive Glycoproteins
- Structural support: provides structural support and organization of tissues;
- Segregation: separates different tissue compartments, creating specific microenvironments for different cell types;
- Cellular signaling: participates in cell signaling, influencing cell proliferation, differentiation, and survival;
- Cell migration: facilitates cell migration, which is essential in developmental processes, wound healing, and immune responses;
- Tissue homeostasis: regulates tissue growth, remodeling, and repair processes [53].
3.2. Role of Fibroblasts in ECM Synthesis and Remodeling
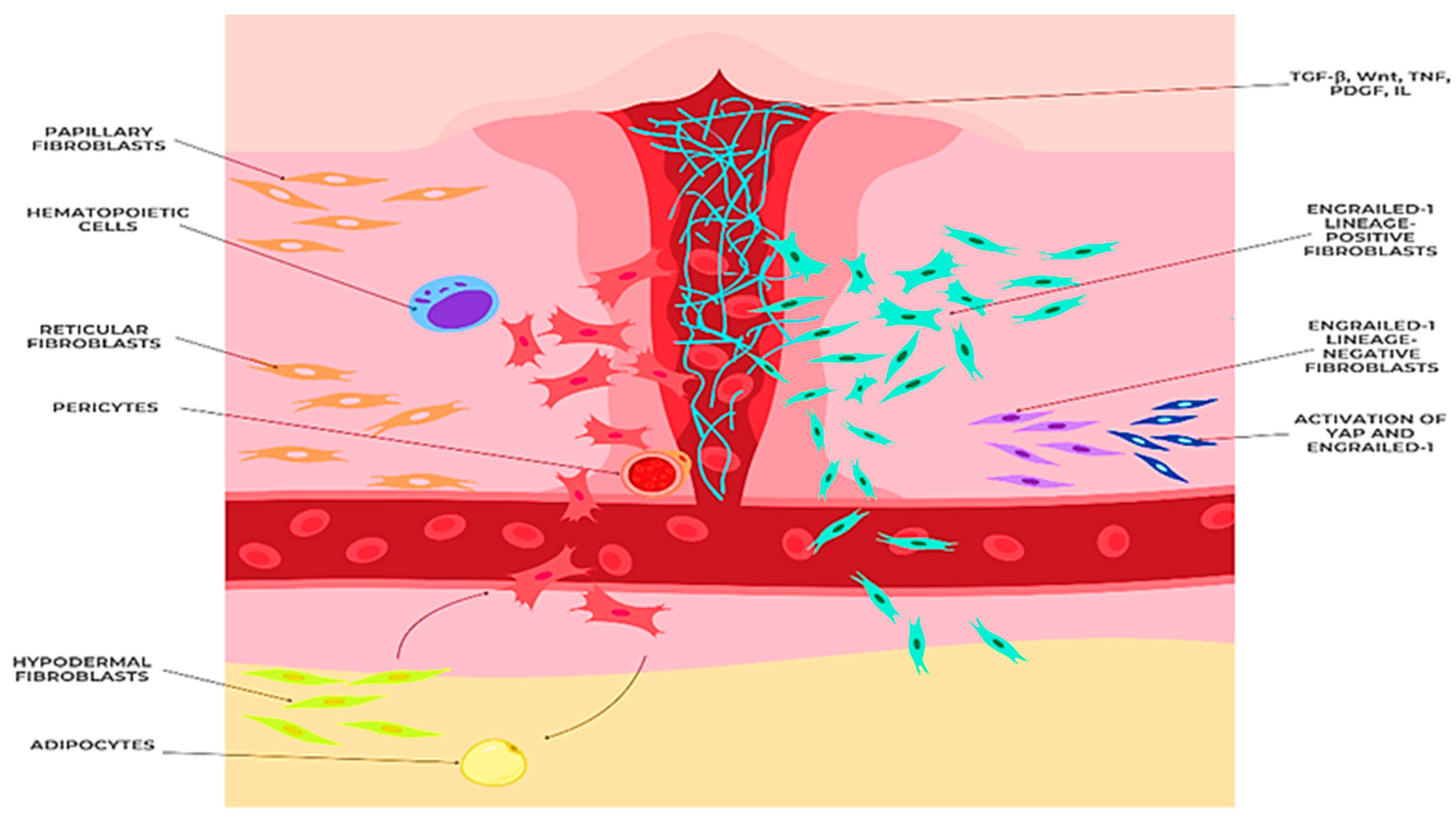
3.3. ECM Composition and Dermal Fibroblast Subpopulations
4. Impact of Aging on Extracellular Matrix Synthesis and Remodeling: A Molecular and Cellular Perspective
4.1. Diminished ECM Production
- Collagen-decreased synthesis rate and altered composition
- Elastin: breakdown and loss of elasticity
- Glycosaminoglycans (GAGs)–reduced hydration and modified biomechanical properties
4.2. Enhanced ECM Degradation
- Upregulation of matrix metalloproteinases (MMPs): Various MMPs, especially MMP-1, MMP-2, and MMP-9, become more active with age [84]. The expression of MMPs is regulated by a variety of factors, including growth factors, cytokines, and UV radiation [85]. Aged fibroblasts exhibit increased expression of MMPs due to increased activity of transcription factors such as AP-1 and NF-κB [86]. Furthermore, the levels of reactive oxygen species (ROS) are elevated in aged skin, which can activate MMPs and promote ECM degradation [87]. This increased MMP activity, often exceeding the capacity of tissue inhibitors of metalloproteinases (TIMPs) to neutralize them, leads to the net degradation of collagen and elastin fibers [88]. The imbalance between MMPs and TIMPs is a key factor in age-related ECM degradation. The expression of TIMPs, particularly TIMP-1 and TIMP-2, is reduced in aged fibroblasts, which further contributes to the increased MMP activity [89]. The imbalance between MMP and TIMP activity makes the ECM structure less stable and accelerates aging phenotypes;
- Accumulation of advanced glycation end products (AGEs): AGEs are formed through the non-enzymatic glycation of ECM proteins, particularly collagen and elastin. AGEs cross-link ECM molecules, increasing their rigidity and susceptibility to degradation [90]. Glycation is a process in which reducing sugars, such as glucose and fructose, react with amino groups in proteins to form Schiff bases, which undergo further reactions to form irreversible AGEs. AGEs accumulate in the skin with age, particularly in long-lived proteins such as collagen and elastin [91]. Furthermore, AGEs stimulate the production of pro-inflammatory cytokines and reactive oxygen species (ROS), further damaging the ECM;
- Chronic low-grade inflammation: Chronic low-grade inflammation is a hallmark of aging and significantly contributes to ECM degradation. Inflammatory mediators, such as cytokines and chemokines, promote the activity of MMPs and inhibit collagen synthesis [84,92]. Inflammation is characterized by elevated levels of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, in the circulation and in tissues [93,94]. This combination further accelerates the breakdown of the ECM.
4.3. Cellular and Microenvironmental Alterations
- Senescent Fibroblasts and the Senescence-Associated Secretory Phenotype (SASP)
- Impaired Cell–Cell Interactions
- Altered Growth Factor Signaling
4.4. Clinical Consequences
- Increased wrinkle formation and reduced skin elasticity: The elastin content, coupled with increased ECM degradation, directly leads to visible signs of aging such as fine lines and deeper wrinkles [100]. The loss of elasticity reduces the skin’s ability to recoil after stretching, leading to persistent wrinkles and disorganization of collagen fibers, which in turn disrupt the smooth structure of the skin and contribute to textural irregularities [101];
- Skin sagging and loss of turgor: The reduced collagen and elastin support results in a loss of skin volume and a decrease in the skin’s ability to resist gravity. This leads to sagging, particularly in areas like the cheeks, jawline, and under the eyes. The decreased turgor, or skin fullness, makes the skin appear thinner and more fragile [102]. Changes in the subcutaneous fat distribution, which also occur with aging, further contribute to the loss of facial volume and sagging;
- Increased skin dryness and roughness: The decline in GAGs, especially hyaluronic acid, reduces the skin’s ability to retain moisture, leading to increased dryness and a rough, uneven texture. This dryness can exacerbate the appearance of wrinkles and fine lines and can compromise the skin’s barrier function, making it more susceptible to irritants and allergens. The altered lipid composition of the stratum corneum, which also occurs with aging, further contributes to the increased skin dryness;
- Impaired wound healing: The age-related decline in fibroblast function and ECM remodeling capacity impairs the skin’s ability to heal wounds effectively. Aged fibroblasts exhibit reduced proliferation and migration, and their ability to synthesize new collagen and other ECM components is compromised. The increased levels of MMPs in aged skin can also disrupt the formation of a stable wound matrix, leading to delayed wound closure and increased risk of scarring [103]. Furthermore, the reduced vascularity in aged skin can impair oxygen and nutrient delivery to the wound site, further delaying the healing process;
- Increased susceptibility to skin damage and infections: The thinner, less elastic, and more fragile skin is more vulnerable to damage from external factors such as UV radiation, mechanical trauma, and chemical irritants [102]. The compromised skin barrier function makes it easier for pathogens to penetrate the skin, which increases the risk of infections. The reduced immune function in aged skin further contributes to an increased susceptibility to infections and delayed wound healing [104].
5. Senescence of Dermal Fibroblasts
5.1. Senescent Cell Secretome (SASP)
5.2. Therapeutic Perspectives: Senolytics and Geroprotectors
- Selective induction of apoptosis in senescent cells;
- Reduction of the senescence-associated secretory phenotype (SASP);
- Promotion of tissue regeneration by creating space for healthy cells to proliferate.
- Nutrient-sensing pathways;
- Cellular senescence;
- Mitochondrial dysfunction;
- Genomic instability;
- Epigenetic alterations.
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hayflick, L.; Moorhead, P.S. The Serial Cultivation of Human Diploid Cell Strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular Senescence: When Bad Things Happen to Good Cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Rodier, F.; Campisi, J. Four Faces of Cellular Senescence. J. Cell Biol. 2011, 192, 547–556. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V.; Kudlay, D.; Kopnin, P. Age-Related Changes in the Fibroblastic Differon of the Dermis: Role in Skin Aging. Int. J. Mol. Sci. 2022, 23, 6135. [Google Scholar] [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound Healing, Fibroblast Heterogeneity, and Fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef]
- Deng, C.-C.; Hu, Y.-F.; Zhu, D.-H.; Cheng, Q.; Gu, J.-J.; Feng, Q.-L.; Zhang, L.-X.; Xu, Y.-P.; Wang, D.; Rong, Z.; et al. Single-Cell RNA-Seq Reveals Fibroblast Heterogeneity and Increased Mesenchymal Fibroblasts in Human Fibrotic Skin Diseases. Nat. Commun. 2021, 12, 3709. [Google Scholar] [CrossRef]
- Kang, J.-H.; Jung, M.-Y.; Choudhury, M.; Leof, E.B. Transforming Growth Factor Beta Induces Fibroblasts to Express and Release the Immunomodulatory Protein PD-L1 into Extracellular Vesicles. FASEB J. 2020, 34, 2213–2226. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, S.-R.; Zhang, R.; Jin, J.-L.; Zhu, Y.-F. The Inhibitory Effect of Salvianolic Acid B on TGF-Β1-Induced Proliferation and Differentiation in Lung Fibroblasts. Exp. Lung Res. 2014, 40, 172–185. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming Growth Factor–β in Tissue Fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Heldin, C.H.; Westermark, B. Mechanism of Action and in Vivo Role of Platelet-Derived Growth Factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef]
- Sun, C.; Tian, X.; Jia, Y.; Yang, M.; Li, Y.; Fernig, D.G. Functions of Exogenous FGF Signals in Regulation of Fibroblast to Myofibroblast Differentiation and Extracellular Matrix Protein Expression. Open Biol. 2022, 12, 210356. [Google Scholar] [CrossRef]
- Sun, Z.; Fukui, M.; Taketani, S.; Kako, A.; Kunieda, S.; Kakudo, N. Predominant Control of PDGF/PDGF Receptor Signaling in the Migration and Proliferation of Human Adipose-derived Stem Cells under Culture Conditions with a Combination of Growth Factors. Exp. Ther. Med. 2024, 27, 156. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of Platelet-Derived Growth Factors in Physiology and Medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef]
- Ion, H.; Carmen, D.; Liliana, H.L.; Raluca, J.; Mihaela, M.; Carina, B.; Oana, A.A.; Alexandra, M.M.; Irina, G. Platelet Derivatives with Dental Medicine Applications. Rom. J. Oral Rehabil. 2020, 12, 142–152. [Google Scholar]
- Jian, K.; Yang, C.; Li, T.; Wu, X.; Shen, J.; Wei, J.; Yang, Z.; Yuan, D.; Zhao, M.; Shi, J. PDGF-BB-Derived Supramolecular Hydrogel for Promoting Skin Wound Healing. J. Nanobiotechnol. 2022, 20, 201. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of Canonical Wnt Signalling Is Required for TGF-β-Mediated Fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef]
- Koch, C.M.; Suschek, C.V.; Lin, Q.; Bork, S.; Goergens, M.; Joussen, S.; Pallua, N.; Ho, A.D.; Zenke, M.; Wagner, W. Specific Age-Associated DNA Methylation Changes in Human Dermal Fibroblasts. PLoS ONE 2011, 6, e16679. [Google Scholar] [CrossRef]
- Tao, H.; Song, Z.-Y.; Ding, X.-S.; Yang, J.-J.; Shi, K.-H.; Li, J. Epigenetic Signatures in Cardiac Fibrosis, Special Emphasis on DNA Methylation and Histone Modification. Heart Fail. Rev. 2018, 23, 789–799. [Google Scholar] [CrossRef]
- Sengupta, P.; Xu, Y.; Wang, L.; Widom, R.; Smith, B.D. Collagen A1(I) Gene (COL1A1) Is Repressed by RFX Family. J. Biol. Chem. 2005, 280, 21004–21014. [Google Scholar] [CrossRef]
- Sanders, Y.Y.; Hagood, J.S.; Liu, H.; Zhang, W.; Ambalavanan, N.; Thannickal, V.J. Histone Deacetylase Inhibition Promotes Fibroblast Apoptosis and Ameliorates Pulmonary Fibrosis in Mice. Eur. Respir. J. 2014, 43, 1448–1458. [Google Scholar] [CrossRef]
- Huang, S.K.; Scruggs, A.M.; Donaghy, J.; Horowitz, J.C.; Zaslona, Z.; Przybranowski, S.; White, E.S.; Peters-Golden, M. Histone Modifications Are Responsible for Decreased Fas Expression and Apoptosis Resistance in Fibrotic Lung Fibroblasts. Cell Death Dis. 2013, 4, e621. [Google Scholar] [CrossRef]
- Mamalis, A.; Koo, E.; Tepper, C.; Jagdeo, J. MicroRNA Expression Analysis of Human Skin Fibroblasts Treated with High-Fluence LED Red Light. J. Biophotonics 2019, 12, e201800207. [Google Scholar] [CrossRef]
- Noskovičová, N.; Petřek, M.; Eickelberg, O.; Heinzelmann, K. Platelet-Derived Growth Factor Signaling in the Lung. From Lung Development and Disease to Clinical Studies. Am. J. Respir. Cell Mol. Biol. 2015, 52, 263–284. [Google Scholar] [CrossRef]
- Swahari, V.; Nakamura, A.; Hollville, E.; Hung, Y.-H.; Kanke, M.; Kurtz, C.L.; Caravia, X.M.; Roiz-Valle, D.; He, S.; Krishnamurthy, J.; et al. miR-29 Is an Important Driver of Aging-Related Phenotypes. Commun. Biol. 2024, 7, 1055. [Google Scholar] [CrossRef]
- Liu, C.; Tong, Z.; Tan, J.; Xin, Z.; Wang, Z.; Tian, L. MicroRNA-21-5p Targeting PDCD4 Suppresses Apoptosis via Regulating the PI3K/AKT/FOXO1 Signaling Pathway in Tongue Squamous Cell Carcinoma. Exp. Ther. Med. 2019, 18, 3543–3551. [Google Scholar] [CrossRef]
- Eissa, M.G.; Artlett, C.M. The MicroRNA miR-155 Is Essential in Fibrosis. Noncoding RNA 2019, 5, 23. [Google Scholar] [CrossRef]
- Dykxhoorn, D.M.; Da Fonseca Ferreira, A.; Gomez, K.; Shi, J.; Zhu, S.; Zhang, L.; Wang, H.; Wei, J.; Zhang, Q.; Macon, C.J.; et al. MicroRNA-29c-3p and -126a Contribute to the Decreased Angiogenic Potential of Aging Endothelial Progenitor Cells. Int. J. Mol. Sci. 2025, 26, 4259. [Google Scholar] [CrossRef]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular Matrix Composition of Connective Tissues: A Systematic Review and Meta-Analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Greene, K.A.; Sankaran, B.; Downey, G.P.; Radisky, D.C.; Radisky, E.S. Directed evolution of the metalloproteinase inhibitor TIMP-1 reveals that its N- and C-terminal domains cooperate in matrix metalloproteinase recognition. J. Biol. Chem. 2019, 294, 9476–9488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Baek, W.; Park, S.; Lee, Y.; Roh, H.; Yun, C.-O.; Roh, T.S.; Lee, W.J. Ethyl Pyruvate Decreases Collagen Synthesis and Upregulates MMP Activity in Keloid Fibroblasts and Keloid Spheroids. Int. J. Mol. Sci. 2024, 25, 5844. [Google Scholar] [CrossRef]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell–Extracellular Matrix Mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [CrossRef]
- Guerrero-Juarez, C.F.; Dedhia, P.H.; Jin, S.; Ruiz-Vega, R.; Ma, D.; Liu, Y.; Yamaga, K.; Shestova, O.; Gay, D.L.; Yang, Z.; et al. Single-Cell Analysis Reveals Fibroblast Heterogeneity and Myeloid-Derived Adipocyte Progenitors in Murine Skin Wounds. Nat. Commun. 2019, 10, 650. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct Fibroblast Lineages Determine Dermal Architecture in Skin Development and Repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef]
- Trempus, C.S.; Papas, B.N.; Sifre, M.I.; Bortner, C.D.; Scappini, E.; Tucker, C.J.; Xu, X.; Johnson, K.L.; Deterding, L.J.; Williams, J.G.; et al. Functional Pdgfra fibroblast heterogeneity in normal and fibrotic mouse lung. JCI Insight 2023, 8, e164380. [Google Scholar] [CrossRef]
- Faraci, E.; Eck, M.; Gerstmayer, B.; Bosio, A.; Vogel, W.F. An Extracellular Matrix-Specific Microarray Allowed the Identification of Target Genes Downstream of Discoidin Domain Receptors. Matrix Biol. 2003, 22, 373–381. [Google Scholar] [CrossRef]
- Essner, J.J.; Chen, E.; Ekker, S.C. Syndecan-2. Int. J. Biochem. Cell Biol. 2006, 38, 152–156. [Google Scholar] [CrossRef]
- Eun, K.; Kim, A.Y.; Ryu, S. Matricellular Proteins in Immunometabolism and Tissue Homeostasis. BMB Rep. 2024, 57, 400–416. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The Extracellular Matrix: Structure, Composition, Age-Related Differences, Tools for Analysis and Applications for Tissue Engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Tan, P.-C.; Zhou, S.-B.; Ou, M.-Y.; He, J.-Z.; Zhang, P.-Q.; Zhang, X.-J.; Xie, Y.; Gao, Y.-M.; Zhang, T.-Y.; Li, Q.-F. Mechanical Stretching Can Modify the Papillary Dermis Pattern and Papillary Fibroblast Characteristics during Skin Regeneration. J. Investig. Dermatol. 2022, 142, 2384–2394.e8. [Google Scholar] [CrossRef]
- Fujisawa, M.; Omori, M.; Doihara, H.; Than, Y.; Swe, H.W.W.; Yoshimura, T.; Matsukawa, A. Elastin and Collagen IV Double Staining: A Refined Method to Detect Blood Vessel Invasion in Breast Cancer. Pathol. Int. 2020, 70, 612–623. [Google Scholar] [CrossRef]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthetic Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]
- Lee, S.S.; Al Halawani, A.; Teo, J.D.; Weiss, A.S.; Yeo, G.C. The Matrix Protein Tropoelastin Prolongs Mesenchymal Stromal Cell Vitality and Delays Senescence During Replicative Aging. Adv. Sci. 2024, 11, 2402168. [Google Scholar] [CrossRef]
- Yao, Y.; Findlay, A.; Stolp, J.; Rayner, B.; Ask, K.; Jarolimek, W. Pan-Lysyl Oxidase Inhibitor PXS-5505 Ameliorates Multiple-Organ Fibrosis by Inhibiting Collagen Crosslinks in Rodent Models of Systemic Sclerosis. Int. J. Mol. Sci. 2022, 23, 5533. [Google Scholar] [CrossRef]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A Role for Advanced Glycation End Products in Molecular Ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vynios, D.H. Metabolism of Cartilage Proteoglycans in Health and Disease. BioMed Res. Int. 2014, 2014, 452315. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, F.; Linhardt, R.J. Glycosaminoglycans. Adv. Exp. Med. Biol. 2021, 1325, 103–116. [Google Scholar] [CrossRef]
- Hwang, C.T.; Halper, J. Proteoglycans and Diseases of Soft Tissues. Adv. Exp. Med. Biol. 2021, 1348, 127–138. [Google Scholar] [CrossRef]
- Mattson, J.M.; Turcotte, R.; Zhang, Y. Glycosaminoglycans Contribute to Extracellular Matrix Fiber Recruitment and Arterial Wall Mechanics. Biomech. Model. Mechanobiol. 2017, 16, 213–225. [Google Scholar] [CrossRef]
- Mizumoto, S.; Kwok, J.C.F.; Whitelock, J.M.; Li, F.; Perris, R. Editorial: Roles of Chondroitin Sulfate and Dermatan Sulfate as Regulators for Cell and Tissue Development. Front. Cell Dev. Biol. 2022, 10, 941178. [Google Scholar] [CrossRef]
- Oz, O.K.; Campbell, A.; Tao, T.W. Reduced Cell Adhesion to Fibronectin and Laminin Is Associated with Altered Glycosylation of Beta 1 Integrins in a Weakly Metastatic Glycosylation Mutant. Int. J. Cancer 1989, 44, 343–347. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Kulczyk, A.W. Polymerizing Laminins in Development, Health, and Disease. J. Biol. Chem. 2024, 300, 107429. [Google Scholar] [CrossRef]
- Yue, B. Biology of the Extracellular Matrix: An Overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Margulis, A.; Nocka, K.H.; Wood, N.L.; Wolf, S.F.; Goldman, S.J.; Kasaian, M.T. MMP Dependence of Fibroblast Contraction and Collagen Production Induced by Human Mast Cell Activation in a Three-Dimensional Collagen Lattice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L236–L247. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The Tissue Inhibitors of Metalloproteinases (TIMPs): An Ancient Family with Structural and Functional Diversity. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Skin Fibrosis. Identification and Isolation of a Dermal Lineage with Intrinsic Fibrogenic Potential. Science 2015, 348, aaa2151. [Google Scholar] [CrossRef]
- Grässel, S.; Unsöld, C.; Schäcke, H.; Bruckner-Tuderman, L.; Bruckner, P. Collagen XVI Is Expressed by Human Dermal Fibroblasts and Keratinocytes and Is Associated with the Microfibrillar Apparatus in the Upper Papillary Dermis. Matrix Biol. 1999, 18, 309–317. [Google Scholar] [CrossRef]
- Janson, D.; Saintigny, G.; Mahé, C.; Ghalbzouri, A.E. Papillary Fibroblasts Differentiate into Reticular Fibroblasts after Prolonged in Vitro Culture. Exp. Dermatol. 2013, 22, 48–53. [Google Scholar] [CrossRef]
- Chylińska, N.; Maciejczyk, M. Hyaluronic Acid and Skin: Its Role in Aging and Wound-Healing Processes. Gels 2025, 11, 281. [Google Scholar] [CrossRef]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in Extracellular Matrix Composition during Aging and Photoaging of the Skin. Matrix Biol. Pluss 2020, 8, 100041. [Google Scholar] [CrossRef]
- Charoenchon, N.; Rhodes, L.E.; Nicolaou, A.; Williamson, G.; Watson, R.E.B.; Farrar, M.D. Ultraviolet Radiation-induced Degradation of Dermal Extracellular Matrix and Protection by Green Tea Catechins: A Randomized Controlled Trial. Clin. Exp. Dermatol. 2022, 47, 1314–1323. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, S.; Lovisa, S.; Ambrose, C.G.; McAndrews, K.M.; Sugimoto, H.; Kalluri, R. Type-I Collagen Produced by Distinct Fibroblast Lineages Reveals Specific Function during Embryogenesis and Osteogenesis Imperfecta. Nat. Commun. 2021, 12, 7199. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, D.H.; Kim, H.J.; Lee, J.Y.; Cho, B.K.; Bang, S.I.; Song, S.Y.; Yamasaki, K.; Nardo, A.D.; Gallo, R.L. Collagen Synthesis Is Suppressed in Dermal Fibroblasts by the Human Antimicrobial Peptide LL-37. J. Investig. Dermatol. 2009, 129, 843–850. [Google Scholar] [CrossRef]
- De Giorgi, F.; Fumagalli, M.; Scietti, L.; Forneris, F. Collagen Hydroxylysine Glycosylation: Non-Conventional Substrates for Atypical Glycosyltransferase Enzymes. Biochem. Soc. Trans. 2021, 49, 855–866. [Google Scholar] [CrossRef]
- Pihlajaniemi, T.; Myllylä, R.; Kivirikko, K.I. Prolyl 4-hydroxylase and its role in collagen synthesis. J. Hepatol. 1991, 13 (Suppl. S3), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Fisher, G.J.; Kim, A.J.; Quan, T. Age-Related Changes in Dermal Collagen Physical Properties in Human Skin. PLoS ONE 2023, 18, e0292791. [Google Scholar] [CrossRef]
- Byun, K.A.; Kim, H.M.; Oh, S.; Batsukh, S.; Son, K.H.; Byun, K. Radiofrequency Treatment Attenuates Age-Related Changes in Dermal-Epidermal Junctions of Animal Skin. Int. J. Mol. Sci. 2024, 25, 5178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, L.T.; Saad, S.; Shi, Y.; Wang, R.; Chou, A.S.Y.; Gill, A.; Yao, Y.; Jarolimek, W.; Pollock, C.A. Lysyl oxidase inhibitors attenuate cyclosporin A-induced nephropathy in mouse. Sci. Rep. 2021, 11, 12437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, C.; Chen, X.; Yin, X.; Jiang, Y.; Zhao, C. Matrix Metalloproteinases on Skin Photoaging. J. Cosmet. Dermatol. 2024, 23, 3847–3862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ozsvar, J.; Yang, C.; Cain, S.A.; Baldock, C.; Tarakanova, A.; Weiss, A.S. Tropoelastin and Elastin Assembly. Front. Bioeng. Biotechnol. 2021, 9, 643110. [Google Scholar] [CrossRef]
- Dierckx, S.; Patrizi, M.; Merino, M.; González, S.; Mullor, J.L.; Nergiz-Unal, R. Collagen peptides affect collagen synthesis and the expression of collagen, elastin, and versican genes in cultured human dermal fibroblasts. Front. Med. 2024, 11, 1397517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heinz, A. Elastic Fibers during Aging and Disease. Ageing Res. Rev. 2021, 66, 101255. [Google Scholar] [CrossRef]
- Shape of Tropoelastin, the Highly Extensible Protein That Controls Human Tissue Elasticity|PNAS. Available online: https://www.pnas.org/doi/10.1073/pnas.1014280108 (accessed on 11 February 2025).
- Cirillo, N.; Prime, S.S. A Scoping Review of the Role of Metalloproteinases in the Pathogenesis of Autoimmune Pemphigus and Pemphigoid. Biomolecules 2021, 11, 1506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cenizo, V.; André, V.; Reymermier, C.; Sommer, P.; Damour, O.; Perrier, E. LOXL as a target to increase the elastin content in adult skin: A dill extract induces the LOXL gene expression. Exp. Dermatol. 2006, 15, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Calderon, T.; Önner, H.; Yavuz, C. 68Ga-PSMA Uptake in Solar Elastosis. Clin. Nucl. Med. 2024, 49, 106–107. [Google Scholar] [CrossRef]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules 2020, 11, 29. [Google Scholar] [CrossRef]
- Oguchi, T.; Ishiguro, N. Differential Stimulation of Three Forms of Hyaluronan Synthase by TGF-β, IL-1β, and TNF-α. Connect. Tissue Res. 2004, 45, 197–205. [Google Scholar] [CrossRef]
- Fink, S.P.; Triggs-Raine, B. Genetic Deficiencies of Hyaluronan Degradation. Cells 2024, 13, 1203. [Google Scholar] [CrossRef]
- Cortes-Medina, M.; Bushman, A.R.; Beshay, P.E.; Adorno, J.J.; Menyhert, M.M.; Hildebrand, R.M.; Agarwal, S.S.; Avendano, A.; Friedman, A.K.; Song, J.W. Chondroitin Sulfate, Dermatan Sulfate, and Hyaluronic Acid Differentially Modify the Biophysical Properties of Collagen-Based Hydrogels. Acta Biomater. 2024, 174, 116–126. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of Topical Hyaluronic Acid for Skin Quality and Signs of Skin Aging: From Literature Review to Clinical Evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The Role of Matrix Metalloproteinases in Aging: Tissue Remodeling and beyond. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864 Pt A, 2015–2025. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Jeong, H.D.; Kim, J.H.; Kwon, G.E.; Lee, S.-T. Expression of Polyamine Oxidase in Fibroblasts Induces MMP-1 and Decreases the Integrity of Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 10487. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.H.; Choi, Y.J.; Kim, D.-W.; Cho, E.; Kang, M.; Kim, D.; Pyo, J.; Kang, K.S. Inhibition of TNF-α-Induced Collagen Degradation and Oxidative Damage by Centipeda Minima and Brevilin A in Human Dermal Fibroblasts. Curr. Issues Mol. Biol. 2025, 47, 376. [Google Scholar] [CrossRef]
- Ishii, T.; Asuwa, N. Collagen and Elastin Degradation by Matrix Metalloproteinases and Tissue Inhibitors of Matrix Metalloproteinase in Aortic Dissection. Hum. Pathol. 2000, 31, 640–646. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, C.; Zhang, S.; Ke, Z.-X.; Chen, D.-X.; Li, Y.-Q.; Li, Q. The Imbalance of MMP-2/TIMP-2 and MMP-9/TIMP-1 Contributes to Collagen Deposition Disorder in Diabetic Non-Injured Skin. Front. Endocrinol. 2021, 12, 734485. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Zheng, W.; Li, H.; Go, Y.; Chan, X.H.; Huang, Q.; Wu, J. Research Advances on the Damage Mechanism of Skin Glycation and Related Inhibitors. Nutrients 2022, 14, 4588. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.-S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF Cytokine Triad Is Associated with Post-Acute Sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Z.; Xue, R.; Singh, G.K.; Shi, K.; Lv, Y.; Yang, L. Combined Effects of TNF-α, IL-1β, and HIF-1α on MMP-2 Production in ACL Fibroblasts under Mechanical Stretch: An in Vitro Study. J. Orthop. Res. 2011, 29, 1008–1014. [Google Scholar] [CrossRef]
- Wlaschek, M.; Maity, P.; Makrantonaki, E.; Scharffetter-Kochanek, K. Connective Tissue and Fibroblast Senescence in Skin Aging. J. Investig. Dermatol. 2021, 141, 985–992. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many Therapeutic Avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, Y.; Zeng, Y.; Yang, D.; Mo, J.; Zheng, Z.; Wang, J.; Zhang, Y.; Zhou, Z.; Zhong, X.; et al. Impaired Angiogenesis in Ageing: The Central Role of the Extracellular Matrix. J. Transl. Med. 2023, 21, 457. [Google Scholar] [CrossRef]
- Amidzadeh, Z.; Yasami-Khiabani, S.; Rahimi, H.; Bonakdar, S.; Shams, D.; Habibi-Anbouhi, M.; Golkar, M.; Shokrgozar, M.A. Enhancement of Keratinocyte Growth Factor Potential in Inducing Adipose-derived Stem Cells Differentiation into Keratinocytes by Collagen-targeting. J. Cell. Mol. Med. 2022, 26, 5929–5942. [Google Scholar] [CrossRef]
- Hodgson, D.; Rowan, A.D.; Falciani, F.; Proctor, C.J. Systems Biology Reveals How Altered TGFβ Signalling with Age Reduces Protection against Pro-Inflammatory Stimuli. PLoS Comput. Biol. 2019, 15, e1006685. [Google Scholar] [CrossRef]
- Quan, T. Human Skin Aging and the Anti-Aging Properties of Retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef]
- Faria, A.V.S.; Andrade, S.S. Decoding the Impact of Ageing and Environment Stressors on Skin Cell Communication. Biogerontology 2025, 26, 3. [Google Scholar] [CrossRef]
- Oh, S.; Rhee, D.-Y.; Batsukh, S.; Son, K.H.; Byun, K. High-Intensity Focused Ultrasound Increases Collagen and Elastin Fiber Synthesis by Modulating Caveolin-1 in Aging Skin. Cells 2023, 12, 2275. [Google Scholar] [CrossRef]
- Meng, H.; Su, J.; Shen, Q.; Hu, W.; Li, P.; Guo, K.; Liu, X.; Ma, K.; Zhong, W.; Chen, S.; et al. A Smart MMP-9-responsive Hydrogel Releasing M2 Macrophage-derived Exosomes for Diabetic Wound Healing. Adv. Healthc. Mater. 2025, 14, 2404966. [Google Scholar] [CrossRef]
- Fullard, N.; Wordsworth, J.; Welsh, C.; Maltman, V.; Bascom, C.; Tasseff, R.; Isfort, R.; Costello, L.; Scanlan, R.-L.; Przyborski, S.; et al. Cell Senescence-Independent Changes of Human Skin Fibroblasts with Age. Cells 2024, 13, 659. [Google Scholar] [CrossRef]
- Chen, D.; Yin, S.; Lu, X.; Fu, H.; Gao, H.; Zhang, S. Research on the Correlation Between Skin Elasticity Evaluation Parameters and Age. Cosmetics 2024, 11, 205. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and Aging: Causes, Consequences, and Therapeutic Avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Ungureanu, L.B.; Grădinaru, I.; Ghiciuc, C.M.; Amălinei, C.; Gelețu, G.L.; Petrovici, C.G.; Stănescu, R. Ștefania Atrophy and Inflammatory Changes in Salivary Glands Induced by Oxidative Stress after Exposure to Drugs and Other Chemical Substances: A Systematic Review. Medicina 2023, 59, 1692. [Google Scholar] [CrossRef]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics Decrease Senescent Cells in Humans: Preliminary Report from a Clinical Trial of Dasatinib plus Quercetin in Individuals with Diabetic Kidney Disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics Improve Physical Function and Increase Lifespan in Old Age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin Is a Senotherapeutic That Extends Health and Lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in Idiopathic Pulmonary Fibrosis: Results from a First-in-Human, Open-Label, Pilot Study. eBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef]
- Orioli, D.; Dellambra, E. Epigenetic Regulation of Skin Cells in Natural Aging and Premature Aging Diseases. Cells 2018, 7, 268. [Google Scholar] [CrossRef]
- Zheng, L.; He, S.; Wang, H.; Li, J.; Liu, Y.; Liu, S. Targeting Cellular Senescence in Aging and Age-Related Diseases: Challenges, Considerations, and the Emerging Role of Senolytic and Senomorphic Therapies. Aging Dis. 2024, 15, 2554–2594. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a Novel Senolytic Agent, Navitoclax, Targeting the Bcl-2 Family of Anti-Apoptotic Factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef]
- Elliehausen, C.J.; Anderson, R.M.; Diffee, G.M.; Rhoads, T.W.; Lamming, D.W.; Hornberger, T.A.; Konopka, A.R. Geroprotector Drugs and Exercise: Friends or Foes on Healthy Longevity? BMC Biol. 2023, 21, 287. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Barzilai, N. New Horizons in Life Extension, Healthspan Extension and Exceptional Longevity. Age Ageing 2022, 51, afac156. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef]
- Cui, J.; Li, H.; Zhang, T.; Lin, F.; Chen, M.; Zhang, G.; Feng, Z. Research Progress on the Mechanism of Curcumin Anti-Oxidative Stress Based on Signaling Pathway. Front. Pharmacol. 2025, 16, 1548073. [Google Scholar] [CrossRef]
- Liao, D.; Shangguan, D.; Wu, Y.; Chen, Y.; Liu, N.; Tang, J.; Yao, D.; Shi, Y. Curcumin protects against doxorubicin induced oxidative stress by regulating the Keap1-Nrf2-ARE and autophagy signaling pathways. Psychopharmacology 2023, 240, 1179–1190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Recent Advances in the Discovery of Senolytics. Mech. Ageing Dev. 2021, 200, 111587. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Rapamycin for Longevity: Opinion Article. Aging 2019, 11, 8048–8067. [Google Scholar] [CrossRef]
- Xu, M.; Palmer, A.K.; Ding, H.; Weivoda, M.M.; Pirtskhalava, T.; White, T.A.; Sepe, A.; Johnson, K.O.; Stout, M.B.; Giorgadze, N.; et al. Targeting Senescent Cells Enhances Adipogenesis and Metabolic Function in Old Age. eLife 2015, 4, e12997. [Google Scholar] [CrossRef]
- Konstantinou, E.; Longange, E.; Kaya, G. Mechanisms of Senescence and Anti-Senescence Strategies in the Skin. Biology 2024, 13, 647. [Google Scholar] [CrossRef]
- Bocklandt, S.; Lin, W.; Sehl, M.E.; Sánchez, F.J.; Sinsheimer, J.S.; Horvath, S.; Vilain, E. Epigenetic Predictor of Age. PLoS ONE 2011, 6, e14821. [Google Scholar] [CrossRef]
- Rayson, A.; Boudiffa, M.; Naveed, M.; Griffin, J.; Dall’Ara, E.; Bellantuono, I. Geroprotectors and Skeletal Health: Beyond the Headlines. Front. Cell Dev. Biol. 2022, 10, 682045. [Google Scholar] [CrossRef]
- Yanar, K. Novel Classification Perspective of Geroprotective and Senolytic Drugs as an Antiaging Strategy. In Molecular Basis and Emerging Strategies for Anti-Aging Interventions; Springer: Berlin/Heidelberg, Germany, 2018; pp. 83–96. ISBN 978-981-13-1698-2. [Google Scholar]
- Mbara, K.C.; Devnarain, N.; Owira, P.M.O. Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection. Pharm. Med. 2022, 36, 331–352. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic Drugs: From Discovery to Translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Raffaele, M.; Vinciguerra, M. The Costs and Benefits of Senotherapeutics for Human Health. Lancet Healthy Longev. 2022, 3, e67–e77. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alqahtani, T.; Venkatesan, K.; Sivadasan, D.; Ahmed, R.; Sirag, N.; Elfadil, H.; Abdullah Mohamed, H.; T.A., H.; Elsayed Ahmed, R.; et al. SASP Modulation for Cellular Rejuvenation and Tissue Homeostasis: Therapeutic Strategies and Molecular Insights. Cells 2025, 14, 608. [Google Scholar] [CrossRef]
- Chin, T.; Lee, X.E.; Ng, P.Y.; Lee, Y.; Dreesen, O. The Role of Cellular Senescence in Skin Aging and Age-Related Skin Pathologies. Front. Physiol. 2023, 14, 1297637. [Google Scholar] [CrossRef]
- Wang, S.T.; Neo, B.H.; Betts, R.J. Glycosaminoglycans: Sweet as Sugar Targets for Topical Skin Anti-Aging. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1227–1246. [Google Scholar] [CrossRef]
- Gonzalo, S. Epigenetic Alterations in Aging. J. Appl. Physiol. 2010, 109, 586–597. [Google Scholar] [CrossRef]
- Moskalev, A.; Chernyagina, E.; De Magalhães, J.P.; Barardo, D.; Thoppil, H.; Shaposhnikov, M.; Budovsky, A.; Fraifeld, V.E.; Garazha, A.; Tsvetkov, V.; et al. Geroprotectors.Org: A New, Structured and Curated Database of Current Therapeutic Interventions in Aging and Age-Related Disease. Aging 2015, 7, 616–628. [Google Scholar] [CrossRef]
- Smer-Barreto, V.; Quintanilla, A.; Elliott, R.J.R.; Dawson, J.C.; Sun, J.; Campa, V.M.; Lorente-Macías, Á.; Unciti-Broceta, A.; Carragher, N.O.; Acosta, J.C.; et al. Discovery of Senolytics Using Machine Learning. Nat. Commun. 2023, 14, 3445. [Google Scholar] [CrossRef]


| Category | Medications | Mechanism of Action | Target Cell Types | Efficacy | References |
|---|---|---|---|---|---|
| Senolytics | Dasatinib, Quercetin | Inducing apoptosis in senescent cells | Senescent cells from various tissues | Significant reduction of sarcopenia | [113] |
| Navitoclax | Blocking BCL-2 signaling to promote apoptosis | Senescent cells, especially from adipose tissue | Improvement of muscle function | [114,115] | |
| Geroprotectors | Rapamycin, Metformin | Inhibiting the mTOR pathway to improve longevity | Cells from different types of tissue | Delaying the aging process | [116,117] |
| Curcumin | Activating the Nrf2 pathway to reduce oxidative stress | Cells in general, especially those involved in inflammation | Reducing inflammation and oxidative stress | [118,119,120] | |
| Resveratrol | Activating the SIRT1 protein to induce longevity | Cells from all tissues, including muscular and nervous | Improving metabolism and cardiovascular function | [121,122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jipu, R.; Serban, I.L.; Goriuc, A.; Jipu, A.G.; Luchian, I.; Amititeloaie, C.; Tarniceriu, C.C.; Hurjui, I.; Butnaru, O.M.; Hurjui, L.L. Targeting Dermal Fibroblast Senescence: From Cellular Plasticity to Anti-Aging Therapies. Biomedicines 2025, 13, 1927. https://doi.org/10.3390/biomedicines13081927
Jipu R, Serban IL, Goriuc A, Jipu AG, Luchian I, Amititeloaie C, Tarniceriu CC, Hurjui I, Butnaru OM, Hurjui LL. Targeting Dermal Fibroblast Senescence: From Cellular Plasticity to Anti-Aging Therapies. Biomedicines. 2025; 13(8):1927. https://doi.org/10.3390/biomedicines13081927
Chicago/Turabian StyleJipu, Raluca, Ionela Lacramioara Serban, Ancuta Goriuc, Alexandru Gabriel Jipu, Ionut Luchian, Carmen Amititeloaie, Claudia Cristina Tarniceriu, Ion Hurjui, Oana Maria Butnaru, and Loredana Liliana Hurjui. 2025. "Targeting Dermal Fibroblast Senescence: From Cellular Plasticity to Anti-Aging Therapies" Biomedicines 13, no. 8: 1927. https://doi.org/10.3390/biomedicines13081927
APA StyleJipu, R., Serban, I. L., Goriuc, A., Jipu, A. G., Luchian, I., Amititeloaie, C., Tarniceriu, C. C., Hurjui, I., Butnaru, O. M., & Hurjui, L. L. (2025). Targeting Dermal Fibroblast Senescence: From Cellular Plasticity to Anti-Aging Therapies. Biomedicines, 13(8), 1927. https://doi.org/10.3390/biomedicines13081927











