Chemerin as a Driver of Cardiovascular Diseases: New Perspectives and Future Directions
Abstract
1. Introduction
2. Methods
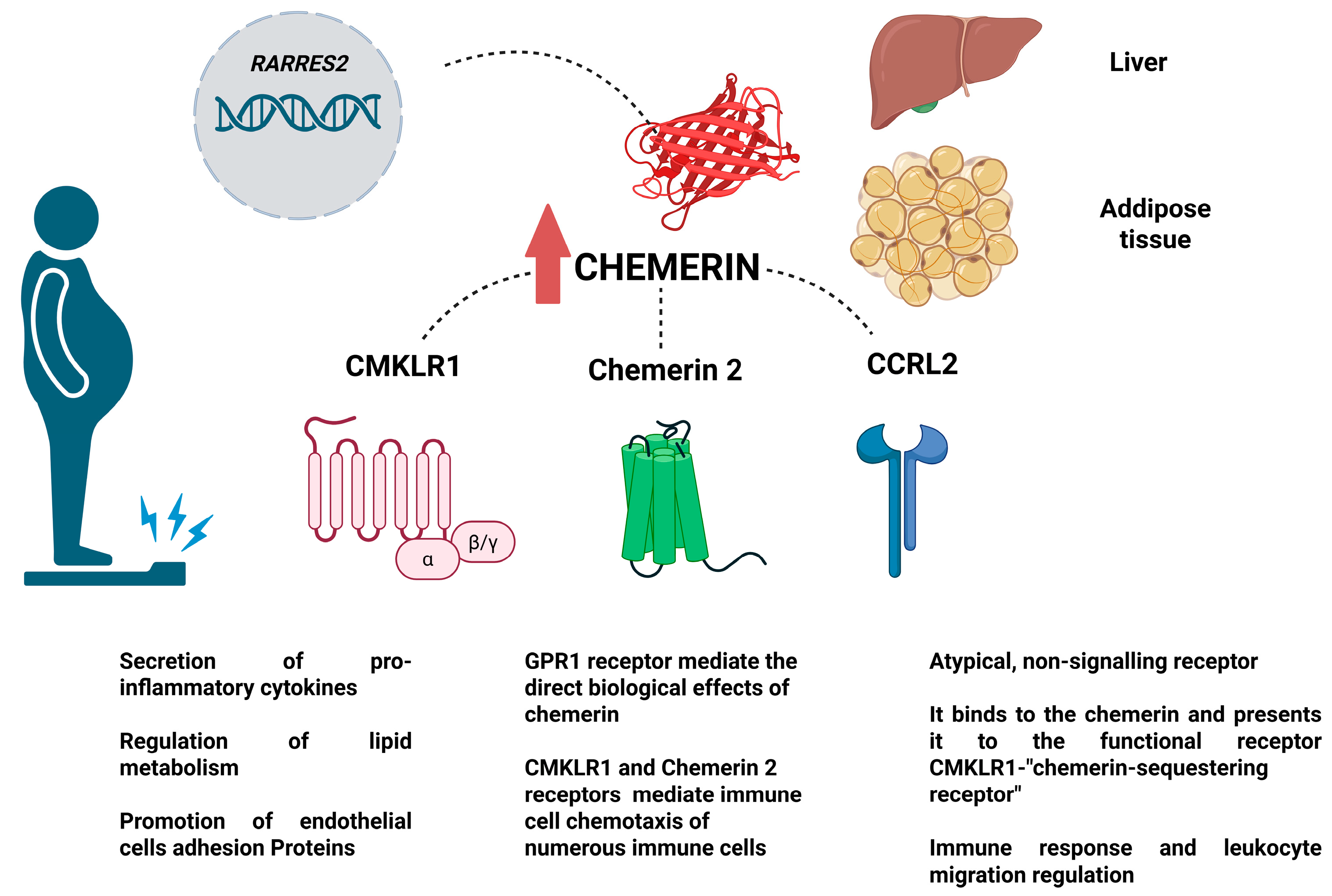
Chemerin—Molecular Effect and Site of Action
3. The Physiological Role of Chemerin in Adipocytes
3.1. Chemerin in Glucose Metabolism
3.2. Chemerin in Lipid Metabolism
3.3. Chemerin in Angiogenesis and Vascular Function
4. Chemerin in Obesity-Induced Inflammation
5. Chemerin and Obesity—Studies
5.1. Chemerin and Obesity—Data from Animals Models and Cells
5.2. Chemerin and Obesity—Data from Humans
6. Chemerin and the Metabolic Syndrome
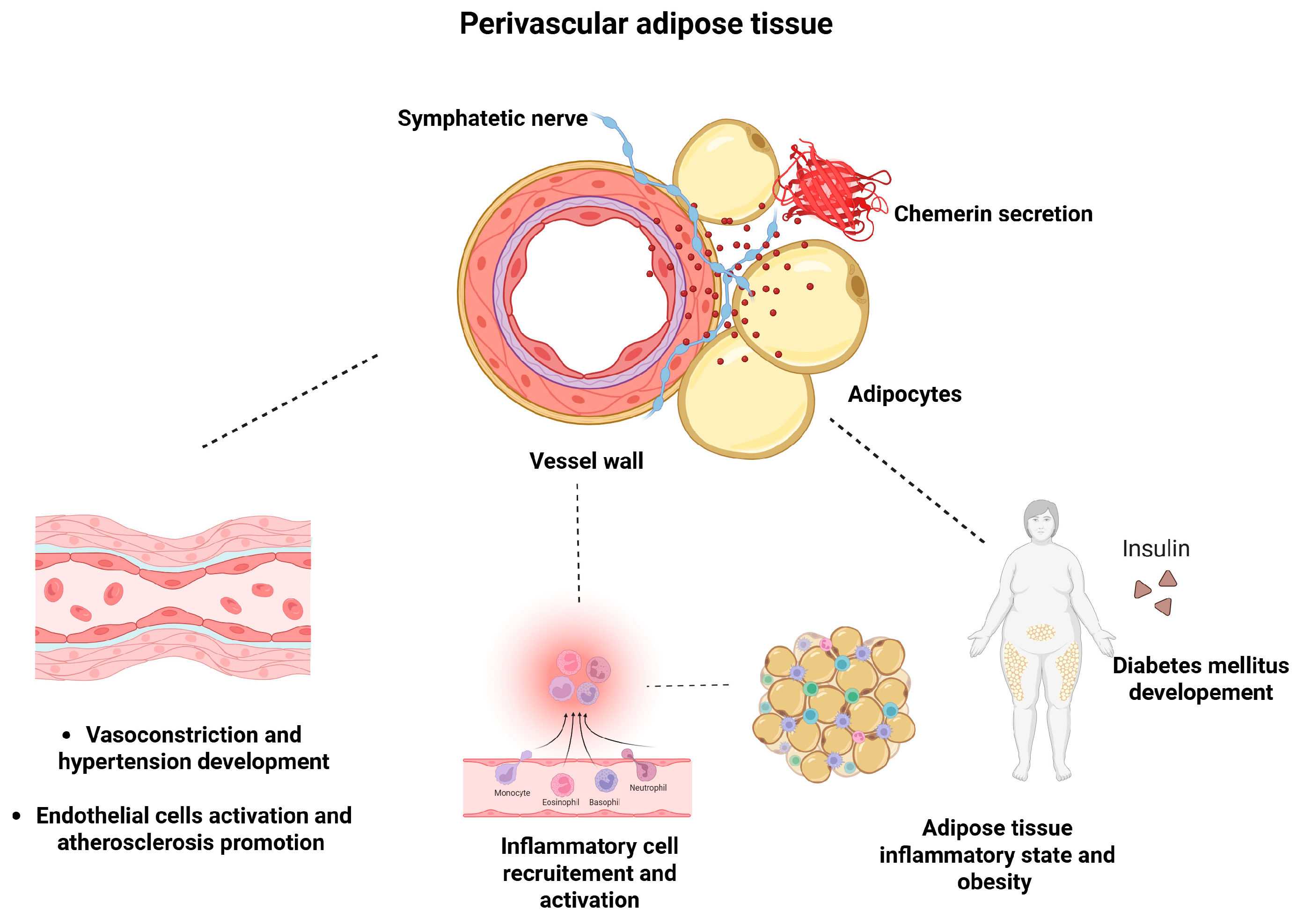
7. The Role of Chemerin in the Pathophysiology of Hypertension
8. Chemerin and Hypertension—Studies
8.1. Chemerin in Experimental Models of Hypertension
8.2. The Role of Chemerin in Human Studies
9. The Role of Chemerin in the Pathophysiology of Atherosclerosis
The Role of Chemerin in the Pathophysiology of Atherosclerosis in the Experimental Models
10. Chemerin’s Role in Human Atherosclerosis
11. Clinical Approaches and Future Directions
11.1. Diagnostic Approach of Chemerin
11.2. Therapeutic Approach
12. Strengths and Limitations
13. Conclusions/Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEI-2 | Angiotensin-converting enzyme type 2 |
| Akt | Protein kinase B |
| AMI | Acute myocardial infarction |
| APC | Antigen-presenting cell |
| ApoA-I | Apolipoprotein A-I |
| ApoB | Apolipoprotein B |
| ASCVD | Atherosclerotic cardiovascular diseases |
| ASO | Antisense oligonucleotide |
| AT | Adipose tissue |
| BMI | Body mass index |
| CAD | Coronary artery disease |
| cAMP | Cyclic adenosine monophosphate |
| CCRL2 | Chemokine receptor-like 2 |
| C-IMT | Carotid intima–media thickness |
| CMKLR1 | Chemerin-like receptor 1 |
| CVD | Cardiovascular disease |
| DC | Dendritic cell |
| DOCA | Deoxycorticosterone acetate |
| EC | Endothelial cell |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| FABP4 | Fatty acid-binding protein 4 |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT4 | Glucose transporter 4 |
| GPR1 | G protein-coupled receptor 1 |
| HDL | High-density lipoprotein |
| HMEC | Human microvascular endothelial cell |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| hs-CRP | High-sensitivity C-reactive protein |
| HUVEC | Human umbilical vein endothelial cell |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| IMT | Intima–media thickness |
| MACEs | Major adverse cardiovascular events |
| MAPK | Mitogen-activated protein kinase |
| MeS | Metabolic syndrome |
| MMP-2 | Gelatinase A |
| MMP-9 | Gelatinase B |
| mTOR | Mammalian target of rapamycin |
| NAFLD | Non-alcoholic fatty liver disease |
| NDO | Non-diabetic obese |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer |
| NOS3 | Endothelial nitric oxide synthase |
| PI3K | Phosphatidylinositol 3-kinase |
| PON1 | Paraoxonase 1 |
| PVAT | Perivascular adipose tissue |
| RARRES2 | Retinoic acid receptor responder protein 2 |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| SD rats | Sprague Dawley rats |
| siRNA | Small interfering RNA |
| T2DM | Type 2 diabetes mellitus |
| TG | Triglyceride |
| TNF-α | Tumor necrosis factor |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VEGF | Vascular endothelial growth factor |
| VLDL | Very low-density lipoproteins |
| VSMC | Vascular smooth muscle cell |
| WAT | White adipose tissue |
| WHR | Waist-to-hip ratio |
| WT mice | C57BL/6 mice |
References
- Imiela, A.M.; Mikolajczyk, T.P.; Siedlinski, M.; Dobrowolski, P.; Konior-Rozlachowska, A.; Wrobel, A.; Biernat-Kaluza, E.; Januszewicz, M.; Guzik, B.; Guzik, T.J.; et al. Th17/Treg imbalance in patients with primary hyperaldosteronism and resistant hypertension. Pol. Arch. Intern. Med. 2022, 132, 132. [Google Scholar] [CrossRef]
- Mitsis, A.; Khattab, E.; Myrianthefs, M.; Tzikas, S.; Kadoglou, N.P.E.; Fragakis, N.; Ziakas, A.; Kassimis, G. Chemerin in the Spotlight: Revealing Its Multifaceted Role in Acute Myocardial Infarction. Biomedicines 2024, 12, 2133. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Lu, X.; Danser, A.H.J.; Verdonk, K. The Role of Chemerin in Metabolic and Cardiovascular Disease: A Literature Review of Its Physiology and Pathology from a Nutritional Perspective. Nutrients 2023, 15, 2878. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Dridi-Brahimi, I.; Vatier, C.; Fellahi, S.; Feve, B. Biological markers of adipose tissue: Adipokines. Ann. Endocrinol. 2024, 85, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; Le Poul, E.; Migeotte, I.; Brezillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef]
- Yue, G.; An, Q.; Xu, X.; Jin, Z.; Ding, J.; Hu, Y.; Du, Q.; Xu, J.; Xie, R. The role of Chemerin in human diseases. Cytokine 2023, 162, 156089. [Google Scholar] [CrossRef]
- Neves, K.B.; Nguyen Dinh Cat, A.; Lopes, R.A.; Rios, F.J.; Anagnostopoulou, A.; Lobato, N.S.; de Oliveira, A.M.; Tostes, R.C.; Montezano, A.C.; Touyz, R.M. Chemerin Regulates Crosstalk Between Adipocytes and Vascular Cells Through Nox. Hypertension 2015, 66, 657–666. [Google Scholar] [CrossRef]
- Parlee, S.D.; Ernst, M.C.; Muruganandan, S.; Sinal, C.J.; Goralski, K.B. Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-alpha. Endocrinology 2010, 151, 2590–2602. [Google Scholar] [CrossRef]
- Ferland, D.J.; Darios, E.S.; Neubig, R.R.; Sjogren, B.; Truong, N.; Torres, R.; Dexheimer, T.S.; Thompson, J.M.; Watts, S.W. Chemerin-induced arterial contraction is Gi- and calcium-dependent. Vasc. Pharmacol. 2017, 88, 30–41. [Google Scholar] [CrossRef]
- Chang, S.S.; Eisenberg, D.; Zhao, L.; Adams, C.; Leib, R.; Morser, J.; Leung, L. Chemerin activation in human obesity. Obesity 2016, 24, 1522–1529. [Google Scholar] [CrossRef]
- Schultz, S.; Saalbach, A.; Heiker, J.T.; Meier, R.; Zellmann, T.; Simon, J.C.; Beck-Sickinger, A.G. Proteolytic activation of prochemerin by kallikrein 7 breaks an ionic linkage and results in C-terminal rearrangement. Biochem. J. 2013, 452, 271–280. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Davenport, A.P. International Union of Basic and Clinical Pharmacology CIII: Chemerin Receptors CMKLR1 (Chemerin1) and GPR1 (Chemerin2) Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2018, 70, 174–196. [Google Scholar] [CrossRef]
- Li, J.; Xiang, L.; Jiang, X.; Teng, B.; Sun, Y.; Chen, G.; Chen, J.; Zhang, J.V.; Ren, P.G. Investigation of bioeffects of G protein-coupled receptor 1 on bone turnover in male mice. J. Orthop. Transl. 2017, 10, 42–51. [Google Scholar] [CrossRef]
- Foster, S.R.; Hauser, A.S.; Vedel, L.; Strachan, R.T.; Huang, X.P.; Gavin, A.C.; Shah, S.D.; Nayak, A.P.; Haugaard-Kedstrom, L.M.; Penn, R.B.; et al. Discovery of Human Signaling Systems: Pairing Peptides to G Protein-Coupled Receptors. Cell 2019, 179, 895–908 e821. [Google Scholar] [CrossRef]
- Fischer, T.F.; Beck-Sickinger, A.G. Chemerin—Exploring a versatile adipokine. Biol. Chem. 2022, 403, 625–642. [Google Scholar] [CrossRef]
- Muruganandan, S.; Parlee, S.D.; Rourke, J.L.; Ernst, M.C.; Goralski, K.B.; Sinal, C.J. Chemerin, a novel peroxisome proliferator-activated receptor gamma (PPARγ) target gene that promotes mesenchymal stem cell adipogenesis. J. Biol. Chem. 2011, 286, 23982–23995. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, J.; Wang, X. Progress in the contrary effects of glucagon-like peptide-1 and chemerin on obesity development. Exp. Biol. Med. 2023, 248, 2020–2029. [Google Scholar] [CrossRef]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef]
- Chazenbalk, G.; Singh, P.; Irge, D.; Shah, A.; Abbott, D.H.; Dumesic, D.A. Androgens inhibit adipogenesis during human adipose stem cell commitment to preadipocyte formation. Steroids 2013, 78, 920–926. [Google Scholar] [CrossRef]
- Villarroya, F.; Giralt, M.; Iglesias, R. Retinoids and adipose tissues metabolism, cell differentiation and gene expression. Int. J. Obes. Relat. Metab. Disord. 1999, 23, 1–6. [Google Scholar] [CrossRef]
- Harris, R.B. Direct and indirect effects of leptin on adipocyte metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 414–423. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Lipson, K.L.; Fonseca, S.G.; Ishigaki, S.; Nguyen, L.X.; Foss, E.; Bortell, R.; Rossini, A.A.; Urano, F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006, 4, 245–254. [Google Scholar] [CrossRef]
- Takahashi, M.; Okimura, Y.; Iguchi, G.; Nishizawa, H.; Yamamoto, M.; Suda, K.; Kitazawa, R.; Fujimoto, W.; Takahashi, K.; Zolotaryov, F.N.; et al. Chemerin regulates beta-cell function in mice. Sci. Rep. 2011, 1, 123. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Asp. Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Santoro, A.; McGraw, T.E.; Kahn, B.B. Insulin action in adipocytes, adipose remodeling, and systemic effects. Cell Metab. 2021, 33, 748–757. [Google Scholar] [CrossRef]
- Ernst, M.C.; Haidl, I.D.; Zuniga, L.A.; Dranse, H.J.; Rourke, J.L.; Zabel, B.A.; Butcher, E.C.; Sinal, C.J. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 2012, 153, 672–682. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Chen, K.L.; Li, H.X.; Zhou, G.H. The adipokine Chemerin induces lipolysis and adipogenesis in bovine intramuscular adipocytes. Mol. Cell. Biochem. 2016, 418, 39–48. [Google Scholar] [CrossRef]
- Takahashi, M.; Takahashi, Y.; Takahashi, K.; Zolotaryov, F.N.; Hong, K.S.; Kitazawa, R.; Iida, K.; Okimura, Y.; Kaji, H.; Kitazawa, S.; et al. Chemerin enhances insulin signaling and potentiates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. FEBS Lett. 2008, 582, 573–578. [Google Scholar] [CrossRef]
- Sell, H.; Divoux, A.; Poitou, C.; Basdevant, A.; Bouillot, J.L.; Bedossa, P.; Tordjman, J.; Eckel, J.; Clement, K. Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J. Clin. Endocrinol. Metab. 2010, 95, 2892–2896. [Google Scholar] [CrossRef]
- Ernst, M.C.; Issa, M.; Goralski, K.B.; Sinal, C.J. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 2010, 151, 1998–2007. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Hu, B.H.; Chen, K.L.; Li, H.X. Chemerin induces lipolysis through ERK1/2 pathway in intramuscular mature adipocytes of dairy bull calves. J. Cell. Biochem. 2019, 120, 1122–1132. [Google Scholar] [CrossRef]
- Herold, J.; Kalucka, J. Angiogenesis in Adipose Tissue: The Interplay Between Adipose and Endothelial Cells. Front. Physiol. 2020, 11, 624903. [Google Scholar] [CrossRef]
- Piqueras, L.; Reynolds, A.R.; Hodivala-Dilke, K.M.; Alfranca, A.; Redondo, J.M.; Hatae, T.; Tanabe, T.; Warner, T.D.; Bishop-Bailey, D. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 63–69. [Google Scholar] [CrossRef]
- Kaur, J.; Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Identification of chemerin receptor (ChemR23) in human endothelial cells: Chemerin-induced endothelial angiogenesis. Biochem. Biophys. Res. Commun. 2010, 391, 1762–1768. [Google Scholar] [CrossRef]
- Kawasaki, K.; Smith, R.S., Jr.; Hsieh, C.M.; Sun, J.; Chao, J.; Liao, J.K. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol. Cell. Biol. 2003, 23, 5726–5737. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Nguyen, M.; Arkell, J.; Jackson, C.J. Human endothelial gelatinases and angiogenesis. Int. J. Biochem. Cell Biol. 2001, 33, 960–970. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Curran, J.E.; Stocker, C.J.; Zaibi, M.S.; Segal, D.; Konstantopoulos, N.; Morrison, S.; Carless, M.; Dyer, T.D.; Cole, S.A.; et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J. Clin. Endocrinol. Metab. 2010, 95, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef]
- Iannone, F.; Lapadula, G. Chemerin/ChemR23 pathway: A system beyond chemokines. Arthritis Res. Ther. 2011, 13, 104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mariani, F.; Roncucci, L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm. Res. 2015, 64, 85–95. [Google Scholar] [CrossRef]
- Skrzeczyńska-Moncznik, J.; Stefańska, A.; Zabel, B.A.; Kapińska-Mrowiecka, M.; Butcher, E.C.; Cichy, J. Chemerin and the recruitment of NK cells to diseased skin. Acta Biochim. Pol. 2009, 56, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Miyabe, Y.; Takayasu, A.; Fukuda, S.; Miyabe, C.; Ebisawa, M.; Yokoyama, W.; Watanabe, K.; Imai, T.; Muramoto, K.; et al. Chemerin activates fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R158. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Wang, H. Correlation of blood glucose, serum chemerin and insulin resistance with NAFLD in patients with type 2 diabetes mellitus. Exp. Ther. Med. 2018, 15, 2936–2940. [Google Scholar] [CrossRef]
- Weigert, J.; Obermeier, F.; Neumeier, M.; Wanninger, J.; Filarsky, M.; Bauer, S.; Aslanidis, C.; Rogler, G.; Ott, C.; Schaffler, A.; et al. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s disease. Inflamm. Bowel Dis. 2010, 16, 630–637. [Google Scholar] [CrossRef]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef]
- Stejskal, D.; Karpisek, M.; Hanulova, Z.; Svestak, M. Chemerin is an independent marker of the metabolic syndrome in a Caucasian population—A pilot study. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2008, 152, 217–221. [Google Scholar] [CrossRef]
- Ortega Martinez de Victoria, E.; Xu, X.; Koska, J.; Francisco, A.M.; Scalise, M.; Ferrante, A.W., Jr.; Krakoff, J. Macrophage content in subcutaneous adipose tissue: Associations with adiposity, age, inflammatory markers, and whole-body insulin action in healthy Pima Indians. Diabetes 2009, 58, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.E.; Weisberg, S.; Vardhana, P.; Tortoriello, D.V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. 2008, 32, 451–463. [Google Scholar] [CrossRef]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Herova, M.; Schmid, M.; Gemperle, C.; Hersberger, M. ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J. Immunol. 2015, 194, 2330–2337. [Google Scholar] [CrossRef]
- Pelczynska, M.; Miller-Kasprzak, E.; Piatkowski, M.; Mazurek, R.; Klause, M.; Suchecka, A.; Bucon, M.; Bogdanski, P. The Role of Adipokines and Myokines in the Pathogenesis of Different Obesity Phenotypes-New Perspectives. Antioxidants 2023, 12, 2046. [Google Scholar] [CrossRef]
- Chylikova, J.; Dvorackova, J.; Tauber, Z.; Kamarad, V. M1/M2 macrophage polarization in human obese adipose tissue. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2018, 162, 79–82. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Lehrke, M.; Becker, A.; Greif, M.; Stark, R.; Laubender, R.P.; von Ziegler, F.; Lebherz, C.; Tittus, J.; Reiser, M.; Becker, C.; et al. Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur. J. Endocrinol. 2009, 161, 339–344. [Google Scholar] [CrossRef]
- Fulop, P.; Seres, I.; Lorincz, H.; Harangi, M.; Somodi, S.; Paragh, G. Association of chemerin with oxidative stress, inflammation and classical adipokines in non-diabetic obese patients. J. Cell. Mol. Med. 2014, 18, 1313–1320. [Google Scholar] [CrossRef]
- Catalan, V.; Gomez-Ambrosi, J.; Rodriguez, A.; Ramirez, B.; Rotellar, F.; Valenti, V.; Silva, C.; Gil, M.J.; Salvador, J.; Fruhbeck, G. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: Tumor necrosis factor-alpha stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg. Obes. Relat. Dis. 2013, 9, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Zylla, S.; Pietzner, M.; Kuhn, J.P.; Volzke, H.; Dorr, M.; Nauck, M.; Friedrich, N. Serum chemerin is associated with inflammatory and metabolic parameters-results of a population-based study. Obesity 2017, 25, 468–475. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Jarvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Lu, W.; Zong, X.F.; Ruan, H.Y.; Liu, Y. Obesity and hypertension. Exp. Ther. Med. 2016, 12, 2395–2399. [Google Scholar] [CrossRef]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of Body Mass Index with Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018, 3, 280–287. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Ard, J.; Fitch, A.; Fruh, S.; Herman, L. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists. Adv. Ther. 2021, 38, 2821–2839. [Google Scholar] [CrossRef]
- Parthsarathy, V.; Holscher, C. The type 2 diabetes drug liraglutide reduces chronic inflammation induced by irradiation in the mouse brain. Eur. J. Pharmacol. 2013, 700, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Ghanim, H.; Vora, M.; Sia, C.L.; Korzeniewski, K.; Dhindsa, S.; Makdissi, A.; Dandona, P. Exenatide exerts a potent antiinflammatory effect. J. Clin. Endocrinol. Metab. 2012, 97, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Misiti, R.; Sicilia, L.; Brunetti, F.S.; Chiefari, E.; Brunetti, A.; Foti, D.P. Hypoxia in Human Obesity: New Insights from Inflammation towards Insulin Resistance-A Narrative Review. Int. J. Mol. Sci. 2024, 25, 9802. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int. J. Obes. 2009, 33, 54–66. [Google Scholar] [CrossRef]
- Man, K.; Kallies, A.; Vasanthakumar, A. Resident and migratory adipose immune cells control systemic metabolism and thermogenesis. Cell. Mol. Immunol. 2022, 19, 421–431. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, Q.; Cao, J.; Xie, N.; Liu, K.; Shou, P.; Qian, F.; Wang, Y.; Shi, Y. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 2016, 7, e2167. [Google Scholar] [CrossRef]
- Faria, S.S.; Correa, L.H.; Heyn, G.S.; de Sant’Ana, L.P.; Almeida, R.D.N.; Magalhaes, K.G. Obesity and Breast Cancer: The Role of Crown-Like Structures in Breast Adipose Tissue in Tumor Progression, Prognosis, and Therapy. J. Breast Cancer 2020, 23, 233–245. [Google Scholar] [CrossRef]
- Xian, Y.; Chen, Z.; Deng, H.; Cai, M.; Liang, H.; Xu, W.; Weng, J.; Xu, F. Exenatide mitigates inflammation and hypoxia along with improved angiogenesis in obese fat tissue. J. Endocrinol. 2019, 242, 79–89. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, M.S.; Choung, J.S.; Kim, S.S.; Oh, H.H.; Choi, C.S.; Ha, S.Y.; Kang, Y.; Kim, Y.; Jun, H.S. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012, 55, 2456–2468. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Y.; Guo, Y.; Yu, X.; Wang, R.; Wang, X. Chemerin loss-of-function attenuates glucagon-like peptide-1 secretion in exercised obese mice. Diabetes Obes. Metab. 2025, 27, 1296–1313. [Google Scholar] [CrossRef]
- Gagnon, J.; Sauve, M.; Zhao, W.; Stacey, H.M.; Wiber, S.C.; Bolz, S.S.; Brubaker, P.L. Chronic Exposure to TNFalpha Impairs Secretion of Glucagon-Like Peptide-1. Endocrinology 2015, 156, 3950–3960. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Ferrell, J.E., Jr. The roles of cyclin A2, B1, and B2 in early and late mitotic events. Mol. Biol. Cell 2010, 21, 3149–3161. [Google Scholar] [CrossRef] [PubMed]
- Corvera, S.; Solivan-Rivera, J.; Yang Loureiro, Z. Angiogenesis in adipose tissue and obesity. Angiogenesis 2022, 25, 439–453. [Google Scholar] [CrossRef]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Naruse, K.; Kobayashi, Y.; Miyabe, M.; Saiki, T.; Enomoto, A.; Takahashi, M.; Matsubara, T. Chemerin promotes angiogenesis in vivo. Physiol. Rep. 2018, 6, e13962. [Google Scholar] [CrossRef]
- Cornier, M.A.; Despres, J.P.; Davis, N.; Grossniklaus, D.A.; Klein, S.; Lamarche, B.; Lopez-Jimenez, F.; Rao, G.; St-Onge, M.P.; Towfighi, A.; et al. Assessing adiposity: A scientific statement from the American Heart Association. Circulation 2011, 124, 1996–2019. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, X.; Xu, J.; Yang, B.; Yu, J.; Gong, Q.; Zhang, X.; Sun, X.; Zhang, Q.; Xia, J.; et al. Association of serum chemerin and inflammatory factors with type 2 diabetes macroangiopathy and waist-to-stature ratio. Bosn. J. Basic. Med. Sci. 2019, 19, 328–335. [Google Scholar] [CrossRef]
- Sell, H.; Laurencikiene, J.; Taube, A.; Eckardt, K.; Cramer, A.; Horrighs, A.; Arner, P.; Eckel, J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 2009, 58, 2731–2740. [Google Scholar] [CrossRef]
- Chakaroun, R.; Raschpichler, M.; Kloting, N.; Oberbach, A.; Flehmig, G.; Kern, M.; Schon, M.R.; Shang, E.; Lohmann, T.; Dressler, M.; et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 2012, 61, 706–714. [Google Scholar] [CrossRef]
- Liu, M.; Lin, X.; Wang, X. Decrease in serum chemerin through aerobic exercise plus dieting and its association with mitigation of cardio-metabolic risk in obese female adolescents. J. Pediatr. Endocrinol. Metab. 2018, 31, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, P.; Jiao, W.; Meng, J.; Feng, J. Gax suppresses chemerin/CMKLR1-induced preadipocyte biofunctions through the inhibition of Akt/mTOR and ERK signaling pathways. J. Cell. Physiol. 2018, 233, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Patel, S.; Jacobe, H.; DiSepio, D.; Ghosn, C.; Malhotra, M.; Teng, M.; Duvic, M.; Chandraratna, R.A. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Investig. Dermatol. 1997, 109, 91–95. [Google Scholar] [CrossRef]
- Albanesi, C.; Scarponi, C.; Pallotta, S.; Daniele, R.; Bosisio, D.; Madonna, S.; Fortugno, P.; Gonzalvo-Feo, S.; Franssen, J.D.; Parmentier, M.; et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 2009, 206, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Savulescu-Fiedler, I.; Mihalcea, R.; Dragosloveanu, S.; Scheau, C.; Baz, R.O.; Caruntu, A.; Scheau, A.E.; Caruntu, C.; Benea, S.N. The Interplay between Obesity and Inflammation. Life 2024, 14, 856. [Google Scholar] [CrossRef]
- Parolini, S.; Santoro, A.; Marcenaro, E.; Luini, W.; Massardi, L.; Facchetti, F.; Communi, D.; Parmentier, M.; Majorana, A.; Sironi, M.; et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007, 109, 3625–3632. [Google Scholar] [CrossRef]
- Lorincz, H.; Katko, M.; Harangi, M.; Somodi, S.; Gaal, K.; Fulop, P.; Paragh, G.; Seres, I. Strong correlations between circulating chemerin levels and lipoprotein subfractions in nondiabetic obese and nonobese subjects. Clin. Endocrinol. 2014, 81, 370–377. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Abou Ziki, M.D.; Mani, A. Metabolic syndrome: Genetic insights into disease pathogenesis. Curr. Opin. Lipidol. 2016, 27, 162–171. [Google Scholar] [CrossRef]
- Pachocka, L.; Mekus, M. Comparison of lifestyle and nutritional status between women with and without metabolic syndrome. Przegląd Epidemiol. 2023, 77, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Funahashi, T.; Nakamura, T. The concept of metabolic syndrome contribution of visceral fat accumulation and its molecular mechanism. J. Atheroscler. Thromb. 2011, 18, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Gebhardt, C.; Scholz, M.; Wohland, T.; Schleinitz, D.; Fasshauer, M.; Bluher, M.; Stumvoll, M.; Kovacs, P.; Tonjes, A. Relationship Between 12 Adipocytokines and Distinct Components of the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2018, 103, 1015–1023. [Google Scholar] [CrossRef]
- Dong, B.; Ji, W.; Zhang, Y. Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern. Med. 2011, 50, 1093–1097. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Kaiser, N.; Halban, P.A.; Donath, M.Y. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef]
- Qu, H.Q.; Li, Q.; Rentfro, A.R.; Fisher-Hoch, S.P.; McCormick, J.B. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS ONE 2011, 6, e21041. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Hasanvand, Z.; Sadeghi, A.; Rezvanfar, M.R.; Goodarzi, M.T.; Rahmannezhad, G.; Mashayekhi, F.J. Association between chemerin rs17173608 and rs4721 gene polymorphisms and gestational diabetes mellitus in Iranian pregnant women. Gene 2018, 649, 87–92. [Google Scholar] [CrossRef]
- Shafer-Eggleton, J.; Adams-Huet, B.; Jialal, I. Chemerin Ratios to HDL-cholesterol and Adiponectin as Biomarkers of Metabolic Syndrome. Endocr. Res. 2020, 45, 241–245. [Google Scholar] [CrossRef]
- Jialal, I.; Devaraj, S.; Kaur, H.; Adams-Huet, B.; Bremer, A.A. Increased chemerin and decreased omentin-1 in both adipose tissue and plasma in nascent metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, E514–E517. [Google Scholar] [CrossRef]
- Bobbert, T.; Schwarz, F.; Fischer-Rosinsky, A.; Maurer, L.; Mohlig, M.; Pfeiffer, A.F.; Mai, K.; Spranger, J. Chemerin and prediction of Diabetes mellitus type 2. Clin. Endocrinol. 2015, 82, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Budavari, A.; Murray, D.; Spiegelman, B.M. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J. Clin. Investig. 1994, 94, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef]
- Kawamoto, R.; Tabara, Y.; Kohara, K.; Miki, T.; Kusunoki, T.; Takayama, S.; Abe, M.; Katoh, T.; Ohtsuka, N. Relationships between lipid profiles and metabolic syndrome, insulin resistance and serum high molecular adiponectin in Japanese community-dwelling adults. Lipids Health Dis. 2011, 10, 79. [Google Scholar] [CrossRef]
- Chou, H.H.; Teng, M.S.; Hsu, L.A.; Er, L.K.; Wu, S.; Ko, Y.L. Circulating chemerin level is associated with metabolic, biochemical and haematological parameters-A population-based study. Clin. Endocrinol. 2021, 94, 927–939. [Google Scholar] [CrossRef]
- Haile, K.; Haile, A.; Timerga, A. Predictors of Lipid Profile Abnormalities Among Patients with Metabolic Syndrome in Southwest Ethiopia: A Cross-Sectional Study. Vasc. Health Risk Manag. 2021, 17, 461–469. [Google Scholar] [CrossRef]
- Ruotolo, G.; Howard, B.V. Dyslipidemia of the metabolic syndrome. Curr. Cardiol. Rep. 2002, 4, 494–500. [Google Scholar] [CrossRef]
- Packard, C.J. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem. Soc. Trans. 2003, 31, 1066–1069. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Barnett, J.; Fong, L.G. High-density lipoprotein inhibits the oxidative modification of low-density lipoprotein. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1990, 1044, 275–283. [Google Scholar] [CrossRef]
- Steinberg, D.; Parthasarathy, S.; Carew, T.E.; Khoo, J.C.; Witztum, J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989, 320, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991, 286, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Dornas, W.; Silva, M. Modulation of the antioxidant enzyme paraoxonase-1 for protection against cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 2611–2622. [Google Scholar] [CrossRef]
- Mathieu, P.; Pibarot, P.; Després, J.P. Metabolic Syndrome The Danger Signal in Atherosclerosis. Vasc. Health Risk Manag. 2006, 2, 285–302. [Google Scholar] [CrossRef]
- Hui, X.; Lam, K.S.; Vanhoutte, P.M.; Xu, A. Adiponectin and cardiovascular health: An update. Br. J. Pharmacol. 2012, 165, 574–590. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Funahashi, T.; Kihara, S.; Shimomura, I. Adiponectin and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 29–33. [Google Scholar] [CrossRef]
- Chu, S.H.; Lee, M.K.; Ahn, K.Y.; Im, J.A.; Park, M.S.; Lee, D.C.; Jeon, J.Y.; Lee, J.W. Chemerin and adiponectin contribute reciprocally to metabolic syndrome. PLoS ONE 2012, 7, e34710. [Google Scholar] [CrossRef]
- Golbidi, S.; Mesdaghinia, A.; Laher, I. Exercise in the metabolic syndrome. Oxid. Med. Cell. Longev. 2012, 2012, 349710. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-Leon, A.M.; Sierra-Perez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Niklowitz, P.; Rothermel, J.; Lass, N.; Barth, A.; Reinehr, T. Link between chemerin, central obesity, and parameters of the Metabolic Syndrome: Findings from a longitudinal study in obese children participating in a lifestyle intervention. Int. J. Obes. 2018, 42, 1743–1752. [Google Scholar] [CrossRef]
- Jurca, R.; Lamonte, M.J.; Church, T.S.; Earnest, C.P.; Fitzgerald, S.J.; Barlow, C.E.; Jordan, A.N.; Kampert, J.B.; Blair, S.N. Associations of muscle strength and fitness with metabolic syndrome in men. Med. Sci. Sports Exerc. 2004, 36, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Silverio, A.M.; Butcher, E.C. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J. Immunol. 2005, 174, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Matsumoto, K.; Hori, K.; Kameshima, S.; Yamaguchi, N.; Okada, S.; Okada, M.; Yamawaki, H. Acute intracerebroventricular injection of chemerin-9 increases systemic blood pressure through activating sympathetic nerves via CMKLR1 in brain. Pflügers Arch. 2020, 472, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Jiang, W.; Lu, B.; Shi, Z. Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients. Clin. Exp. Hypertens. 2014, 36, 326–332. [Google Scholar] [CrossRef]
- Meric, M.; Soylu, K.; Avci, B.; Yuksel, S.; Gulel, O.; Yenercag, M.; Coksevim, M.; Uzun, A. Evaluation of plasma chemerin levels in patients with non-dipper blood pressure patterns. Med. Sci. Monit. 2014, 20, 698–705. [Google Scholar] [CrossRef][Green Version]
- Wojcik, M.; Koziol-Kozakowska, A.; Janus, D.; Furtak, A.; Malek, A.; Sztefko, K.; Starzyk, J.B. Circulating chemerin level may be associated with early vascular pathology in obese children without overt arterial hypertension—Preliminary results. J. Pediatr. Endocrinol. Metab. 2020, 33, 729–734. [Google Scholar] [CrossRef]
- Watts, S.W.; Dorrance, A.M.; Penfold, M.E.; Rourke, J.L.; Sinal, C.J.; Seitz, B.; Sullivan, T.J.; Charvat, T.T.; Thompson, J.M.; Burnett, R.; et al. Chemerin connects fat to arterial contraction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1320–1328. [Google Scholar] [CrossRef]
- Darios, E.S.; Winner, B.M.; Charvat, T.; Krasinksi, A.; Punna, S.; Watts, S.W. The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H498–H507. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Yang, P.; Read, C.; Kuc, R.E.; Yang, L.; Taylor, E.J.; Taylor, C.W.; Maguire, J.J.; Davenport, A.P. Chemerin Elicits Potent Constrictor Actions via Chemokine-Like Receptor 1 (CMKLR1), not G-Protein-Coupled Receptor 1 (GPR1), in Human and Rat Vasculature. J. Am. Heart Assoc. 2016, 5, e004421. [Google Scholar] [CrossRef]
- Kunimoto, H.; Kazama, K.; Takai, M.; Oda, M.; Okada, M.; Yamawaki, H. Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1017–H1028. [Google Scholar] [CrossRef] [PubMed]
- Lobato, N.S.; Neves, K.B.; Filgueira, F.P.; Fortes, Z.B.; Carvalho, M.H.; Webb, R.C.; Oliveira, A.M.; Tostes, R.C. The adipokine chemerin augments vascular reactivity to contractile stimuli via activation of the MEK-ERK1/2 pathway. Life Sci. 2012, 91, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Wang, J.; Guo, L.; Cai, W.; Wu, Y.; Chen, W.; Tang, X. Chemerin stimulates aortic smooth muscle cell proliferation and migration via activation of autophagy in VSMCs of metabolic hypertension rats. Am. J. Transl. Res. 2019, 11, 1327. [Google Scholar]
- Man, A.W.C.; Zhou, Y.; Reifenberg, G.; Camp, A.; Munzel, T.; Daiber, A.; Xia, N.; Li, H. Deletion of adipocyte NOS3 potentiates high-fat diet-induced hypertension and vascular remodelling via chemerin. Cardiovasc. Res. 2023, 119, 2755–2769. [Google Scholar] [CrossRef] [PubMed]
- Ferland, D.J.; Flood, E.D.; Garver, H.; Yeh, S.T.; Riney, S.; Mullick, A.E.; Fink, G.D.; Watts, S.W. Different blood pressure responses in hypertensive rats following chemerin mRNA inhibition in dietary high fat compared to dietary high-salt conditions. Physiol. Genom. 2019, 51, 553–561. [Google Scholar] [CrossRef]
- Imiela, A.M.; MikoŁAjczyk, T.P.; Kołodziejczyk-Kruk, S.; Kądziela, J.; Spiewak, M.; Januszewicz, M.; Kabat, M.; Sterliński, I.; Wąs, M.; Wróbel, A.; et al. Plasmacytoid dendritic cell content is associated with plasma aldosterone concentration in patients with primary aldosteronism. Am. J. Hypertens. 2025, hpaf019. [Google Scholar] [CrossRef]
- Mocker, A.; Hilgers, K.F.; Cordasic, N.; Wachtveitl, R.; Menendez-Castro, C.; Woelfle, J.; Hartner, A.; Fahlbusch, F.B. Renal Chemerin Expression is Induced in Models of Hypertensive Nephropathy and Glomerulonephritis and Correlates with Markers of Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 6240. [Google Scholar] [CrossRef]
- Cheng, C.K.; Ding, H.; Jiang, M.; Yin, H.; Gollasch, M.; Huang, Y. Perivascular adipose tissue: Fine-tuner of vascular redox status and inflammation. Redox Biol. 2023, 62, 102683. [Google Scholar] [CrossRef]
- Kostopoulos, C.G.; Spiroglou, S.G.; Varakis, J.N.; Apostolakis, E.; Papadaki, H.H. Chemerin and CMKLR1 expression in human arteries and periadventitial fat a possible role for local chemerin in atherosclerosis? BMC Cardiovasc. Disord. 2014, 14, 56. [Google Scholar] [CrossRef]
- Ferland, D.J.; Mullick, A.E.; Watts, S.W. Chemerin as a Driver of Hypertension: A Consideration. Am. J. Hypertens. 2020, 33, 975–986. [Google Scholar] [CrossRef]
- Flood, E.D.; Watts, S.W. Endogenous Chemerin from PVAT Amplifies Electrical Field-Stimulated Arterial Contraction: Use of the Chemerin Knockout Rat. Int. J. Mol. Sci. 2020, 21, 6392. [Google Scholar] [CrossRef]
- Stauss, H.M.; Gödecke, A.; Mrowka, R.; Schrader, J.; Persson, P.B. Enhanced blood pressure variability in eNOS knockout mice. Hypertension 1999, 33, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Li, H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W.; Darios, E.S.; Mullick, A.E.; Garver, H.; Saunders, T.L.; Hughes, E.D.; Filipiak, W.E.; Zeidler, M.G.; McMullen, N.; Sinal, C.J.; et al. The chemerin knockout rat reveals chemerin dependence in female, but not male, experimental hypertension. FASEB J. 2018, 32, fj201800479. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, G.; Dong, J.; Liu, Y.; Zong, H.; Liu, H.; Boden, G.; Li, L. Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J. Investig. Med. 2010, 58, 883–886. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Liu, H.; Li, Z.; Li, L.; Wu, X.; Lei, Q.; Yin, A.; Tong, J.; Liu, K.; et al. Third-Trimester Maternal Serum Chemerin and Hypertension After Preeclampsia: A Prospective Cohort Study. J. Am. Heart Assoc. 2023, 12, e027930. [Google Scholar] [CrossRef]
- Tang, C.; Chen, G.; Wu, F.; Cao, Y.; Yang, F.; You, T.; Liu, C.; Li, M.; Hu, S.; Ren, L.; et al. Endothelial CCRL2 induced by disturbed flow promotes atherosclerosis via chemerin-dependent beta2 integrin activation in monocytes. Cardiovasc. Res. 2023, 119, 1811–1824. [Google Scholar] [CrossRef]
- Jia, J.; Yu, F.; Xiong, Y.; Wei, W.; Ma, H.; Nisi, F.; Song, X.; Yang, L.; Wang, D.; Yuan, G.; et al. Chemerin enhances the adhesion and migration of human endothelial progenitor cells and increases lipid accumulation in mice with atherosclerosis. Lipids Health Dis. 2020, 19, 207. [Google Scholar] [CrossRef]
- Wang, B.; Kou, W.; Ji, S.; Shen, R.; Ji, H.; Zhuang, J.; Zhao, Y.; Li, B.; Peng, W.; Yu, X.; et al. Prognostic value of plasma adipokine chemerin in patients with coronary artery disease. Front. Cardiovasc. Med. 2022, 9, 968349. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, Y.; Hong, J.; Gu, W.; Dai, M.; Shi, J.; Zhai, Y.; Wang, W.; Li, X.; Ning, G. The association of serum chemerin level with risk of coronary artery disease in Chinese adults. Endocrine 2012, 41, 281–288. [Google Scholar] [CrossRef]
- Xiaotao, L.; Xiaoxia, Z.; Yue, X.; Liye, W. Serum chemerin levels are associated with the presence and extent of coronary artery disease. Coron. Artery Dis. 2012, 23, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.J.; Kim, N.K.; Kim, M.K.; Kim, H.S.; Hur, S.H.; Yoon, H.J.; Kim, Y.N.; Park, K.G. Relationship between Chemerin Levels and Cardiometabolic Parameters and Degree of Coronary Stenosis in Korean Patients with Coronary Artery Disease. Diabetes Metab. J. 2011, 35, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tao, Y.; Chen, Y.; Xu, W.; Qian, Z.; Lu, X. Serum Chemerin as a Novel Prognostic Indicator in Chronic Heart Failure. J. Am. Heart Assoc. 2019, 8, e012091. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, Y.; Chen, S.; Wu, X.; Nong, W. Correlation between adiponectin, chemerin, vascular endothelial growth factor and epicardial fat volume in patients with coronary artery disease. Exp. Ther. Med. 2020, 19, 1095–1102. [Google Scholar] [CrossRef]
- McKenney-Drake, M.L.; Rodenbeck, S.D.; Bruning, R.S.; Kole, A.; Yancey, K.W.; Alloosh, M.; Sacks, H.S.; Sturek, M. Epicardial Adipose Tissue Removal Potentiates Outward Remodeling and Arrests Coronary Atherogenesis. Ann. Thorac. Surg. 2017, 103, 1622–1630. [Google Scholar] [CrossRef]
- Gibbons GH, D.V. The emerging concept of vascular remodeling. N. Engl. J. Med. 1994, 330, 1431–1438. [Google Scholar] [CrossRef]
- Motawi, T.M.K.; Mahdy, S.G.; El-Sawalhi, M.M.; Ali, E.N.; El-Telbany, R.F.A. Serum levels of chemerin, apelin, vaspin, and omentin-1 in obese type 2 diabetic Egyptian patients with coronary artery stenosis. Can. J. Physiol. Pharmacol. 2018, 96, 38–44. [Google Scholar] [CrossRef]
- Carracedo, M.; Witasp, A.; Qureshi, A.R.; Laguna-Fernandez, A.; Brismar, T.; Stenvinkel, P.; Back, M. Chemerin inhibits vascular calcification through ChemR23 and is associated with lower coronary calcium in chronic kidney disease. J. Intern. Med. 2019, 286, 449–457. [Google Scholar] [CrossRef]
- Lachine, N.A.; Elnekiedy, A.A.; Megallaa, M.H.; Khalil, G.I.; Sadaka, M.A.; Rohoma, K.H.; Kassab, H.S. Serum chemerin and high-sensitivity C reactive protein as markers of subclinical atherosclerosis in Egyptian patients with type 2 diabetes. Ther. Adv. Endocrinol. Metab. 2016, 7, 47–56. [Google Scholar] [CrossRef]
- Gu, P.; Cheng, M.; Hui, X.; Lu, B.; Jiang, W.; Shi, Z. Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J. Hypertens. 2015, 33, 1624–1632. [Google Scholar] [CrossRef]
- Zhao, D.; Bi, G.; Feng, J.; Huang, R.; Chen, X. Association of Serum Chemerin Levels with Acute Ischemic Stroke and Carotid Artery Atherosclerosis in a Chinese Population. Med. Sci. Monit. 2015, 21, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, Y.; Yidilisi, A.; Xu, Y.; Dong, Q.; Jiang, J. Causal Associations Between Circulating Adipokines and Cardiovascular Disease: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e2572–e2580. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Otani, K.; Okada, M.; Yamawaki, H. Chemokine-like Receptor 1 in Brain of Spontaneously Hypertensive Rats Mediates Systemic Hypertension. Int. J. Mol. Sci. 2021, 22, 11812. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, E.T.; Mesbah, N.M.; Ghattas, M.H.; Saleh, S.M.; Abo-Elmatty, D.M. Association of chemerin Rs17173608 and vaspin Rs2236242 gene polymorphisms with metabolic syndrome in Egyptian women. Endocr. Res. 2016, 41, 43–48. [Google Scholar] [CrossRef]
- Batista, A.P.; Barbosa, K.F.; Masioli, C.Z.; Queiroz, E.M.; Marinho, C.C.; Candido, A.P.C.; Machado-Coelho, G.L.L. High levels of chemerin associated with variants in the NOS3 and APOB genes in rural populations of Ouro Preto, Minas Gerais, Brazil. Braz. J. Med. Biol. Res. 2020, 53, e9113. [Google Scholar] [CrossRef]
- Afify, A.A.; Fathy, G.; Elzawahry, M.; Taha, S.I. Assessment of serum chemerin levels in acanthosis nigricans: A case-control study. J. Cosmet. Dermatol. 2022, 21, 6414–6421. [Google Scholar] [CrossRef]
- Lu, B.; Zhao, M.; Jiang, W.; Ma, J.; Yang, C.; Shao, J.; Gu, P. Independent Association of Circulating Level of Chemerin With Functional and Early Morphological Vascular Changes in Newly Diagnosed Type 2 Diabetic Patients. Medicine 2015, 94, e1990. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, B.W.; Song, Y.M.; Kim, W.J.; Chang, H.J.; Choi, D.H.; Yu, H.T.; Kang, E.; Cha, B.S.; Lee, H.C. Potential association between coronary artery disease and the inflammatory biomarker YKL-40 in asymptomatic patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2012, 11, 84. [Google Scholar] [CrossRef]
- Ji, Q.; Lin, Y.; Liang, Z.; Yu, K.; Liu, Y.; Fang, Z.; Liu, L.; Shi, Y.; Zeng, Q.; Chang, C.; et al. Chemerin is a novel biomarker of acute coronary syndrome but not of stable angina pectoris. Cardiovasc. Diabetol. 2014, 13, 145. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Adverse Cardiac Remodelling after Acute Myocardial Infarction: Old and New Biomarkers. Dis. Markers 2020, 2020, 1215802. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
| Study | Species | Year | Conclusions |
|---|---|---|---|
| Obesity | |||
| Goralski et al. [17] | C57/BL/6J, Lep (ob/ob) mice; mouse 3T3-L1 preadipocytes | 2007 | Maturing 3T3-L1 cells increase chemerin/CMKLR1 mRNA and secrete more bioactive chemerin. White adipocytes source and target chemerin signaling. |
| Bozaoglu et al. [49] | Psammomys obesus rats | 2007 | Chemerin is synthesized by mature adipocytes but not preadipocytes. In obese and T2D P. obesus, chemerin and CMKLR1 expression were elevated in adipose tissue compared to lean, normoglycemic P. obesus. |
| Ernst et al. [33] | Lep (ob/ob), Lepr (db/db), C57BL/6 mice | 2010 | Significantly higher serum chemerin levels and elevated mRNA levels of chemerin and its receptors in liver, skeletal muscle, WAT were observed in mouse models of obesity/diabetes. |
| Muruganandan et al. [16] | C57BL/6 mice | 2011 | CMKLR1 deletion inhibits adipogenesis during differentiation. Chemerin/CMKLR1 deletion leads to loss of cyclin A2/B2 mRNA and protein, inhibiting adipogenesis at the G2/M phase. |
| Ernst et al. [29] | CMKLR1 knockout and wild-type mice | 2012 | Fasting blood glucose, serum insulin, and food consumption were decreased in CMKLR1−/− mice compared to the control group. |
| Nakamura et al. [86] | Male C57BL/6 mice (WT), Male Sprague Dawley (SD) rats | 2018 | Chemerin promotes angiogenesis. Corneal assay—chemerin led to a significant increase in corneal neovascularization. Chemerin significantly accelerated neovascularization in the rat aortic ring assay. |
| Jiang et al. [92] | Female athymic Balb/c nude mice | 2018 | Chemerin promoted preadipocyte proliferation and the expression of VEGF, FABP4, and CMKLR1, as well as the phosphorylation of proteins in the Akt/mTOR and ERK1/2 pathways, in a concentration-dependent manner. |
| Hypertension | |||
| Lobato N.S. et al. [142] | Aortic rings of Wistar rats incubated with chemerin | 2012 | Chemerin potentiates vasoconstriction induced by other vasoconstrictors such as endothelin and phenylephrine or norepinephrine. |
| Watts S.W. et al. [138] | Sprague Dawley rats, Wistar Kyoto rats, stroke-prone spontaneously hypertensive rats | 2013 | Chemerin has been shown to have significant vasoconstrictor effects in humans and rats. |
| Kunimoto H. et al. [141] | Kurabo human aortic SMCs, BALB/c mice, Wister rats mesenteric artery SMCs | 2015 | Chemerin/CMKLR1 stimulates SMC proliferation and migration via reactive oxygen species-dependent phosphorylation of Akt/ERK, which may lead to vascular structural remodeling and an increase in systolic blood pressure. |
| Kunimoto H. et al. [141] | Kurabo human aortic SMCs, BALB/c mice, Wister rats mesenteric artery SMCs | 2015 | Chemerin potentiates vasoconstriction induced by other vasoconstrictors such as endothelin and phenylephrine or norepinephrine. |
| Darios E.S. et al. [139] | Sprague Dawley rats | 2016 | Chemerin has been shown to have significant vasoconstrictor effects in humans and rats. |
| Kennedy A.J. et al. [140] | Sprague Dawley rats | 2016 | The potent vasoconstrictor effects of chemerin are mediated by CMKLR1, not GPR1. |
| Watts S.W. et al. [154] | CRISPR/Cas9-induced chemerin knockout Sprague Dawley rats | 2018 | Chemerin expression shows sex differences. Chemerin is able to modify blood pressure in response to a hypertensive challenge. Male chemerin knockout rats had higher blood pressure than male wild-type rats, while female knockout rats had lower blood pressure than female wild-type rats. |
| Wen J. et al. [143] | Wistar rats; high-salt, high-fat diet | 2019 | Chemerin stimulates SMC proliferation and migration via autophagy, which may lead to vascular structural remodeling in metabolic hypertension. |
| Mocker A. et al. [147] | 2-kidney 1-clip hypertensive rats, Thy1.1 nephritic rats | 2019 | Renal chemerin expression is associated with processes of inflammation and fibrosis related to renal damage. |
| Ferland D.J. et al. [145] | Dahl S rats; high fat vs. high salt diet | 2019 | Chemerin secreted from adipose tissue is an important pathological factor in hypertension associated with high fat, not high salt diet. |
| Yamamoto A. et al. [134] | Wistar rats; intracerebroventricular injection of chemerin-9 | 2020 | Chemerin, injected intraventricularly, increases blood pressure in circulatory system via CMKLR1 located in brain. |
| Yamamoto A. et al. [173] | Wistar Kyoto rats, spontaneously hypertensive rats; intracerebroventricular injection of CMKLR1 | 2021 | Increased protein expression of CMKLR1 in paraventricular nucleus is at least partly responsible for systemic hypertension in spontaneously hypertensive rats. |
| Andy W.C. Man et al. [144] | Adipocyte-specific nitric oxide synthase-knockout mice; high-fat diet | 2023 | Adipocyte NOS3 may play an important role in regulating chemerin in adipose tissue. Adipocyte NOS3 is essential for maintaining vascular homeostasis, and its dysfunction contributes to obesity-induced vascular remodeling and hypertension. |
| Atherosclerosis | |||
| Nakamura N. et al. [86] | C57BL/6 mice, Sprague Dawley rats | 2018 | Chemerin promotes migration and angiogenic activities mainly through CMKLR1. |
| Jia J. et al. [158] | ApoE−/− mice | 2020 | Chemerin enhances the adhesion and migration abilities of endothelial progenitor cells and increases the instability of plaques and abnormal lipid accumulation presumably through the p38 MAPK pathway. |
| Tang C. et al. [157] | ApoE−/− mice | 2023 | Chemerin promotes atherosclerotic plaque formation via a CCRL2 receptor-β2 integrin axis. |
| Author | Country | Year | Study Design | Total Patients | Main Conclusions |
|---|---|---|---|---|---|
| Obesity | |||||
| Sell H. et al. [32] | France, Germany | 2010 | prospective | 60 | Serum chemerin levels are significantly higher in morbidly obese patients compared to non-obese individuals. |
| Dong B. et al. [105] | China | 2011 | prospective | 164 | In patients with MetS, higher serum chemerin levels are associated with higher BMI, systolic blood pressure, serum triglycerides, and hs-CRP levels. |
| Chakaroun R. et al. [90] | Germany | 2012 | cross-sectional and interventional | 740 | Moderate weight loss 6 months after a calorie-restricted diet significantly reduces serum chemerin levels. Individuals with type 2 diabetes have chemerin serum concentrations 15% higher than healthy individuals. |
| Lorincz H. et al. [98] | Hungary | 2014 | observational study | 88 | Serum chemerin levels are inversely correlated with levels of HDL-C. |
| Fulop P. et al. [60] | Hungary | 2014 | case-control | 88 | Serum chemerin levels are positively correlated with oxidative stress and inflammation markers. |
| Lorincz H. et al. [98] | Hungary | 2014 | observational study | 88 | Obese patients have higher serum chemerin levels than healthy controls. |
| Fulop P. et al. [60] | Hungary | 2014 | case-control | 88 | Serum chemerin levels correlated positively with leptin levels and negatively with adiponectin levels. |
| Lorincz H. et al. [98] | Hungary | 2014 | observational study | 88 | The proportion of small dense LDL subfraction is higher in obese patients than in the control group. Serum chemerin levels are inversely correlated with mean LDL size. |
| Mehanna E.T. et al. [174] | Egypt | 2016 | cross-sectional | 200 | MetS patients show a significantly higher frequency of the minor allele of chemerin rs17173608 polymorphism. |
| Zylla S. et al. [64] | Germany | 2017 | prospective | 3986 | Chemerin promotes inflammation by contributing to the development of leukocyte populations, regardless of body mass. |
| Yang M. et al. [88] | China | 2019 | case-control | 100 | Serum chemerin levels are higher in the group with waist-to-stature > 0.55 compared to the group with waist-to-stature ≤ 0.55. |
| Shafer-Eggleton J. et al. [110] | USA | 2020 | prospective | 50 | In MetS, the chemerin:HDL-C ratio is significantly elevated and shows a positive correlation with MetS severity. |
| Batista A.P. et al. [175] | Brazil | 2020 | cross-sectional | 508 | High serum chemerin levels are associated with elevated triglyceride levels and insulin resistance. |
| Afify A.A. et al. [176] | Egypt | 2022 | case-control | 75 | Compared to the control group, serum chemerin concentrations are significantly elevated in participants with obesity. |
| Hypertension | |||||
| Yang M. et al. [155] | China | 2010 | cross-sectional | 174 | Serum chemerin levels are significantly increased in hypertensive patients. |
| Bozaoglu K. et al. [41] | US, Australia | 2010 | cross-sectional family-based genetic epidemiological study | 1354 | Chemerin acts as a stimulator of angiogenesis and contributes to vascular damage in hypertension. |
| Gu P. et al. [135] | China | 2014 | case-control | 347 | Chemerin in hypertensive patients is associated with inflammatory markers, as well as with key components of MetS: obesity, plasma triglycerides, and HOMA-IR. |
| Lu B. et al. [177] | China | 2015 | observational study | 393 | Serum chemerin levels are independently associated with arterial function index and early atherosclerotic changes. Elevated serum chemerin levels in hypertensive patients are associated with endothelial dysfunction. |
| Atherosclerosis | |||||
| Lehrke M. et al. [59] | Germany | 2009 | cross-sectional | 303 | Serum chemerin levels are associated with levels of hs-CRP, interleukin 6, TNF-α, resistin, and leptin. Chemerin does not predict coronary atherosclerosis. |
| Hah Y.J. et al. [162] | Korea | 2011 | cross-sectional | 131 | Serum chemerin levels have a significant correlation with the degree of coronary artery stenosis in patients with CAD. Chemerin was not an independent risk factor for multiple vessel disease. |
| Yan Q. et al. [160] | China | 2012 | case-control | 430 | Higher serum chemerin levels are associated with metabolic parameters, increased risk of CAD per se, and number of diseased vessels. |
| Xiaotao L. et al. [161] | China | 2012 | cross-sectional | 188 | Higher serum chemerin levels are associated with the presence of CAD. Chemerin levels may reflect the extent of coronary atherosclerosis in the sense of calcium score. |
| Kim H.M. et al. [178] | Korea | 2012 | cross-sectional | 70 | Serum chemerin levels were not significantly different between asymptomatic type 2 diabetic patients with CAD and without CAD. |
| Gu P. et al. [170] | China | 2015 | cross-sectional | 356 | Serum chemerin levels were independently associated with the index of arterial function and early atherosclerosis in essentially hypertensive patients. |
| Zhao D. et al. [171] | China | 2015 | case-control | 140 | Serum chemerin levels may be an independent risk factor for acute ischemic stroke and carotid artery plaque instability. |
| Lachine N.A. et al. [169] | Egypt | 2016 | cross-sectional | 180 | Serum chemerin could be considered a marker of subclinical atherosclerosis in patients with type 2 diabetes. |
| Motawi T.M.K. et al. [167] | Egypt | 2018 | cross-sectional | 90 | Serum chemerin levels could statistically significantly differentiate between non-obese, non-diabetic patients with CAD and obese, diabetic patients with CAD. |
| Carracedo M. et al. [168] | Sweden | 2019 | case-control | 163 | Chemerin signaling through CMKLR1 in vascular smooth muscle cells protects against vascular calcification in chronic kidney disease. |
| Zhou X. et al. [163] | China | 2019 | prospective cohort study | 834 | Serum chemerin levels correlate with risk of major adverse cardiac events in patients with chronic heart failure. |
| Wu Q. et al. [164] | China | 2020 | case-control | 100 | Epicardial fat volume, adiponectin, chemerin, and VEGF are independent risk factors for vascular remodeling. The expression of adiponectin, chemerin, and VEGF can reflect epicardial fat volume. |
| Wang B. et al. [159] | China | 2022 | cohort study | 152 | Serum chemerin levels are significantly increased in patients with coronary artery disease compared to controls. High chemerin levels in those patients increase the risk of major adverse cardiac events. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imiela, A.M.; Stępnicki, J.; Zawadzka, P.S.; Bursa, A.; Pruszczyk, P. Chemerin as a Driver of Cardiovascular Diseases: New Perspectives and Future Directions. Biomedicines 2025, 13, 1481. https://doi.org/10.3390/biomedicines13061481
Imiela AM, Stępnicki J, Zawadzka PS, Bursa A, Pruszczyk P. Chemerin as a Driver of Cardiovascular Diseases: New Perspectives and Future Directions. Biomedicines. 2025; 13(6):1481. https://doi.org/10.3390/biomedicines13061481
Chicago/Turabian StyleImiela, Anna M., Jan Stępnicki, Patrycja Sandra Zawadzka, Angelika Bursa, and Piotr Pruszczyk. 2025. "Chemerin as a Driver of Cardiovascular Diseases: New Perspectives and Future Directions" Biomedicines 13, no. 6: 1481. https://doi.org/10.3390/biomedicines13061481
APA StyleImiela, A. M., Stępnicki, J., Zawadzka, P. S., Bursa, A., & Pruszczyk, P. (2025). Chemerin as a Driver of Cardiovascular Diseases: New Perspectives and Future Directions. Biomedicines, 13(6), 1481. https://doi.org/10.3390/biomedicines13061481






