Pathogenesis and Therapeutic Perspectives of Tubular Injury in Diabetic Kidney Disease: An Update
Abstract
1. Introduction
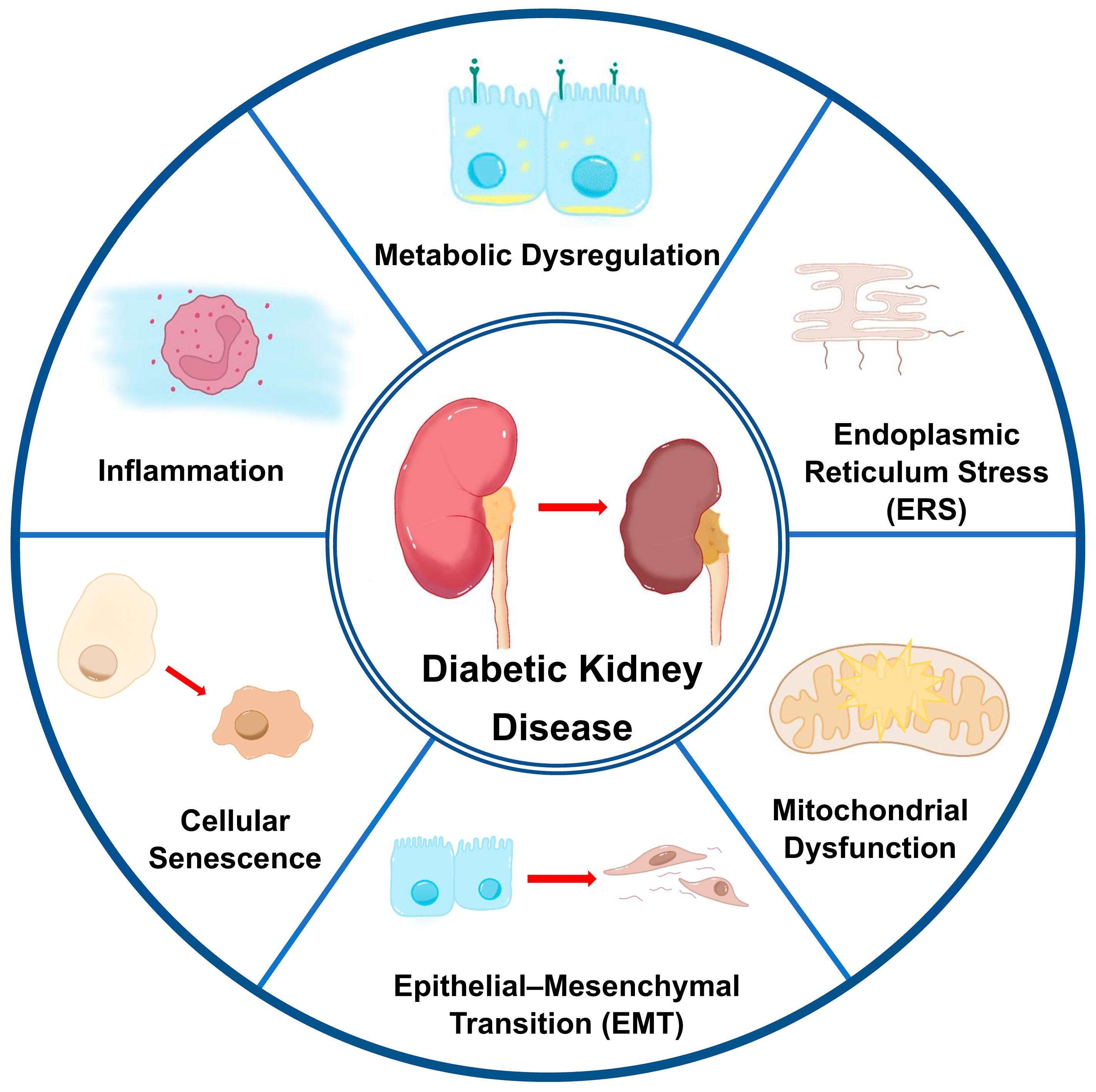
2. Pathogenesis of Tubular Injury in DKD
2.1. Metabolic Dysregulation
2.1.1. Hyperglycemia and Advanced Glycation End Products (AGEs)
2.1.2. Disorders of Lipid Metabolism
2.2. Inflammation
2.3. Cellular Stress Responses
2.3.1. Oxidative Stress
2.3.2. Endoplasmic Reticulum Stress (ERS)
2.3.3. Mitochondrial Dysfunction
2.4. Epithelial–Mesenchymal Transition (EMT)
2.5. Cellular Senescence
2.6. Gut–Kidney Axis
3. Therapeutic Perspectives
3.1. Hypoglycemic Agents
3.1.1. Metformin
3.1.2. Sodium–Glucose Cotransporter 2 Inhibitors (SGLT2i)
3.1.3. Dipeptidyl Peptidase 4 Inhibitors (DPP-4i)
3.1.4. Glucagon-like Peptide-1 Receptor Agonists (GLP-1RA)
3.2. Hypotensive Drags
3.2.1. Renin–Angiotensin System Inhibitors (RASi)
3.2.2. Endothelin Receptor Antagonists (ERAs)
3.2.3. Mineralocorticoid Receptor Antagonists (MRAs)
3.3. Lipid-Modulating Drugs
3.4. Stem Cell Therapy
3.5. Gene Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, L.; Liang, X.; Qiao, Y.; Chen, B.; Wang, P.; Liu, Z. Mitochondrial dysfunction in diabetic tubulopathy. Metabolism 2022, 131, 155195. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, L.; Alvarez, P.J.; Reaven, N.L.; Funk, S.E.; McGaughey, K.J.; Romero, A.; Brenner, M.S.; Onuigbo, M. All-cause costs increase exponentially with increased chronic kidney disease stage. Am. J. Manag. Care 2017, 23, S163–S172. [Google Scholar] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.M.; Nee, R.; Ceckowski, K.A.; Knight, K.R.; Abbott, K.C. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin. Kidney J. 2017, 10, 257–262. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Agarwal, R.; Alpers, C.E.; Bakris, G.L.; Brosius, F.C.; Kolkhof, P.; Uribarri, J. Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Int. 2022, 102, 248–260. [Google Scholar] [CrossRef]
- Linh, H.T.; Iwata, Y.; Senda, Y.; Sakai-Takemori, Y.; Nakade, Y.; Oshima, M.; Nakagawa-Yoneda, S.; Ogura, H.; Sato, K.; Minami, T.; et al. Intestinal Bacterial Translocation Contributes to Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2022, 33, 1105–1119. [Google Scholar] [CrossRef]
- Rico-Fontalvo, J.; Aroca, G.; Cabrales, J.; Daza-Arnedo, R.; Yánez-Rodríguez, T.; Martínez-Ávila, M.C.; Uparella-Gulfo, I.; Raad-Sarabia, M. Molecular Mechanisms of Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 8668. [Google Scholar] [CrossRef]
- Nakagawa, T.; Tanabe, K.; Croker, B.P.; Johnson, R.J.; Grant, M.B.; Kosugi, T.; Li, Q. Endothelial dysfunction as a potential con-tributor in diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 36–44. [Google Scholar] [CrossRef]
- Shahzad, K.; Fatima, S.; Khawaja, H.; Elwakiel, A.; Gadi, I.; Ambreen, S.; Zimmermann, S.; Mertens, P.R.; Biemann, R.; Isermann, B. Podocyte-specific Nlrp3 inflammasome activation promotes diabetic kidney disease. Kidney Int. 2022, 102, 766–779. [Google Scholar] [CrossRef]
- Jefferson, J.; Shankland, S.; Pichler, R. Proteinuria in diabetic kidney disease: A mechanistic viewpoint. Kidney Int. 2008, 74, 22–36. [Google Scholar] [CrossRef]
- He, X.; Cheng, R.; Huang, C.; Takahashi, Y.; Yang, Y.; Benyajati, S.; Chen, Y.; Zhang, X.A.; Ma, J.X. A novel role of LRP5 in tubu-lointerstitial fibrosis through activating TGF-β/Smad signaling. Signal Transduct. Target. Ther. 2020, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Lytvyn, Y.; Bjornstad, P.; van Raalte, D.H.; Heerspink, H.L.; Cherney, D.Z.I. The New Biology of Diabetic Kidney Disease—Mechanisms and Therapeutic Implications. Endocr. Rev. 2020, 41, 202–231. [Google Scholar] [CrossRef] [PubMed]
- Folz, R.; Laiteerapong, N. The legacy effect in diabetes: Are there long-term benefits? Diabetologia 2021, 64, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Ma, C.; Chen, Y.; Xiang, J.; Li, L.; Li, Y.; Kang, L.; Liang, Z.; Yang, S. HSPA8 dampens SCAP/INSIG split and SREBP activation by reducing PKR-mediated INSIG phosphorylation. Cell Rep. 2025, 44, 115339. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Neumiller, J.J.; Johnson, E.J.; Dieter, B.; Tuttle, K.R. Sodium–Glucose Cotransporter 2 Inhibition and Diabetic Kidney Disease. Diabetes 2019, 68, 248–257. [Google Scholar] [CrossRef]
- Vieira, A.B.; Cavanaugh, S.M.; Ciambarella, B.T.; Machado, M.V. Sodium-glucose co-transporter 2 inhibitors: A pleiotropic drug in humans with promising results in cats. Front. Vet. Sci. 2025, 12, 1480977. [Google Scholar] [CrossRef]
- Ghezzi, C.; Loo, D.D.F.; Wright, E.M. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia 2018, 61, 2087–2097. [Google Scholar] [CrossRef]
- Tabatabai, N.M.; Sharma, M.; Blumenthal, S.S.; Petering, D.H. Enhanced expressions of sodium–glucose cotransporters in the kidneys of diabetic Zucker rats. Diabetes Res. Clin. Pract. 2009, 83, e27–e30. [Google Scholar] [CrossRef][Green Version]
- Marks, J.; Carvou, N.J.C.; Debnam, E.S.; Srai, S.K.; Unwin, R.J. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J. Physiol. 2003, 553 Pt 1, 137–145. [Google Scholar] [CrossRef]
- Rahmoune, H.; Thompson, P.W.; Ward, J.M.; Smith, C.D.; Hong, G.; Brown, J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non–insulin-dependent diabetes. Diabetes 2005, 54, 3427–3434. [Google Scholar] [CrossRef]
- Czajka, A.; Malik, A.N. Hyperglycemia induced damage to mitochondrial respiration in renal mesangial and tubular cells: Implications for diabetic nephropathy. Redox Biol. 2016, 10, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Geis, L.; Kurtz, A. Oxygen sensing in the kidney. Nephrol. Dial. Transplant. 2025, 40, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Chen, S.; Shi, Y.; Wang, P.; Wu, Y.; Li, G. Dietary advanced glycation end products (dAGEs): An insight between modern diet and health. Food Chem. 2023, 415, 135735. [Google Scholar] [CrossRef]
- Dozio, E.; Caldiroli, L.; Molinari, P.; Castellano, G.; Delfrate, N.W.; Romanelli, M.M.C.; Vettoretti, S. Accelerated AGEing: The Impact of Advanced Glycation End Products on the Prognosis of Chronic Kidney Disease. Antioxidants 2023, 12, 584. [Google Scholar] [CrossRef]
- Schalkwijk, C.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Vlassara, H.; Striker, G.E. AGE restriction in diabetes mellitus: A paradigm shift. Nat. Rev. Endocrinol. 2011, 7, 526–539. [Google Scholar] [CrossRef]
- Sanajou, D.; Haghjo, A.G.; Argani, H.; Aslani, S. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions. Eur. J. Pharmacol. 2018, 833, 158–164. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Inagaki, Y.; Okamoto, T.; Amano, S.; Koga, K.; Takeuchi, M. Advanced glycation end products inhibit de novo protein synthesis and induce TGF-β overexpression in proximal tubular cells. Kidney Int. 2003, 63, 464–473. [Google Scholar] [CrossRef]
- Wang, S.-N.; Lapage, J.; Hirschberg, R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int. 2000, 57, 1002–1014. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, É. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology 2019, 20, 279–301. [Google Scholar] [CrossRef]
- Sergi, D.; Boulestin, H.; Campbell, F.M.; Williams, L.M. The Role of Dietary Advanced Glycation End Products in Metabolic Dysfunction. Mol. Nutr. Food Res. 2021, 65, e1900934. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamy, R.; Naka, Y.; Schmidt, A.M. Glycation, inflammation, and RAGE: A scaffold for the macrovascular complications of diabetes and beyond. Circ. Res. 2003, 93, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Sano, Y.; Haruki, H.; Kanda, T. Advanced glycation end-products induce basement membrane hypertrophy in endoneurial microvessels and disrupt the blood–nerve barrier by stimulating the release of TGF-β and vascular endothelial growth factor (VEGF) by pericytes. Diabetologia 2011, 54, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, K.; Cai, G.Y.; Chen, X.M.; Yang, J.R.; Lin, L.R.; Yang, J.; Huo, B.G.; Zhan, J.; He, Y.N. Receptor for advanced glycation end-products promotes premature senescence of proximal tubular epithelial cells via activation of endoplasmic reticulum stress-dependent p21 signaling. Cell. Signal. 2014, 26, 110–121. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and Oxidative Stress: An Integral, Updated and Critical Overview of Their Metabolic Interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Yamagishi, S.-I.; Nakamura, K.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Agents that block advanced glycation end product (AGE)-RAGE (receptor for AGEs)-oxidative stress system: A novel therapeutic strategy for diabetic vascular complications. Expert Opin. Investig. Drugs 2008, 17, 983–996. [Google Scholar] [CrossRef]
- Wan, L.; Bai, X.; Zhou, Q.; Chen, C.; Wang, H.; Liu, T.; Xue, J.; Wei, C.; Xie, L. The advanced glycation end-products (AGEs)/ROS/NLRP3 inflammasome axis contributes to delayed diabetic corneal wound healing and nerve regeneration. Int. J. Biol. Sci. 2022, 18, 809–825. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.-A.; Han, S.H.; Chinga, F.; Park, A.S.D.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Yang, X.; Okamura, D.M.; Lu, X.; Chen, Y.; Moorhead, J.; Varghese, Z.; Ruan, X.Z. CD36 in chronic kidney disease: Novel insights and therapeutic opportunities. Nat. Rev. Nephrol. 2017, 13, 769–781. [Google Scholar] [CrossRef]
- Khan, S.; Cabral, P.D.; Schilling, W.P.; Schmidt, Z.W.; Uddin, A.N.; Gingras, A.; Madhavan, S.M.; Garvin, J.L.; Schelling, J.R. Kidney Proximal Tubule Lipoapoptosis Is Regulated by Fatty Acid Transporter-2 (FATP2). J. Am. Soc. Nephrol. 2018, 29, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, L.; Jin, B.; Wu, Y.; Xu, L.; Chang, X.; Hu, L.; Wang, G.; Huang, Y.; Song, L.; et al. Metrnl Alleviates Lipid Accumulation by Modulating Mitochondrial Homeostasis in Diabetic Nephropathy. Diabetes 2023, 72, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, J.; DeHoog, R.J.; Pennathur, S.; Anderton, C.R.; Venkatachalam, M.A.; Alexandrov, T.; Eberlin, L.S.; Sharma, K. DESI-MSI and METASPACE indicates lipid abnormalities and altered mitochondrial membrane components in diabetic renal proximal tubules. Metabolomics 2020, 16, 11. [Google Scholar] [CrossRef]
- Yang, W.; Luo, Y.; Yang, S.; Zeng, M.; Zhang, S.; Liu, J.; Han, Y.; Liu, Y.; Zhu, X.; Wu, H.; et al. Ectopic lipid accumulation: Potential role in tubular injury and inflammation in diabetic kidney disease. Clin. Sci. 2018, 132, 2407–2422. [Google Scholar] [CrossRef]
- Shao, W.; Espenshade, P.J. Expanding Roles for SREBP in Metabolism. Cell Metab. 2012, 16, 414–419. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Q.; Lv, M.; Song, K.; Dai, Y.; Huang, Y.; Zhang, L.; Zhang, C.; Gao, H. Involvement of FATP2-mediated tubular lipid metabolic reprogramming in renal fibrogenesis. Cell Death Dis. 2020, 11, 994. [Google Scholar] [CrossRef]
- Sakashita, M.; Tanaka, T.; Inagi, R. Metabolic Changes and Oxidative Stress in Diabetic Kidney Disease. Antioxidants 2021, 10, 1143. [Google Scholar] [CrossRef]
- Kishi, S.; Nagasu, H.; Kidokoro, K.; Kashihara, N. Oxidative stress and the role of redox signalling in chronic kidney disease. Nat. Rev. Nephrol. 2024, 20, 101–119. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, Y.; Zhao, Y.; Huang, S.; Xin, X.; Jiang, L.; Wang, H.; Wu, W.; Qu, L.; Xiang, C.; et al. Nephropathy Is Aggravated by Fatty Acids in Diabetic Kidney Disease through Tubular Epithelial Cell Necroptosis and Is Alleviated by an RIPK-1 Inhibitor. Kidney Dis. 2023, 9, 408–423. [Google Scholar] [CrossRef]
- Zhou, K.; Yao, P.; He, J.; Zhao, H. Lipophagy in nonliver tissues and some related diseases: Pathogenic and therapeutic im-plications. J. Cell. Physiol. 2019, 234, 7938–7947. [Google Scholar] [CrossRef]
- Han, Y.; Xiong, S.; Zhao, H.; Yang, S.; Yang, M.; Zhu, X.; Jiang, N.; Xiong, X.; Gao, P.; Wei, L.; et al. Lipophagy deficiency exacerbates ectopic lipid accumulation and tubular cells injury in diabetic nephropathy. Cell Death Dis. 2021, 12, 1031. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Debnath, J. Autophagy at the crossroads of catabolism and anabolism. Nat. Rev. Mol. Cell Biol. 2015, 16, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef]
- Sanchez, A.P.; Sharma, K. Transcription factors in the pathogenesis of diabetic nephropathy. Expert Rev. Mol. Med. 2009, 11, e13. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, J.F.; Mora-Fernández, C.; de Fuentes, M.M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Zhao, L.; Hwang, D.H.; Jialal, I. High glucose induces toll-like receptor expression in human monocytes: Mechanism of activation. Diabetes 2008, 57, 3090–3098. [Google Scholar] [CrossRef]
- Fessler, M.B.; Rudel, L.L.; Brown, J.M. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 2009, 20, 379–385. [Google Scholar] [CrossRef]
- Dasu, M.R.; Devaraj, S.; Park, S.; Jialal, I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010, 33, 861–868. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zheng, D.-N.; Ji, C.; Qian, H.; Jin, J.; He, Q. MicroRNA-630 alleviates inflammatory reactions in rats with diabetic kidney disease by targeting toll-like receptor 4. World J. Diabetes 2024, 15, 488–501. [Google Scholar] [CrossRef]
- Chen, L.; Li, D.; Zhan, Z.; Quan, J.; Peng, J.; Huang, Z.; Yi, B. Sirtuin 2 exacerbates renal tubule injury and inflammation in diabetic mice via deacetylation of c-Jun/c-Fos. Cell. Mol. Life Sci. 2025, 82, 45. [Google Scholar] [CrossRef]
- Huang, Y.; He, W.; Zhang, Y.; Zou, Z.; Han, L.; Luo, J.; Wang, Y.; Tang, X.; Li, Y.; Bao, Y.; et al. Targeting SIRT2 in Aging-Associated Fibrosis Pathophysiology. Aging Dis. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, X.; Wang, X.; Zhang, C.; Gao, J.; Yu, G.; He, Q.; Yang, J.; Liu, X.; Wei, Y.; et al. Insights into the modulation of bacterial NADase activity by phage proteins. Nat. Commun. 2024, 15, 2692. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Lai, K.N. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol. Dial. Transplant. 2012, 27, 3049–3056. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.; Yiu, W.H.; Chan, K.W.; Li, Y.; Li, B.; Lok, S.W.; Taketo, M.M.; Igarashi, P.; Chan, L.Y.; Leung, J.C.; et al. Activated renal tubular Wnt/β-catenin signaling triggers renal inflammation during overload proteinuria. Kidney Int. 2018, 93, 1367–1383. [Google Scholar] [CrossRef]
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. A review of the molecular mechanisms of hyperglycemia-induced free radical generation leading to oxidative stress. J. Cell. Physiol. 2019, 234, 1300–1312. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Lin, S.; King, L.; Liu, L. The Potential Role of Advanced Glycation End Products in the Development of Kidney Disease. Nutrients 2025, 17, 758. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Russell, G.B.; Miller, M.E.; Rich, S.S.; Mauer, M. Early progression of diabetic nephropathy correlates with methylglyoxal-derived advanced glycation end products. Diabetes Care 2013, 36, 3234–3239. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Zhang, J.X.; Li, L.; Wu, Q.Y.; Ruan, X.Z.; Chen, P.P.; Ma, K.L. Deficiency of GADD45α-R-Loop Pathway and Kidney Injury in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Jia, D.; Gao, B. Preventive treatment of tripdiolide ameliorates kidney injury in diabetic mice by modulating the Nrf2/NF-κB pathway. Front. Pharmacol. 2025, 16, 1492834. [Google Scholar] [CrossRef]
- Zhang, B.; Geng, H.; Zhao, K.; Omorou, M.; Liu, S.; Ye, Z.; Zhang, F.; Luan, H.; Zhang, X. FSTL1 aggravates high glucose-induced oxidative stress and transdifferentiation in HK-2 cells. Sci. Rep. 2025, 15, 434. [Google Scholar] [CrossRef]
- Hou, Y.; Tan, E.; Shi, H.; Ren, X.; Wan, X.; Wu, W.; Chen, Y.; Niu, H.; Zhu, G.; Li, J.; et al. Mitochondrial oxidative damage reprograms lipid metabolism of renal tubular epithelial cells in the diabetic kidney. Cell. Mol. Life Sci. 2024, 81, 23. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Chambers, J.E.; Ron, D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat. Rev. Drug Discov. 2022, 21, 115–140. [Google Scholar] [CrossRef]
- Xie, Y.; E, J.; Cai, H.; Zhong, F.; Xiao, W.; Gordon, R.E.; Wang, L.; Zheng, Y.-L.; Zhang, A.; Lee, K.; et al. Reticulon-1A mediates diabetic kidney disease progression through endoplasmic reticulum-mitochondrial contacts in tubular epithelial cells. Kidney Int. 2022, 102, 293–306. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, D.; Su, Y.; Liu, H.; Su, Q.; Shen, T.; Zhang, M.; Mi, X.; Zhang, Y.; Yue, S.; et al. PDIA4 targets IRE1α/sXBP1 to alleviate NLRP3 inflammasome activation and renal tubular injury in diabetic kidney disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2025, 1871, 167645. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.R.; Chen, X.M.; Cai, G.Y.; Lin, L.R.; He, Y.N. Impact of ER stress-regulated ATF4/p16 signaling on the premature senescence of renal tubular epithelial cells in diabetic nephropathy. Am. J. Physiol. Cell Physiol. 2015, 308, C621–C630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Bian, C.; Gao, J.; Ren, H. Endoplasmic reticulum stress in diabetic kidney disease: Adaptation and apoptosis after three UPR pathways. Apoptosis 2023, 28, 977–996. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nan, P.; Gong, Y.; Tian, L.; Zheng, Y.; Wu, Z. Endoplasmic reticulum stress-triggered ferroptosis via the XBP1-Hrd1-Nrf2 pathway induces EMT progression in diabetic nephropathy. Biomed. Pharmacother. 2023, 164, 114897. [Google Scholar] [CrossRef] [PubMed]
- Elwakiel, A.; Mathew, A.; Isermann, B. The role of endoplasmic reticulum–mitochondria-associated membranes in diabetic kidney disease. Cardiovasc. Res. 2024, 119, 2875–2883. [Google Scholar] [CrossRef]
- Chen, J.; Liu, D.; Lei, L.; Liu, T.; Pan, S.; Wang, H.; Liu, Y.; Qiao, Y.; Liu, Z.; Feng, Q. CNPY2 Aggravates Renal Tubular Cell Ferroptosis in Diabetic Nephropathy by Regulating PERK/ATF4/CHAC1 Pathway and MAM Integrity. Adv. Sci. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Hong, F.; Liu, B.; Wu, B.X.; Morreall, J.; Roth, B.; Davies, C.; Sun, S.; Diehl, J.A.; Li, Z. CNPY2 is a key initiator of the PERK–CHOP pathway of the unfolded protein response. Nat. Struct. Mol. Biol. 2017, 24, 834–839. [Google Scholar] [CrossRef]
- Amen, O.M.; Sarker, S.D.; Ghildyal, R.; Arya, A. Endoplasmic Reticulum Stress Activates Unfolded Protein Response Signaling and Mediates Inflammation, Obesity, and Cardiac Dysfunction: Therapeutic and Molecular Approach. Front. Pharmacol. 2019, 10, 977. [Google Scholar] [CrossRef]
- Fang, M.; Shen, Z.; Huang, S.; Zhao, L.; Chen, S.; Mak, T.W.; Wang, X. The ER UDPase ENTPD5 promotes protein N-glycosylation, the Warburg effect, and proliferation in the PTEN pathway. Cell 2010, 143, 711–724. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.; Wang, G.; Bo, L.; Jin, B.; Dai, L.; Lu, Q.; Cai, X.; Hu, L.; Liu, L.; et al. The UDPase ENTPD5 regulates ER stress-associated renal injury by mediating protein N-glycosylation. Cell Death Dis. 2023, 14, 166. [Google Scholar] [CrossRef]
- Gao, G.; Su, X.; Liu, S.; Wang, P.; Chen, J.J.; Liu, T.; Xu, J.; Zhang, Z.; Zhang, X.; Xie, Z. Cornuside as a promising therapeutic agent for diabetic kidney disease: Targeting regulation of Ca2+ disorder-mediated renal tubular epithelial cells apoptosis. Int. Immunopharmacol. 2025, 149, 114190. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, Z.; Jia, X.; Xiong, Y.; Li, B.; Zhang, L.; Li, X.; Li, X.; Fang, Y.; Dong, X.; et al. (+)-Catechin ameliorates diabetic nephropathy injury by inhibiting endoplasmic reticulum stress-related NLRP3-mediated inflammation. Food Funct. 2024, 15, 5450–5465. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, L.; Yang, H.; Chen, X.; Zheng, H.; Liao, X. SIK2 protects against renal tubular injury and the progression of diabetic kidney disease. Transl. Res. 2023, 253, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, P.; Park, J.; del Pozo, C.H.; Li, L.; Doke, T.; Huang, S.; Zhao, J.; Kang, H.M.; Shrestra, R.; Balzer, M.S.; et al. The Nuclear Receptor ESRRA Protects from Kidney Disease by Coupling Metabolism and Differentiation. Cell Metab. 2021, 33, 379–394.e8. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hu, J.; Chen, Z.; Yang, K.; Zhu, Z.; Hao, Y.; Zhang, Z.; Li, W.; Peng, Z.; Cao, Y.; et al. RBBP6-Mediated ERRα Degradation Contributes to Mitochondrial Injury in Renal Tubular Cells in Diabetic Kidney Disease. Adv. Sci. 2024, 11, e2405153. [Google Scholar] [CrossRef]
- Li, H.; Leung, J.C.K.; Yiu, W.H.; Chan, L.Y.Y.; Li, B.; Lok, S.W.Y.; Xue, R.; Zou, Y.; Lai, K.N.; Tang, S.C.W. Tubular β-catenin alleviates mitochondrial dysfunction and cell death in acute kidney injury. Cell Death Dis. 2022, 13, 1061. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, M.; Zheng, X.; Li, S.; Fan, Y.; Wang, Y.; Peng, H.; Chen, S.; Yang, J.; Tan, L.; et al. YAP1 preserves tubular mitochondrial quality control to mitigate diabetic kidney disease. Redox Biol. 2024, 78, 103435. [Google Scholar] [CrossRef]
- Souza, C.S.; Deluque, A.L.; Oliveira, B.M.; Maciel, A.L.D.; Giovanini, C.; Boer, P.A.; de Paula, F.J.A.; Costa, R.S.; Franscecato, H.D.C.; de Almeida, L.F.; et al. Vitamin D deficiency contributes to the diabetic kidney disease progression via increase ZEB1/ZEB2 expressions. Nutr. Diabetes 2023, 13, 9. [Google Scholar] [CrossRef]
- Han, F.; Wu, S.; Dong, Y.; Liu, Y.; Sun, B.; Chen, L. Aberrant expression of NEDD4L disrupts mitochondrial homeostasis by downregulating CaMKKβ in diabetic kidney disease. J. Transl. Med. 2024, 22, 465. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef]
- Takasu, M.; Kishi, S.; Nagasu, H.; Kidokoro, K.; Brooks, C.R.; Kashihara, N. The Role of Mitochondria in Diabetic Kidney Disease and Potential Therapeutic Targets. Kidney Int. Rep. 2024, 10, 328–342. [Google Scholar] [CrossRef]
- Zhang, J.X.; Chen, P.P.; Li, X.Q.; Li, L.; Wu, Q.Y.; Wang, G.H.; Ruan, X.Z.; Ma, K.L. Deficiency of thiosulfate sulfurtransferase mediates the dysfunction of renal tubular mitochondrial fatty acid oxidation in diabetic kidney disease. Cell Death Differ. 2024, 31, 1636–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, D.; Kang, X.; Zhou, R.; Sun, Y.; Lian, F.; Tong, X. Signaling Pathways Involved in Diabetic Renal Fibrosis. Front. Cell Dev. Biol. 2021, 9, 696542. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Q.; Li, C.; Liang, K.; Xiang, Y.; Hsiao, H.; Nguyen, T.K.; Park, P.K.; Egranov, S.D.; Ambati, C.R.; et al. PTEN-induced partial epithelial-mesenchymal transition drives diabetic kidney disease. J. Clin. Investig. 2019, 129, 1129–1151. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Y. Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat. Rev. Nephrol. 2016, 12, 68–70. [Google Scholar] [CrossRef]
- Chiu, I.-J.; Ajay, A.K.; Chen, C.-H.; Jadhav, S.; Zhao, L.; Cao, M.; Ding, Y.; Shah, K.M.; Shah, S.I.; Hsiao, L.-L. Suppression of aldehyde dehydrogenase 2 in kidney proximal tubules contributes to kidney fibrosis through Transforming Growth Factor-β signaling. Kidney Int. 2025, 107, 84–98. [Google Scholar] [CrossRef]
- Yu, T.; Mai, Z.; Zhang, S.; Wang, S.; Yang, W.; Ruan, Z.; Li, P.; Guo, F.; Zhang, Y.; Li, J.; et al. ACVR1 mediates renal tubular EMT in kidney fibrosis via AKT activation. Cell Signal. 2025, 125, 111521. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Shi, H.; Liu, X.; Li, Z.; Zhang, J.; Wang, X.; Wang, W.; Tong, X. CRTC2 activates the epithelial-mesenchymal transition of diabetic kidney disease through the CREB-Smad2/3 pathway. Mol. Med. 2023, 29, 146. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, R.; Wang, Y.; Liang, Y.; Yang, Z.; Wang, T.; Xu, X.; Liu, F. Implications of immunoglobulin G deposit in glomeruli in Chinese patients with diabetic nephropathy. J. Diabetes 2020, 12, 521–531. [Google Scholar] [CrossRef]
- Azushima, K.; Kovalik, J.-P.; Yamaji, T.; Ching, J.; Chng, T.W.; Guo, J.; Liu, J.-J.; Nguyen, M.; Sakban, R.B.; George, S.E.; et al. Abnormal lactate metabolism is linked to albuminuria and kidney injury in diabetic nephropathy. Kidney Int. 2023, 104, 1135–1149. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Lin, R.; Huang, Y.; Wang, Z.; Xu, S.; Wang, L.; Chen, F.; Zhang, J.; Pan, K.; et al. Lactate drives epitheli-al-mesenchymal transition in diabetic kidney disease via the H3K14la/KLF5 pathway. Redox Biol. 2024, 75, 103246. [Google Scholar] [CrossRef]
- Xu, Z.; Jia, K.; Wang, H.; Gao, F.; Zhao, S.; Li, F.; Hao, J. METTL14-regulated PI3K/Akt signaling pathway via PTEN affects HDAC5-mediated epithelial–mesenchymal transition of renal tubular cells in diabetic kidney disease. Cell Death Dis. 2021, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lin, H.; Jin, F.; Luo, Y.; Yang, P.; Song, J.; Yao, W.; Lin, W.; Yuan, D.; Zuo, A.; et al. Jia Wei Qingxin Lotus Seed Drink ameliorates epithelial mesenchymal transition injury in diabetic kidney disease via inhibition of JMJD1C/SP1/ZEB1 signaling pathway. Phytomedicine 2024, 135, 156142. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, S.; He, X.; Wei, W.; Huang, K. Quercetin prevents the USP22-Snail1 signaling pathway to ameliorate diabetic tubulointerstitial fibrosis. Food Funct. 2024, 15, 11990–12006. [Google Scholar] [CrossRef]
- de Magalhães, J.P. Cellular senescence in normal physiology. Science 2024, 384, 1300–1301. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Passos, J.F. Stress, cell senescence and organismal ageing. Mech. Ageing Dev. 2018, 170, 2–9. [Google Scholar] [CrossRef]
- Lans, H.; Hoeijmakers, J.H.J. Genome stability, progressive kidney failure and aging. Nat. Genet. 2012, 44, 836–838. [Google Scholar] [CrossRef]
- Elwakiel, A.; Gupta, D.; Rana, R.; Manoharan, J.; Al-Dabet, M.M.; Ambreen, S.; Fatima, S.; Zimmermann, S.; Mathew, A.; Li, Z.; et al. Factor XII signaling via uPAR-integrin β1 axis promotes tubular senescence in diabetic kidney disease. Nat. Commun. 2024, 15, 7963. [Google Scholar] [CrossRef]
- Feng, Q.; Su, C.; Yang, C.; Wu, M.; Li, X.; Lin, X.; Zeng, Y.; He, J.; Wang, Y.; Guo, L.; et al. RXRα/MR signaling promotes diabetic kidney disease by facilitating renal tubular epithelial cells senescence and metabolic reprogramming. Transl. Res. 2024, 274, 101–117. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Wang, X.; Bai, L.; Yang, X.; Chen, J.; He, Y.; Chen, K. Glis1 inhibits RTEC cellular senescence and renal fibrosis by downregulating histone lactylation in DKD. Life Sci. 2025, 361, 123293. [Google Scholar] [CrossRef]
- Zhao, X.-P.; Chang, S.-Y.; Pang, Y.; Liao, M.-C.; Peng, J.; Ingelfinger, J.R.; Chan, J.S.D.; Zhang, S.-L. Hedgehog interacting protein activates sodium–glucose cotransporter 2 expression and promotes renal tubular epithelial cell senescence in a mouse model of type 1 diabetes. Diabetologia 2023, 66, 223–240. [Google Scholar] [CrossRef]
- Maas, C.; Renné, T. Coagulation factor XII in thrombosis and inflammation. Blood 2018, 131, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Amor, C.; Feucht, J.; Leibold, J.; Ho, Y.-J.; Zhu, C.; Alonso-Curbelo, D.; Mansilla-Soto, J.; Boyer, J.A.; Li, X.; Giavridis, T.; et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020, 583, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, J.; Li, R. Alterations of gut microbiota in biopsy-proven diabetic nephropathy and a long history of diabetes without kidney damage. Sci. Rep. 2023, 13, 12150. [Google Scholar] [CrossRef]
- Yan, S.; Wang, H.; Feng, B.; Ye, L.; Chen, A. Causal relationship between gut microbiota and diabetic nephropathy: A two-sample Mendelian randomization study. Front. Immunol. 2024, 15, 1332757. [Google Scholar] [CrossRef]
- Wu, I.-W.; Liao, Y.-C.; Tsai, T.-H.; Lin, C.-H.; Shen, Z.-Q.; Chan, Y.-H.; Tu, C.-W.; Chou, Y.-J.; Lo, C.-J.; Yeh, C.-H.; et al. Machine-learning assisted discovery unveils novel interplay between gut microbiota and host metabolic disturbance in diabetic kidney disease. Gut Microbes 2025, 17, 2473506. [Google Scholar] [CrossRef]
- Cheng, T.-H.; Ma, M.-C.; Liao, M.-T.; Zheng, C.-M.; Lu, K.-C.; Liao, C.-H.; Hou, Y.-C.; Liu, W.-C.; Lu, C.-L. Indoxyl Sulfate, a Tubular Toxin, Contributes to the Development of Chronic Kidney Disease. Toxins 2020, 12, 684. [Google Scholar] [CrossRef]
- Altunkaynak, H.O.; Karaismailoglu, E.; Massy, Z.A. The Ability of AST-120 to Lower the Serum Indoxyl Sulfate Level Improves Renal Outcomes and the Lipid Profile in Diabetic and Nondiabetic Animal Models of Chronic Kidney Disease: A Meta-Analysis. Toxins 2024, 16, 544. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- Si, H.; Chen, Y.; Hu, D.; Yao, S.; Yang, J.; Wen, X. Agraminan type fructan from Achyranthes bidentata prevents the kidney injury in diabetic mice by regulating gut microbiota. Carbohydr. Polym. 2024, 339, 122275. [Google Scholar] [CrossRef]
- Shen, Z.; Cui, T.; Liu, Y.; Wu, S.; Han, C.; Li, J. Astragalus membranaceus and Salvia miltiorrhiza ameliorate diabetic kidney disease via the “gut-kidney axis”. Phytomedicine 2023, 121, 155129. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Zhang, C.; Meng, X. Mechanism and application of metformin in kidney diseases: An update. Biomed. Pharmacother. 2021, 138, 111454. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-C.; Tang, S.-Q.; Liu, Y.-T.; Li, A.-M.; Zhan, M.; Yang, M.; Song, N.; Zhang, W.; Wu, X.-Q.; Peng, C.-H.; et al. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice. Cell Death Dis. 2021, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, H.; Zuo, B.; Shi, K.; Zhang, X.; Zhang, C.; Sun, D. Metformin attenuates renal tubulointerstitial fibrosis via upgrading autophagy in the early stage of diabetic nephropathy. Sci. Rep. 2021, 11, 16362. [Google Scholar] [CrossRef]
- Matsui, T.; Sotokawauchi, A.; Nishino, Y.; Koga, Y.; Yamagishi, S.-I. Empagliflozin ameliorates renal and metabolic derangements in obese type 2 diabetic mice by blocking advanced glycation end product–receptor axis. Mol. Med. 2025, 31, 88. [Google Scholar] [CrossRef]
- Cai, X.; Cao, H.; Wang, M.; Yu, P.; Liang, X.; Liang, H.; Xu, F.; Cai, M. SGLT2 inhibitor empagliflozin ameliorates tubulointerstitial fibrosis in DKD by downregulating renal tubular PKM2. Cell. Mol. Life Sci. 2025, 82, 159. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Perco, P.; Mulder, S.; Leierer, J.; Hansen, M.K.; Heinzel, A.; Mayer, G. Canagliflozin reduces inflammation and fibrosis biomarkers: A potential mechanism of action for beneficial effects of SGLT2 inhibitors in diabetic kidney disease. Diabetologia 2019, 62, 1154–1166. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, T.; Du, L.; Yu, K.; Zeng, S.; Li, M.; Chi, Y.; Li, Y. Empagliflozin attenuates renal damage in diabetic nephropathy by modulating mitochondrial quality control via Prdx3-PINK1 pathway. Biochem. Pharmacol. 2025, 235, 116821. [Google Scholar] [CrossRef]
- Chang, S.-Y.; Liao, M.-C.; Miyata, K.N.; Pang, Y.; Zhao, X.-P.; Peng, J.; Rivard, A.; Ingelfinger, J.R.; Chan, J.S.; Zhang, S.-L. Canagliflozin inhibits hedgehog interacting protein (Hhip) induction of tubulopathy in diabetic Akita mice. Transl. Res. 2025, 277, 13–26. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, L.; Xiao, J.-J.; Liu, Q.; Ni, L.; Hu, J.-W.; Yu, H.; Wu, X.; Zhang, B.-F. Empagliflozin attenuates the renal tubular ferroptosis in diabetic kidney disease through AMPK/NRF2 pathway. Free Radic. Biol. Med. 2023, 195, 89–102. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, C.; Yan, Q.; Li, Z.; Chen, D.; Feng, B.; Song, J. Dapagliflozin improves diabetic kidney disease by inhibiting ferroptosis through β-hydroxybutyrate production. Ren. Fail. 2025, 47, 2438857. [Google Scholar] [CrossRef] [PubMed]
- Kaifu, K.; Ueda, S.; Nakamura, N.; Matsui, T.; Yamada-Obara, N.; Ando, R.; Kaida, Y.; Nakata, M.; Matsukuma-Toyonaga, M.; Higashimoto, Y.; et al. Advanced glycation end products evoke inflammatory reactions in proximal tubular cells via autocrine production of dipeptidyl peptidase-4. Microvasc. Res. 2018, 120, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Lee, S.-Y.; Du, C.-X.; Ku, H.-C. Soluble dipeptidyl peptidase-4 induces epithelial–mesenchymal transition through tumor growth factor-β receptor. Pharmacol. Rep. 2023, 75, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Daza-Arnedo, R.; Rico-Fontalvo, J.-E.; Pájaro-Galvis, N.; Leal-Martínez, V.; Abuabara-Franco, E.; Raad-Sarabia, M.; Montejo-Hernández, J.; Cardona-Blanco, M.; Cabrales-Juan, J.; Uparella-Gulfo, I.; et al. Dipeptidyl Peptidase-4 Inhibitors and Diabetic Kidney Disease: A Narrative Review. Kidney Med. 2021, 3, 1065–1073. [Google Scholar] [CrossRef]
- Seo, J.B.; Choi, Y.-K.; Woo, H.-I.; Jung, Y.-A.; Lee, S.; Lee, S.; Park, M.; Lee, I.-K.; Jung, G.-S.; Park, K.-G. Gemigliptin Attenuates Renal Fibrosis Through Down-Regulation of the NLRP3 Inflammasome. Diabetes Metab. J. 2019, 43, 830–839. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Wang, Y.; He, W.; Wei, W.; Mei, X.; Yang, M.; Wang, Y. Exenatide Attenuates Obesity-Induced Mitochondrial Dysfunction by Activating SIRT1 in Renal Tubular Cells. Front. Endocrinol. 2021, 12, 622737. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, S.; Wu, W.; Lin, Y.; Wang, T.; Sun, H.; A-Ni-Wan, A.S.; Li, Y.; Wang, C.; Li, X.; et al. GLP-1 Receptor Agonists Alleviate Diabetic Kidney Injury via β-Klotho-Mediated Ferroptosis Inhibition. Adv. Sci. 2025, 12, e2409781. [Google Scholar] [CrossRef]
- Su, K.; Yi, B.; Yao, B.-Q.; Xia, T.; Yang, Y.-F.; Zhang, Z.-H.; Chen, C. Liraglutide attenuates renal tubular ectopic lipid deposition in rats with diabetic nephropathy by inhibiting lipid synthesis and promoting lipolysis. Pharmacol. Res. 2020, 156, 104778. [Google Scholar] [CrossRef]
- Yi, B.; Su, K.; Cai, Y.-L.; Chen, X.-L.; Bao, Y.; Wen, Z.-Y. Liraglutide ameliorates diabetic kidney disease by modulating gut microbiota and L-5-Oxoproline. Eur. J. Pharmacol. 2024, 983, 176905. [Google Scholar] [CrossRef]
- Shen, R.; Qin, S.; Lv, Y.; Liu, D.; Ke, Q.; Shi, C.; Jiang, L.; Yang, J.; Zhou, Y. GLP-1 receptor agonist attenuates tubular cell ferroptosis in diabetes via enhancing AMPK-fatty acid metabolism pathway through macropinocytosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 167060. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.T.; Cao, Z.; Long, D.M.; Panagiotopoulos, S.; Jerums, G.; Cooper, M.E.; Forbes, J.M. Interactions between angiotensin II and NF-κB–dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J. Am. Soc. Nephrol. 2004, 15, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Long, W.; Zhang, H.; Zhao, M.; Gao, M.; Guo, W.; Yu, L. Irbesartan ameliorates diabetic nephropathy by activating the Nrf2/Keap1 pathway and suppressing NLRP3 inflammasomes in vivo and in vitro. Int. Immunopharmacol. 2024, 131, 111844. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Luan, G.; Wu, S.; Song, Y.; Shen, S.; Wu, K.; Qian, S.; Jia, W.; Yin, J.; Ren, T.; et al. Single-cell atlas reveals multi-faced responses of losartan on tubular mitochondria in diabetic kidney disease. J. Transl. Med. 2025, 23, 90. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, A.; Liu, C.; Liu, Y.; Qiao, L.; Xu, Z.; Bi, S.; Tian, J.; Yu, B.; Lin, Z.; et al. Identification of key antifibrotic targets FPR1, TAS2R5, and LRP2BP of valsartan in diabetic nephropathy: A transcriptomics-driven study integrating machine learning, molecular docking, and dynamics simulations. Int. J. Biol. Macromol. 2025, 297, 139842. [Google Scholar] [CrossRef]
- Nasr, N.M.A.E.; Saleh, D.O.; Hashad, I.M. Role of olmesartan in ameliorating diabetic nephropathy in rats by targeting the AGE/PKC, TLR4/P38-MAPK and SIRT-1 autophagic signaling pathways. Eur. J. Pharmacol. 2022, 928, 175117. [Google Scholar] [CrossRef]
- Barton, M.; Sorokin, A. Endothelin and the Glomerulus in Chronic Kidney Disease. Semin. Nephrol. 2015, 35, 156–167. [Google Scholar] [CrossRef]
- Raina, R.; Chauvin, A.; Chakraborty, R.; Nair, N.; Shah, H.; Krishnappa, V.; Kusumi, K. The Role of Endothelin and Endothelin Antagonists in Chronic Kidney Disease. Kidney Dis. 2020, 6, 22–34. [Google Scholar] [CrossRef]
- Kang, W.-L.; Xu, G.-S. Atrasentan increased the expression of klotho by mediating miR-199b-5p and prevented renal tubular injury in diabetic nephropathy. Sci. Rep. 2016, 6, 19979. [Google Scholar] [CrossRef]
- Whaley-Connell, A.T.; Habibi, J.; Nistala, R.; DeMarco, V.G.; Pulakat, L.; Hayden, M.R.; Joginpally, T.; Ferrario, C.M.; Parrish, A.R.; Sowers, J.R. Mineralocorticoid receptor-dependent proximal tubule injury is mediated by a redox-sensitive mTOR/S6K1 pathway. Am. J. Nephrol. 2012, 35, 90–100. [Google Scholar] [CrossRef]
- Ostrominski, J.W.; Claggett, B.L.; Miao, Z.M.; Filippatos, G.; Desai, A.S.; Jhund, P.S.; Henderson, A.; Brinker, M.; Schloemer, P.; Viswanathan, P.; et al. Efficacy and Safety of Finerenone in Type 2 Diabetes: A Pooled Analysis of Trials of Heart Failure and Chronic Kidney Disease. Diabetes Care 2025, 48, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liang, X.; Liu, Y.; Li, B.; Hong, M.; Wang, X.; Chen, B.; Liu, Z.; Wang, P. Non-steroidal mineralocorticoid receptor antagonist finerenone ameliorates mitochondrial dysfunction via PI3K/Akt/eNOS signaling pathway in diabetic tubulopathy. Redox Biol. 2023, 68, 102946. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Sánchez-Navaro, A.; Wu, J.; Klötzer, K.A.; Ma, Z.; Poudel, B.; Doke, T.; Balzer, M.S.; Frederick, J.; Cernecka, H.; et al. Single-cell transcriptomics and chromatin accessibility profiling elucidate the kidney-protective mechanism of mineralocorticoid receptor antagonists. J. Clin. Investig. 2024, 134, e157165. [Google Scholar] [CrossRef]
- Barayev, O.; Hawley, C.E.; Wellman, H.; Gerlovin, H.; Hsu, W.; Paik, J.M.; Mandel, E.I.; Liu, C.K.; Djoussé, L.; Gaziano, J.M.; et al. Statins, Mortality, and Major Adverse Cardiovascular Events Among US Veterans With Chronic Kidney Disease. JAMA Netw. Open 2023, 6, e2346373. [Google Scholar] [CrossRef]
- Hasan, I.H.; Shaheen, S.Y.; Alhusaini, A.M.; Mahmoud, A.M. Simvastatin mitigates diabetic nephropathy by upregulating farnesoid X receptor and Nrf2/HO-1 signaling and attenuating oxidative stress and inflammation in rats. Life Sci. 2024, 340, 122445. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, Y.; Cai, R.; Gao, J.; Xu, Q.; Zhang, L.; Kang, M.; Jia, H.; Chen, Q.; Liu, Y.; et al. Atorvastatin ameliorates diabetic nephropathy through inhibiting oxidative stress and ferroptosis signaling. Eur. J. Pharmacol. 2024, 976, 176699. [Google Scholar] [CrossRef]
- Tang, C.; Deng, X.; Qu, J.; Miao, Y.; Tian, L.; Zhang, M.; Li, X.; Sun, B.; Chen, L. Fenofibrate Attenuates Renal Tubular Cell Apoptosis by Up-Regulating MCAD in Diabetic Kidney Disease. Drug Des. Dev. Ther. 2023, ume 17, 1503–1514. [Google Scholar] [CrossRef]
- Islam, N.; Griffin, T.P.; Sander, E.; Rocks, S.; Qazi, J.; Cabral, J.; McCaul, J.; McMorrow, T.; Griffin, M.D. Human mesenchymal stromal cells broadly modulate high glucose-induced inflammatory responses of renal proximal tubular cell monolayers. Stem Cell Res. Ther. 2019, 10, 329. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Su, R.; Zhen, J.; Liu, X.; Liu, G. Small extracellular vesicles-shuttled miR-23a-3p from mesenchymal stem cells alleviate renal fibrosis and inflammation by inhibiting KLF3/STAT3 axis in diabetic kidney disease. Int. Immunopharmacol. 2024, 139, 112667. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.-L.; Huang, L.-L.; Yang, J.; Hou, Y.-Y.; Bai, Y.-H. Inhibition of SLC3A2 Deletion-Mediated Ferroptosis by Bone Marrow Stromal Cells to Alleviate Inflammation and Fibrosis in Diabetic Kidney Disease. Inflammation 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Lee, S.E.; Jang, J.E.; Kim, H.S.; Jung, M.K.; Ko, M.S.; Kim, M.-O.; Park, H.S.; Oh, W.; Choi, S.J.; Jin, H.J.; et al. Mesenchymal stem cells prevent the progression of diabetic nephropathy by improving mitochondrial function in tubular epithelial cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Konari, N.; Nagaishi, K.; Kikuchi, S.; Fujimiya, M. Mitochondria transfer from mesenchymal stem cells structurally and functionally repairs renal proximal tubular epithelial cells in diabetic nephropathy in vivo. Sci. Rep. 2019, 9, 5184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zheng, S.; Wu, J.; He, J.; Ouyang, Y.; Ao, C.; Lang, R.; Jiang, Y.; Yang, Y.; Xiao, H.; et al. Human umbilical cord mesenchymal stem cell-derived exosomes ameliorate renal fibrosis in diabetic nephropathy by targeting Hedgehog/SMO signaling. FASEB J. 2024, 38, e23599. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. Ther. 2019, 10, 95. [Google Scholar] [CrossRef]
- Jung, H.R.; Lee, J.; Hong, S.P.; Shin, N.; Cho, A.; Shin, D.J.; Choi, J.W.; Kim, J.I.; Lee, J.P.; Cho, S.Y. Targeting the m6A RNA methyltransferase METTL3 attenuates the development of kidney fibrosis. Exp. Mol. Med. 2024, 56, 355–369. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, J.; Xu, A.; Huang, J.; Zhang, H.; Xie, G.; Zhong, K.; Wu, Y.; Ye, P.; Wang, H.; et al. N6-methyladenosine triggers renal fibrosis via enhancing translation and stability of ZEB2 mRNA. J. Biol. Chem. 2024, 300, 107598. [Google Scholar] [CrossRef]
- Qu, P.; Li, L.; Jin, Q.; Liu, D.; Qiao, Y.; Zhang, Y.; Sun, Q.; Ran, S.; Li, Z.; Liu, T.; et al. Histone methylation modification and diabetic kidney disease: Potential molecular mechanisms and therapeutic approaches (Review). Int. J. Mol. Med. 2024, 54, 104. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, Z.; Li, H.; Fan, J.; Yang, S.; Chen, C.; Wang, D.W. MiR-30c protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition in db/db mice. Aging Cell 2017, 16, 387–400. [Google Scholar] [CrossRef]
- Huang, Y.; Tong, J.; He, F.; Yu, X.; Fan, L.; Hu, J.; Tan, J.; Chen, Z. miR-141 regulates TGF-β1-induced epithelial-mesenchymal transition through repression of HIPK2 expression in renal tubular epithelial cells. Int. J. Mol. Med. 2015, 35, 311–318. [Google Scholar] [CrossRef]
- Cheng, L.; Qiu, X.; He, L.; Liu, L. MicroRNA-122-5p ameliorates tubular injury in diabetic nephropathy via FIH-1/HIF-1α pathway. Ren. Fail. 2022, 44, 293–303. [Google Scholar] [CrossRef]
- Rathan, V.P.; Bhuvaneshwari, K.; Adit, G.N.; Kavyashree, S.; Thulasi, N.; Geetha, A.; Milan, K.; Ramkumar, K. Therapeutic potential of SMAD7 targeting miRNA in the pathogenesis of diabetic nephropathy. Arch. Biochem. Biophys. 2025, 764, 110265. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kulkarni, A. Metformin: Past, Present, and Future. Curr. Diab. Rep. 2024, 24, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Yi, Y.; Kwon, E.J.; Yun, G.; Park, S.; Jeong, J.C.; Na, K.Y.; Chin, H.J.; Yoo, S.; Kim, S.; Oh, T.J.; et al. Impact of metformin on cardiovascular and kidney outcome based on kidney function status in type 2 diabetic patients: A multicentric, retrospective cohort study. Sci. Rep. 2024, 14, 2081. [Google Scholar] [CrossRef]
- Agur, T.; Steinmetz, T.; Goldman, S.; Zingerman, B.; Bielopolski, D.; Nesher, E.; Fattal, I.; Meisel, E.; Rozen-Zvi, B. The impact of metformin on kidney disease progression and mortality in diabetic patients using SGLT2 inhibitors: A real-world cohort study. Cardiovasc. Diabetol. 2025, 24, 97. [Google Scholar] [CrossRef]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Shen, S.; Wang, X.; Dong, L.; Li, Q.; Ren, W.; Li, Y.; Bai, J.; Gong, Q.; et al. Safety and effectiveness of metformin plus lifestyle intervention compared with lifestyle intervention alone in preventing progression to diabetes in a Chinese population with impaired glucose regulation: A multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2023, 11, 567–577. [Google Scholar] [CrossRef]
- Richy, F.F.; Sabidó-Espin, M.; Guedes, S.; Corvino, F.A.; Gottwald-Hostalek, U. Incidence of lactic acidosis in patients with type 2 diabetes with and without renal impairment treated with metformin: A retrospective cohort study. Diabetes Care 2014, 37, 2291–2295. [Google Scholar] [CrossRef]
- Yen, F.-S.; Hwu, C.-M.; Liu, J.-S.; Wu, Y.-L.; Chong, K.; Hsu, C.-C. Sodium–Glucose Cotransporter-2 Inhibitors and the Risk for Dialysis and Cardiovascular Disease in Patients With Stage 5 Chronic Kidney Disease. Ann. Intern. Med. 2024, 177, 693–700. [Google Scholar] [CrossRef]
- Pan, H.C.; Chen, J.Y.; Chen, H.Y.; Yeh, F.Y.; Huang, T.T.; Sun, C.Y.; Wang, S.I.; Wei, J.C.; Wu, V.C. Sodium-Glucose Cotransport Protein 2 Inhibitors in Patients With Type 2 Diabetes and Acute Kidney Disease. JAMA Netw. Open 2024, 7, e2350050. [Google Scholar] [CrossRef]
- Nuffield Department of Population Health Renal Studies Group. SGLT2 Inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.; Bakris, G.; Baeres, F.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Parving, H.-H.; Andress, D.L.; Correa-Rotter, R.; Hou, F.-F.; Kitzman, D.W.; Kohan, D.; Makino, H.; McMurray, J.J.V.; Melnick, J.Z.; et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 1937–1947. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Iqbal, N.; Ambery, P.; Logue, J.; Mallappa, A.; Sjöström, C.D. Perspectives in weight control in diabetes—SGLT2 inhibitors and GLP-1–glucagon dual agonism. Diabetes Res. Clin. Pract. 2023, 199, 110669. [Google Scholar] [CrossRef]
- Ashfaq, A.; Meineck, M.; Pautz, A.; Arioglu-Inan, E.; Weinmann-Menke, J.; Michel, M.C. A systematic review on renal effects of SGLT2 inhibitors in rodent models of diabetic nephropathy. Pharmacol. Ther. 2023, 249, 108503. [Google Scholar] [CrossRef]
- Huang, B.; Yen, C.L.; Wu, C.Y.; Tsai, C.Y.; Chen, J.J.; Hsiao, C.C.; Chen, Y.C.; Hsieh, I.C.; Yang, H.Y. SGLT2 inhibitors reduce the risk of renal failure in CKD stage 5 patients with Type 2 DM. Sci. Rep. 2025, 15, 5872. [Google Scholar]
- Maigret, L.; Basle, L.; Chatelet, V.; Ecotiere, L.; Perrin, P.; Golbin, L.; Bertrand, D.; Anglicheau, D.; Poulain, C.; Garrouste, C.; et al. Sodium-Glucose Cotransporter-2 Inhibitor in Diabetic and Nondiabetic Renal Transplant Recipients. Kidney Int. Rep. 2024, 10, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Cui, W.; Liu, R.; Wang, S.; Ke, H.; Lei, X.; Chen, L. Structural mechanism of SGLT1 inhibitors. Nat. Commun. 2022, 13, 6440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, N.; Zhou, H. SGLT1: A Potential Drug Target for Cardiovascular Disease. Drug Des. Dev. Ther. 2023, ume 17, 2011–2023. [Google Scholar] [CrossRef]
- Pitt, B.; Bhatt, D.L. Does SGLT1 Inhibition Add Benefit to SGLT2 Inhibition in Type 2 Diabetes? Circulation 2021, 144, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.-W.; Zhao, L.-J.; Sun, J.-F. Synthesis and clinical application of representative small-molecule dipeptidyl Peptidase-4 (DPP-4) inhibitors for the treatment of type 2 diabetes mellitus (T2DM). Eur. J. Med. Chem. 2024, 272, 116464. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Byun, J.; Yoon, D.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Lee, K.-W.; Park, R.W.; Kim, H.J. Renal Protective Effect of DPP-4 Inhibitors in Type 2 Diabetes Mellitus Patients: A Cohort Study. J. Diabetes Res. 2016, 2016, 1423191. [Google Scholar] [CrossRef]
- Samaan, E.; Ramadan, N.M.; Abdulaziz, H.M.M.; Ibrahim, D.; El-Sherbiny, M.; ElBayar, R.; Ghattas, Y.; Abdlmalek, J.; Bayali, O.; Elhusseini, Y.; et al. DPP-4i versus SGLT2i as modulators of PHD3/HIF-2α pathway in the diabetic kidney. Biomed. Pharmacother. 2023, 167, 115629. [Google Scholar] [CrossRef]
- Bu, T.; Sun, Z.; Pan, Y.; Deng, X.; Yuan, G. Glucagon-Like Peptide-1: New Regulator in Lipid Metabolism. Diabetes Metab. J. 2024, 48, 354–372. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, A.; Li, D.; Wu, Y.; Wang, C.-Z.; Wan, J.-Y.; Yuan, C.-S. Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: Systematic review and network meta-analysis. BMJ 2024, 384, e076410. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Simms-Williams, N.; Treves, N.; Yin, H.; Lu, S.; Yu, O.; Pradhan, R.; Renoux, C.; Suissa, S.; Azoulay, L. Effect of combination treatment with glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors on incidence of cardiovascular and serious renal events: Population based cohort study. BMJ 2024, 385, e078242. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L.; Pollock, D.M. Endothelin System in Hypertension and Chronic Kidney Disease. Hypertension 2024, 81, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Kohan, D.E.; Barton, M. Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int. 2014, 86, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.M.; Badve, S.V.; Heerspink, H.J.L.; Wong, M.G. Endothelin receptor antagonists in kidney protection for diabetic kidney disease and beyond? Nephrology 2023, 28, 97–108. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Shao, H.; Li, X.-X.; Yu, F.; Xu, M. Inhibition of CPU0213, a Dual Endothelin Receptor Antagonist, on Apoptosis via Nox4-Dependent ROS in HK-2 Cells. Cell. Physiol. Biochem. 2016, 39, 183–192. [Google Scholar] [CrossRef]
- Rovin, B.H.; Barratt, J.; Heerspink, H.J.L.; Alpers, C.E.; Bieler, S.; Chae, D.-W.; Diva, U.A.; Floege, J.; Gesualdo, L.; Inrig, J.K.; et al. Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-year results from a randomised, active-controlled, phase 3 trial. Lancet 2023, 402, 2077–2090. [Google Scholar] [CrossRef]
- Moedt, E.; Wasehuus, V.S.; Heerspink, H.J.L. Selective endothelin A receptor antagonism in chronic kidney disease: Improving clinical application. Nephrol. Dial. Transplant. 2025, 40 (Suppl. S1), i37–i46. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, S.; Hou, J.; Chen, G.; Xu, Z.-G. Endothelin receptor antagonists for the treatment of diabetic nephropathy: A meta-analysis and systematic review. World J. Diabetes 2020, 11, 553–566. [Google Scholar] [CrossRef]
- Savarese, G.; Lindberg, F.; Filippatos, G.; Butler, J.; Anker, S.D. Mineralocorticoid receptor overactivation: Targeting systemic impact with non-steroidal mineralocorticoid receptor antagonists. Diabetologia 2024, 67, 246–262. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney disease—Mechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar] [CrossRef]
- Pandey, A.K.; Bhatt, D.L.; Cosentino, F.; Marx, N.; Rotstein, O.; Pitt, B.; Pandey, A.; Butler, J.; Verma, S. Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Eur. Heart J. 2022, 43, 2931–2945. [Google Scholar] [CrossRef] [PubMed]
- Hanouneh, M.; Le, D.; Jaar, B.G.; Tamargo, C.; Cervantes, C.E. Real-Life Experience on the Effect of SGLT2 Inhibitors vs. Finerenone vs. Combination on Albuminuria in Chronic Kidney Disease. Diagnostics 2024, 14, 1357. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Oliveira, A.C.; Vasques-Novoa, F.; Leite, A.R.; Mendonça, L.; Zannad, F.; Butler, J.; Leite-Moreira, A.; Saraiva, F.; Neves, J.S. Mineralocorticoid Receptor Antagonist Combined with SGLT2 Inhibitor versus SGLT2 Inhibitor Alone in Chronic Kidney Disease: A Meta-Analysis of Randomized Trials. Am. J. Nephrol. 2025, 56, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, Q.; Ma, L.; Fu, P. The Role of Cholesterol Homeostasis in Diabetic Kidney Disease. Curr. Med. Chem. 2021, 28, 7413–7426. [Google Scholar] [CrossRef]
- Han, Y.-Z.; Du, B.-X.; Zhu, X.-Y.; Wang, Y.-Z.; Zheng, H.-J.; Liu, W.-J. Lipid metabolism disorder in diabetic kidney disease. Front. Endocrinol. 2024, 15, 1336402. [Google Scholar] [CrossRef]
- Wu, L.; Liu, C.; Chang, D.-Y.; Zhan, R.; Zhao, M.; Lam, S.M.; Shui, G.; Zhao, M.-H.; Zheng, L.; Chen, M. The Attenuation of Diabetic Nephropathy by Annexin A1 via Regulation of Lipid Metabolism through AMPK/PPARα/CPT1b Pathway. Diabetes 2021, 70, 2192–2203. [Google Scholar] [CrossRef]
- Gu, W.; Wang, X.; Zhao, H.; Geng, J.; Li, X.; Zheng, K.; Guan, Y.; Hou, X.; Wang, C.; Song, G. Resveratrol ameliorates diabetic kidney injury by reducing lipotoxicity and modulates expression of components of the junctional adhesion molecule-like/sirtuin 1 lipid metabolism pathway. Eur. J. Pharmacol. 2022, 918, 174776. [Google Scholar] [CrossRef]
- Li, M.; Zhu, H.-Y.; Zhao, S.-Y.; Li, X.-D.; Tong, S.-M.; Ma, J.; Xu, A.-J.; Zhang, J. Baicalin alleviates lipid metabolism disorders in diabetic kidney disease via targeting FKBP51. Phytomedicine 2025, 139, 156473. [Google Scholar] [CrossRef]
- Luo, M.; Hu, Z.; Yang, J.; Yang, J.; Sheng, W.; Lin, C.; Li, D.; He, Q. Diosgenin Improves Lipid Metabolism in Diabetic Nephropathy via Regulation of miR-148b-3p/DNMT1/FOXO1 Axis. Nephron 2025, 149, 226–239. [Google Scholar] [CrossRef]
- Huang, T.-S.; Wu, T.; Wu, Y.-D.; Li, X.-H.; Tan, J.; Shen, C.-H.; Xiong, S.-J.; Feng, Z.-Q.; Gao, S.-F.; Li, H.; et al. Long-term statins administration exacerbates diabetic nephropathy via ectopic fat deposition in diabetic mice. Nat. Commun. 2023, 14, 390. [Google Scholar] [CrossRef]
- Hickson, L.J.; Abedalqader, T.; Ben-Bernard, G.; Mondy, J.M.; Bian, X.; Conley, S.M.; Zhu, X.; Herrmann, S.M.; Kukla, A.; Lorenz, E.C.; et al. A systematic review and meta-analysis of cell-based interventions in experimental diabetic kidney disease. STEM CELLS Transl. Med. 2021, 10, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, C. Mesenchymal Stem Cell Therapy: Therapeutic Opportunities and Challenges for Diabetic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 10540. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Remuzzi, G.; Griffin, M.D.; Cockwell, P.; Maxwell, A.P.; Casiraghi, F.; Rubis, N.; Peracchi, T.; Villa, A.; Todeschini, M.; et al. Safety and Preliminary Efficacy of Mesenchymal Stromal Cell (ORBCEL-M) Therapy in Diabetic Kidney Disease: A Randomized Clinical Trial (NEPHSTROM). J. Am. Soc. Nephrol. 2023, 34, 1733–1751. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.; Carta, G.; Pereira, D.d.C.; Gupta, R.; Murphy, C.; Feifel, E.; Kern, G.; Lechner, J.; Cavallo, A.L.; Gupta, S.; et al. Generation and characterization of iPSC-derived renal proximal tubule-like cells with extended stability. Sci. Rep. 2021, 11, 11575. [Google Scholar] [CrossRef]
- Kato, M.; Natarajan, R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nat. Rev. Nephrol. 2019, 15, 327–345. [Google Scholar] [CrossRef]
- Smyth, L.J.; Dahlström, E.H.; Syreeni, A.; Kerr, K.; Kilner, J.; Doyle, R.; Brennan, E.; Nair, V.; Fermin, D.; Nelson, R.G.; et al. Epigenome-wide meta-analysis identifies DNA methylation biomarkers associated with diabetic kidney disease. Nat. Commun. 2022, 13, 7891. [Google Scholar] [CrossRef]
| Therapeutic Class | Drug Example | Protective Mechanisms | References |
|---|---|---|---|
| Hypoglycemic Agents | Metformin | Activate mitophagy, reduce mitochondrial damage and ROS generation (AMPK-PINK1-Parkin pathway), and inhibit oxidative stress and apoptosis (AMPK and mTOR pathway) | [132,133,134] |
| SGLT2 Inhibitors (e.g., empagliflozin, dapagliflozin, canagliflozin) | Reduce glucose reabsorption, suppress inflammation (TNFR1/IL-6/MMP7), inhibit oxidative stress (AGE-RAGE-ROS), modulate metabolism (PKM2), protect mitochondria (Prdx3), inhibit ferroptosis (AMPK/NRF2), and counteract senescence (HHIP suppression) | [135,136,137,138,139,140,141] | |
| DPP-4 Inhibitors (e.g., sitagliptin, vildagliptin, alogliptin) | Inhibit DPP-4 activity, suppress inflammation (AGE-DPP4 axis), inhibit EMT (TGFBR), attenuate fibrosis (TGF-β1 signaling), and reduce apoptosis (PI3K/AKT, NF-κB) | [142,143,144,145] | |
| GLP-1R Agonists (e.g., exenatide, semaglutide, liraglutide) | Enhance insulin secretion, inhibit oxidative stress, reduce inflammation, improve mitochondrial function, inhibit ferroptosis (AMPK pathway), and regulate lipid metabolism | [146,147,148,149,150,151] | |
| Hypotensive Drugs | RAS Inhibitors (e.g., ACEi, ARBs like irbesartan, losartan, valsartan, olmesartan) | Inhibit NF-κB, reduce inflammation and fibrosis, activate Nrf2/Keap1, suppress NLRP3 inflammasome, remodel mitochondria, and modulate AGE-RAGE-TGFβ-PI3K pathways | [152,153,154,155,156] |
| Endothelin Receptor Antagonists (e.g., atrasentan) | Block ET-1 signaling, reduce oxidative stress (NADPH oxidase inhibition), suppress EMT (TGF-β-SMAD), and protect mitochondria (UCP2, SOD2 upregulation) | [157,158,159] | |
| Mineralocorticoid Receptor Antagonists (e.g., finerenone) | Inhibit mTOR/S6K1, activate PI3K/Akt/eNOS, improve mitophagy, reduce oxidative stress and fibrosis, and counteract senescence (RXRα/MR pathway) | [118,160,161,162,163] | |
| Lipid-Modulating Drugs | Statins (e.g., simvastatin, atorvastatin), Fenofibrate | Reduce oxidative stress, inflammation, and ferroptosis; modulate lipid metabolism via AMPK/FOXA2/MCAD pathways | [164,165,166,167] |
| Stem Cell Therapy | Mesenchymal Stem Cells (MSCs) | Reduce inflammation, fibrosis, and apoptosis, transfer mitochondria, restore autophagy, and modulate KLF3/STAT3 pathways | [168,169,170,171,172,173,174] |
| Gene Therapy | METTL3 Inhibitors, miRNAs (e.g., miR-30c, miR-141, miR-122-5p) | Modify epigenetic regulation (m6A methylation), inhibit EMT/fibrosis, restore SMAD7, and suppress TGF-β1 pathways | [175,176,177,178,179,180,181] |
| Drugs | Clinical Trials | Study Design | Number of Patients | Primary Outcomes | Secondary Outcomes | Reference |
|---|---|---|---|---|---|---|
| Empagliflozin | EMPA-KIDNEY study | Randomized, double-blind, placebo-controlled trial in CKD patients with or without diabetes; empagliflozin 10 mg daily; median follow-up 2.0 years. | 6609 | 28% reduction in progression of kidney disease or CV death (HR 0.72, p < 0.001). | Lower all-cause hospitalization; no significant effect on CV death or HF hospitalization. | [192] |
| Dapagliflozin | DAPA-CKD study | Randomized, placebo-controlled trial in CKD patients with or without T2D; dapagliflozin 10 mg daily; median follow-up 2.4 years. | 4304 | 39% reduction in sustained GFR decline, kidney failure, or renal/CV death (HR 0.61, p < 0.001). | 29% reduction in CV death or HF hospitalization; 31% reduction in all-cause mortality. | [193] |
| Liraglutide | LEADER study | Randomized, double-blind trial in T2D with high CV risk; liraglutide 1.8 mg (or the maximum tolerated dose) daily; median follow-up 3.8 years. | 9340 | 13% reduction in CV death, nonfatal MI, or stroke (HR 0.87, p = 0.01). | 22% reduction in CV death; 15% reduction in all-cause mortality; fewer MI and stroke. | [194] |
| Semaglutide | FLOW study | Multicenter, randomized, double-blind, placebo-controlled trial in patients with T2D and CKD; semaglutide 1.0 mg weekly; median follow-up 3.4 years. | 3533 | 24% reduction in major kidney disease events (HR 0.76, p = 0.0003). | Slower eGFR decline; 18% reduction in MACE; 20% reduction in all-cause mortality; fewer serious adverse events. | [195] |
| Atrasentan | SONAR study | Double-blind, placebo-controlled trial in CKD with T2D; atrasentan 0.75 mg daily; median follow-up 2.2 years. | 2648 | 35% reduction in doubling serum creatinine or kidney failure (HR 0.65, p = 0.0047). | No significant difference in CV death or HF hospitalization. | [196] |
| Finerenone | FIDELIO-DKD study | Randomized, double-blind trial in CKD with T2D; maximum dose on the manufacturer’s label; median follow-up 2.6 years. | 5734 | 18% reduction in kidney failure, GFR decline, or renal death (HR 0.82, p = 0.001). | 14% reduction in CV events (HR 0.86, p = 0.03); higher discontinuation due to hyperkalemia. | [197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, J.; Ma, S.; Tang, H.; Zhang, C. Pathogenesis and Therapeutic Perspectives of Tubular Injury in Diabetic Kidney Disease: An Update. Biomedicines 2025, 13, 1424. https://doi.org/10.3390/biomedicines13061424
Geng J, Ma S, Tang H, Zhang C. Pathogenesis and Therapeutic Perspectives of Tubular Injury in Diabetic Kidney Disease: An Update. Biomedicines. 2025; 13(6):1424. https://doi.org/10.3390/biomedicines13061424
Chicago/Turabian StyleGeng, Jiamian, Sijia Ma, Hui Tang, and Chun Zhang. 2025. "Pathogenesis and Therapeutic Perspectives of Tubular Injury in Diabetic Kidney Disease: An Update" Biomedicines 13, no. 6: 1424. https://doi.org/10.3390/biomedicines13061424
APA StyleGeng, J., Ma, S., Tang, H., & Zhang, C. (2025). Pathogenesis and Therapeutic Perspectives of Tubular Injury in Diabetic Kidney Disease: An Update. Biomedicines, 13(6), 1424. https://doi.org/10.3390/biomedicines13061424






