Unraveling the Roles of Macrophages in Vascularized Composite Allotransplantation
Abstract
1. Introduction
2. Overview of Leukocyte Differentiation and Function
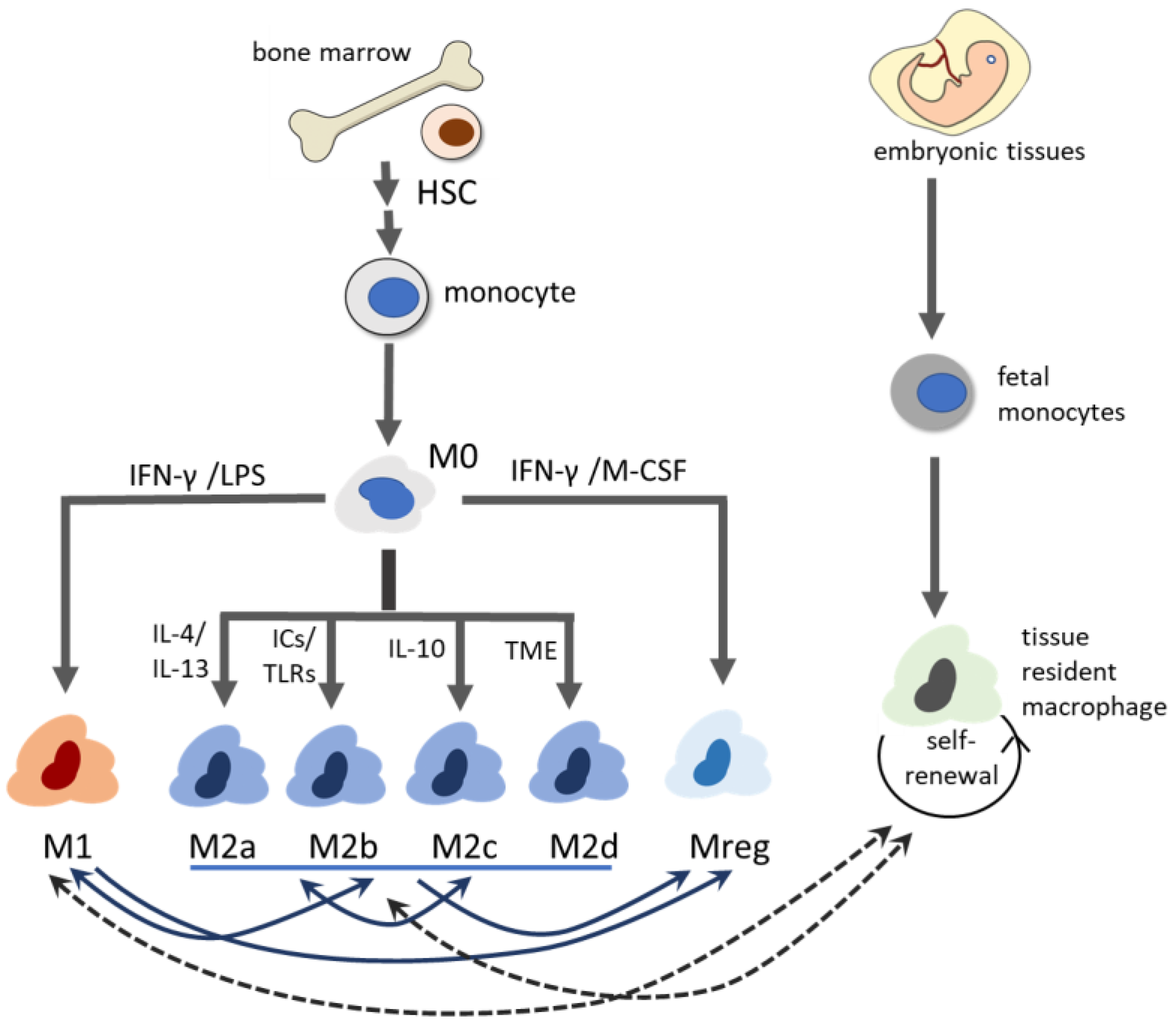
3. Heterogeneity of Macrophages
3.1. M1 Macrophage
3.2. M2 Macrophage
3.3. Regulatory Macrophage
3.4. Tissue-Resident Macrophage (TRM)
3.5. Emerging Insights into Macrophage Heterogeneity During Transplantation Rejection
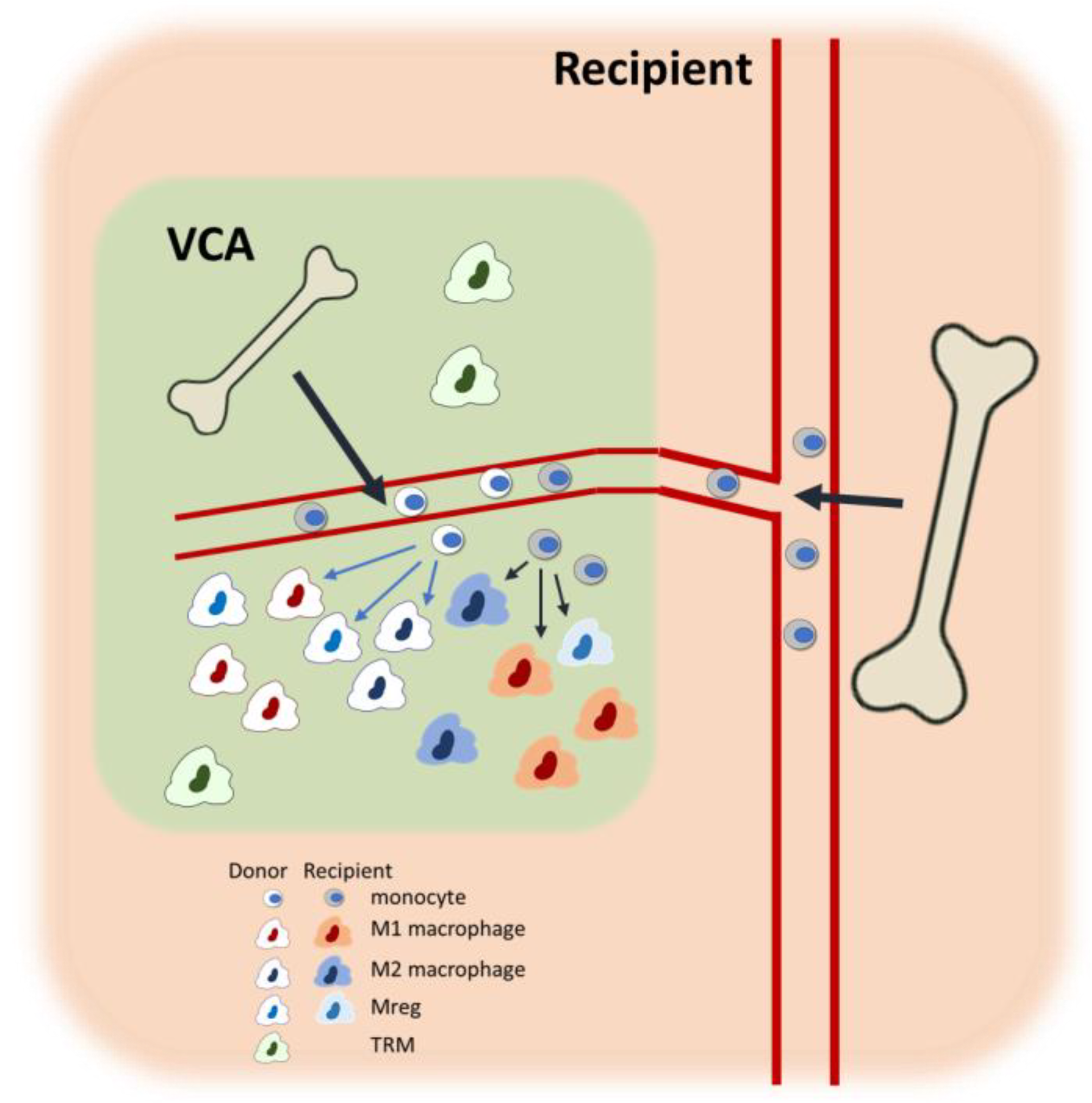
4. Current Understanding of Macrophages in VCA
4.1. Overview of VCA Immunobiology
4.2. Macrophages in Ischemia-Reperfusion Injury
4.3. Macrophages in Acute Rejection of VCA
4.4. Macrophages in Chronic Rejection of VCA
4.5. Macrophages to Promote VCA Graft Survival
5. Discussion and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VCA | Vascularized composite allotransplantation |
| IRI | Ischemia–reperfusion injury |
| AR | Acute rejection |
| CR | Chronic rejection |
| HSC | Hematopoietic stem cell |
| NK | Natural killer cell |
| DC | Dendritic cell |
| ROS | Reactive oxygen species |
| NET | Neutrophil extracellular trap |
| M-CSF | Macrophage colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| Mreg | Regulatory macrophage |
| TRM | Tissue-resident macrophage |
| PRR | Pattern recognition receptor |
| DAMP | Danger-associated molecular pattern |
| IFN-γ | Interferon γ |
| LPS | Lipopolysaccharide |
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| MHC | Major histocompatibility complex |
| Th1 | T helper 1 |
| TGF-β | Transforming growth factor-β |
| TLR | Toll-like receptor |
| scRNAseq | Single-cell RNA sequencing |
| Treg | Regulatory T cell |
| TCMR | T cell-mediated rejection |
| POD | Postoperative day |
| CRP | C-reactive protein |
| ECM | Extracellular matrix |
| POD | Postoperative day |
| IDO | Indoleamine 2,3-dioxygenase |
| TAIC | Transplant acceptance-inducing cell |
| SPIO | superparamagnetic iron nanoparticle |
References
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Priceman, S.J.; Sung, J.L.; Shaposhnik, Z.; Burton, J.B.; Torres-Collado, A.X.; Moughon, D.L.; Johnson, M.; Lusis, A.J.; Cohen, D.A.; Iruela-Arispe, M.L.; et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: Combating tumor evasion of antiangiogenic therapy. Blood 2010, 115, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Reed, E.F. The divergent roles of macrophages in solid organ transplantation. Curr. Opin. Organ Transplant. 2015, 20, 446–453. [Google Scholar] [CrossRef]
- Lackner, K.; Ebner, S.; Watschinger, K.; Maglione, M. Multiple Shades of Gray-Macrophages in Acute Allograft Rejection. Int. J. Mol. Sci. 2023, 24, 8257. [Google Scholar] [CrossRef]
- Fischer, S.; Lian, C.G.; Kueckelhaus, M.; Strom, T.B.; Edelman, E.R.; Clark, R.A.; Murphy, G.F.; Chandraker, A.K.; Riella, L.V.; Tullius, S.G.; et al. Acute rejection in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2014, 19, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Knoedler, L.; Knoedler, S.; Panayi, A.C.; Lee, C.A.A.; Sadigh, S.; Huelsboemer, L.; Stoegner, V.A.; Schroeter, A.; Kern, B.; Mookerjee, V.; et al. Cellular activation pathways and interaction networks in vascularized composite allotransplantation. Front. Immunol. 2023, 14, 1179355. [Google Scholar] [CrossRef]
- Tchiloemba, B.; Kauke, M.; Haug, V.; Abdulrazzak, O.; Safi, A.F.; Kollar, B.; Pomahac, B. Long-term Outcomes After Facial Allotransplantation: Systematic Review of the Literature. Transplantation 2021, 105, 1869–1880. [Google Scholar] [CrossRef]
- Etra, J.W.; Shores, J.T.; Sander, I.B.; Brandacher, G.; Lee, W.P.A. Trauma-induced Rejection in Vascularized Composite Allotransplantation. Ann. Surg. 2020, 271, e113–e114. [Google Scholar] [CrossRef]
- Kollar, B.; Rizzo, N.M.; Borges, T.J.; Haug, V.; Abdulrazzak, O.; Kauke, M.; Safi, A.F.; Lian, C.G.; Marty, F.M.; Rutherford, A.E.; et al. Accelerated chronic skin changes without allograft vasculopathy: A 10-year outcome report after face transplantation. Surgery 2020, 167, 991–998. [Google Scholar] [CrossRef]
- Kaufman, C.L.; Kanitakis, J.; Weissenbacher, A.; Brandacher, G.; Mehra, M.R.; Amer, H.; Zelger, B.G.; Zelger, B.; Pomahac, B.; McDiarmid, S.; et al. Defining chronic rejection in vascularized composite allotransplantation-The American Society of Reconstructive Transplantation and International Society of Vascularized Composite Allotransplantation chronic rejection working group: 2018 American Society of Reconstructive Transplantation meeting report and white paper Research goals in defining chronic rejection in vascularized composite allotransplantation. SAGE Open Med. 2020, 8, 2050312120940421. [Google Scholar] [CrossRef]
- Unadkat, J.V.; Bourbeau, D.; Afrooz, P.N.; Solari, M.G.; Washington, K.M.; Pulikkottil, B.J.; Weber, D.J.; Lee, W.P. Functional outcomes following multiple acute rejections in experimental vascularized composite allotransplantation. Plast. Reconstr. Surg. 2013, 131, 720e–730e. [Google Scholar] [CrossRef] [PubMed]
- Puscz, F.; Dadras, M.; Dermietzel, A.; Jacobsen, F.; Lehnhardt, M.; Behr, B.; Hirsch, T.; Kueckelhaus, M. A chronic rejection model and potential biomarkers for vascularized composite allotransplantation. PLoS ONE 2020, 15, e0235266. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Guo, Y.; Feng, C.; Hou, Y.; Xie, X.; Zhao, Y. The Dual Regulatory Roles of Macrophages in Acute Allogeneic Organ Graft Rejection. Engineering 2022, 10, 21–29. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or adaptive immunity? The example of natural killer cells. Science 2011, 331, 44–49. [Google Scholar] [CrossRef]
- Florez-Sampedro, L.; Song, S.; Melgert, B.N. The diversity of myeloid immune cells shaping wound repair and fibrosis in the lung. Regeneration 2018, 5, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Perobelli, S.M.; Galvani, R.G.; Gonçalves-Silva, T.; Xavier, C.R.; Nóbrega, A.; Bonomo, A. Plasticity of neutrophils reveals modulatory capacity. Braz. J. Med. Biol. Res. 2015, 48, 665–675. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, X.; Daha, M.R.; van Kooten, C. Reversible differentiation of pro- and anti-inflammatory macrophages. Mol. Immunol. 2013, 53, 179–186. [Google Scholar] [CrossRef]
- Valledor, A.F.; Borràs, F.E.; Cullell-Young, M.; Celada, A. Transcription factors that regulate monocyte/macrophage differentiation. J. Leukoc. Biol. 1998, 63, 405–417. [Google Scholar] [CrossRef]
- Kurotaki, D.; Osato, N.; Nishiyama, A.; Yamamoto, M.; Ban, T.; Sato, H.; Nakabayashi, J.; Umehara, M.; Miyake, N.; Matsumoto, N.; et al. Essential role of the IRF8-KLF4 transcription factor cascade in murine monocyte differentiation. Blood 2013, 121, 1839–1849. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, X.; Fang, J.; Li, Y.; Fan, M. Macrophages polarization is mediated by the combination of PRR ligands and distinct inflammatory cytokines. Int. J. Clin. Exp. Pathol. 2015, 8, 10964–10974. [Google Scholar]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Deng, Z.; Shi, F.; Zhou, Z.; Sun, F.; Sun, M.H.; Sun, Q.; Chen, L.; Li, D.; Jiang, C.Y.; Zhao, R.Z.; et al. M1 macrophage mediated increased reactive oxygen species (ROS) influence wound healing via the MAPK signaling in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 366, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.S.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Panzer, S.E. Macrophages in Transplantation: A Matter of Plasticity, Polarization, and Diversity. Transplantation 2022, 106, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Karim, M.R.; Kuramochi, M.; Izawa, T.; Kuwamura, M.; Yamate, J. Appearance of Heterogeneous Macrophages During Development of Isoproterenol-Induced Rat Myocardial Fibrosis. Toxicol. Pathol. 2021, 49, 1048–1061. [Google Scholar] [CrossRef] [PubMed]
- Toki, D.; Zhang, W.; Hor, K.L.; Liuwantara, D.; Alexander, S.I.; Yi, Z.; Sharma, R.; Chapman, J.R.; Nankivell, B.J.; Murphy, B.; et al. The role of macrophages in the development of human renal allograft fibrosis in the first year after transplantation. Am. J. Transplant. 2014, 14, 2126–2136. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Jiang, H.; Pan, J.; Huang, X.R.; Wang, Y.C.; Huang, H.F.; To, K.F.; Nikolic-Paterson, D.J.; Lan, H.Y.; Chen, J.H. Macrophage-to-Myofibroblast Transition Contributes to Interstitial Fibrosis in Chronic Renal Allograft Injury. J. Am. Soc. Nephrol. 2017, 28, 2053–2067. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Duan, Z.; Kang, J.; Chen, K.; Li, G.; Weng, C.; Zhang, D.; Zhang, L.; Wang, J.; et al. The effects of the M2a macrophage-induced axonal regeneration of neurons by arginase 1. Biosci. Rep. 2020, 40, BSR20193031. [Google Scholar] [CrossRef]
- Kiseleva, V.; Vishnyakova, P.; Elchaninov, A.; Fatkhudinov, T.; Sukhikh, G. Biochemical and molecular inducers and modulators of M2 macrophage polarization in clinical perspective. Int. Immunopharmacol. 2023, 122, 110583. [Google Scholar] [CrossRef]
- Yue, Y.; Huang, S.; Wang, L.; Wu, Z.; Liang, M.; Li, H.; Lv, L.; Li, W.; Wu, Z. M2b Macrophages Regulate Cardiac Fibroblast Activation and Alleviate Cardiac Fibrosis After Reperfusion Injury. Circ. J. 2020, 84, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, C.J.; Pinhal-Enfield, G.; Elson, G.; Cronstein, B.N.; Hasko, G.; Outram, S.; Leibovich, S.J. The Adenosine-Dependent Angiogenic Switch of Macrophages to an M2-Like Phenotype is Independent of Interleukin-4 Receptor Alpha (IL-4Rα) Signaling. Inflammation 2013, 36, 921–931. [Google Scholar] [CrossRef]
- Anders, C.B.; Lawton, T.M.; Smith, H.L.; Garret, J.; Doucette, M.M.; Ammons, M.C.B. Use of integrated metabolomics, transcriptomics, and signal protein profile to characterize the effector function and associated metabotype of polarized macrophage phenotypes. J. Leukoc. Biol. 2021, 111, 667–693. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, R.; Roth-Walter, F.; Ohradanova-Repic, A.; Flicker, S.; Hufnagl, K.; Fischer, M.B.; Stockinger, H.; Jensen-Jarolim, E. IgG4 drives M2a macrophages to a regulatory M2b-like phenotype: Potential implication in immune tolerance. Allergy 2019, 74, 483–494. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Diskin, C.; Pålsson-McDermott, E.M. Metabolic Modulation in Macrophage Effector Function. Front. Immunol. 2018, 9, 270. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Q.; Yang, T.; Ding, W.; Zhao, Y. Cellular metabolism and macrophage functional polarization. Int. Rev. Immunol. 2015, 34, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.E.; García, A.J. Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies. Acta Biomater. 2021, 133, 4–16. [Google Scholar] [CrossRef]
- Chen, S.; Wang, K.; Fan, Z.; Zhou, T.; Li, R.; Zhang, B.; Chen, J.; Chi, J.; Wei, K.; Liu, J.; et al. Modulation of anti-cardiac fibrosis immune responses by changing M2 macrophages into M1 macrophages. Mol. Med. 2024, 30, 88. [Google Scholar] [CrossRef]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513. [Google Scholar] [CrossRef]
- Stöger, J.L.; Gijbels, M.J.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.L.; Daemen, M.J.A.P.; Lutgens, E.; de Winther, M.P.J. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Oshi, M.; Tokumaru, Y.; Asaoka, M.; Yan, L.; Satyananda, V.; Matsuyama, R.; Matsuhashi, N.; Futamura, M.; Ishikawa, T.; Yoshida, K.; et al. M1 Macrophage and M1/M2 ratio defined by transcriptomic signatures resemble only part of their conventional clinical characteristics in breast cancer. Sci. Rep. 2020, 10, 16554. [Google Scholar] [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int. 2021, 21, 389. [Google Scholar] [CrossRef]
- Edwards, J.P.; Zhang, X.; Frauwirth, K.A.; Mosser, D.M. Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 2006, 80, 1298–1307. [Google Scholar] [CrossRef]
- Hutchinson, J.A.; Riquelme, P.; Brem-Exner, B.G.; Schulze, M.; Matthäi, M.; Renders, L.; Kunzendorf, U.; Geissler, E.K.; Fändrich, F. Transplant acceptance-inducing cells as an immune-conditioning therapy in renal transplantation. Transpl. Int. 2008, 21, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, J.; Cao, P.; Sun, Z.; Wang, W. The characteristics of regulatory macrophages and their roles in transplantation. Int. Immunopharmacol. 2021, 91, 107322. [Google Scholar] [CrossRef]
- Navarro-Barriuso, J.; Mansilla, M.J.; Martínez-Cáceres, E.M. Searching for the Transcriptomic Signature of Immune Tolerance Induction-Biomarkers of Safety and Functionality for Tolerogenic Dendritic Cells and Regulatory Macrophages. Front. Immunol. 2018, 9, 2062. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Zhuang, Q.; Peng, B.; Zhu, Y.; Ye, Q.; Ming, Y. The Evolving Roles of Macrophages in Organ Transplantation. J. Immunol. Res. 2019, 2019, 5763430. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, P.; Tomiuk, S.; Kammler, A.; Fändrich, F.; Schlitt, H.J.; Geissler, E.K.; Hutchinson, J.A. IFN-γ-induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Mol. Ther. 2013, 21, 409–422. [Google Scholar] [CrossRef]
- Carretero-Iglesia, L.; Bouchet-Delbos, L.; Louvet, C.; Drujont, L.; Segovia, M.; Merieau, E.; Chiffoleau, E.; Josien, R.; Hill, M.; Cuturi, M.C.; et al. Comparative Study of the Immunoregulatory Capacity of In Vitro Generated Tolerogenic Dendritic Cells, Suppressor Macrophages, and Myeloid-Derived Suppressor Cells. Transplantation 2016, 100, 2079–2089. [Google Scholar] [CrossRef]
- Hutchinson, J.A.; Riquelme, P.; Sawitzki, B.; Tomiuk, S.; Miqueu, P.; Zuhayra, M.; Oberg, H.H.; Pascher, A.; Lützen, U.; Janßen, U.; et al. Cutting Edge: Immunological Consequences and Trafficking of Human Regulatory Macrophages Administered to Renal Transplant Recipients. J. Immunol. 2011, 187, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.A.; Gövert, F.; Riquelme, P.; Bräsen, J.H.; Brem-Exner, B.G.; Matthäi, M.; Schulze, M.; Renders, L.; Kunzendorf, U.; Geissler, E.K.; et al. Administration of donor-derived transplant acceptance-inducing cells to the recipients of renal transplants from deceased donors is technically feasible. Clin. Transplant. 2009, 23, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Blériot, C.; Chakarov, S.; Ginhoux, F. Determinants of Resident Tissue Macrophage Identity and Function. Immunity 2020, 52, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Revelo, X.S.; Parthiban, P.; Chen, C.; Barrow, F.; Fredrickson, G.; Wang, H.; Yücel, D.; Herman, A.; van Berlo, J.H. Cardiac Resident Macrophages Prevent Fibrosis and Stimulate Angiogenesis. Circ. Res. 2021, 129, 1086–1101. [Google Scholar] [CrossRef]
- Louwe, P.A.; Badiola Gomez, L.; Webster, H.; Perona-Wright, G.; Bain, C.C.; Forbes, S.J.; Jenkins, S.J. Recruited macrophages that colonize the post-inflammatory peritoneal niche convert into functionally divergent resident cells. Nat. Commun. 2021, 12, 1770. [Google Scholar] [CrossRef]
- Gundra, U.M.; Girgis, N.M.; Gonzalez, M.A.; San Tang, M.; Van Der Zande, H.J.P.; Lin, J.-D.; Ouimet, M.; Ma, L.J.; Poles, J.; Vozhilla, N.; et al. Vitamin A mediates conversion of monocyte-derived macrophages into tissue-resident macrophages during alternative activation. Nat. Immunol. 2017, 18, 642–653. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Wu, X.; Xu, X.; Gong, J.; Chen, Y.; Gong, J. Rubicon promotes the M2 polarization of Kupffer cells via LC3-associated phagocytosis-mediated clearance to improve liver transplantation. Cell. Immunol. 2022, 378, 104556. [Google Scholar] [CrossRef]
- Kopecky, B.J.; Dun, H.; Amrute, J.M.; Lin, C.-Y.; Bredemeyer, A.L.; Terada, Y.; Bayguinov, P.O.; Koenig, A.L.; Frye, C.C.; Fitzpatrick, J.A.J.; et al. Donor Macrophages Modulate Rejection After Heart Transplantation. Circulation 2022, 146, 623–638. [Google Scholar] [CrossRef]
- Bos, S.; Hunter, B.; McDonald, D.; Merces, G.; Sheldon, G.; Pradère, P.; Majo, J.; Pulle, J.; Vanstapel, A.; Vanaudenaerde, B.M.; et al. High-dimensional tissue profiling of immune cell responses in chronic lung allograft dysfunction. J. Heart Lung Transplant. 2025, 44, 645–658. [Google Scholar] [CrossRef]
- Barbetta, A.; Rocque, B.; Bangerth, S.; Street, K.; Weaver, C.; Chopra, S.; Kim, J.; Sher, L.; Gaudilliere, B.; Akbari, O.; et al. Spatially resolved immune exhaustion within the alloreactive microenvironment predicts liver transplant rejection. Sci. Adv. 2024, 10, eadm8841. [Google Scholar] [CrossRef]
- Salem, F.; Perin, L.; Sedrakyan, S.; Angeletti, A.; Ghiggeri, G.M.; Coccia, M.C.; Ross, M.; Fribourg, M.; Cravedi, P. The spatially resolved transcriptional profile of acute T cell-mediated rejection in a kidney allograft. Kidney Int. 2022, 101, 131–136. [Google Scholar] [CrossRef]
- Kauke-Navarro, M.; Crisler, W.J.; Younis, N.; Khetani, R.S.; Sadigh, S.; Teague, J.E.; Ho Sui, S.J.; Ko, C.; Zhan, Q.; Steuart, S.; et al. B-cell infiltration distinguishes mucosal from skin patterns of rejection in facial vascularized composite allografts. Am. J. Transplant. 2025, 25, 1193–1207. [Google Scholar] [CrossRef] [PubMed]
- Travaglini, K.J.; Nabhan, A.N.; Penland, L.; Sinha, R.; Gillich, A.; Sit, R.V.; Chang, S.; Conley, S.D.; Mori, Y.; Seita, J.; et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020, 587, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, J.; Liu, D.; Zhou, S.; Liao, J.; Liao, G.; Yang, S.; Guo, Z.; Li, Y.; Li, S.; et al. Single-cell analysis reveals immune landscape in kidneys of patients with chronic transplant rejection. Theranostics 2020, 10, 8851–8862. [Google Scholar] [CrossRef]
- Abedini-Nassab, R.; Taheri, F.; Emamgholizadeh, A.; Naderi-Manesh, H. Single-Cell RNA Sequencing in Organ and Cell Transplantation. Biosensors 2024, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, J.; Zhang, Y.; Li, J.; Chen, M.; Gao, Y.; Dai, M.; Lin, S.; He, X.; Wu, C.; et al. Single-Cell RNA Sequencing Identifies Intra-Graft Population Heterogeneity in Acute Heart Allograft Rejection in Mouse. Front. Immunol. 2022, 13, 832573. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Chen, J.; Ma, L.; Huang, X.; Tang, S.C.W.; Lan, H.; Jiang, H.; Chen, J. Single-Cell RNA Sequencing Reveals the Immunological Profiles of Renal Allograft Rejection in Mice. Front. Immunol. 2021, 12, 693608. [Google Scholar] [CrossRef]
- Wang, J.; Luo, P.; Zhao, J.; Tan, J.; Huang, F.; Ma, R.; Huang, P.; Huang, M.; Huang, Y.; Wei, Q.; et al. Profiling the Resident and Infiltrating Monocyte/Macrophages during Rejection following Kidney Transplantation. J. Immunol. Res. 2020, 2020, 5746832. [Google Scholar] [CrossRef]
- Sun, J.A.; Adil, A.; Biniazan, F.; Haykal, S. Immunogenicity and tolerance induction in vascularized composite allotransplantation. Front. Transplant. 2024, 3, 1350546. [Google Scholar] [CrossRef]
- Messner, F.; Grahammer, J.; Hautz, T.; Brandacher, G.; Schneeberger, S. Ischemia/reperfusion injury in vascularized tissue allotransplantation: Tissue damage and clinical relevance. Curr. Opin. Organ Transplant. 2016, 21, 503–509. [Google Scholar] [CrossRef]
- Paradis, S.; Charles, A.L.; Meyer, A.; Lejay, A.; Scholey, J.W.; Chakfé, N.; Zoll, J.; Geny, B. Chronology of mitochondrial and cellular events during skeletal muscle ischemia-reperfusion. Am. J. Physiol. Cell Physiol. 2016, 310, C968–C982. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, F.; Okamoto, O.; Katagiri, K.; Fujiwara, S.; Wei, F.C. Prolonged ischemia increases severity of rejection in skin flap allotransplantation in rats. Microsurgery 2010, 30, 132–137. [Google Scholar] [CrossRef]
- Datta, N.; Devaney, S.G.; Busuttil, R.W.; Azari, K.; Kupiec-Weglinski, J.W. Prolonged Cold Ischemia Time Results in Local and Remote Organ Dysfunction in a Murine Model of Vascularized Composite Transplantation. Am. J. Transplant. 2017, 17, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, H.M.; Gauthier, J.M.; Terada, Y.; Li, W.; Krupnick, A.S.; Gelman, A.E.; Kreisel, D. Updated Views on Neutrophil Responses in Ischemia-Reperfusion Injury. Transplantation 2022, 106, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Etra, J.W.; Raimondi, G.; Brandacher, G. Mechanisms of rejection in vascular composite allotransplantation. Curr. Opin. Organ Transplant. 2018, 23, 28–33. [Google Scholar] [CrossRef]
- Weissenbacher, A.; Loupy, A.; Chandraker, A.; Schneeberger, S. Donor-specific antibodies and antibody-mediated rejection in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2016, 21, 510–515. [Google Scholar] [CrossRef]
- Ito, A.; Shimura, H.; Nitahara, A.; Tomiyama, K.; Ito, M.; Kanekura, T.; Okumura, K.; Yagita, H.; Kawai, K. NK cells contribute to the skin graft rejection promoted by CD4+ T cells activated through the indirect allorecognition pathway. Int. Immunol. 2008, 20, 1343–1349. [Google Scholar] [CrossRef]
- Kummer, L.; Zaradzki, M.; Vijayan, V.; Arif, R.; Weigand, M.A.; Immenschuh, S.; Wagner, A.H.; Larmann, J. Vascular Signaling in Allogenic Solid Organ Transplantation—The Role of Endothelial Cells. Front. Physiol. 2020, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Wang, Y.L.; Anggelia, M.R.; Chuang, W.Y.; Cheng, H.Y.; Mao, Q.; Zelken, J.A.; Lin, C.H.; Zheng, X.X.; Lee, W.P.; et al. Combined Anti-CD154/CTLA4Ig Costimulation Blockade-Based Therapy Induces Donor-Specific Tolerance to Vascularized Osteomyocutaneous Allografts. Am. J. Transplant. 2016, 16, 2030–2041. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Ghetu, N.; Huang, W.C.; Wang, Y.L.; Wallace, C.G.; Wen, C.J.; Chen, H.C.; Shih, L.Y.; Lin, C.F.; Hwang, S.M.; et al. Syngeneic adipose-derived stem cells with short-term immunosuppression induce vascularized composite allotransplantation tolerance in rats. Cytotherapy 2014, 16, 369–380. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Tay, S.K.L.; Wen, C.J.; Lin, C.F.; Wang, A.Y.; Shih, L.Y.; Liu, S.C.; Kobayashi, E.; Lin, C.H.; Wei, F.C. Bioimaging of alloantigen-stimulated regulatory T cells in rat vascularized composite allotransplantation. PLoS ONE 2018, 13, e0203624. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, R.; Taddeo, A.; Waldner, M.; Klein, H.J.; Fuchs, N.; Kamat, P.; Targosinski, S.; Barth, A.A.; Drach, M.C.; Gorantla, V.S.; et al. Adipose-derived stromal cell therapy combined with a short course nonmyeloablative conditioning promotes long-term graft tolerance in vascularized composite allotransplantation. Am. J. Transplant. 2020, 20, 1272–1284. [Google Scholar] [CrossRef]
- Lin, C.H.; Anggelia, M.R.; Cheng, H.Y.; Wang, A.Y.L.; Chuang, W.Y.; Lin, C.H.; Lee, W.P.A.; Wei, F.C.; Brandacher, G. The intragraft vascularized bone marrow component plays a critical role in tolerance induction after reconstructive transplantation. Cell. Mol. Immunol. 2021, 18, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Anggelia, M.R.; Cheng, H.Y.; Hsieh, Y.H.; Chuang, W.Y.; Yang, H.Y.; Lin, C.H. The intragraft vascularized bone marrow induces secondary donor-specific mystacial pad allograft tolerance. Front. Immunol. 2022, 13, 1059271. [Google Scholar] [CrossRef]
- Radu, C.A.; Horn, D.; Kiefer, J.; Rebel, M.; Gebhard, M.M.; Ryssel, H.; Köllensperger, E.; Fändrich, F.; Germann, G.; Kremer, T. Donor-derived transplant acceptance-inducing cells in composite tissue allotransplantation. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 1684–1691. [Google Scholar] [CrossRef]
- Datta, S.; Fitzpatrick, A.M.; Haykal, S. Preservation solutions for attenuation of ischemia-reperfusion injury in vascularized composite allotransplantation. SAGE Open Med. 2021, 9, 20503121211034924. [Google Scholar] [CrossRef]
- Friedman, O.; Carmel, N.; Sela, M.; Abu Jabal, A.; Inbal, A.; Ben Hamou, M.; Krelin, Y.; Gur, E.; Shani, N. Immunological and inflammatory mapping of vascularized composite allograft rejection processes in a rat model. PLoS ONE 2017, 12, e0181507. [Google Scholar] [CrossRef] [PubMed]
- Duru, Ç.; Biniazan, F.; Hadzimustafic, N.; D’Elia, A.; Shamoun, V.; Haykal, S. Review of machine perfusion studies in vascularized composite allotransplant preservation. Front. Transplant. 2023, 2, 1323387. [Google Scholar] [CrossRef]
- Bonastre, J.; Landín, L.; Bolado, P.; Casado-Sánchez, C.; López-Collazo, E.; Díez, J. Effect of Cold Preservation on Chronic Rejection in a Rat Hindlimb Transplantation Model. Plast. Reconstr. Surg. 2016, 138, 628–637. [Google Scholar] [CrossRef]
- Oh, J.Y.; Lee, H.J.; Ko, A.Y.; Ko, J.H.; Kim, M.K.; Wee, W.R. Analysis of macrophage phenotype in rejected corneal allografts. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7779–7784. [Google Scholar] [CrossRef]
- Shao, X. Roles of M1 and M2 macrophage infiltration in post-renal transplant antibody-mediated rejection. Transpl. Immunol. 2024, 85, 102076. [Google Scholar] [CrossRef] [PubMed]
- Hautz, T.; Zelger, B.; Brandacher, G.; Mueller, H.; Grahammer, J.; Zelger, B.; Lee, A.; Cavadas, P.; Margreiter, R.; Pratschke, J.; et al. Histopathologic characterization of mild rejection (grade I) in skin biopsies of human hand allografts. Transpl. Int. 2012, 25, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hautz, T.; Zelger, B.; Grahammer, J.; Krapf, C.; Amberger, A.; Brandacher, G.; Landin, L.; Müller, H.; Schön, M.P.; Cavadas, P.; et al. Molecular markers and targeted therapy of skin rejection in composite tissue allotransplantation. Am. J. Transplant. 2010, 10, 1200–1209. [Google Scholar] [CrossRef]
- Baran, W.; Koziol, M.; Woźniak, Z.; Banasik, M.; Boratyńska, M.; Kunze, A.; Schakel, K. Increased Numbers of 6-sulfo LacNAc (slan) Dendritic Cells in Hand Transplant Recipients. Ann. Transplant. 2015, 20, 649–654. [Google Scholar] [CrossRef]
- Kawada, J.I.; Takeuchi, S.; Imai, H.; Okumura, T.; Horiba, K.; Suzuki, T.; Torii, Y.; Yasuda, K.; Imanaka-Yoshida, K.; Ito, Y. Immune cell infiltration landscapes in pediatric acute myocarditis analyzed by CIBERSORT. J. Cardiol. 2021, 77, 174–178. [Google Scholar] [CrossRef]
- Zhang, L.; Arenas Hoyos, I.; Helmer, A.; Banz, Y.; Zubler, C.; Lese, I.; Hirsiger, S.; Constantinescu, M.; Rieben, R.; Gultom, M.; et al. Transcriptome profiling of immune rejection mechanisms in a porcine vascularized composite allotransplantation model. Front. Immunol. 2024, 15, 1390163. [Google Scholar] [CrossRef] [PubMed]
- Volanakis, J.E. Human C-reactive protein: Expression, structure, and function. Mol. Immunol. 2001, 38, 189–197. [Google Scholar] [CrossRef]
- Kiefer, J.; Zeller, J.; Schneider, L.; Thomé, J.; McFadyen, J.D.; Hoerbrand, I.A.; Lang, F.; Deiss, E.; Bogner, B.; Schaefer, A.L.; et al. C-reactive protein orchestrates acute allograft rejection in vascularized composite allotransplantation via selective activation of monocyte subsets. J. Adv. Res. 2025, 72, 401–420. [Google Scholar] [CrossRef]
- Kimmel, H.; Rahn, M.; Gilbert, T.W. The clinical effectiveness in wound healing with extracellular matrix derived from porcine urinary bladder matrix: A case series on severe chronic wounds. J. Am. Coll. Certif. Wound Spec. 2010, 2, 55–59. [Google Scholar] [CrossRef]
- Kruper, G.J.; VandeGriend, Z.P.; Lin, H.-S.; Zuliani, G.F. Salvage of Failed Local and Regional Flaps with Porcine Urinary Bladder Extracellular Matrix Aided Tissue Regeneration. Case Rep. Otolaryngol. 2013, 2013, 917183. [Google Scholar] [CrossRef]
- Sommerfeld, S.D.; Zhou, X.; Mejías, J.C.; Oh, B.C.; Maestas, D.R., Jr.; Furtmüller, G.J.; Laffont, P.A.; Elisseeff, J.H.; Brandacher, G. Biomaterials-based immunomodulation enhances survival of murine vascularized composite allografts. Biomater. Sci. 2023, 11, 4022–4031. [Google Scholar] [CrossRef] [PubMed]
- Grebe, S.O.; Kuhlmann, U.; Fogl, D.; Luyckx, V.A.; Mueller, T.F. Macrophage activation is associated with poorer long-term outcomes in renal transplant patients. Clin. Transplant. 2011, 25, 744–754. [Google Scholar] [CrossRef]
- Ng, Z.Y.; Lellouch, A.G.; Rosales, I.A.; Geoghegan, L.; Gama, A.R.; Colvin, R.B.; Lantieri, L.A.; Randolph, M.A.; Cetrulo, C.L., Jr. Graft vasculopathy of vascularized composite allografts in humans: A literature review and retrospective study. Transpl. Int. 2019, 32, 831–838. [Google Scholar] [CrossRef]
- Longo, B.; Pomahac, B.; Giacalone, M.; Cardillo, M.; Cervelli, V. 18 years of face transplantation: Adverse outcomes and challenges. J. Plast. Reconstr. Aesthetic Surg. 2023, 87, 187–199. [Google Scholar] [CrossRef]
- Unadkat, J.V.; Schneeberger, S.; Horibe, E.H.; Goldbach, C.; Solari, M.G.; Washington, K.M.; Gorantla, V.S.; Cooper, G.M.; Thomson, A.W.; Lee, W.P. Composite tissue vasculopathy and degeneration following multiple episodes of acute rejection in reconstructive transplantation. Am. J. Transplant. 2010, 10, 251–261. [Google Scholar] [CrossRef]
- Lee, C.A.A.; Wang, D.; Kauke-Navarro, M.; Russell-Goldman, E.; Xu, S.; Mucciarone, K.N.; Sohrabi, S.; Lian, C.G.; Pomahac, B.; Murphy, G.F. Insights from immunoproteomic profiling of a rejected full-face transplant. Am. J. Transplant. 2023, 23, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhao, Y.; Xiao, X.; Fan, Y.; Kloc, M.; Liu, W.; Ghobrial, R.M.; Lan, P.; He, X.; Li, X.C. Graft-Infiltrating Macrophages Adopt an M2 Phenotype and Are Inhibited by Purinergic Receptor P2X7 Antagonist in Chronic Rejection. Am. J. Transplant. 2016, 16, 2563–2573. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Li, W. M2 Macrophages Serve as Critical Executor of Innate Immunity in Chronic Allograft Rejection. Front. Immunol. 2021, 12, 648539. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, X.; Lu, J.; Li, Z.; Jia, H.; Chen, M.; Chang, Y.; Liu, Y.; Li, P.; Zhang, B.; et al. Mesenchymal stem cells transfected with sFgl2 inhibit the acute rejection of heart transplantation in mice by regulating macrophage activation. Stem Cell Res. Ther. 2020, 11, 241. [Google Scholar] [CrossRef]
- Li, Y.; Ding, X.; Tian, X.; Zheng, J.; Ding, C.; Li, X.; Hu, X.; Qiao, Y.; Wang, Y.; Xue, W. Islet transplantation modulates macrophage to induce immune tolerance and angiogenesis of islet tissue in type I diabetes mice model. Aging 2020, 12, 24023–24032. [Google Scholar] [CrossRef]
- Mfarrej, B.; Jofra, T.; Morsiani, C.; Gagliani, N.; Fousteri, G.; Battaglia, M. Key role of macrophages in tolerance induction via T regulatory type 1 (Tr1) cells. Clin. Exp. Immunol. 2020, 201, 222–230. [Google Scholar] [CrossRef]
- Haribhai, D.; Ziegelbauer, J.; Jia, S.; Upchurch, K.; Yan, K.; Schmitt, E.G.; Salzman, N.H.; Simpson, P.; Hessner, M.J.; Chatila, T.A.; et al. Alternatively Activated Macrophages Boost Induced Regulatory T and Th17 Cell Responses during Immunotherapy for Colitis. J. Immunol. 2016, 196, 3305–3317. [Google Scholar] [CrossRef]
- Warnecke, G.; Hutchinson, J.A.; Riquelme, P.; Kruse, B.; Thissen, S.; Avsar, M.; Zehle, G.; Steinkamp, T.; Peters, C.; Baumann, R.; et al. Postoperative intravenous infusion of donor-derived transplant acceptance-inducing cells as an adjunct immunosuppressive therapy in a porcine pulmonary allograft model. Transpl. Int. 2009, 22, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, P.; Haarer, J.; Kammler, A.; Walter, L.; Tomiuk, S.; Ahrens, N.; Wege, A.K.; Goecze, I.; Zecher, D.; Banas, B.; et al. TIGIT(+) iTregs elicited by human regulatory macrophages control T cell immunity. Nat. Commun. 2018, 9, 2858. [Google Scholar] [CrossRef] [PubMed]
- Nordham, K.D.; Ninokawa, S. The history of organ transplantation. Proceedings 2022, 35, 124–128. [Google Scholar] [CrossRef]
- Hariharan, S.; Rogers, N.; Naesens, M.; Pestana, J.M.; Ferreira, G.F.; Requião-Moura, L.R.; Foresto, R.D.; Kim, S.J.; Sullivan, K.; Helanterä, I.; et al. Long-term Kidney Transplant Survival Across the Globe. Transplantation 2024, 108, e254–e263. [Google Scholar] [CrossRef]
- Kaufman, C.L.; Ouseph, R.; Marvin, M.R.; Manon-Matos, Y.; Blair, B.; Kutz, J.E. Monitoring and long-term outcomes in vascularized composite allotransplantation. Curr. Opin. Organ Transplant. 2013, 18, 652–658. [Google Scholar] [CrossRef]
- Shores, J.T.; Malek, V.; Lee, W.P.A.; Brandacher, G. Outcomes after hand and upper extremity transplantation. J. Mater. Sci. Mater. Med. 2017, 28, 72. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.C.; Kopecky, B.J. Targeting Macrophages in Organ Transplantation: A Step Toward Personalized Medicine. Transplantation 2024, 108, 2045–2056. [Google Scholar] [CrossRef]
- Kannegieter, N.M.; Hesselink, D.A.; Dieterich, M.; Kraaijeveld, R.; Rowshani, A.T.; Leenen, P.J.; Baan, C.C. The Effect of Tacrolimus and Mycophenolic Acid on CD14+ Monocyte Activation and Function. PLoS ONE 2017, 12, e0170806. [Google Scholar] [CrossRef]
- van den Bosch, T.P.; Kannegieter, N.M.; Hesselink, D.A.; Baan, C.C.; Rowshani, A.T. Targeting the Monocyte-Macrophage Lineage in Solid Organ Transplantation. Front. Immunol. 2017, 8, 153. [Google Scholar] [CrossRef]
- Kannegieter, N.M.; Hesselink, D.A.; Dieterich, M.; de Graav, G.N.; Kraaijeveld, R.; Rowshani, A.T.; Leenen, P.J.M.; Baan, C.C. Pharmacodynamic Monitoring of Tacrolimus-Based Immunosuppression in CD14+ Monocytes After Kidney Transplantation. Ther. Drug Monit. 2017, 39, 463–471. [Google Scholar] [CrossRef]
- Dangi, A.; Natesh, N.R.; Husain, I.; Ji, Z.; Barisoni, L.; Kwun, J.; Shen, X.; Thorp, E.B.; Luo, X. Single cell transcriptomics of mouse kidney transplants reveals a myeloid cell pathway for transplant rejection. JCI Insight 2020, 5, e141321. [Google Scholar] [CrossRef] [PubMed]
- Messner, F.; Sardu, C.; Petruzzo, P. Grasping time—Longevity of vascularized composite allografts. Curr. Opin. Organ Transplant. 2024, 29, 376–381. [Google Scholar] [CrossRef]
- Ramirez, A.E.; Cheng, H.Y.; Lao, W.W.; Wang, Y.L.; Wen, C.J.; Wallace, C.G.; Lin, C.F.; Shih, L.Y.; Chuang, S.H.; Wei, F.C. A novel rat full-thickness hemi-abdominal wall/hindlimb osteomyocutaneous combined flap: Influence of allograft mass and vascularized bone marrow content on vascularized composite allograft survival. Transpl. Int. 2014, 27, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Malone, A.F.; Wu, H.; Fronick, C.; Fulton, R.; Gaut, J.P.; Humphreys, B.D. Harnessing Expressed Single Nucleotide Variation and Single Cell RNA Sequencing to Define Immune Cell Chimerism in the Rejecting Kidney Transplant. J. Am. Soc. Nephrol. 2020, 31, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.; Cannet, C.; Fringeli-Tanner, M.; Baumann, D.; Pally, C.; Bruns, C.; Zerwes, H.-G.; Andriambeloson, E.; Bigaud, M. Macrophage labeling by SPIO as an early marker of allograft chronic rejection in a rat model of kidney transplantation. Magn. Reson. Med. 2003, 49, 459–467. [Google Scholar] [CrossRef]
- Cho, H.; Kwon, H.-Y.; Lee, S.H.; Lee, H.-G.; Kang, N.-Y.; Chang, Y.-T. Development of a Fluorescent Probe for M2 Macrophages via Gating-Oriented Live-Cell Distinction. J. Am. Chem. Soc. 2023, 145, 2951–2957. [Google Scholar] [CrossRef]
- Al Faraj, A.; Shaik, A.S.; Afzal, S.; Al Sayed, B.; Halwani, R. MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int. J. Nanomed. 2014, 9, 1491–1503. [Google Scholar] [CrossRef]
- Filz von Reiterdank, I.; Dinicu, A.T.; Rosales, I.; Cetrulo, C.L., Jr.; Coert, J.H.; Mink van der Molen, A.B.; Uygun, K. Supercooling preservation of vascularized composite allografts through CPA optimization, thermal tracking, and stepwise loading techniques. Sci. Rep. 2024, 14, 22339. [Google Scholar] [CrossRef]
- Rostami, S.; Xu, M.; Datta, S.; Haykal, S. Evaluation of Early Markers of Ischemia-reperfusion Injury and Preservation Solutions in a Modified Hindlimb Model of Vascularized Composite Allotransplantation. Transplant. Direct 2022, 8, e1251. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Khan, U.Z.; Qing, L.; Wu, P.; Tang, J. Improving the ischemia-reperfusion injury in vascularized composite allotransplantation: Clinical experience and experimental implications. Front. Immunol. 2022, 13, 998952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Bailey, S.R.; Lei, B.; Paulos, C.M.; Atkinson, C.; Tomlinson, S. Targeted Complement Inhibition Protects Vascularized Composite Allografts From Acute Graft Injury and Prolongs Graft Survival When Combined with Subtherapeutic Cyclosporine A Therapy. Transplantation 2017, 101, e75–e85. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Huang, S.S.; Wei, F.C.; Hung, L.M. Resveratrol attenuates ischemia—Reperfusion-induced leukocyte—Endothelial cell adhesive interactions and prolongs allograft survival across the MHC barrier. Circ J 2007, 71, 423–428. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Anggelia, M.R.; Lin, C.H.; Wei, F.C. Toward transplantation tolerance with adipose tissue-derived therapeutics. Front. Immunol. 2023, 14, 1111813. [Google Scholar] [CrossRef]
- Ellis, B.W.; Traktuev, D.O.; Merfeld-Clauss, S.; Can, U.I.; Wang, M.; Bergeron, R.; Zorlutuna, P.; March, K.L. Adipose stem cell secretome markedly improves rodent heart and human induced pluripotent stem cell-derived cardiomyocyte recovery from cardioplegic transport solution exposure. Stem Cells 2021, 39, 170–182. [Google Scholar] [CrossRef]
- Cho, D.I.; Kim, M.R.; Jeong, H.Y.; Jeong, H.C.; Jeong, M.H.; Yoon, S.H.; Kim, Y.S.; Ahn, Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp. Mol. Med. 2014, 46, e70. [Google Scholar] [CrossRef]
- Garcia, M.R.; Ledgerwood, L.; Yang, Y.; Xu, J.; Lal, G.; Burrell, B.; Ma, G.; Hashimoto, D.; Li, Y.; Boros, P.; et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J. Clin. Invest. 2010, 120, 2486–2496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.-Y.; Anggelia, M.R.; Lin, C.-H. Unraveling the Roles of Macrophages in Vascularized Composite Allotransplantation. Biomedicines 2025, 13, 1425. https://doi.org/10.3390/biomedicines13061425
Cheng H-Y, Anggelia MR, Lin C-H. Unraveling the Roles of Macrophages in Vascularized Composite Allotransplantation. Biomedicines. 2025; 13(6):1425. https://doi.org/10.3390/biomedicines13061425
Chicago/Turabian StyleCheng, Hui-Yun, Madonna Rica Anggelia, and Cheng-Hung Lin. 2025. "Unraveling the Roles of Macrophages in Vascularized Composite Allotransplantation" Biomedicines 13, no. 6: 1425. https://doi.org/10.3390/biomedicines13061425
APA StyleCheng, H.-Y., Anggelia, M. R., & Lin, C.-H. (2025). Unraveling the Roles of Macrophages in Vascularized Composite Allotransplantation. Biomedicines, 13(6), 1425. https://doi.org/10.3390/biomedicines13061425





