Heterosynaptic Regulation of α2A-Adrenoceptors on Glutamate/GABA Release in the Prefrontal Cortex of Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Immunohistochemistry
2.3. Whole-Cell Patch-Clamp Recordings
2.4. Depletion of Catecholamine
2.5. Confocal Microscopy
2.6. Data Analysis and Statistics
3. Results
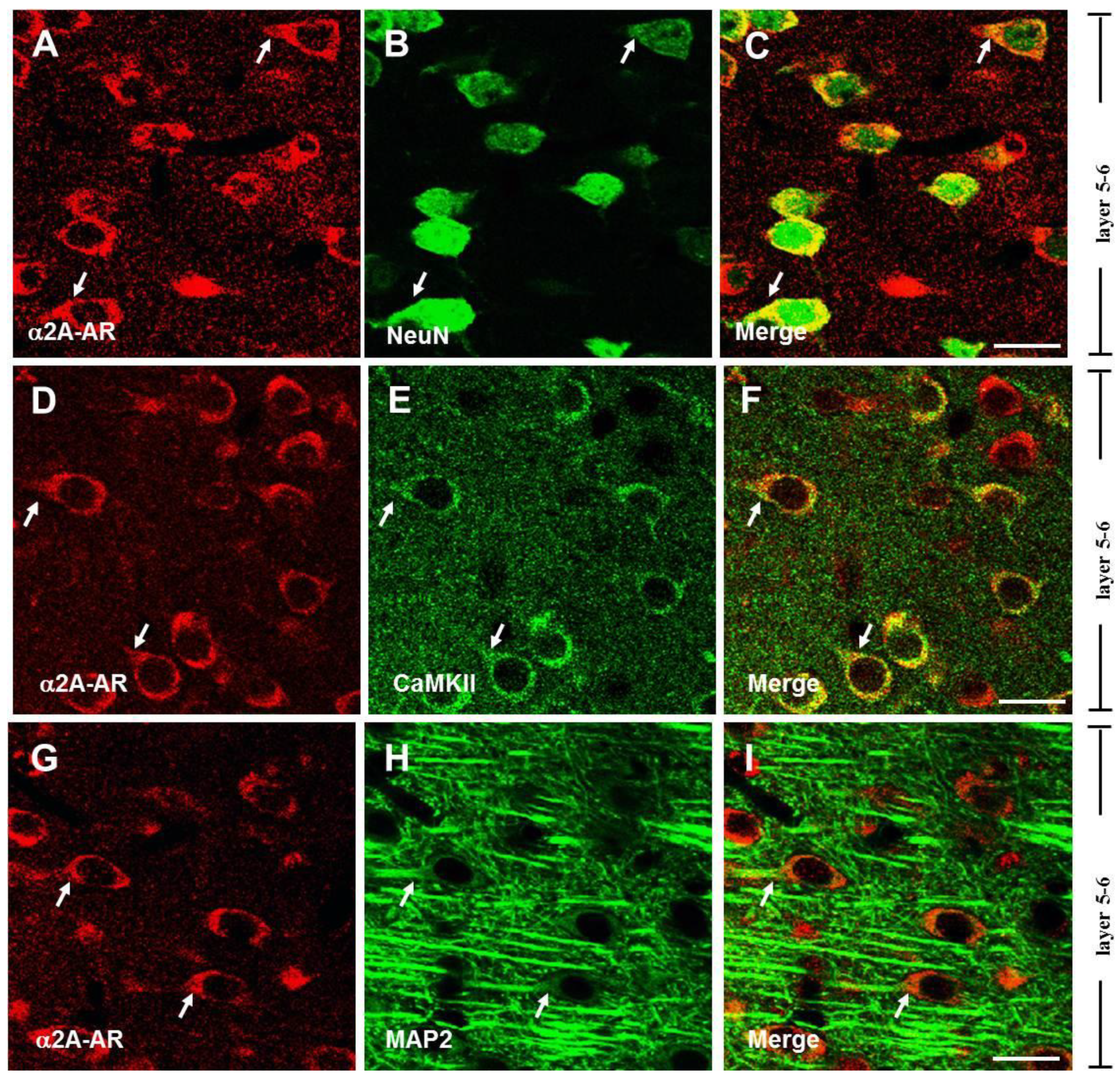
3.1. The α2A-ARs Are Expressed in the Pyramidal Neurons of PFC
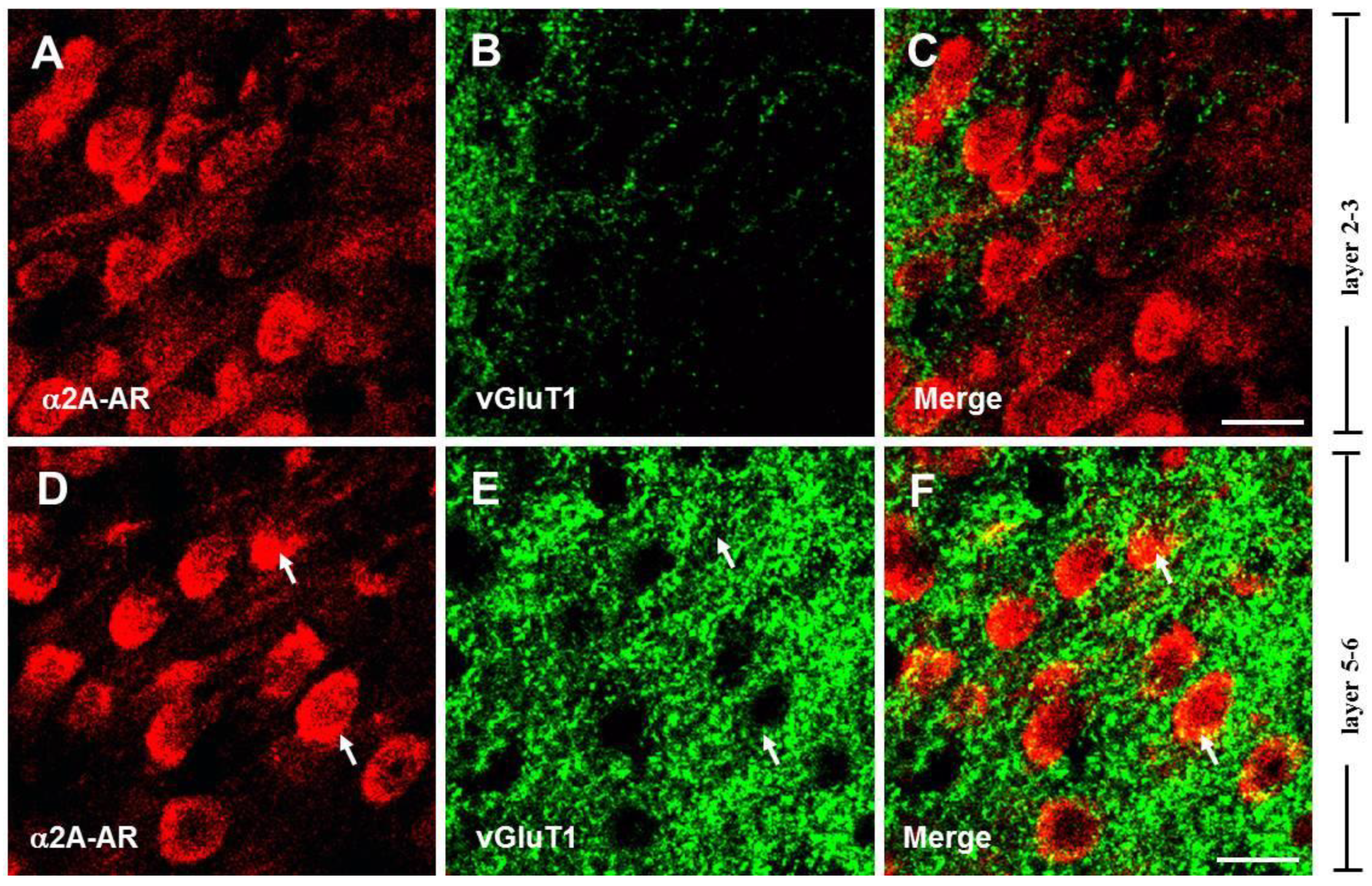
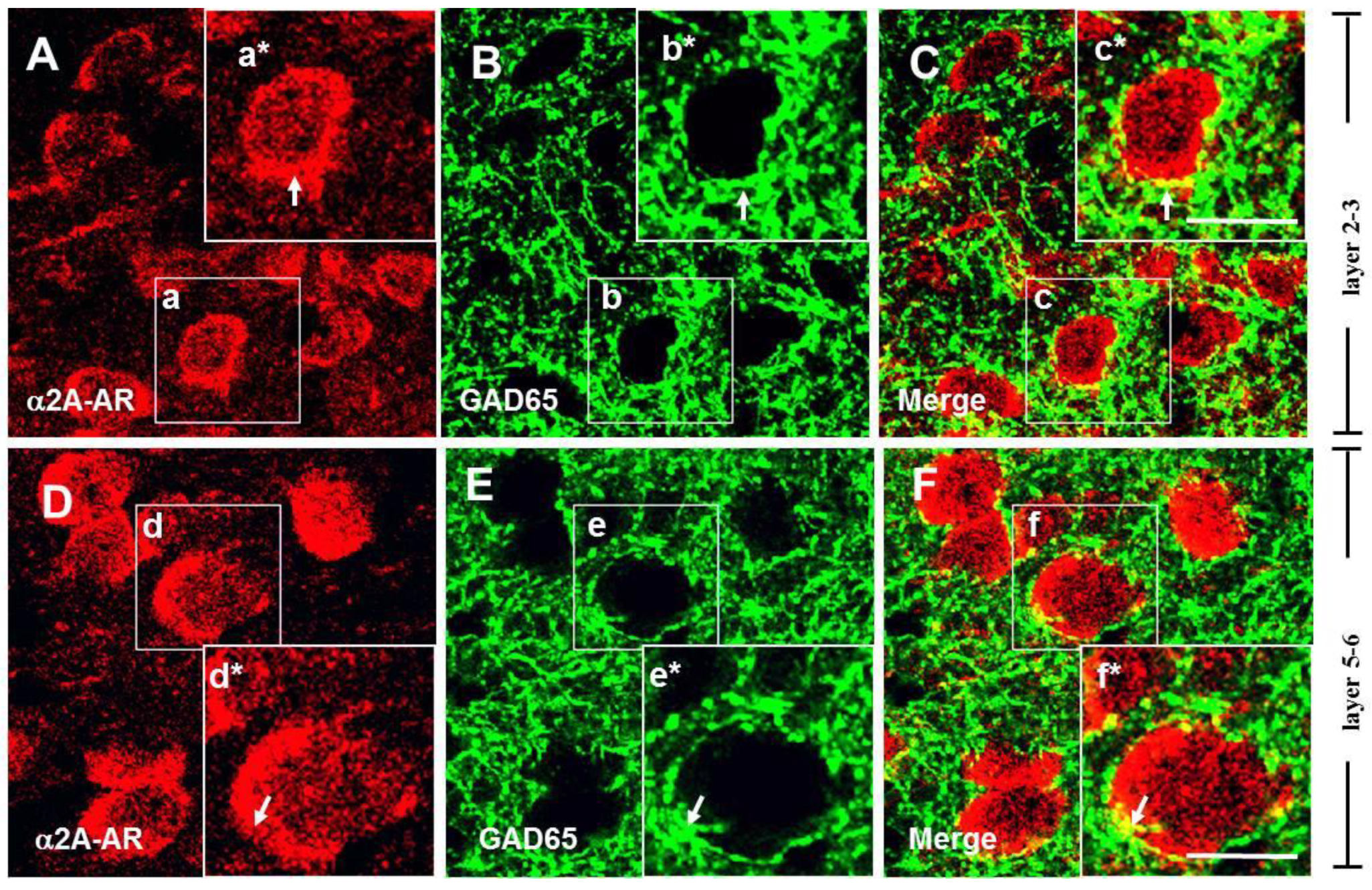
3.2. The α2A-ARs Are Located at the Glutamatergic and GABAergic Terminals in the PFC
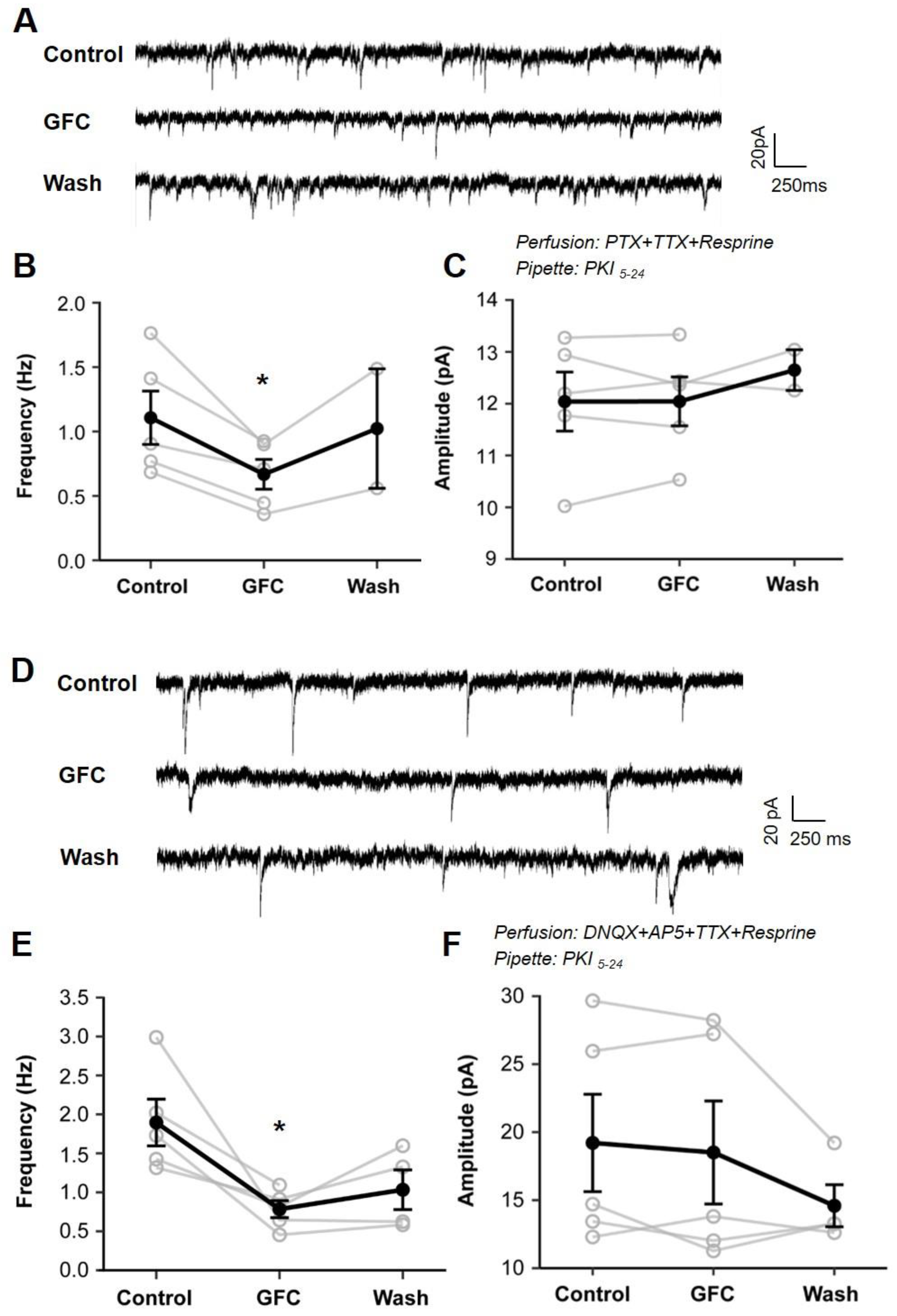
3.3. Stimulation of Presynaptic α2A-ARs Suppresses Glutamate/GABA Release in the PFC
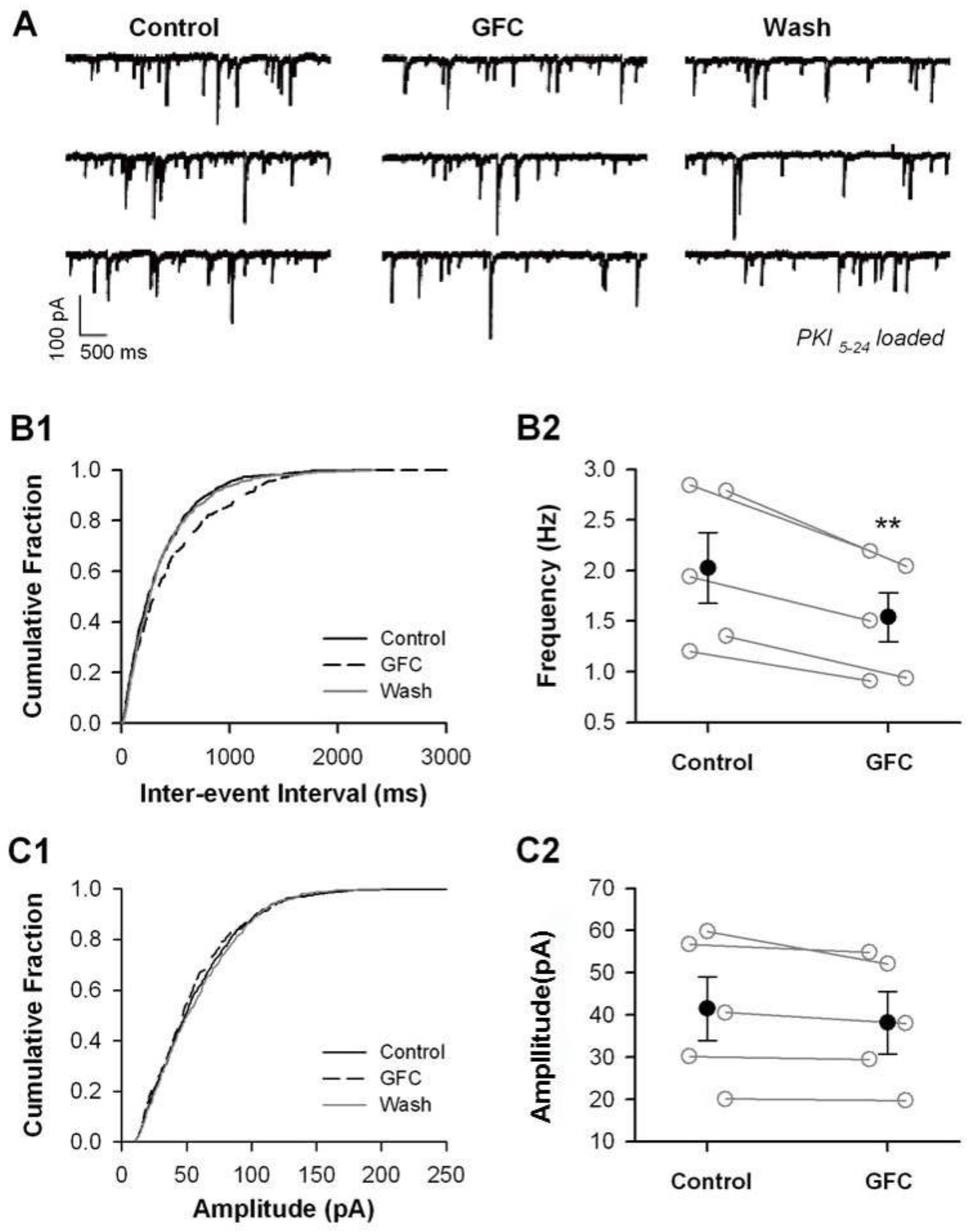
3.4. Suppression of Glutamate/GABA Release by Stimulating Presynaptic α2A-ARs at Non-NE Terminals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Funahashi, S.; Bruce, C.J.; Goldman-Rakic, P.S. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J. Neurophysiol. 1989, 61, 331–349. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Rakic, P.S. Cellular basis of working memory. Neuron 1995, 14, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Baddeley, A. Working memory: Looking back and looking forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef]
- Simons, J.S.; Spiers, H.J. Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 2003, 4, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal cortex and depression. Neuropsychopharmacology 2022, 47, 225–246. [Google Scholar] [CrossRef]
- Dias, R.; Robbins, T.W.; Roberts, A.C. Dissociable forms of inhibitory control within prefrontal cortex with an analog of the Wisconsin Card Sort Test: Restriction to novel situations and independence from “on-line” processing. J. Neurosci. 1997, 17, 9285–9297. [Google Scholar] [CrossRef]
- Kopp, B.; Tabeling, S.; Moschner, C.; Wessel, K. Cognitive functions of the prefrontal cortex: Neuroscience and clinic. Nervenarzt 2008, 79, 143–152. [Google Scholar] [CrossRef]
- Sakurai, T.; Gamo, N.J. Cognitive functions associated with developing prefrontal cortex during adolescence and developmental neuropsychiatric disorders. Neurobiol. Dis. 2019, 131, 104322. [Google Scholar] [CrossRef]
- LeDuke, D.O.; Borio, M.; Miranda, R.; Tye, K.M. Anxiety and depression: A top-down, bottom-up model of circuit function. Ann. N. Y. Acad. Sci. 2023, 1525, 70–87. [Google Scholar] [CrossRef]
- Rossetti, Z.L.; Carboni, S. Noradrenaline and dopamine elevations in the rat prefrontal cortex in spatial working memory. J. Neurosci. 2005, 25, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ramos, B.P.; Paspalas, C.D.; Shu, Y.; Simen, A.; Duque, A.; Vijayraghavan, S.; Brennan, A.; Dudley, A.; Nou, E.; et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 2007, 129, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Li, B.M.; Mao, Z.M.; Wang, M.; Mei, Z.T. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology 1999, 21, 601–610. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Goldman-Rakic, P.S. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science 1985, 230, 1273–1276. [Google Scholar] [CrossRef]
- Wang, C.C.; Shyu, B.C. Differential projections from the mediodorsal and centrolateral thalamic nuclei to the frontal cortex in rats. Brain Res. 2004, 995, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Rama, P.; Linnankoski, I.; Tanila, H.; Pertovaara, A.; Carlson, S. Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol. Biochem. Behav. 1996, 55, 415–422. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Li, B.M. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol. Psychiatry 2005, 57, 1377–1384. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Cai, J.X.; Goldman-Rakic, P.S. The alpha-2 adrenergic agonist guanfacine improves memory in aged monkeys without sedative or hypotensive side effects: Evidence for alpha-2 receptor subtypes. J. Neurosci. 1988, 8, 4287–4298. [Google Scholar] [CrossRef]
- Ma, C.L.; Arnsten, A.F.; Li, B.M. Locomotor hyperactivity induced by blockade of prefrontal cortical alpha2-adrenoceptors in monkeys. Biol. Psychiatry 2005, 57, 192–195. [Google Scholar] [CrossRef]
- Li, B.M.; Mei, Z.T. Delayed-response deficit induced by local injection of the alpha 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav. Neural Biol. 1994, 62, 134–139. [Google Scholar] [CrossRef]
- Franowicz, J.S.; Kessler, L.E.; Borja, C.M.; Kobilka, B.K.; Limbird, L.E.; Arnsten, A.F. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J. Neurosci. 2002, 22, 8771–8777. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.A.; Franowicz, J.S.; Studholme, C.; van Dyck, C.H.; Arnsten, A.F. The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychopharmacology 2000, 23, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Jakala, P.; Riekkinen, M.; Sirvio, J.; Koivisto, E.; Kejonen, K.; Vanhanen, M.; Riekkinen, P., Jr. Guanfacine, but not clonidine, improves planning and working memory performance in humans. Neuropsychopharmacology 1999, 20, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.; Venkatesan, C.; Go, C.G.; Forman, R.; Kurose, H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb. Cortex 1998, 8, 269–277. [Google Scholar] [CrossRef]
- Labarrera, C.; Deitcher, Y.; Dudai, A.; Weiner, B.; Kaduri Amichai, A.; Zylbermann, N.; London, M. Adrenergic Modulation Regulates the Dendritic Excitability of Layer 5 Pyramidal Neurons In Vivo. Cell Rep. 2018, 23, 1034–1044. [Google Scholar] [CrossRef]
- Barth, A.M.; Vizi, E.S.; Zelles, T.; Lendvai, B. Alpha2-adrenergic receptors modify dendritic spike generation via HCN channels in the prefrontal cortex. J. Neurophysiol. 2008, 99, 394–401. [Google Scholar] [CrossRef]
- Starke, K. Presynaptic autoreceptors in the third decade: Focus on alpha2-adrenoceptors. J. Neurochem. 2001, 78, 685–693. [Google Scholar] [CrossRef]
- Harsing, L.G., Jr.; Vizi, E.S. Evidence that two stereochemically different alpha-2 adrenoceptors modulate norepinephrine release in rat cerebral cortex. J. Pharmacol. Exp. Ther. 1991, 256, 44–49. [Google Scholar] [CrossRef]
- Yamauchi, M.; Imanishi, T.; Koyama, T. A combination of mirtazapine and milnacipran augments the extracellular levels of monoamines in the rat brain. Neuropharmacology 2012, 62, 2278–2287. [Google Scholar] [CrossRef]
- Mongeau, R.; Weiss, M.; de Montigny, C.; Blier, P. Effect of acute, short- and long-term milnacipran administration on rat locus coeruleus noradrenergic and dorsal raphe serotonergic neurons. Neuropharmacology 1998, 37, 905–918. [Google Scholar] [CrossRef]
- Jackisch, R.; Haaf, A.; Jeltsch, H.; Lazarus, C.; Kelche, C.; Cassel, J.C. Modulation of 5-hydroxytryptamine release in hippocampal slices of rats: Effects of fimbria-fornix lesions on 5-HT1B-autoreceptor and alpha2-heteroreceptor function. Brain Res. Bull. 1999, 48, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Lejeune, F.; Gobert, A. Reciprocal autoreceptor and heteroreceptor control of serotonergic, dopaminergic and noradrenergic transmission in the frontal cortex: Relevance to the actions of antidepressant agents. J. Psychopharmacol. 2000, 14, 114–138. [Google Scholar] [CrossRef] [PubMed]
- Forray, M.I.; Bustos, G.; Gysling, K. Noradrenaline inhibits glutamate release in the rat bed nucleus of the stria terminalis: In vivo microdialysis studies. J. Neurosci. Res. 1999, 55, 311–320. [Google Scholar] [CrossRef]
- Jimenez-Rivera, C.A.; Figueroa, J.; Vazquez-Torres, R.; Velez-Hernandez, M.E.; Schwarz, D.; Velasquez-Martinez, M.C.; Arencibia-Albite, F. Presynaptic inhibition of glutamate transmission by alpha2 receptors in the VTA. Eur. J. Neurosci. 2012, 35, 1406–1415. [Google Scholar] [CrossRef]
- Shields, A.D.; Wang, Q.; Winder, D.G. alpha2A-adrenergic receptors heterosynaptically regulate glutamatergic transmission in the bed nucleus of the stria terminalis. Neuroscience 2009, 163, 339–351. [Google Scholar] [CrossRef]
- Zha, D.J.; Wang, Z.M.; Lin, Y.; Liu, T.; Qiao, L.; Lu, L.J.; Li, Y.Q.; Qiu, J.H. Effects of noradrenaline on the GABA response in rat isolated spiral ganglion neurons in culture. J. Neurochem. 2007, 103, 57–66. [Google Scholar] [CrossRef]
- Philbin, K.E.; Bateman, R.J.; Mendelowitz, D. Clonidine, an alpha2-receptor agonist, diminishes GABAergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. 2010, 1347, 65–70. [Google Scholar] [CrossRef]
- Yi, F.; Liu, S.S.; Luo, F.; Zhang, X.H.; Li, B.M. Signaling mechanism underlying alpha2A -adrenergic suppression of excitatory synaptic transmission in the medial prefrontal cortex of rats. Eur. J. Neurosci. 2013, 38, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Egli, R.E.; Kash, T.L.; Choo, K.; Savchenko, V.; Matthews, R.T.; Blakely, R.D.; Winder, D.G. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 2005, 30, 657–668. [Google Scholar] [CrossRef]
- Hayar, A.; Guyenet, P.G. Alpha2-adrenoceptor-mediated presynaptic inhibition in bulbospinal neurons of rostral ventrolateral medulla. Am. J. Physiol. 1999, 277, H1069–H1080. [Google Scholar] [CrossRef]
- Hirono, M.; Nagao, S.; Obata, K. Developmental alpha(2)-adrenergic regulation of noradrenergic synaptic facilitation at cerebellar GABAergic synapses. Neuroscience 2014, 256, 242–251. [Google Scholar] [CrossRef] [PubMed]
- He, X.T.; Yu, J.; Li, B.M.; Zhang, X.H. The expression of α2A-adrenoceptors in the calcium-binding protein immunoreactive interneurons in rat prefrontal cortex. Sheng Li Xue Bao 2014, 66, 537–544. [Google Scholar] [PubMed]
- Milner, T.A.; Rosin, D.L.; Lee, A.; Aicher, S.A. Alpha2A-adrenergic receptors are primarily presynaptic heteroreceptors in the C1 area of the rat rostral ventrolateral medulla. Brain Res. 1999, 821, 200–211. [Google Scholar] [CrossRef]
- Milner, T.A.; Lee, A.; Aicher, S.A.; Rosin, D.L. Hippocampal alpha2a-adrenergic receptors are located predominantly presynaptically but are also found postsynaptically and in selective astrocytes. J. Comp. Neurol. 1998, 395, 310–327. [Google Scholar] [CrossRef]
- Cai, W.; Liu, S.S.; Li, B.M.; Zhang, X.H. Presynaptic HCN channels constrain GABAergic synaptic transmission in pyramidal cells of the medial prefrontal cortex. Biol. Open 2022, 11, bio058840. [Google Scholar] [CrossRef]
- Ji, X.H.; Ji, J.Z.; Zhang, H.; Li, B.M. Stimulation of alpha2-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology 2008, 33, 2263–2271. [Google Scholar] [CrossRef]
- Liu, X.B.; Murray, K.D. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: Location, location, location. Epilepsia 2012, 53 (Suppl. S1), 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, C.; Szábo, G.; Sun, Q.Q. Distribution of CaMKIIα expression in the brain in vivo, studied by CaMKIIα-GFP mice. Brain Res. 2013, 1518, 9–25. [Google Scholar] [CrossRef]
- Wolf, H.K.; Buslei, R.; Schmidt-Kastner, R.; Schmidt-Kastner, P.K.; Pietsch, T.; Wiestler, O.D.; Blumcke, I. NeuN: A useful neuronal marker for diagnostic histopathology. J. Histochem. Cytochem. 1996, 44, 1167–1171. [Google Scholar] [CrossRef]
- Muller, J.F.; Mascagni, F.; McDonald, A.J. Pyramidal cells of the rat basolateral amygdala: Synaptology and innervation by parvalbumin-immunoreactive interneurons. J. Comp. Neurol. 2006, 494, 635–650. [Google Scholar] [CrossRef]
- Noraberg, J.; Zimmer, J. Ethanol induces MAP2 changes in organotypic hippocampal slice cultures. Neuroreport 1998, 9, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- Chiu, K.M.; Lin, T.Y.; Lu, C.W.; Wang, S.J. Inhibitory effect of glutamate release from rat cerebrocortical nerve terminals by alpha2 adrenoceptor agonist dexmedetomidine. Eur. J. Pharmacol. 2011, 670, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Fujiyama, F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci. Res. 2002, 42, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Fremeau, R.T., Jr.; Voglmaier, S.; Seal, R.P.; Edwards, R.H. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004, 27, 98–103. [Google Scholar] [CrossRef]
- Nakamura, M.; Suk, K.; Lee, M.G.; Jang, I.S. alpha(2A) adrenoceptor-mediated presynaptic inhibition of GABAergic transmission in rat tuberomammillary nucleus neurons. J. Neurochem. 2013, 125, 832–842. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hashimoto, T.; Morris, H.M. Cell and receptor type-specific alterations in markers of GABA neurotransmission in the prefrontal cortex of subjects with schizophrenia. Neurotox. Res. 2008, 14, 237–248. [Google Scholar] [CrossRef]
- Duffy, S.N.; Nguyen, P.V. Postsynaptic application of a peptide inhibitor of cAMP-dependent protein kinase blocks expression of long-lasting synaptic potentiation in hippocampal neurons. J. Neurosci. 2003, 23, 1142–1150. [Google Scholar] [CrossRef]
- Altman, J.D.; Trendelenburg, A.U.; MacMillan, L.; Bernstein, D.; Limbird, L.; Starke, K.; Kobilka, B.K.; Hein, L. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol. Pharmacol. 1999, 56, 154–161. [Google Scholar] [CrossRef]
- Stewart, J. Pathways to relapse: The neurobiology of drug- and stress-induced relapse to drug-taking. J. Psychiatry Neurosci. 2000, 25, 125–136. [Google Scholar]
- Trendelenburg, A.U.; Klebroff, W.; Hein, L.; Starke, K. A study of presynaptic alpha2-autoreceptors in alpha2A/D-, alpha2B- and alpha2C-adrenoceptor-deficient mice. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 364, 117–130. [Google Scholar] [CrossRef]
- Glass, M.J.; Huang, J.; Aicher, S.A.; Milner, T.A.; Pickel, V.M. Subcellular localization of alpha-2A-adrenergic receptors in the rat medial nucleus tractus solitarius: Regional targeting and relationship with catecholamine neurons. J. Comp. Neurol. 2001, 433, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Blackmer, T.; Larsen, E.C.; Bartleson, C.; Kowalchyk, J.A.; Yoon, E.J.; Preininger, A.M.; Alford, S.; Hamm, H.E.; Martin, T.F. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat. Neurosci. 2005, 8, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Blackmer, T.; Larsen, E.C.; Takahashi, M.; Martin, T.F.; Alford, S.; Hamm, H.E. G protein betagamma subunit-mediated presynaptic inhibition: Regulation of exocytotic fusion downstream of Ca2+ entry. Science 2001, 292, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Delaney, A.J.; Crane, J.W.; Sah, P. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron 2007, 56, 880–892. [Google Scholar] [CrossRef]
- Dong, C.J.; Guo, Y.; Wheeler, L.; Hare, W.A. Alpha2 adrenergic receptor-mediated modulation of cytosolic Ca++ signals at the inner plexiform layer of the rat retina. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1410–1415. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Jiao, Y.; He, X.; Tao, X.; Li, B.; Zhang, X. Heterosynaptic Regulation of α2A-Adrenoceptors on Glutamate/GABA Release in the Prefrontal Cortex of Rats. Biomedicines 2025, 13, 1322. https://doi.org/10.3390/biomedicines13061322
Wei Y, Jiao Y, He X, Tao X, Li B, Zhang X. Heterosynaptic Regulation of α2A-Adrenoceptors on Glutamate/GABA Release in the Prefrontal Cortex of Rats. Biomedicines. 2025; 13(6):1322. https://doi.org/10.3390/biomedicines13061322
Chicago/Turabian StyleWei, Yaru, Yuhan Jiao, Xiaoting He, Xiaodong Tao, Baoming Li, and Xuehan Zhang. 2025. "Heterosynaptic Regulation of α2A-Adrenoceptors on Glutamate/GABA Release in the Prefrontal Cortex of Rats" Biomedicines 13, no. 6: 1322. https://doi.org/10.3390/biomedicines13061322
APA StyleWei, Y., Jiao, Y., He, X., Tao, X., Li, B., & Zhang, X. (2025). Heterosynaptic Regulation of α2A-Adrenoceptors on Glutamate/GABA Release in the Prefrontal Cortex of Rats. Biomedicines, 13(6), 1322. https://doi.org/10.3390/biomedicines13061322





