Dementia Deaths Most Commonly Result from Heart and Lung Disease: Evidence from the South Carolina Alzheimer’s Disease Registry
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Exposure Assessment
2.3. Outcome Assessment
2.4. Time, Event, and Censoring
2.5. Covariates
2.6. Inclusion and Exclusion Criteria
2.7. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Leading Causes of Death
3.3. Survival Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COD | Cause of Death |
| LCOD | Leading Cause of Death |
| AD/ADRD | Alzheimer’s Disease/Alzheimer’s Disease or Related Dementias |
| CVD | Cardiovascular Disease |
| CVD_C | Cardiovascular Disease (operationalized) |
| COPD | Chronic Obstructive Pulmonary Disease |
| COP_C | Chronic Obstructive Pulmonary Disease (operationalized) |
| VaD | Vascular Dementia |
| SC | South Carolina |
| GERD | Gastroesophageal Reflux Disease |
Appendix A

References
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; Moberg, M.E.; et al. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef]
- Mensah, G.A.; Habtegiorgis Abate, Y.; Abbasian, M.; Abd-Allah, F.; Abdollahi, A.; Abdollahi, M.; Morad Abdulah, D.; Abdullahi, A.; Abebe, A.M.; Abedi, A.; et al. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report from the American Heart Association. Circulation 2021, 143, E254–E743. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.H.; Saeed, A.; Bambs, C.E.; Swanson, J.; Emechebe, N.; Mansuri, F.; Talreja, K.; Reis, S.E.; Kip, K. Usefulness of the American Heart Association’s Ideal Cardiovascular Health Measure to Predict Long-term Major Adverse Cardiovascular Events (From the Heart SCORE Study). Am. J. Cardiol. 2021, 138, 20–25. [Google Scholar] [CrossRef]
- Van De Vorst, I.E.; Koek, H.L.; De Vries, R.; Bots, M.L.; Reitsma, J.B.; Vaartjes, I. Effect of Vascular Risk Factors and Diseases on Mortality in Individuals with Dementia: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2016, 64, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, R.F.; Schneider, A.L.C.; Zhou, Y.; Coresh, J.; Green, E.; Gupta, N.; Knopman, D.S.; Mintz, A.; Rahmim, A.; Sharrett, A.R.; et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA J. Am. Med. Assoc. 2017, 317, 1443–1450. [Google Scholar] [CrossRef]
- Kuller, L.H.; Lopez, O.L.; Mackey, R.H.; Rosano, C.; Edmundowicz, D.; Becker, J.T.; Newman, A.B. Subclinical Cardiovascular Disease and Death, Dementia, and Coronary Heart Disease in Patients 80þ Years. J. Am. Coll. Cardiol. 2016, 67, 1013–1022. [Google Scholar] [CrossRef]
- Carnevale, D.; Perrotta, M.; Lembo, G.; Trimarco, B. Pathophysiological Links Among Hypertension and Alzheimer’s Disease. High Blood Press. Cardiovasc. Prev. 2016, 23, 3–7. [Google Scholar] [CrossRef]
- Saeed, A.; Lopez, O.; Cohen, A.; Reis, S.E. Cardiovascular Disease and Alzheimer’s Disease: The Heart–Brain Axis. J. Am. Heart Assoc. 2023, 12, e030780. [Google Scholar] [CrossRef]
- Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein e and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Love, S.; Miners, J.S. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol. 2016, 131, 645–658. [Google Scholar] [CrossRef]
- Santos, C.Y.; Snyder, P.J.; Wu, W.C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 7, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Exposure to Wildfire Smoke Raises Risk of Dementia. Available online: https://aaic.alz.org/releases-2024/exposure-wildfire-smoke-raises-dementia-risk.asp (accessed on 7 January 2025).
- Multiple Cause of Death, 1999–2020 Results Form n.d. Available online: https://wonder.cdc.gov/controller/datarequest/D77;jsessionid=F794E73B8B74833BAAADF423424E (accessed on 12 January 2025).
- Stats of the States—Heart Disease Mortality. Available online: https://www.cdc.gov/nchs/pressroom/sosmap/heart_disease_mortality/heart_disease.htm (accessed on 7 January 2025).
- Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- South Carolina Alzheimer’s Disease Registry—2023 Annual Report. Available online: https://osa-sc.org/wp-content/uploads/2024/03/alzheimers_registry_report_2023.pdf (accessed on 12 January 2025).
- ICD-10-CM TABULAR LIST of DISEASES and INJURIES: Instructional Notations. Available online: https://ftp.cdc.gov/pub/health_statistics/nchs/publications/ICD10CM/2022/icd10cm-tabular-2022-April-1.pdf (accessed on 11 May 2024).
- Parris, B.A.; O’Farrell, H.E.; Fong, K.M.; Yang, I.A. Chronic obstructive pulmonary disease (COPD) and lung cancer: Common pathways for pathogenesis. J. Thorac. Dis. 2019, 11, S2155–S2172. [Google Scholar] [CrossRef]
- Whittaker, H.; Rothnie, K.J.; Quint, J.K. Cause-specific mortality in COPD subpopulations: A cohort study of 339,647 people in England. Thorax 2023, 79, 202–208. [Google Scholar] [CrossRef]
- Oelsner, E.C.; Carr, J.J.; Enright, P.L.; Hoffman, E.A.; Folsom, A.R.; Kawut, S.M.; Kronmal, R.A.; Lederer, D.J.; Lima, J.A.C.; Lovasi, G.S. Per cent emphysema is associated with respiratory and lung cancer mortality in the general population: A cohort study. Thorax 2016, 71, 624–632. [Google Scholar] [CrossRef]
- Garcia-Ptacek, S.; Kåreholt, I.; Cermakova, P.; Rizzuto, D.; Religa, D.; Eriksdotter, M. Causes of Death According to Death Certificates in Individuals with Dementia: A Cohort from the Swedish Dementia Registry. J. Am. Geriatr. Soc. 2016, 64, e137–e142. [Google Scholar] [CrossRef]
- Lim, U.; Wang, S.; Park, S.Y.; Bogumil, D.; Wu, A.H.; Cheng, I.; Haiman, C.A.; Le Marchand, L.; Wilkens, L.R.; White, L.; et al. Risk of Alzheimer’s disease and related dementia by sex and race/ethnicity: The Multiethnic Cohort Study. Alzheimer’s Dement. 2022, 18, 1625–1634. [Google Scholar] [CrossRef]
- Belloy, M.E.; Andrews, S.J.; Le Guen, Y.; Cuccaro, M.; Farrer, L.A.; Napolioni, V.; Greicius, M.D. APOE Genotype and Alzheimer Disease Risk Across Age, Sex, and Population Ancestry. JAMA Neurol. 2023, 80, 1284–1294. [Google Scholar] [CrossRef]
- Hiller, L.; Marshall, A.; Dunn, J. Assessing violations of the proportional hazards assumption in Cox regression: Does the chosen method matter? Trials 2015, 16, P134. [Google Scholar] [CrossRef]
- Kochanek, K.D.; Kochanek, M.A.; Murphy, S.L.; Xu, J.; Arias, E. Mortality in the United States, 2022: Key Findings Data from the National Vital Statistics System; National Center for Health Statistics: Hyattsville, MD, USA, 2024. [Google Scholar]
- Brunnström, H.R.; Englund, E.M. Cause of death in patients with dementia disorders. Eur. J. Neurol. 2009, 16, 488–492. [Google Scholar] [CrossRef]
- Dodd, J.W.; Getov, S.V.; Jones, P.W. Cognitive function in COPD. Eur. Respir. J. 2010, 35, 913–922. [Google Scholar] [CrossRef]
- Antonelli-Incalzi, R.; Corsonello, A.; Pedone, C.; Trojano, L.; Acanfora, D.; Spada, A.; Izzo, O.; Rengo, F. Drawing impairment predicts mortality in severe COPD. Chest 2006, 130, 1687–1694. [Google Scholar] [CrossRef]
- Buckley, E.; Jonsson, A.; Flood, Z.; Lavelle, M.; O’Sullivan, N.; Nurdin, N.; Dowling, P.; Duggan, E.; Callaly, E.; Byrne, C.; et al. Potentially inappropriate medication use and mortality in patients with cognitive impairment. Eur. J. Clin. Pharmacol. 2022, 78, 2013–2020. [Google Scholar] [CrossRef]
- Fried, T.R.; O’Leary, J.; Towle, V.; Goldstein, M.K.; Trentalange, M.; Martin, D.K. Health outcomes associated with polypharmacy in community-dwelling older adults: A systematic review. J. Am. Geriatr. Soc. 2014, 62, 2261–2272. [Google Scholar] [CrossRef]
- Patel, A.R.C.; Kowlessar, B.S.; Donaldson, G.C.; Mackay, A.J.; Singh, R.; George, S.N.; Garcha, D.S.; Wedzicha, J.A.; Hurst, J.R. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1091–1099. [Google Scholar] [CrossRef]
- Hurst, J.R.; Vestbo, J.; Anzueto, A.; Locantore, N.; Müllerova, H.; Tal-Singer, R.; Miller, B.; Lomas, D.A.; Agusti, A.; MacNee, W.; et al. Susceptibility to Exacerbation in Chronic Obstructive Pulmonary Disease Abstract. N. Engl. J. Med. 2010, 363, 1128–1138. [Google Scholar] [CrossRef]
- Hasegawa, W.; Yamauchi, Y.; Yasunaga, H.; Sunohara, M.; Jo, T.; Matsui, H.; Fushimi, K.; Takami, K.; Nagase, T. Factors affecting mortality following emergency admission for chronic obstructive pulmonary disease. BMC Pulm. Med. 2014, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Cavedo, E.; Chiesa, P.A.; Houot, M.; Ferretti, M.T.; Grothe, M.J.; Teipel, S.J.; Lista, S.; Habert, M.O.; Potier, M.C.; Dubois, B.; et al. Sex differences in functional and molecular neuroimaging biomarkers of Alzheimer’s disease in cognitively normal older adults with subjective memory complaints. Alzheimer’s Dement. 2018, 14, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, L.; Barnes, L.L.; Thambisetty, M.; Beecham, G.; Kunkle, B.; Bush, W.S.; Gifford, K.A.; Chibnik, L.B.; Mukherjee, S.; de Jager, P.L.; et al. Sex differences in the genetic predictors of Alzheimer’s pathology. Brain 2019, 142, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 2007, 29, 125–132. [Google Scholar] [CrossRef]
- Hebert, L.E.; Scherr, P.A.; Mccann, J.J.; Beckett, L.A.; Evans, D.A. Is the Risk of Developing Alzheimer’s Disease Greater for Women than for Men? Am. J. Epidemiol. 2001, 153, 132–136. [Google Scholar] [CrossRef]
- Jagger, C.; Clarke, M.; Stone, A. Predictors of survival with Alzheimer’s disease: A community-based study. Psychol. Med. 1995, 25, 171–177. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, S.; Huang, J.; Li, C.; Shang, H. Predictors for survival in patients with Alzheimer’s disease: A large comprehensive meta-analysis. Transl. Psychiatry 2024, 14, 184. [Google Scholar] [CrossRef]
- Seshadri, S.; Wolf, P.A.; Beiser, A.; Au, R.; McNulty, K.; White, R.; D’Agostino, R.B. Lifetime risk of dementia and Alzheimer’s disease: The impact of mortality on risk estimates in the Framingham Study. Neurology 1997, 49, 1498–1504. [Google Scholar] [CrossRef]
- Fang, M.; Hu, J.; Weiss, J.; Knopman, D.S.; Albert, M.; Windham, B.G.; Walker, K.A.; Sharrett, A.R.; Gottesman, R.F.; Lutsey, P.L.; et al. Lifetime risk and projected burden of dementia. Nat. Med. 2025, 31, 772–776. [Google Scholar] [CrossRef]
- Mayeda, E.R.; Glymour, M.M.; Quesenberry, C.P.; Johnson, J.K.; Pérez-Stable, E.J.; Whitmer, R.A. Survival after dementia diagnosis in five racial/ethnic groups. Alzheimer’s Dement. 2017, 13, 761–769. [Google Scholar] [CrossRef]
- Mehta, K.M.; Yaffe, K.; Pérez-Stable, E.J.; Stewart, A.; Barnes, D.; Kurland, B.F.; Miller, B.L. Race/ethnic differences in AD survival in US Alzheimer’s Disease Centers. Neurology 2008, 70, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Reuser, M.; Willekens, F.J.; Bonneux, L. Higher education delays and shortens cognitive impairment. A multistate life table analysis of the US Health and Retirement Study. Eur. J. Epidemiol. 2011, 26, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Helzner, E.P.; Scarmeas, N.; Cosentino, S.; Tang, M.X.; Schupf, N.; Stern, Y. Survival in Alzheimer disease: A multiethnic, population-based study of incident cases. Neurology 2008, 71, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.P.; Schultz, S.A.; Austin, B.P.; Boots, E.A.; Dowling, N.M.; Gleason, C.E.; Bendlin, B.B.; Sager, M.A.; Hermann, B.P.; Zetterberg, H.; et al. Effect of cognitive reserve on age-related changes in cerebrospinal fluid biomarkers of Alzheimer disease. JAMA Neurol. 2015, 72, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Laine, R.D.; Greenwald, R. An Exchange: Part. I: Does Money Matter? A Meta-Analysis of Studies of the Effects of Differential School Inputs on Student Outcomes. Educ. Res. 1994, 23, 5–14. [Google Scholar] [CrossRef]
- Barnes, L.L.; Bennett, D.A. Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Aff. 2014, 33, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Bynum, J.P.W.; Benloucif, S.; Martindale, J.; O’Malley, A.J.; Davis, M.A. Regional variation in diagnostic intensity of dementia among older U.S. adults: An observational study. Alzheimer’s Dement. 2024, 20, 6755–6764. [Google Scholar] [CrossRef]
- Schulz, R.; Belle, S.H.; Czaja, S.J.; Mcginnis, K.A.; Stevens, A.; Zhang, S. Long-term Care Placement of Dementia Patients and Caregiver Health and Well-being. JAMA 2004, 292, 961–967. [Google Scholar] [CrossRef]
- Yaffe, K.; Fox, P.; Newcomer, R.; Sands, L.; Lindquist, K.; Dane, K.; Covinsky, K.E. Patient and Caregiver Characteristics and Nursing Home Placement in Patients with Dementia. JAMA 2002, 287, 2090–2097. [Google Scholar] [CrossRef]
- Mausbach, B.T.; Coon, D.W.; Depp, C.; Rabinowitz, Y.G.; Wilson-Arias, E.; Kraemer, H.C.; Thompson, L.W.; Lane, G.; Gallagher-Thompson, D. Ethnicity and time to institutionalization of dementia patients: A comparison of Latina and Caucasian female family caregivers. J. Am. Geriatr. Soc. 2004, 52, 1077–1084. [Google Scholar] [CrossRef]
- Stokes, A.C.; Weiss, J.; Lundberg, D.J.; Xie, W.; Kim, J.K.; Preston, S.H.; Crimmins, E.M. Estimates of the Association of Dementia with US Mortality Levels Using Linked Survey and Mortality Records. JAMA Neurol. 2020, 77, 1543–1550. [Google Scholar] [CrossRef]
- Barnes, L.L.; Leurgans, S.; Aggarwal, N.T.; Shah, R.C.; Arvanitakis, Z.; James, B.D.; Buchman, A.S.; Bennett, D.A.; Schneider, J.A. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology 2015, 85, 528–534. [Google Scholar] [CrossRef]
- Chen, R.; Charpignon, M.L.; Raquib, R.V.; Wang, J.; Meza, E.; Aschmann, H.E.; Devost, M.A.; Mooney, A.; Bibbins-Domingo, K.; Riley, A.R.; et al. Excess Mortality with Alzheimer Disease and Related Dementias as an Underlying or Contributing Cause during the COVID-19 Pandemic in the US. JAMA Neurol. 2023, 80, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Take Brain Health to Heart | South Carolina Department of Public Health. Available online: https://dph.sc.gov/diseases-conditions/conditions/cognitive-impairment-dementia-and-alzheimers-disease/take-brain (accessed on 15 May 2025).
- Miller, M.C.; Mishio Bawa, E.; Absher, J.R.; Bonilha, L.; Ross, L.A.; Chai, H.W.; Milano, N.J.; Adams, R.J. Longevity in the South Carolina Alzheimer’s disease registry. Front. Neurol. 2024, 15, 1425495. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total | AD | VaD | Mixed | Other | p-Value * |
|---|---|---|---|---|---|---|
| All Deaths (%) | 122,252 (100) | 85,233 (69.72) | 11,874 (9.71) | 4935 (4.04) | 20,210 (16.53) | <0.0001 |
| Age Group | <0.0001 | |||||
| <65 | 9908 (8.66) | 4644 (5.83) | 1503 (13.50) | 262 (5.74) | 3499 (18.39) | |
| 65–74 | 18,381 (16.07) | 11,258 (14.14) | 2382 (21.39) | 802 (17.56) | 3939 (20.70) | |
| 75–84 | 40,368 (35.30) | 28,628 (35.95) | 3864 (34.70) | 1783 (39.04) | 6093 (32.02) | |
| >85 | 45,713 (39.97) | 35,106 (44.08) | 3387 (30.41) | 1720 (37.66) | 5500 (28.90) | |
| Sex | <0.0001 | |||||
| Male | 47,490 (39.73) | 30,725 (36.95) | 5183 (44.68) | 2009 (40.90) | 9573 (48.19) | |
| Female | 72,034 (60.27) | 52,420 (63.05) | 6418 (55.32) | 2903 (59.10) | 10,293 (51.81) | |
| Race | <0.0001 | |||||
| White | 85,427 (69.88) | 61,420 (72.06) | 7104 (59.83) | 3341 (67.70) | 13,562 (67.11) | |
| African American/Black | 27,247 (22.29) | 17,108 (20.07) | 3849 (32.42) | 1287 (26.08) | 5003 (24.76) | |
| Other | 9578 (7.83) | 6705 (7.87) | 921 (7.76) | 307 (6.22) | 1645 (8.14) |
| Cause of Death | All Deaths N (%) | Alzheimer’s N (%) | VaD N (%) | Mixed N (%) | Other N (%) |
|---|---|---|---|---|---|
| Alzheimer’s disease, unspecified | 17,133 (13.25) | 15,104 (16.36) | 475 (4.01) | 581 (11.57) | 973 (4.84) |
| Unspecified dementia | 15,686 (12.13) | 12,382 (13.41) | 920 (7.76) | 655 (13.05) | 1729 (8.59) |
| Atherosclerotic heart disease of native coronary artery | 5545 (4.29) | 3844 (4.16) | 637 (5.38) | 242 (4.82) | 822 (4.09) |
| Cerebrovascular disease/stroke | 5055 (3.91) | 3067 (3.32) | 902 (7.61) | 378 (7.53) | 708 (3.52) |
| Chronic obstructive pulmonary disease | 4913 (3.80) | 3513 (3.81) | 443 (3.74) | 140 (2.79) | 817 (4.06) |
| Acute myocardial infarction | 3637 (2.81) | 2479 (2.69) | 403 (3.40) | 123 (2.45) | 632 (3.14) |
| Parkinson’s disease | 3252 (2.52) | 1302 (1.41) | 124 (1.05) | 49 (0.98) | 1777 (8.83) |
| Heart Failure/I500 | 3037 (2.35) | 2219 (2.40) | 285 (2.41) | 96 (1.91) | 437 (2.17) |
| Malignant neoplasm of part of bronchus or lung | 3086 (2.39) | 2012 (2.18) | 296 (2.50) | 80 (1.59) | 698 (3.47) |
| Pneumonia, unspecified | 2208 (1.71) | 1558 (1.69) | 185 (1.56) | 91 (1.81) | 374 (1.86) |
| Other | 65,739 (50.85) | 44,820 (48.56) | 7179 (60.59) | 2585 (51.49) | 11,155 (55.44) |
| Cause of Death | All Deaths N (%) | Alzheimer’s N (%) | VaD N (%) | Mixed N (%) | Other N (%) |
|---|---|---|---|---|---|
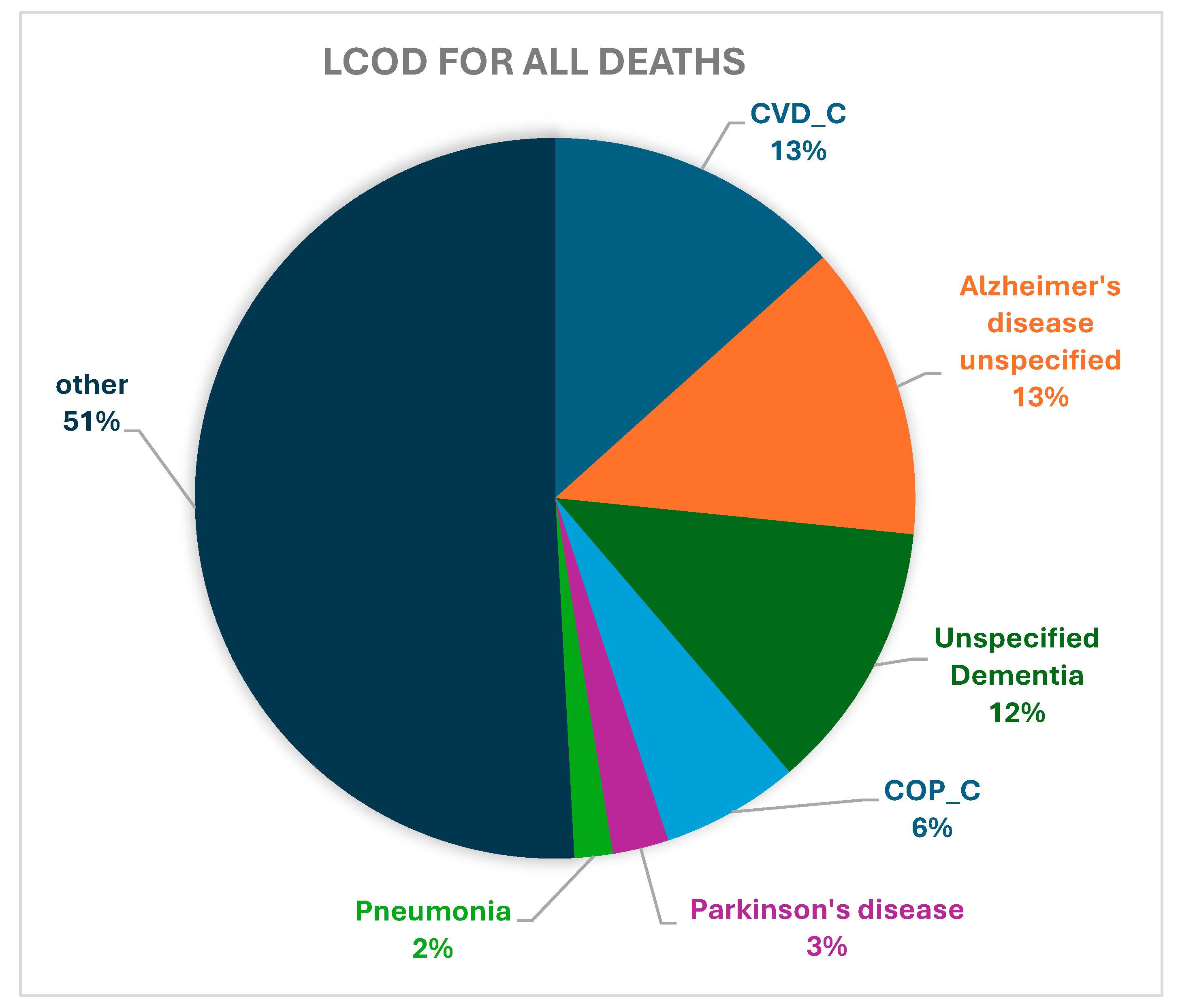
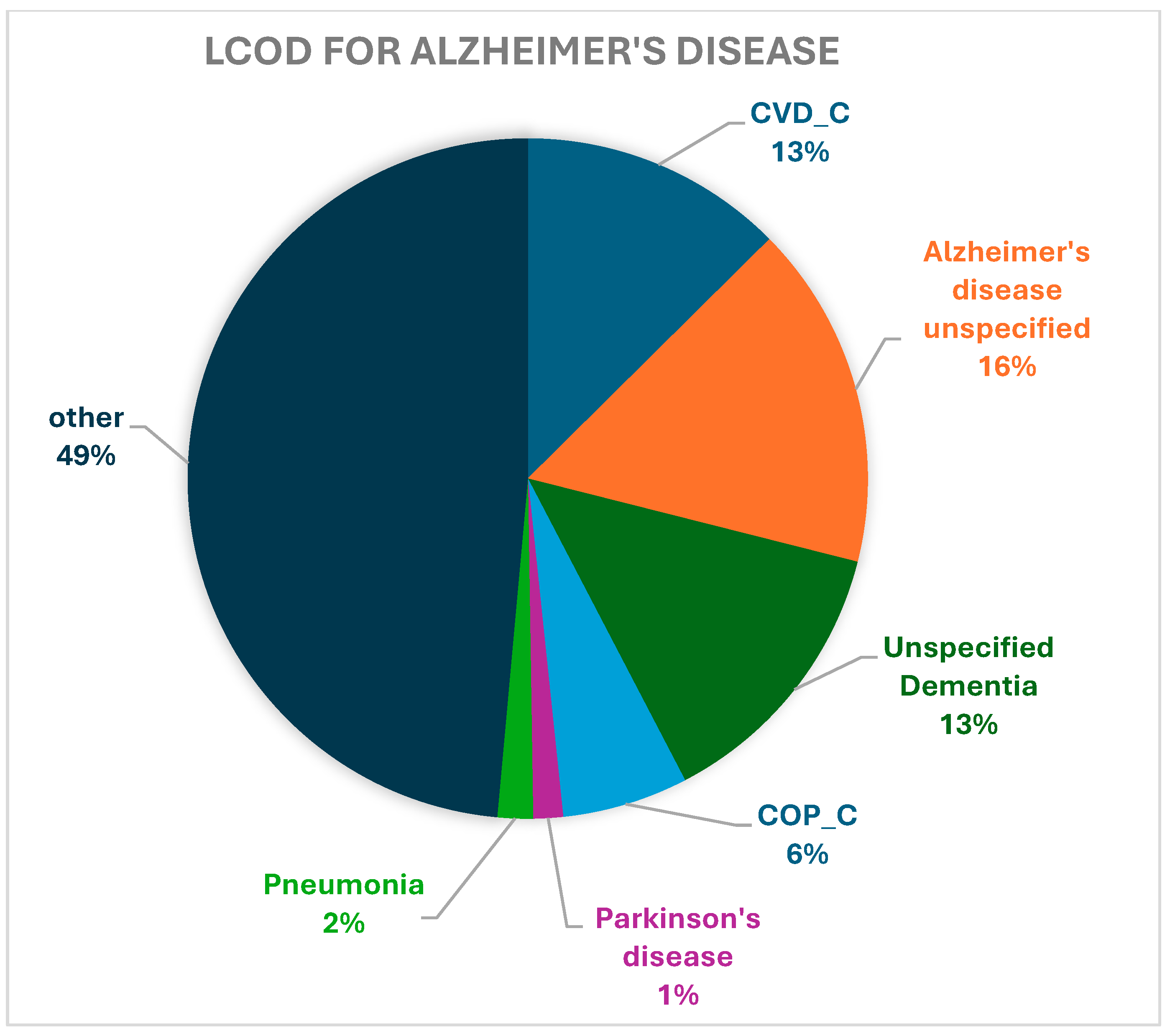
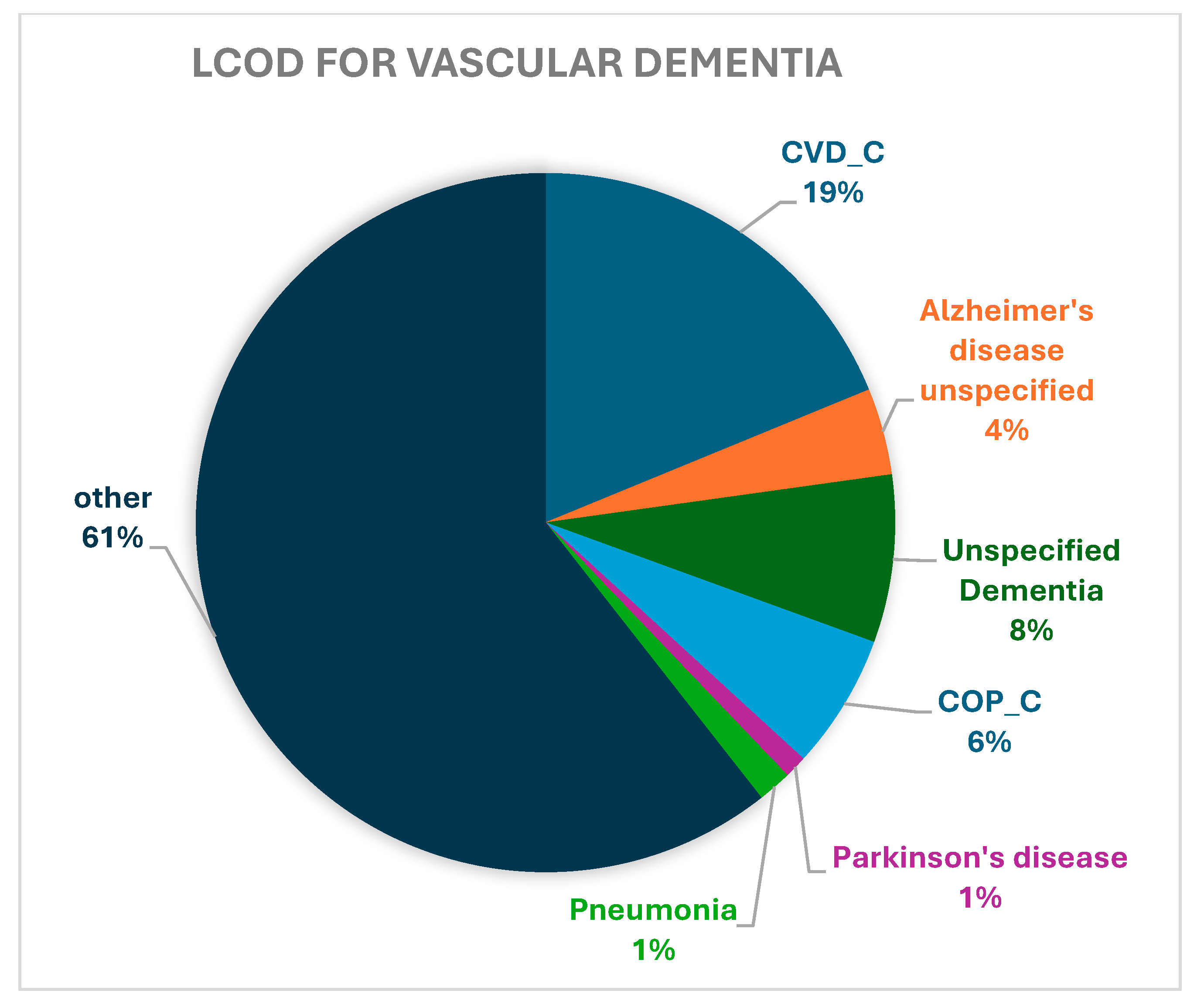
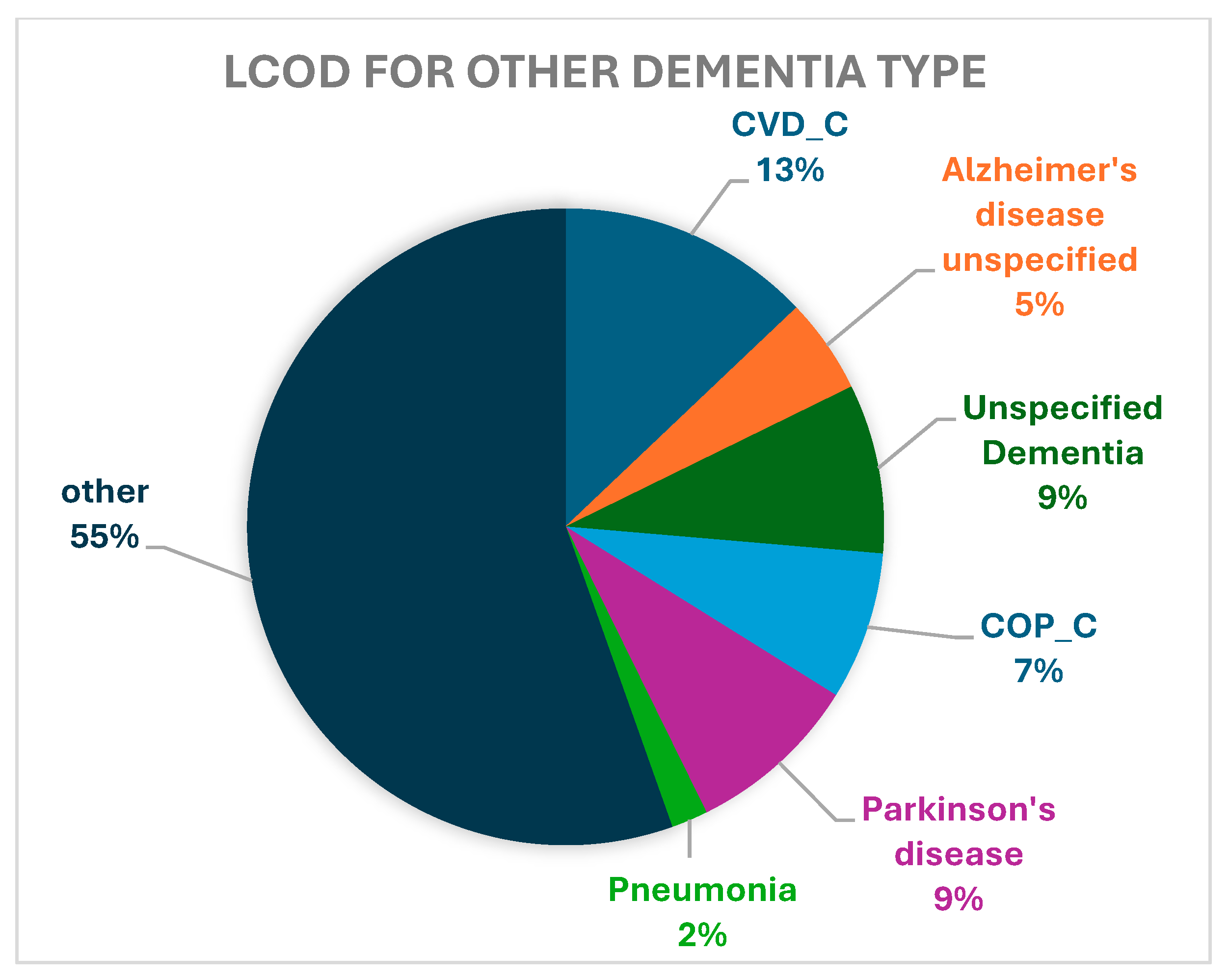
| CVD_C | 17,274 (13.36) | 11,609 (12.58) | 2227 (18.79) | 839 (16.71) | 2599 (12.92) |
| Alzheimer’s disease, unspecified | 17,133 (13.25) | 15,104 (16.36) | 475 (4.01) | 581 (11.57) | 973 (4.84) |
| Unspecified dementia | 15,686 (12.13) | 12,382 (13.41) | 920 (7.76) | 655 (13.05) | 1729 (8.59) |
| COP_C | 7999 (6.19) | 5525 (5.99) | 739 (6.24) | 220 (4.38) | 1515 (7.53) |
| Parkinson’s disease | 3252 (2.52) | 1302 (1.41) | 124 (1.05) | 49 (0.98) | 1777 (8.83) |
| Pneumonia, unspecified | 2208 (1.71) | 1558 (1.69) | 185 (1.56) | 91 (1.81) | 374 (1.86) |
| Other | 65,739 (50.85) | 44,820 (48.56) | 7179 (60.59) | 2585 (51.49) | 11,155 (55.44) |
| LCOD | Risk of Death | ||||
|---|---|---|---|---|---|
| All Deaths aHR * (95% CI) | AD aHR (95% CI) | VaD aHR (95% CI) | Mixed aHR (95% CI) | Other aHR (95% CI) | |
| Alzheimer’s Disease | 0.790 (0.771–0.810) | 0.812 (0.790–0.836) | 0.777 (0.693–0.871) | 0.768 (0.680–0.868) | 0.830 (0.764–0.901) |
| Dementia | 0.905 (0.883–0.928) | 0.918 (0.891–0.945) | 0.911 (0.834–0.995) | 0.838 (0.747–0.940) | 0.968 (0.905–1.036) |
| COP_C | 1.057 (1.025–1.090) | 1.096 (1.056–1.138) | 0.920 (0.838–1.011) | 0.953 (0.808–1.124) | 1.041 (0.969–1.117) |
| Parkinson’s Disease | 0.856 (0.818–0.895) | 0.805 (0.751–0.863) | 0.797 (0.644–0.986) | 0.703 (0.499–0.989) | 0.894 (0.833–0.960) |
| Pneumonia, unspecified | 0.989 (0.942–1.040) | 0.977 (0.920–1.037) | 1.089 (0.927–1.278) | 0.975 (0.768–1.239) | 1.029 (0.917–1.155) |
| Sex | |||||
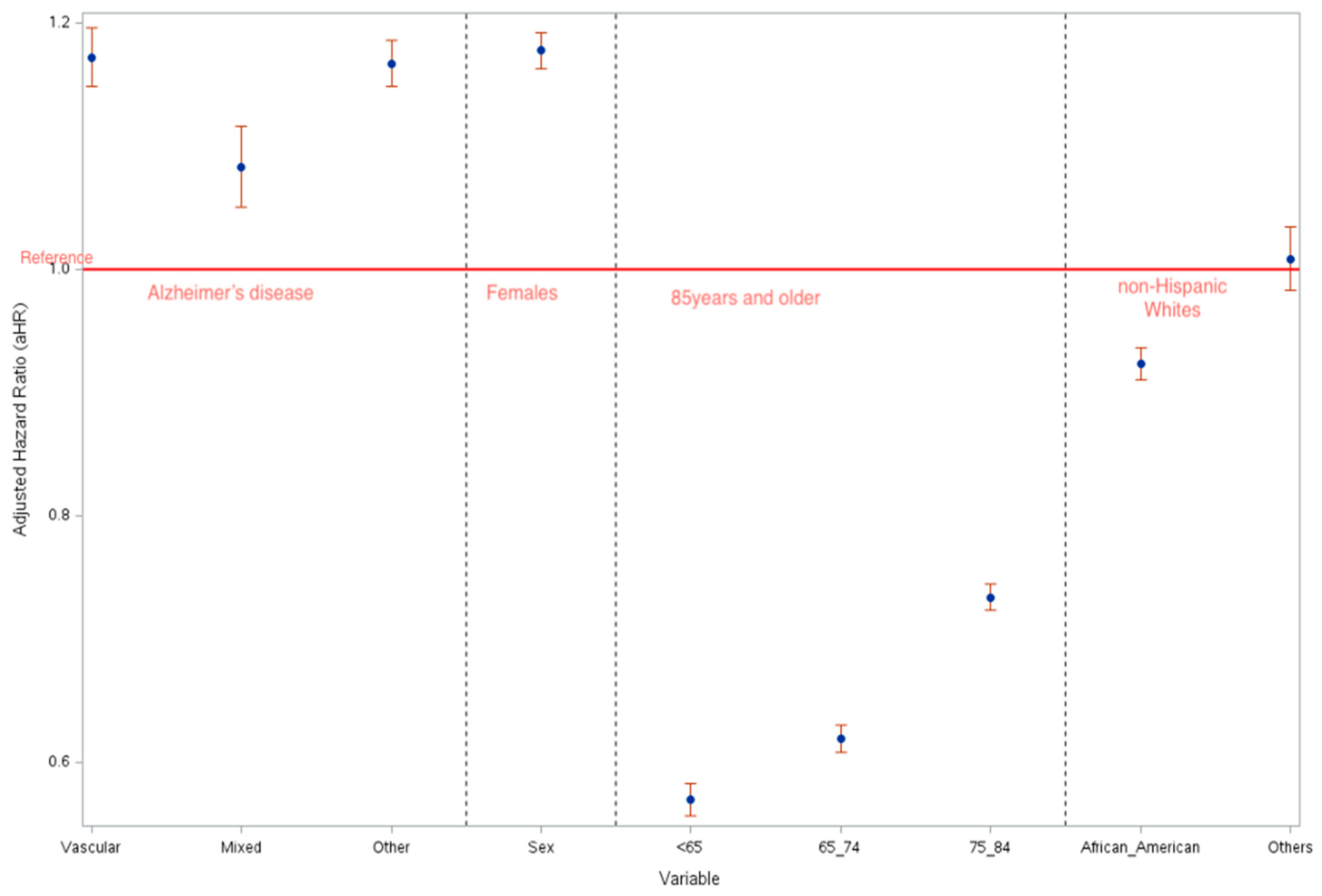
| Males vs. Females | 1.219 (1.196–1.243) | 1.235 (1.208–1.263) | 1.145 (1.071–1.225) | 1.190 (1.082–1.308) | 1.159 (1.104–1.218) |
| Age Group | |||||
| <65 vs. 85 and above | 0.495 (0.474–0.516) | 0.474 (0.448–0.501) | 0.554 (0.491–0.625) | 0.446 (0.354–0.562) | 0.503 (0.461–0.549) |
| 65–74 vs. 85 and above | 0.582 (0.565–0.599) | 0.565 (0.546–0.585) | 0.652 (0.593–0.717) | 0.620 (0.540–0.711) | 0.587 (0.548–0.629) |
| 75–84 vs. 85 and above | 0.707 (0.692–0.721) | 0.693 (0.677–0.710) | 0.760 (0.702–0.821) | 0.755 (0.682–0.836) | 0.735 (0.695–0.778) |
| Race | |||||
| African American vs. White | 0.881 (0.861–0.902) | 0.855 (0.831–0.880) | 0.909 (0.844–0.978) | 0.775 (0.694–0.866) | 0.994 (0.936–1.055) |
| Others vs. White | 1.054 (1.011–1.098) | 1.045 (0.995–1.098) | 1.062 (0.921–1.223) | 0.816 (0.658–1.013) | 1.123 (1.021–1.236) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoatika, D.A.; Absher, J.R.; Khan, M.T.F.; Miller, M.C. Dementia Deaths Most Commonly Result from Heart and Lung Disease: Evidence from the South Carolina Alzheimer’s Disease Registry. Biomedicines 2025, 13, 1321. https://doi.org/10.3390/biomedicines13061321
Amoatika DA, Absher JR, Khan MTF, Miller MC. Dementia Deaths Most Commonly Result from Heart and Lung Disease: Evidence from the South Carolina Alzheimer’s Disease Registry. Biomedicines. 2025; 13(6):1321. https://doi.org/10.3390/biomedicines13061321
Chicago/Turabian StyleAmoatika, Daniel A., John R. Absher, Md Tareq Ferdous Khan, and Maggi C. Miller. 2025. "Dementia Deaths Most Commonly Result from Heart and Lung Disease: Evidence from the South Carolina Alzheimer’s Disease Registry" Biomedicines 13, no. 6: 1321. https://doi.org/10.3390/biomedicines13061321
APA StyleAmoatika, D. A., Absher, J. R., Khan, M. T. F., & Miller, M. C. (2025). Dementia Deaths Most Commonly Result from Heart and Lung Disease: Evidence from the South Carolina Alzheimer’s Disease Registry. Biomedicines, 13(6), 1321. https://doi.org/10.3390/biomedicines13061321







