Stress-Induced Sleep Dysregulation: The Roles of Astrocytes and Microglia in Neurodegenerative and Psychiatric Disorders
Abstract
1. Introduction
2. Stress and Sleep Dysregulation: Mechanistic Insights
3. Neuroinflammation and Sleep Architecture: The Impact of Chronic Stress on Sleep Stages and Neuroinflammatory Signaling
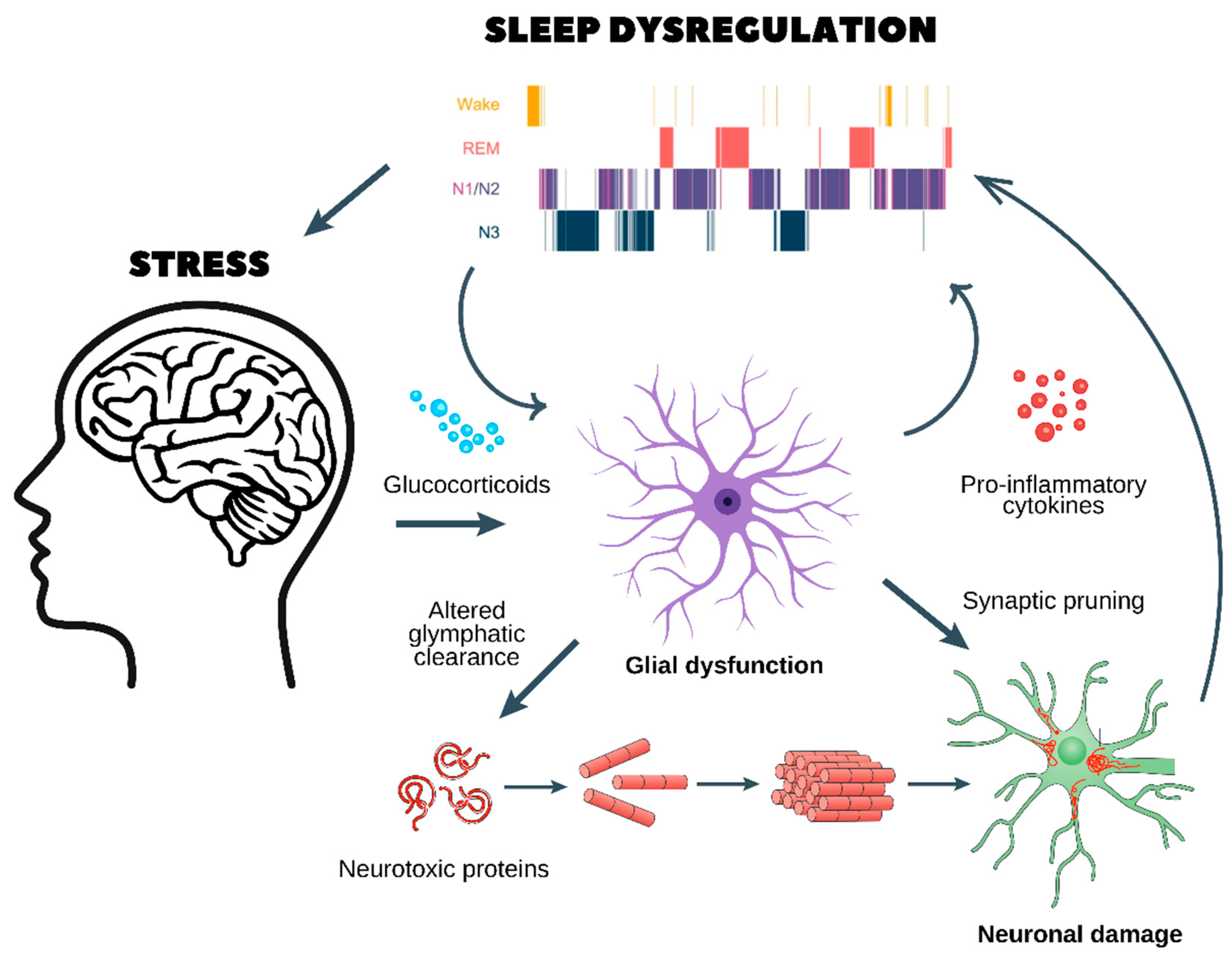
- Chronic stress and glucocorticoid release
- ○
- Prolonged stress stimulates the release of glucocorticoids, which dysregulate glial function and impair glymphatic clearance.
- ○
- This results in the accumulation of neurotoxic proteins (e.g., amyloid-beta and tau aggregates), which are normally cleared during sleep.
- Glial dysfunction and neuroinflammation
- ○
- Dysfunctional astrocytes and microglia enter a pro-inflammatory state, releasing cytokines that further disrupt sleep architecture, as seen in the hypnogram at the top.
- ○
- Sleep fragmentation exacerbates glial dysfunction, creating a self-reinforcing loop.
- Synaptic pruning and neuronal damage
- ○
- Chronic neuroinflammation leads to abnormal synaptic pruning, weakening essential neuronal connections.
- ○
- This results in neuronal damage, reducing cognitive function and increasing vulnerability to neurodegenerative diseases (e.g., Alzheimer’s and Parkinson’s) and psychiatric disorders (e.g., depression, anxiety, and schizophrenia).
- A vicious cycle of stress and sleep dysregulation
- ○
- Poor sleep quality worsens HPA axis dysregulation, leading to sustained stress and further neuronal dysfunction.
- ○
- Over time, this cycle promotes cognitive decline, mood disorders, and an increased risk of age-related neurodegeneration.
4. Astrocytic Control of Sleep Homeostasis
5. Stress-Induced Dysregulation of Glial Functions
6. Psychiatric Disorders and Stress-Induced Sleep Dysregulation
7. Key Topics and Future Directions
7.1. The Role of the Gut–Brain Axis: Microbiota’s Influence on Glial Function, Stress, and Sleep
7.2. Sex Differences: Impact of Hormonal Variations on Glial Responses to Stress and Sleep Dysregulation
7.3. Future Directions
8. Conclusions
Funding
Conflicts of Interest
References
- Pace-Schott, E.F.; Hobson, J.A. The Neurobiology of Sleep: Genetics, cellular physiology and subcortical networks. Nat. Rev. Neurosci. 2002, 3, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, N.; Yu, P.K.; Siegel, N.S. Sleep physiology, pathophysiology, and sleep hygiene. Prog. Cardiovasc. Dis. 2023, 77, 59–69. [Google Scholar] [CrossRef]
- Anghel, L.; Ciubară, A.; Nechita, A.; Nechita, L.; Manole, C.; Baroiu, L.; Ciubară, A.B.; Mușat, C.L. Sleep Disorders Associated with Neurodegenerative Diseases. Diagnostics 2023, 13, 2898. [Google Scholar] [CrossRef]
- Fifel, K.; Videnovic, A. Circadian and Sleep Dysfunctions in Neurodegenerative Disorders—An Update. Front. Neurosci. 2021, 14, 627330. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Leary, J.; Osborne, D.M.; McNay, E.C. Role of Glia in Stress-Induced enhancement and impairment of memory. Front. Integr. Neurosci. 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Camberos-Barraza, J.; Camacho-Zamora, A.; Bátiz-Beltrán, J.C.; Osuna-Ramos, J.F.; Rábago-Monzón, Á.R.; Valdez-Flores, M.A.; Angulo-Rojo, C.E.; Guadrón-Llanos, A.M.; Picos-Cárdenas, V.J.; Calderón-Zamora, L.; et al. Sleep, glial function, and the endocannabinoid system: Implications for neuroinflammation and sleep disorders. Int. J. Mol. Sci. 2024, 25, 3160. [Google Scholar] [CrossRef]
- Osuna-Ramos, J.F.; Camberos-Barraza, J.; Torres-Mondragón, L.E.; Rábago-Monzón, Á.R.; Camacho-Zamora, A.; Valdez-Flores, M.A.; Angulo-Rojo, C.E.; Guadrón-Llanos, A.M.; Picos-Cárdenas, V.J.; Calderón-Zamora, L.; et al. Interplay between the Glymphatic System and the Endocannabinoid System: Implications for Brain Health and Disease. Int. J. Mol. Sci. 2023, 24, 17458. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Hristovska, I.; Robert, M.; Combet, K.; Honnorat, J.; Comte, J.-C.; Pascual, O. Sleep decreases neuronal activity control of microglial dynamics in mice. Nat. Commun. 2022, 13, 6273. [Google Scholar] [CrossRef]
- Quan, L.; Uyeda, A.; Muramatsu, R. Central nervous system regeneration: The roles of glial cells in the potential molecular mechanism underlying remyelination. Inflamm. Regen. 2022, 42, 7. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Karin, O.; Raz, M.; Tendler, A.; Bar, A.; Kohanim, Y.K.; Milo, T.; Alon, U. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol. Syst. Biol. 2020, 16, e9510. [Google Scholar] [CrossRef]
- Camacho-Zamora, A.; Camberos-Barraza, J.; Rábago-Monzón, Á.R.; Sosa-Arámbula, H.J.; De la Herrán-Arita, A.K. Psychosomatic influences on insomnia: Mechanisms, diagnosis, and treatment strategies. J. Clin. Basic Psychosom. 2024, 3, 32–51. [Google Scholar] [CrossRef]
- Hinds, J.A.; Sanchez, E.R. The role of the hypothalamus–pituitary–adrenal (HPA) axis in test-induced anxiety: Assessments, physiological responses, and molecular details. Stresses 2022, 2, 146–155. [Google Scholar] [CrossRef]
- Speranza, L.; Filiz, K.D.; Lippiello, P.; Ferraro, M.G.; Pascarella, S.; Miniaci, M.C.; Volpicelli, F. Enduring neurobiological consequences of early-life stress: Insights from rodent behavioral paradigms. Biomedicines 2024, 12, 1978. [Google Scholar] [CrossRef]
- Landgraf, D.; McCarthy, M.J.; Welsh, D.K. Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Curr. Psychiatry Rep. 2014, 16, 483. [Google Scholar] [CrossRef]
- Bolsius, Y.G.; Zurbriggen, M.D.; Kim, J.K.; Kas, M.J.; Meerlo, P.; Aton, S.J.; Havekes, R. The role of clock genes in sleep, stress and memory. Biochem. Pharmacol. 2021, 191, 114493. [Google Scholar] [CrossRef]
- Sharan, P.; Vellapandian, C. Hypothalamic-Pituitary-Adrenal (HPA) Axis: Unveiling the Potential Mechanisms Involved in Stress-Induced Alzheimer’s Disease and Depression. Cureus 2024, 16, e67595. [Google Scholar] [CrossRef]
- Raven, F.; Van der Zee, E.A.; Meerlo, P.; Havekes, R. The role of sleep in regulating structural plasticity and synaptic strength: Implications for memory and cognitive function. Sleep Med. Rev. 2018, 39, 3–11. [Google Scholar] [CrossRef]
- Coulson, R.L.; Mourrain, P.; Wang, G.X. The intersection of sleep and synaptic translation in synaptic plasticity deficits in neurodevelopmental disorders. J. Comp. Physiol. B 2024, 194, 253–263. [Google Scholar] [CrossRef]
- Das, A.; Mohan Raj, P.S.; Vishwakarma, L.C. Sleep and Neuroinflammation. In Circadian Rhythms, Sleep and Inflammation; Springer Nature: Cham, Switzerland, 2025; pp. 41–55. [Google Scholar]
- Serna-Rodríguez, M.F.; Bernal-Vega, S.; de la Barquera, J.A.O.-S.; Camacho-Morales, A.; Pérez-Maya, A.A. The role of damage associated molecular pattern molecules (DAMPs) and permeability of the blood-brain barrier in depression and neuroinflammation. J. Neuroimmunol. 2022, 371, 577951. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Wang, Y.; Cheng, R.; Deng, L.; Chen, L.; Dong, Y. Different IL-1β levels differentially mediate neuroprotection or neurodegeneration and may be related to BDNF. Cytokine 2025, 188, 156877. [Google Scholar] [CrossRef] [PubMed]
- Fulek, M.; Wieckiewicz, M.; Szymanska-Chabowska, A.; Gac, P.; Poreba, R.; Markiewicz-Gorka, I.; Wojakowska, A.; Mazur, G.; Martynowicz, H. Inflammatory Markers and Sleep Architecture in Sleep Bruxism—A Case-Control Study. J. Clin. Med. 2024, 13, 687. [Google Scholar] [CrossRef] [PubMed]
- Olini, N.; Rothfuchs, I.; Azzinnari, D.; Pryce, C.R.; Kurth, S.; Huber, R. Chronic social stress leads to altered sleep homeostasis in mice. Behav. Brain Res. 2017, 327, 167–173. [Google Scholar] [CrossRef]
- Frazer, M.A.; Cabrera, Y.; Guthrie, R.S.; Poe, G.R. Shining a light on the mechanisms of sleep for memory consolidation. Curr. Sleep Med. Rep. 2021, 7, 221–231. [Google Scholar] [CrossRef]
- Pelkonen, A.; Pistono, C.; Klecki, P.; Gómez-Budia, M.; Dougalis, A.; Konttinen, H.; Stanová, I.; Fagerlund, I.; Leinonen, V.; Korhonen, P.; et al. Functional characterization of human pluripotent stem cell-derived models of the brain with microelectrode arrays. Cells 2021, 11, 106. [Google Scholar] [CrossRef]
- Sriram, S.; Carstens, K.; Dewing, W.; Fiacco, T.A. Astrocyte regulation of extracellular space parameters across the sleep-wake cycle. Front. Cell. Neurosci. 2024, 18, 1401698. [Google Scholar] [CrossRef]
- Guo, Q.; Gobbo, D.; Zhao, N.; Zhang, H.; Awuku, N.-O.; Liu, Q.; Fang, L.-P.; Gampfer, T.M.; Meyer, M.R.; Zhao, R.; et al. Adenosine triggers early astrocyte reactivity that provokes microglial responses and drives the pathogenesis of sepsis-associated encephalopathy in mice. Nat. Commun. 2024, 15, 6340. [Google Scholar] [CrossRef]
- Kamphuis, J.; Lancel, M.; Koolhaas, J.M.; Meerlo, P. Deep sleep after social stress: NREM sleep slow-wave activity is enhanced in both winners and losers of a conflict. Brain Behav. Immun. 2015, 47, 149–154. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Gibbons, A.J. Neuroinflammation, sleep, and circadian rhythms. Front. Cell. Infect. Microbiol. 2022, 12, 853096. [Google Scholar] [CrossRef]
- Gamage, R.; Rossetti, I.; Niedermayer, G.; Münch, G.; Buskila, Y.; Gyengesi, E. Chronic neuroinflammation during aging leads to cholinergic neurodegeneration in the mouse medial septum. J. NeuroInflamm. 2023, 20, 235. [Google Scholar] [CrossRef] [PubMed]
- Torres-Mondragón, L.E.; León-Pimentel, L.C.; Pérez-Tamayo, D.E.; Arita, A.K.D.l.H. The endocannabinoid system: A new frontier in addressing psychosomatic challenges. J. Clin. Basic Psychosom. 2024, 2, 2288. [Google Scholar] [CrossRef]
- Gorlova, A.; Svirin, E.; Pavlov, D.; Cespuglio, R.; Proshin, A.; Schroeter, C.A.; Lesch, K.-P.; Strekalova, T. Understanding the Role of Oxidative Stress, Neuroinflammation and Abnormal Myelination in Excessive Aggression Associated with Depression: Recent Input from Mechanistic Studies. Int. J. Mol. Sci. 2023, 24, 915. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Kaltschmidt, C. NF- B in the Nervous System. Cold Spring Harb. Perspect. Biol. 2009, 1, a001271. [Google Scholar] [CrossRef] [PubMed]
- De la Herrán-Arita, A.K.; Equihua-Benítez, A.C.; Drucker-Colín, R. Orexin/Hypocretin antagonists in insomnia: From Bench to clinic. In Milestones in Drug Therapy; Springer: Berlin, Germany, 2014; pp. 191–207. [Google Scholar] [CrossRef]
- De la Herrán-Arita, A.K.; Guerra-Crespo, M.; Drucker-Colín, R. Narcolepsy and Orexins: An example of progress in sleep research. Front. Neurol. 2011, 2, 10208. [Google Scholar] [CrossRef]
- Geloso, M.C.; D’ambrosi, N. Microglial Microglial pruning: Relevance for synaptic dysfunction in multiple sclerosis and related experimental models. Cells 2021, 10, 686. [Google Scholar] [CrossRef]
- Mordelt, A.; de Witte, L.D. Microglia-mediated synaptic pruning as a key deficit in neurodevelopmental disorders: Hype or hope? Curr. Opin. Neurobiol. 2023, 79, 102674. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Mazzucchelli, G.N.; Villemagne, V.L.; Brown, B.M.; Porter, T.; Weinborn, M.; Bucks, R.S.; Milicic, L.; Sohrabi, H.R.; Taddei, K.; et al. Genetic variation in Aquaporin-4 moderates the relationship between sleep and brain Aβ-amyloid burden. Transl. Psychiatry 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.M.U.; Landolt, H.-P.; Berger, W.; Nedergaard, M.; Knudsen, G.M.; Holst, S.C. Haplotype of the astrocytic water channel AQP4 is associated with slow wave energy regulation in human NREM sleep. PLoS Biol. 2020, 18, e3000623. [Google Scholar] [CrossRef]
- Vasciaveo, V.; Iadarola, A.; Casile, A.; Dante, D.; Morello, G.; Minotta, L.; Tamagno, E.; Cicolin, A.; Guglielmotto, M. Sleep fragmentation affects glymphatic system through the different expression of AQP4 in wild type and 5xFAD mouse models. Acta Neuropathol. Commun. 2023, 11, 16. [Google Scholar] [CrossRef]
- Park, J.; Chung, W.-S. Astrocyte-dependent circuit remodeling by synapse phagocytosis. Curr. Opin. Neurobiol. 2023, 81, 102732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; Chen, Y.; Li, Q.; Marshall, C.; Wu, T.; Hu, G.; Xiao, M. Aquaporin 4 deletion exacerbates brain impairments in a mouse model of chronic sleep disruption. CNS Neurosci. Ther. 2019, 26, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Wu, T.; Tsai, M.-C.; Graykowski, D.; Gandham, V.D.; Rose, C.M.; Bakalarski, C.E.; Ngu, H.; Wang, Y.; Pandey, S.; et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2022, 2, 837–850. [Google Scholar] [CrossRef]
- Gomez-Arboledas, A.; Acharya, M.M.; Tenner, A.J. The role of complement in synaptic pruning and neurodegeneration. ImmunoTargets Ther. 2021, 10, 373–386. [Google Scholar] [CrossRef]
- Ma, C.; Li, B.; Silverman, D.; Ding, X.; Li, A.; Xiao, C.; Huang, G.; Worden, K.; Muroy, S.; Chen, W.; et al. Microglia regulate sleep through calcium-dependent modulation of norepinephrine transmission. Nat. Neurosci. 2024, 27, 249–258. [Google Scholar] [CrossRef]
- Lezmy, J. How astrocytic ATP shapes neuronal activity and brain circuits. Curr. Opin. Neurobiol. 2023, 79, 102685. [Google Scholar] [CrossRef]
- Gallopin, T.; Luppi, P.-H.; Cauli, B.; Urade, Y.; Rossier, J.; Hayaishi, O.; Lambolez, B.; Fort, P. The endogenous somnogen adenosine excites a subset of sleep-promoting neurons via A2A receptors in the ventrolateral preoptic nucleus. Neuroscience 2005, 134, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Xiong, W.; Zhang, D.; Soylu, H.; Sun, C.; Albensi, B.C.; E Parkinson, F. Regulation of adenosine levels during cerebral ischemia. Acta Pharmacol. Sin. 2012, 34, 60–66. [Google Scholar] [CrossRef]
- Garcia-Gil, M.; Camici, M.; Allegrini, S.; Pesi, R.; Tozzi, M.G. Metabolic aspects of adenosine functions in the brain. Front. Pharmacol. 2021, 12, 672182. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, J.; Zhou, J.; Lu, W.; Zhou, H.; Long, L.; Hu, Z.; Ni, L.; Wang, Y.; Chen, J.; et al. AMPK mediates glucocorticoids Stress-Induced downregulation of the glucocorticoid receptor in cultured rat prefrontal cortical astrocytes. PLoS ONE 2016, 11, e0159513. [Google Scholar] [CrossRef]
- Todd, A.C.; Hardingham, G.E. The regulation of astrocytic glutamate transporters in health and neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 9607. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Codeluppi, S.A.; Banasr, M. Astroglial dysfunctions in mood disorders and rodent stress models: Consequences on behavior and potential as treatment target. Int. J. Mol. Sci. 2024, 25, 6357. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.G.; Cerpa, W. Building a bridge between NMDAR-mediated excitotoxicity and mitochondrial dysfunction in chronic and acute diseases. Cell. Mol. Neurobiol. 2021, 41, 1413–1430. [Google Scholar] [CrossRef]
- Heard, K.J.; Shokhirev, M.N.; Becronis, C.; Fredlender, C.; Zahid, N.; Le, A.T.; Ji, Y.; Skime, M.; Nelson, T.; Hall-Flavin, D.; et al. Chronic cortisol differentially impacts stem cell-derived astrocytes from major depressive disorder patients. Transl. Psychiatry 2021, 11, 608. [Google Scholar] [CrossRef]
- Dolotov, O.V.; Inozemtseva, L.S.; Myasoedov, N.F.; Grivennikov, I.A. Stress-Induced Depression and Alzheimer’s Disease: Focus on astrocytes. Int. J. Mol. Sci. 2022, 23, 4999. [Google Scholar] [CrossRef]
- Xu, X.; Xuan, S.; Chen, S.; Liu, D.; Xiao, Q.; Tu, J. Increased excitatory amino acid transporter 2 levels in basolateral amygdala astrocytes mediate chronic stress–induced anxiety-like behavior. Neural Regen. Res. 2024, 20, 1721–1734. [Google Scholar] [CrossRef]
- Davinelli, S.; Medoro, A.; Savino, R.; Scapagnini, G. Sleep and Oxidative Stress: Current Perspectives on the Role of NRF2. Cell. Mol. Neurobiol. 2024, 44, 52. [Google Scholar] [CrossRef]
- Li, H.; Tofigh, A.M.; Amirfakhraei, A.; Chen, X.; Tajik, M.; Xu, D.; Motevalli, S. Modulation of astrocyte activity and improvement of oxidative stress through blockage of NO/NMDAR pathway improve posttraumatic stress disorder (PTSD)-like behavior induced by social isolation stress. Brain Behav. 2022, 12, e2620. [Google Scholar] [CrossRef]
- Boonpraman, N.; Yoon, S.; Kim, C.Y.; Moon, J.; Yi, S.S. NOX4 as a critical effector mediating neuroinflammatory cytokines, myeloperoxidase and osteopontin, specifically in astrocytes in the hippocampus in Parkinson’s disease. Redox Biol. 2023, 62, 102698. [Google Scholar] [CrossRef]
- Herbet, M.; Korga, A.; Gawrońska-Grzywacz, M.; Izdebska, M.; Piątkowska-Chmiel, I.; Poleszak, E.; Wróbel, A.; Matysiak, W.; Jodłowska-Jędrych, B.; Dudka, J. Chronic variable stress is responsible for lipid and DNA oxidative disorders and activation of oxidative stress response genes in the brain of rats. Oxidative Med. Cell. Longev. 2017, 2017, 7313090. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Li, Y.; Jiang, Y.; Huang, J.H.; Wang, F. Glymphatic dysfunction induced oxidative stress and Neuro-Inflammation in major depression disorders. Antioxidants 2022, 11, 2296. [Google Scholar] [CrossRef] [PubMed]
- Kofuji, P.; Araque, A. G-Protein-Coupled receptors in Astrocyte–Neuron communication. Neuroscience 2020, 456, 71–84. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Blutstein, T.; Lee, S.; Erneux, C.; Halassa, M.M.; Haydon, P. Astrocytic IP3/CA2+ signaling modulates theta rhythm and REM sleep. Front. Neural Circuits 2017, 11, 3. [Google Scholar] [CrossRef]
- Franklin, T.C.; Wohleb, E.S.; Zhang, Y.; Fogaça, M.; Hare, B.; Duman, R.S. Persistent increase in microglial RAGE contributes to chronic Stress–Induced priming of depressive-like behavior. Biol. Psychiatry 2018, 83, 50–60. [Google Scholar] [CrossRef]
- Kim, J.; Choi, I.; Jeong, J.; Jang, I.; Lee, M.; Suk, K. Astrocytes in the ventrolateral preoptic area promote sleep. J. Neurosci. 2020, 40, 8994–9011. [Google Scholar] [CrossRef]
- Saper, C.B.; Romanovsky, A.A.; Scammell, T.E. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat. Neurosci. 2012, 15, 1088–1095. [Google Scholar] [CrossRef]
- Bolton, J.L.; Short, A.K.; Othy, S.; Kooiker, C.L.; Shao, M.; Gunn, B.G.; Beck, J.; Bai, X.; Law, S.M.; Savage, J.C.; et al. Early stress-induced impaired microglial pruning of excitatory synapses on immature CRH-expressing neurons provokes aberrant adult stress responses. Cell Rep. 2022, 38, 110600. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Li, H.; Wang, H.; Zou, R.; Lu, X.; Wang, J.; Nie, B.; Wu, J.; Li, S.; et al. Microglia-dependent excessive synaptic pruning leads to cortical underconnectivity and behavioral abnormality following chronic social defeat stress in mice. Brain Behav. Immun. 2022, 109, 23–36. [Google Scholar] [CrossRef]
- Dayananda, K.K.; Ahmed, S.; Wang, D.; Polis, B.; Islam, R.; Kaffman, A. Early life stress impairs synaptic pruning in the developing hippocampus. Brain Behav. Immun. 2022, 107, 16–31. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2015, 41, 3–23. [Google Scholar] [CrossRef]
- Khairova, R.A.; Machado-Vieira, R.; Du, J.; Manji, H.K. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int. J. Neuropsychopharmacol. 2009, 12, 561. [Google Scholar] [CrossRef]
- Golia, M.T.; Poggini, S.; Alboni, S.; Garofalo, S.; Albanese, N.C.; Viglione, A.; Ajmone-Cat, M.A.; St-Pierre, A.; Brunello, N.; Limatola, C.; et al. Interplay between inflammation and neural plasticity: Both immune activation and suppression impair LTP and BDNF expression. Brain Behav. Immun. 2019, 81, 484–494. [Google Scholar] [CrossRef]
- Vicente, M.C.; Paneghini, J.L.; Stabile, A.M.; Amorim, M.; Silva, C.E.A.; Patrone, L.G.A.; Cunha, T.M.; Bícego, K.C.; Almeida, M.C.; Carrettiero, D.C.; et al. Inhibition of Pro-Inflammatory Microglia with Minocycline Improves Cognitive and Sleep-Wake Dysfunction Under Respiratory Stress in a Sporadic Model for Alzheimer’s Disease. J. Alzheimers Dis. 2023, 95, 317–337. [Google Scholar] [CrossRef]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Z.; Van Patten, R.; Nakhla, M.Z.; Twamley, E.W.; Filoteo, J.V.; Schiehser, D.M. REM Sleep Behavior Disorder in Parkinson’s Disease: Effects on Cognitive, Psychiatric, and Functional outcomes. J. Int. Neuropsychol. Soc. 2020, 26, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Tekriwal, A.; Kern, D.S.; Tsai, J.; Ince, N.F.; Wu, J.; Thompson, J.A.; Abosch, A. REM sleep behaviour disorder: Prodromal and mechanistic insights for Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2016, 88, 445–451. [Google Scholar] [CrossRef]
- Iovino, L.; Tremblay, M.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef]
- Salin, P.; Melon, C.; Chassain, C.; Gubellini, P.; Pages, G.; Pereira, B.; Fur, Y.L.; Durif, F.; Goff, L.K. Interhemispheric reactivity of the subthalamic nucleus sustains progressive dopamine neuron loss in asymmetrical parkinsonism. Neurobiol. Dis. 2024, 191, 106398. [Google Scholar] [CrossRef]
- Maiti, P.; Manna, J.; Dunbar, G.L. Current understanding of the molecular mechanisms in Parkinson’s disease: Targets for potential treatments. Transl. Neurodegener. 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Rathitharan, G.; Meyer, J.H.; Furukawa, Y.; Ang, L.; Boileau, I.; Guttman, M.; Hornykiewicz, O.; Kish, S.J. Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain 2017, 140, 2460–2474. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Sa, M.; Ju, Y.H.; Park, M.G.; Lee, C.J. Revisiting the role of astrocytic MAOB in Parkinson’s disease. Int. J. Mol. Sci. 2022, 23, 4453. [Google Scholar] [CrossRef]
- Corsi, G.; Picard, K.; di Castro, M.A.; Garofalo, S.; Tucci, F.; Chece, G.; del Percio, C.; Golia, M.T.; Raspa, M.; Scavizzi, F.; et al. Microglia modulate hippocampal synaptic transmission and sleep duration along the light/dark cycle. Glia 2021, 70, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, A.; Andronie-Cioara, F.L.; Nistor-Cseppento, D.C.; Pascalau, N.; Rus, M.; Vasca, E.; Jurcau, M.C. The involvement of neuroinflammation in the onset and progression of Parkinson’s disease. Int. J. Mol. Sci. 2023, 24, 14582. [Google Scholar] [CrossRef]
- Isik, S.; Kiyak, B.Y.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia mediated neuroinflammation in Parkinson’s disease. Cells 2023, 12, 1012. [Google Scholar] [CrossRef]
- Karki, P.; Smith, K.; Johnson, J.; Aschner, M.; Lee, E.Y. Genetic Dys-regulation of Astrocytic Glutamate Transporter EAAT2 and its Implications in Neurological Disorders and Manganese Toxicity. Neurochem. Res. 2014, 40, 380–388. [Google Scholar] [CrossRef]
- Ng, W.; Ng, S. Remodeling of astrocyte secretome in amyotrophic lateral sclerosis: Uncovering novel targets to combat astrocyte-mediated toxicity. Transl. Neurodegener. 2022, 11, 54. [Google Scholar] [CrossRef]
- You, J.; Youssef, M.; Santos, J.; Lee, J.; Park, J. Microglia and astrocytes in amyotrophic lateral sclerosis: Disease-Associated States, pathological roles, and therapeutic potential. Biology 2023, 12, 1307. [Google Scholar] [CrossRef]
- Benkler, C.; Ben-Zur, T.; Barhum, Y.; Offen, D. Altered astrocytic response to activation in SOD1G93A mice and its implications on amyotrophic lateral sclerosis pathogenesis. Glia 2012, 61, 312–326. [Google Scholar] [CrossRef]
- Izrael, M.; Slutsky, S.G.; Revel, M. Rising Stars: Astrocytes as a therapeutic target for ALS disease. Front. Neurosci. 2020, 14, 824. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.; Wilson, S.; Paterson, L. Sleep disorders as core symptoms of depression. Dialogues Clin. Neurosci. 2008, 10, 329–336. [Google Scholar] [CrossRef]
- Miyanishi, K.; Hotta-Hirashima, N.; Miyoshi, C.; Hayakawa, S.; Kakizaki, M.; Kanno, S.; Ikkyu, A.; Funato, H.; Yanagisawa, M. Microglia modulate sleep/wakefulness under baseline conditions and under acute social defeat stress in adult mice. Neurosci. Res. 2023, 202, 8–19. [Google Scholar] [CrossRef]
- Li, B.; Zhang, D.; Verkhratsky, A. Astrocytes in post-traumatic stress disorder. Neurosci. Bull. 2022, 38, 953–965. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.; Baskerville, R.; Rogero, M.; Castell, L. Emerging evidence for the widespread role of glutamatergic dysfunction in neuropsychiatric diseases. Nutrients 2022, 14, 917. [Google Scholar] [CrossRef]
- Hassamal, S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Cai, G.; Zheng, A.; Wang, Y.; Jia, J.; Fang, H.; Yang, Y.; Hu, M.; Ding, Q. Tumor necrosis factor-alpha regulates the Hypocretin system via mRNA degradation and ubiquitination. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1812, 565–571. [Google Scholar] [CrossRef]
- De La Herrán-Arita, A.K.; García-García, F. Narcolepsy as an Immune-Mediated Disease. Sleep Disord. 2014, 2014, 792687. [Google Scholar] [CrossRef]
- De La Herrán-Arita, A.K.; Drucker-Colín, R. Models for narcolepsy with cataplexy drug discovery. Expert Opin. Drug Discov. 2012, 7, 155–164. [Google Scholar] [CrossRef]
- Gold, A.; Sylvia, L. The role of sleep in bipolar disorder. Nat. Sci. Sleep 2016, 8, 207–214. [Google Scholar] [CrossRef]
- Harvey, A.G.; Talbot, L.S.; Gershon, A. Sleep disturbance in bipolar disorder across the lifespan. Clin. Psychol. Sci. Pract. 2009, 16, 256–277. [Google Scholar] [CrossRef] [PubMed]
- González-Arias, C.; Sánchez-Ruiz, A.; Esparza, J.; Sánchez-Puelles, C.; Arancibia, L.; Ramírez-Franco, J.; Gobbo, D.; Kirchhoff, F.; Perea, G. Dysfunctional serotonergic neuron-astrocyte signaling in depressive-like states. Mol. Psychiatry 2023, 28, 3856–3873. [Google Scholar] [CrossRef]
- Puentes-Orozco, M.; Albarracin, S.L.; Velásquez, M.M. Neuroinflammation and major depressive disorder: Astrocytes at the crossroads. Front. Cell. Neurosci. 2024, 18, 1504555. [Google Scholar] [CrossRef] [PubMed]
- Manninen, T.; Saudargiene, A.; Linne, M. Astrocyte-mediated spike-timing-dependent long-term depression modulates synaptic properties in the developing cortex. PLoS Comput. Biol. 2020, 16, e1008360. [Google Scholar] [CrossRef]
- So, C.J.; Miller, K.E.; Gehrman, P.R. Sleep disturbances associated with posttraumatic stress disorder. Psychiatr. Ann. 2023, 53, 491–495. [Google Scholar] [CrossRef]
- Pace-Schott, E.F.; Germain, A.; Milad, M.R. Sleep and REM sleep disturbance in the pathophysiology of PTSD: The role of extinction memory. Biol. Mood Anxiety Disord. 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ullah, H.; Gu, J.; Li, K. Immune-metabolic mechanisms of post-traumatic stress disorder and atherosclerosis. Front. Physiol. 2023, 14, 1123692. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and beyond. Neuropsychopharmacology 2016, 42, 254–270. [Google Scholar] [CrossRef]
- Tuan, L.; Lee, L. Microglia-mediated synaptic pruning is impaired in sleep-deprived adolescent mice. Neurobiol. Dis. 2019, 130, 104517. [Google Scholar] [CrossRef]
- Camberos-Barraza, J.; Guadrón-Llanos, A.M.; De la Herrán-Arita, A.K. The Gut Microbiome-Neuroglia Axis: Implications for Brain Health, Inflammation, and Disease. Neuroglia 2024, 5, 254–273. [Google Scholar] [CrossRef]
- Corbett, G.T.; Roy, A.; Pahan, K. Sodium phenylbutyrate enhances astrocytic neurotrophin synthesis via protein kinase C (PKC)-mediated activation of CAMP-response element-binding protein (CREB). J. Biol. Chem. 2013, 288, 8299–8312. [Google Scholar] [CrossRef]
- Chakraborty, P.; Gamage, H.K.; Laird, A.S. Butyrate as a potential therapeutic agent for neurodegenerative disorders. Neurochem. Int. 2024, 176, 105745. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Miyoshi, C.; Obana, N.; Yajima, K.; Hotta-Hirashima, N.; Ikkyu, A.; Kanno, S.; Soga, T.; Fukuda, S.; Yanagisawa, M. Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Sci. Rep. 2020, 10, 19554. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Milosavljevic, S.; Pocivavsek, A. The stress of losing sleep: Sex-specific neurobiological outcomes. Neurobiol. Stress 2023, 24, 100543. [Google Scholar] [CrossRef]
- Hodes, G.E.; Bangasser, D.; Sotiropoulos, I.; Kokras, N.; Dalla, C. Sex Differences in stress Response: Classical mechanisms and Beyond. Curr. Neuropharmacol. 2023, 22, 475–494. [Google Scholar] [CrossRef]
- Yap, S.; Luki, J.; Hanstock, C.C.; Seres, P.; Shandro, T.; Hanstock, S.E.C.; Lirette, A.; Zhao, H.; Aitchison, K.J.; Melledo, J.L. Decreased medial prefrontal cortex glutamate levels in perimenopausal women. Front. Psychiatry 2021, 12, 763562. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Tang, C. Estrogen-immuno-neuromodulation disorders in menopausal depression. J. Neuroinflammation 2024, 21, 159. [Google Scholar] [CrossRef]
- Madore, C.; Yin, Z.; Leibowitz, J.; Butovsky, O. Microglia, lifestyle stress, and neurodegeneration. Immunity 2020, 52, 222–240. [Google Scholar] [CrossRef]
- Bojarskaite, L.; Bjørnstad, D.M.; Pettersen, K.H.; Cunen, C.; Hermansen, G.H.; Åbjørsbråten, K.S.; Chambers, A.R.; Sprengel, R.; Vervaeke, K.; Tang, W.; et al. Astrocytic Ca2+ signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat. Commun. 2020, 11, 3240. [Google Scholar] [CrossRef]
- Bang, J.; Kim, H.Y.; Lee, H. Optogenetic and chemogenetic approaches for studying astrocytes and gliotransmitters. Exp. Neurobiol. 2016, 25, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Dopp, J.; Ortega, A.; Davie, K.; Poovathingal, S.; Baz, E.; Liu, S. Single-cell transcriptomics reveals that glial cells integrate homeostatic and circadian processes to drive sleep–wake cycles. Nat. Neurosci. 2024, 27, 359–372. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, Z.; Wang, Q.; Sima, X.; Zhao, W.; He, C.; Yang, W.; Chen, H.; Gong, B.; Song, S.; et al. Single-cell and spatial transcriptome assays reveal heterogeneity in gliomas through stress responses and pathway alterations. Front. Immunol. 2024, 15, 1452172. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal axis: Development, programming actions of hormones, and Maternal-Fetal interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr. Rev. 2021, 41, 470–490. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Kopach, O.; Braga, A.; Nizari, S.; Hosford, P.S.; Sagi-Kiss, V.; Hadjihambi, A.; Konstantinou, C.; Esteras, N.; Del Arroyo, A.G.; et al. Adenosine signalling to astrocytes coordinates brain metabolism and function. Nature 2024, 632, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, X. Adenosine inhibits activity of Hypocretin/Orexin neurons by the A1 receptor in the lateral hypothalamus: A possible Sleep-Promoting effect. J. Neurophysiol. 2006, 97, 837–848. [Google Scholar] [CrossRef]
- De la Herran-Arita, A.K.; Lopez-Lazcano, H.R.; Miranda-Maciel, R.; Moya-Sanchez, D.N. Novel Pharmacological Strategies for the Management of Insomnia. J. Neurol. Exp. Neural Sci. 2017, 1, 1–6. [Google Scholar] [CrossRef]
- Carbone, M.; Duty, S.; Rattray, M. Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem. Int. 2011, 60, 31–38. [Google Scholar] [CrossRef]
- Fontana, A.C.K. Current approaches to enhance glutamate transporter function and expression. J. Neurochem. 2015, 134, 982–1007. [Google Scholar] [CrossRef]
- Fan, R.; Xu, F.; Previti, M.L.; Davis, J.; Grande, A.M.; Robinson, J.K.; Van Nostrand, W.E. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J. Neurosci. 2007, 27, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Gleixner, A.M.; Hutchison, D.F.; Sannino, S.; Bhatia, T.N.; Leak, L.C.; Flaherty, P.T.; Wipf, P.; Brodsky, J.L.; Leak, R.K. N-Acetyl-l-Cysteine Protects Astrocytes against Proteotoxicity without Recourse to Glutathione. Mol. Pharmacol. 2017, 92, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D. Potential of glial cell modulators in the management of substance use disorders. CNS Drugs 2020, 34, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Romero-Miguel, D.; Casquero-Veiga, M.; MacDowell, K.S.; Torres-Sanchez, S.; Garcia-Partida, J.A.; Lamanna-Rama, N.; Romero-Miranda, A.; Berrocoso, E.; Leza, J.C.; Desco, M.; et al. A characterization of the effects of minocycline treatment during adolescence on structural, metabolic, and oxidative stress parameters in a maternal immune stimulation model of neurodevelopmental brain disorders. Int. J. Neuropsychopharmacol. 2021, 24, 734–748. [Google Scholar] [CrossRef]
- Cheng, S.; Hou, J.; Zhang, C.; Xu, C.; Wang, L.; Zou, X.; Yu, H.; Shi, Y.; Yin, Z.; Chen, G. Minocycline reduces neuroinflammation but does not ameliorate neuron loss in a mouse model of neurodegeneration. Sci. Rep. 2015, 5, 10535. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rábago-Monzón, Á.R.; Osuna-Ramos, J.F.; Armienta-Rojas, D.A.; Camberos-Barraza, J.; Camacho-Zamora, A.; Magaña-Gómez, J.A.; De la Herrán-Arita, A.K. Stress-Induced Sleep Dysregulation: The Roles of Astrocytes and Microglia in Neurodegenerative and Psychiatric Disorders. Biomedicines 2025, 13, 1121. https://doi.org/10.3390/biomedicines13051121
Rábago-Monzón ÁR, Osuna-Ramos JF, Armienta-Rojas DA, Camberos-Barraza J, Camacho-Zamora A, Magaña-Gómez JA, De la Herrán-Arita AK. Stress-Induced Sleep Dysregulation: The Roles of Astrocytes and Microglia in Neurodegenerative and Psychiatric Disorders. Biomedicines. 2025; 13(5):1121. https://doi.org/10.3390/biomedicines13051121
Chicago/Turabian StyleRábago-Monzón, Ángel R., Juan F. Osuna-Ramos, David A. Armienta-Rojas, Josué Camberos-Barraza, Alejandro Camacho-Zamora, Javier A. Magaña-Gómez, and Alberto K. De la Herrán-Arita. 2025. "Stress-Induced Sleep Dysregulation: The Roles of Astrocytes and Microglia in Neurodegenerative and Psychiatric Disorders" Biomedicines 13, no. 5: 1121. https://doi.org/10.3390/biomedicines13051121
APA StyleRábago-Monzón, Á. R., Osuna-Ramos, J. F., Armienta-Rojas, D. A., Camberos-Barraza, J., Camacho-Zamora, A., Magaña-Gómez, J. A., & De la Herrán-Arita, A. K. (2025). Stress-Induced Sleep Dysregulation: The Roles of Astrocytes and Microglia in Neurodegenerative and Psychiatric Disorders. Biomedicines, 13(5), 1121. https://doi.org/10.3390/biomedicines13051121






