Correlative Analysis of Tumor-Informed Circulating Tumor DNA (ctDNA) and the Survival Outcomes of Patients with Pancreatic Adenocarcinoma
Abstract
1. Introduction
2. Methodology
2.1. Study Cohort and Sample Collection
2.2. Personalized mPCR-Based NGS Assay for ctDNA Detection
3. Results
3.1. Patient Characteristics
3.2. ctDNA as Detection for MRD
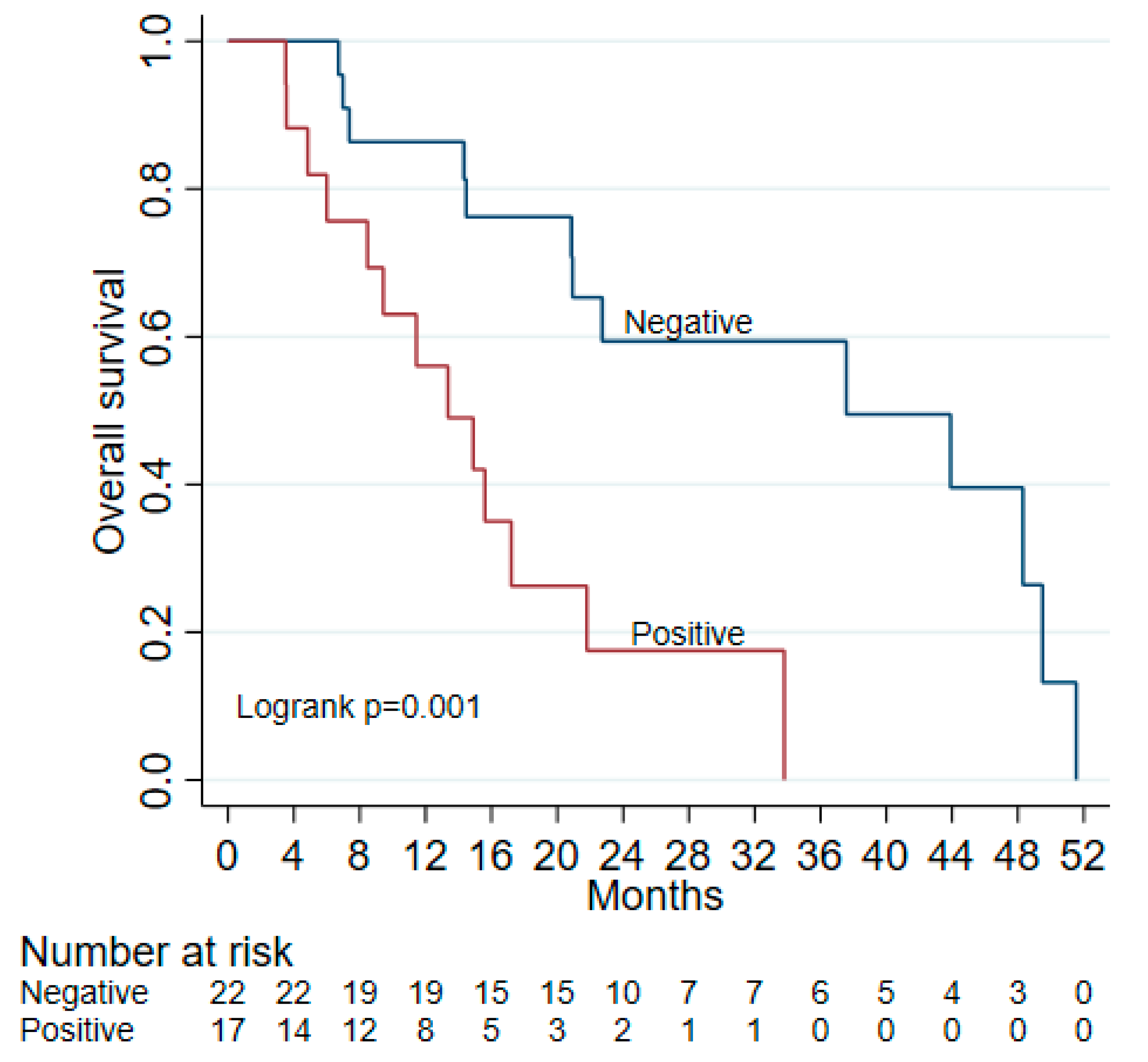
3.3. ctDNA as a Prognostic Marker
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Esmail, A.; Kasi, A.; Esnaola, N.F.; Xiu, J.; Baca, Y.; Weinberg, B.A. Comparative molecular profiling of pancreatic ductal adenocarcinoma of the head versus body and tail. NPJ Precis. Oncol. 2024, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Bugazia, D.; Al-Najjar, E.; Esmail, A.; Abdelrahim, S.; Abboud, K.; Abdelrahim, A.; Umoru, G.; Rayyan, H.A.; Abudayyeh, A.; Al Moustafa, A.E.; et al. Pancreatic ductal adenocarcinoma: The latest on diagnosis, molecular profiling, and systemic treatments. Front. Oncol. 2024, 14, 1386699. [Google Scholar] [CrossRef]
- Pancreatic Cancer Statistics; American Cancer Society: Atlanta, GA, USA, 2024.
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Badheeb, M.; Abdelrahim, A.; Esmail, A.; Umoru, G.; Abboud, K.; Al-Najjar, E.; Rasheed, G.; Alkhulaifawi, M.; Abudayyeh, A.; Abdelrahim, M. Pancreatic tumorigenesis: Precursors, genetic risk factors and screening. Curr. Oncol. 2022, 29, 8693–8719. [Google Scholar] [CrossRef] [PubMed]
- Esmail, A.; Badheeb, M.; Abdelrahim, M. Pancreatic Tumorigenesis: Precursors, Genetic Risk Factors and Screening. In Pancreatic Cancer-Updates in Pathogenesis, Diagnosis and Therapies; IntechOpen: London, UK, 2023. [Google Scholar]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef]
- Kwaśniewska, D.; Fudalej, M.; Nurzyński, P.; Badowska-Kozakiewicz, A.; Czerw, A.; Cipora, E.; Sygit, K.; Bandurska, E.; Deptała, A. How A Patient with Resectable or Borderline Resectable Pancreatic Cancer should Be Treated-A Comprehensive Review. Cancers 2023, 15, 4275. [Google Scholar] [CrossRef]
- Chikhladze, S.; Lederer, A.K.; Kousoulas, L.; Reinmuth, M.; Sick, O.; Fichtner-Feigl, S.; Wittel, U.A. Adjuvant chemotherapy after surgery for pancreatic ductal adenocarcinoma: Retrospective real-life data. World J. Surg. Oncol. 2019, 17, 185. [Google Scholar] [CrossRef]
- Lee, J.C.; Ahn, S.; Cho, I.K.; Lee, J.; Kim, J.; Hwang, J.H. Management of recurrent pancreatic cancer after surgical resection: A protocol for systematic review, evidence mapping and meta-analysis. BMJ Open 2018, 8, e017249. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Mori, S.; Kubota, K. Neoadjuvant and Adjuvant Chemotherapy for Pancreatic Adenocarcinoma: Literature Review and Our Experience of NAC-GS. Cancers 2024, 16, 910. [Google Scholar] [CrossRef] [PubMed]
- Vivarelli, M.; Mocchegiani, F.; Nicolini, D.; Vecchi, A.; Conte, G.; Dalla Bona, E.; Rossi, R.; Benedetti Cacciaguerra, A. Neoadjuvant Treatment in Resectable Pancreatic Cancer. Is It Time for Pushing on It? Front. Oncol. 2022, 12, 914203. [Google Scholar] [CrossRef]
- Sugumar, K.; Hue, J.J.; De La Serna, S.; Rothermel, L.D.; Ocuin, L.M.; Hardacre, J.M.; Ammori, J.B.; Winter, J.M. The importance of time-to-adjuvant treatment on survival with pancreatic cancer: A systematic review and meta-analysis. Cancer Rep. 2021, 4, e1390. [Google Scholar] [CrossRef]
- Khorana, A.A.; McKernin, S.E.; Berlin, J.; Hong, T.S.; Maitra, A.; Moravek, C.; Mumber, M.; Schulick, R.; Zeh, H.J.; Katz, M.H.G. Potentially Curable Pancreatic Adenocarcinoma: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2019, 37, 2082–2088. [Google Scholar] [CrossRef]
- Smaglo, B.G. Role for Neoadjuvant Systemic Therapy for Potentially Resectable Pancreatic Cancer. Cancers 2023, 15, 2377. [Google Scholar] [CrossRef]
- Lee, T.; Teng, T.Z.J.; Shelat, V.G. Carbohydrate antigen 19-9—Tumor marker: Past, present, and future. World J. Gastrointest. Surg. 2020, 12, 468–490. [Google Scholar] [CrossRef]
- Kim, S.; Park, B.K.; Seo, J.H.; Choi, J.; Choi, J.W.; Lee, C.K.; Chung, J.B.; Park, Y.; Kim, D.W. Carbohydrate antigen 19-9 elevation without evidence of malignant or pancreatobiliary diseases. Sci. Rep. 2020, 10, 8820. [Google Scholar] [CrossRef]
- Tsen, A.; Barbara, M.; Rosenkranz, L. Dilemma of elevated CA 19-9 in biliary pathology. Pancreatology 2018, 18, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Campos, N.M.F.; Almeida, V.; Curvo Semedo, L. Peritoneal disease: Key imaging findings that help in the differential diagnosis. Br. J. Radiol. 2022, 95, 20210346. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, C.; Hu, Y.; Zhang, W.; Yang, X.; Qi, Y.; Zhu, C.; Chen, X.; Shen, X.; Ji, W. Clinical application of molecular residual disease detection by circulation tumor DNA in solid cancers and a comparison of technologies: Review article. Cancer Biol. Ther. 2023, 24, 2274123. [Google Scholar] [CrossRef]
- Watanabe, F.; Suzuki, K.; Aizawa, H.; Endo, Y.; Takayama, Y.; Kakizawa, N.; Kato, T.; Noda, H.; Rikiyama, T. Circulating tumor DNA in molecular assessment feasibly predicts early progression of pancreatic cancer that cannot be identified via initial imaging. Sci. Rep. 2023, 13, 4809. [Google Scholar] [CrossRef]
- Botta, G.P.; Abdelrahim, M.; Aushev, V.N.; Esmail, A.; Drummond, B.; Sharma, S.; Kalashnikova, E.; Hook, N.; Chandana, S.R.; Tejani, M.A.; et al. Association of personalized and tumor-informed ctDNA with patient survival outcomes in pancreatic adenocarcinoma. J. Clin. Oncol. 2022, 40, 517. [Google Scholar] [CrossRef]
- Botta, G.P.; Abdelrahim, M.; Drengler, R.L.; Aushev, V.N.; Esmail, A.; Laliotis, G.; Brewer, C.M.; George, G.V.; Abbate, S.M.; Chandana, S.R.; et al. Association of personalized and tumor-informed ctDNA with patient survival outcomes in pancreatic adenocarcinoma. Oncologist 2024, 29, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Marrugo-Ramírez, J.; Mir, M.; Samitier, J. Blood-Based Cancer Biomarkers in Liquid Biopsy: A Promising Non-Invasive Alternative to Tissue Biopsy. Int. J. Mol. Sci. 2018, 19, 2877. [Google Scholar] [CrossRef]
- Crowley, E.; Di Nicolantonio, F.; Loupakis, F.; Bardelli, A. Liquid biopsy: Monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 2013, 10, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Vymetalkova, V.; Cervena, K.; Bartu, L.; Vodicka, P. Circulating Cell-Free DNA and Colorectal Cancer: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3356. [Google Scholar] [CrossRef]
- Merker, J.D.; Oxnard, G.R.; Compton, C.; Diehn, M.; Hurley, P.; Lazar, A.J.; Lindeman, N.; Lockwood, C.M.; Rai, A.J.; Schilsky, R.L.; et al. Circulating Tumor DNA Analysis in Patients With Cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J. Clin. Oncol. 2018, 36, 1631–1641. [Google Scholar] [CrossRef]
- Samandari, M.; Julia, M.G.; Rice, A.; Chronopoulos, A.; Del Rio Hernandez, A.E. Liquid biopsies for management of pancreatic cancer. Transl. Res. J. Lab. Clin. Med. 2018, 201, 98–127. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Okamura, R.; Fanta, P.; Patel, C.; Lanman, R.B.; Raymond, V.M.; Kato, S.; Kurzrock, R. Clinical correlates of blood-derived circulating tumor DNA in pancreatic cancer. J. Hematol. Oncol. 2019, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Hadano, N.; Murakami, Y.; Uemura, K.; Hashimoto, Y.; Kondo, N.; Nakagawa, N.; Sueda, T.; Hiyama, E. Prognostic value of circulating tumour DNA in patients undergoing curative resection for pancreatic cancer. Br. J. Cancer 2016, 115, 59–65. [Google Scholar] [CrossRef]
- Grunvald, M.W.; Jacobson, R.A.; Kuzel, T.M.; Pappas, S.G.; Masood, A. Current Status of Circulating Tumor DNA Liquid Biopsy in Pancreatic Cancer. Int. J. Mol. Sci. 2020, 21, 7651. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Rhee, T.M.; Pietrasz, D.; Bachet, J.B.; Laurent-Puig, P.; Kong, S.Y.; Takai, E.; Yachida, S.; Shibata, T.; Lee, J.W.; et al. Circulating tumor DNA as a prognostic indicator in resectable pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 16971. [Google Scholar] [CrossRef]
- Lapin, M.; Edland, K.H.; Tjensvoll, K.; Oltedal, S.; Austdal, M.; Garresori, H.; Rozenholc, Y.; Gilje, B.; Nordgård, O. Comprehensive ctDNA Measurements Improve Prediction of Clinical Outcomes and Enable Dynamic Tracking of Disease Progression in Advanced Pancreatic Cancer. Clin. Cancer Res. 2023, 29, 1267–1278. [Google Scholar] [CrossRef]

| ctDNA (−) | ctDNA (+) | |
|---|---|---|
| Male sex | 12 (55%) | 9 (53%) |
| Median age | 65 (48–82) | 68 (40–83) |
| Site of tumor | ||
| Head | 17 (77%) | 13 (76%) |
| Body/tail | 5 (23%) | 4 (24%) |
| Underwent resection | 19 (86%) | 11 (65%) |
| Margins clear on resection | 18 (95%) | 7 (64%) |
| Lymphovascular invasion present | 6 (32%) | 10 (91%) |
| ctDNA | CA19-9 | Combined | |
|---|---|---|---|
| Sensitivity | 0.913 | 0.826 | 0.98 |
| Specificity | 0.813 | 0.8 | 0.96 |
| Positive predictive value (PPV) | 0.875 | 0.863 | 0.97 |
| Negative predictive value (NPV) | 0.867 | 0.75 | 0.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Esmail, A.; Hassanain, H.; Dhillon, V.; Abdelrahim, W.; Al-Najjar, E.; Khasawneh, B.; Abdelrahim, M. Correlative Analysis of Tumor-Informed Circulating Tumor DNA (ctDNA) and the Survival Outcomes of Patients with Pancreatic Adenocarcinoma. Biomedicines 2025, 13, 1124. https://doi.org/10.3390/biomedicines13051124
Zhang Y, Esmail A, Hassanain H, Dhillon V, Abdelrahim W, Al-Najjar E, Khasawneh B, Abdelrahim M. Correlative Analysis of Tumor-Informed Circulating Tumor DNA (ctDNA) and the Survival Outcomes of Patients with Pancreatic Adenocarcinoma. Biomedicines. 2025; 13(5):1124. https://doi.org/10.3390/biomedicines13051124
Chicago/Turabian StyleZhang, Yuqi, Abdullah Esmail, Hala Hassanain, Vikramjit Dhillon, Waseem Abdelrahim, Ebtesam Al-Najjar, Bayan Khasawneh, and Maen Abdelrahim. 2025. "Correlative Analysis of Tumor-Informed Circulating Tumor DNA (ctDNA) and the Survival Outcomes of Patients with Pancreatic Adenocarcinoma" Biomedicines 13, no. 5: 1124. https://doi.org/10.3390/biomedicines13051124
APA StyleZhang, Y., Esmail, A., Hassanain, H., Dhillon, V., Abdelrahim, W., Al-Najjar, E., Khasawneh, B., & Abdelrahim, M. (2025). Correlative Analysis of Tumor-Informed Circulating Tumor DNA (ctDNA) and the Survival Outcomes of Patients with Pancreatic Adenocarcinoma. Biomedicines, 13(5), 1124. https://doi.org/10.3390/biomedicines13051124









