Abstract
Issues: The clinical and paraclinical characteristics of prolactinomas differ mainly according to sex and tumor size. Drug treatment with dopamine agonists (ADs) has a crucial role in the management of prolactinomas. The use of surgery also has its indications. Purpose of the work: We aimed to establish the therapeutic strategy and the follow-up profiles of prolactinoma while analyzing the predictive factors of remission; we also looked for correlations between the size of the prolactinoma and the various clinical and paraclinical parameters. Materials and methods: This was a retrospective, descriptive, and analytical study of 77 cases of prolactinomas collected and monitored at the endocrinology and diabetology department of the Hedi Chaker CHU in Sfax between 2000 and 2017. Our patients were divided into three groups according to the size of their prolactinomas. Statistical correlations were sought between tumor size and clinical and biological parameters. Results: The mean age of our patients was 38.3 ± 14.2 years. The sample comprised 51 women (66.2%) and 26 men (33.7%). Anterior pituitary syndrome was observed in 75.3% of cases. The number of antehypophyseal deficits was significantly correlated with tumor size. Comparing the three groups, we noted that age, discovery circumstances, metabolic parameters, hypopituitarism, and pituitary extensions on imaging were significantly different. Therapeutically, our results showed that the favorable evolution of prolactinomas was correlated with tumor size and the duration of treatment. Conclusions: Tumor size appears to be a cornerstone in hormonal and radiological interpretation on the one hand and in the therapeutic decision on the other.
1. Introduction
Prolactinoma is the main etiology of pathologic hyperprolactinemia [1]. Depending on the size of the tumor, we can distinguish microprolactinomas which are the most common (90% of cases). Macroprolactinomas are rarer. Among the latter, a distinction is made for giant prolactinomas, which are larger than 40 mm [2,3]. Macroprolactinomas are seen more frequently in men. Drug treatment with dopamine agonists (ADs) has a crucial role in the management of prolactinomas. However, the therapeutic strategy for prolactinomas has hitherto remained mainly dependent on the size of the tumor. The prognosis of these patients is not well established since it depends on several factors. In this context, we conducted this study to establish the prognostic factors of prolactin adenomas. The main objective of the study was to look for correlations between the size of the prolactinoma and the various clinical, paraclinical, and follow-up parameters.
2. Methods
This study is a retrospective, descriptive, and analytical investigation conducted at the Endocrinology Department of Hedi Chaker University Hospital in Sfax. It focused on cases of prolactinomas collected and monitored over an 18-year period, from 1 January 2000 to 31 December 2017.
2.1. Study Design and Population
We included all patients diagnosed with prolactinomas during the study period. Diagnosis was established based on elevated prolactin levels and confirmatory imaging findings on magnetic resonance imaging (MRI). To ensure consistency, patients with incomplete medical records or insufficient follow-up data were excluded.
The study population was divided into three groups based on tumor size, as determined by MRI:
Group 1 (G1): Microprolactinomas (<10 mm in size)—27 cases (35%).
Group 2 (G2): Macroprolactinomas (10–40 mm)—32 cases (41.6%).
Group 3 (G3): Giant prolactinomas (>40 mm)—18 cases (23.4%).
2.2. Main Steps of the Study
The main steps of our study consisted of the following:
- A descriptive overview of the clinical, hormonal, and radiological features of prolactinomas and their comparison between the three groups.
- An evaluation of treatment strategies and outcomes across the three groups.
- An analytical study to identify predictive factors of prolactinoma remission.
2.3. Data Collection
For each patient, detailed clinical, biological, imaging, and anthropometric data were collected.
2.3.1. Clinical Parameters
Data on the presenting symptoms (e.g., galactorrhea, amenorrhea, infertility, or visual disturbances), demographics (age and sex), and anthropometric parameters including body mass index (BMI), weight, and height were collected.
2.3.2. Biological Parameters
The hormonal profile included serum prolactin, follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone, estradiol, and cortisol levels.
Central hypothyroidism is defined as low free T4 with inappropriately low or normal TSH.
Hypogonadotropic hypogonadism is defined as low sex hormone levels (testosterone in men; estradiol in women) with low or inappropriately normal gonadotropin levels (FSH and LH).
Corticotroph deficiency is defined as low morning cortisol levels (<5 µg/dL) or insufficient response to a standard ACTH stimulation test.
A metabolic panel includes total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and fasting glucose.
2.3.3. Imaging Parameters
Imaging parameters included tumor size, location, and invasion based on MRI findings.
2.4. Treatment and Evaluation of Outcomes
We evaluated the indications and outcomes of both medical and surgical treatments. Medical treatment primarily consisted of dopamine agonists (DAs) such as bromocriptine, cabergoline, and quinagolide. Surgical intervention was considered for patients with DA resistance, intolerance, or complications requiring immediate tumor debulking.
2.5. Definitions of Treatment Outcomes
Remission: Defined as the disappearance of the prolactinoma on MRI with a normal prolactin level sustained for at least 24 months with minimal dose DA therapy.
Favorable Outcome: Characterized by the normalization of prolactin levels and tumor size reduction by more than 50% upon a follow-up MRI.
Relapse: Categorized as early (re-increase in prolactin >30 ng/mL within one year of normalization) or late (re-increase >30 ng/mL after one year of normalization).
Resistance to Dopamine Agonists (DAs): Defined as failure to achieve prolactin normalization or tumor size reduction ≥ 50% after three months of DA therapy at maximum tolerated doses (15 mg/day bromocriptine, 4 mg/week cabergoline, or 300 mg/day quinagolide).
Dosage Timing: Hormonal and imaging evaluations were conducted at regular intervals: 1 month, 6 months, 1 year, 5 years, and 10 years post-initiation of treatment. This enabled tracking of both short-term and long-term outcomes.
2.6. Statistical Analysis
The data entry was carried out on a computerized form by the SPSS software (version 20). A correlation study was carried out in this work between the different qualitative and quantitative variables. The difference between the results was considered significant when the p-value was <0.05. The link between two continuous variables was tested by Pearson’s correlation in the case of Gaussian distribution and by Spearman’s correlation whenever the normality of the distributions was not respected. We also used the Chi-square test for the correlation between the qualitative values with recourse to the Fisher test in the event of small numbers. Student’s tests and ANOVA tests were used to seek a correlation between quantitative variables.
3. Results
3.1. Epidemiological Data
Our study involved 77 cases of prolactin adenomas including 26 men (33.7%) and 51 women (66.2%). The mean age of our patients was 38.3 ± 14.2 years with extremes ranging from 14 to 75 years. Our results showed that the larger the tumor, the more advanced the age of diagnosis. Indeed, in G1, the mean age was 31.4 years versus 41.5 and 42.9 years in G2 and G3, respectively. The statistical study showed the existence of a positive and significant correlation between the age of discovery and the size of the prolactinoma (p = 0.003 and Pearson’s r coefficient = 0.348).
3.2. Anthropometric Data
Obesity was more common in G3. Paralleling this finding, mean waist size was significantly higher in G3 irrespective of sex. Anthropometric data are summarized in Table 1.

Table 1.
Anthropometric parameters by group.
3.3. Biological Data
3.3.1. Metabolic Parameters
Metabolic syndrome and carbohydrate tolerance disorders were significantly more frequent in G3 versus G1 and G2. (Table 2).

Table 2.
Metabolic disorders by group.
3.3.2. Prolactinemia
The mean prolactinemia was higher in G3 (10,569.1 ng/mL) versus G2 (523.4 ng/mL) and G3 (164.1 ng/mL) with a statistically significant difference (p = 0.002). This same result was also demonstrated with the medians (Figure 1).
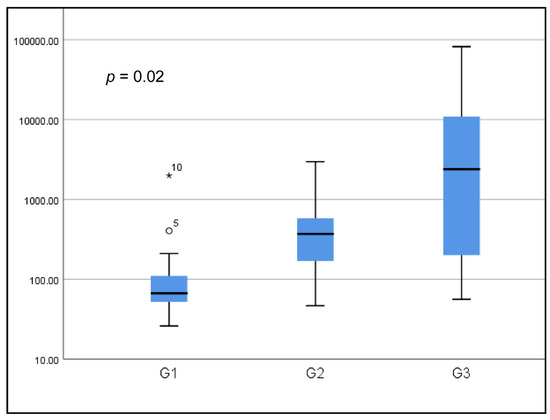
Figure 1.
Boxplot of prolactin levels according to groups (logarithmic scale).
Overall, the tumor size category impacted prolactinemia level distribution (p = 0.002). Prolactinemia < 100 ng/mL was more frequently reported in G1 (78.3%) with a statistically significant difference (p < 0.005). Only three patients (11.5%) of G1 presented prolactinemia > 200 ng/mL. Concerning prolactinemia between 100 and 200 ng/mL, the values were equally distributed between the three groups. Prolactinemia of between 200 and 1000 ng/mL was observed mainly in G2 (84.2%) (Figure 2). Prolactinemia > 1000 ng/mL was significantly more common in group 3 (p < 0.005).
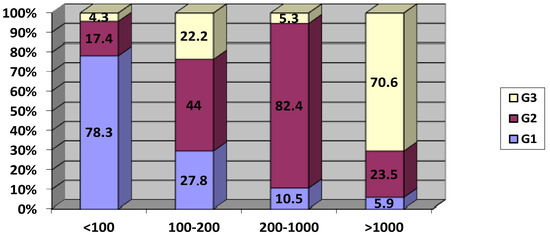
Figure 2.
Distribution of tumor size category according to serum prolactin levels (ng/mL).
An overall statistically significant positive correlation was found between the size of the prolactinoma and the prolactinemia (p = 0.001 and r = 0.4). Intriguingly, this correlation was not significant when tested in each group separately (Figure 3).
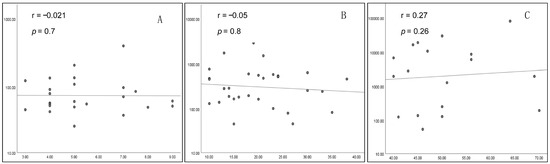
Figure 3.
Correlation between tumor size (mm) and prolactinemia (log scale) ((A) G1; (B) G2; (C) G3).
3.3.3. Other Hormonal Dosages
In both sexes, the mean FSH and LH levels were lower in G3 versus G1 and G2 (p < 0.05). Mean testosteronemia in men was also lower in G3 versus G1 and G2 but this difference was not significant (Table 3).

Table 3.
Comparison of the means of FSH, LH, and testosterone between the 3 groups.
The comparison between the three groups showed that the mean number of antehypophyseal deficits was significantly greater in G3 (2.17) versus G2 (1.25) and G1 (0.70) with a statistically significant difference (p = 0.008). The number of prolactinomas with only one affected axis was higher in G1 (14 cases) versus G2 (12 cases) and G3 (3 cases). A statistically significant difference was found concerning the frequency of each anterior pituitary deficit in the three groups (Table 4).

Table 4.
Frequency of anterior pituitary deficits by group.
In each group, it was observed that the more the size of the prolactinoma increased, the more the number of AH deficits was important (p < 0.001). This finding was irrespective of the group.
3.4. Treatment
Recourse to medical treatment alone was more frequent in G1 (92.1%) versus G2 and G3 (62.5% and 61.1%, respectively). A positive and statistically significant correlation was established between the duration of treatment and the size of the prolactinoma (p = 0.020). The use of surgical treatment was greater in G2 and G3 (37.5% and 38.8%) versus G1 (7.4%). The difference was statistically significant between the three groups (p = 0.024) (Table 5).

Table 5.
Frequency of treatment used by group.
3.5. Follow-Up Data
Normalization of prolactinemia was observed in 47 patients (61%) after an average of 16.5 months (1–120 months). It mainly depended on tumor size and duration of treatment. In fact, it was more frequent in G1 (70.3%) versus G2 (59.3%) and G3 (38.8%) patients. At one month of treatment, there was no difference in the normalization of prolactinemia between the groups. Beyond 6 months, G1 and G2 patients tended to normalize their prolactinemia more frequently than G3. This difference reaches statistical significance after 5 years. (Table 6).

Table 6.
Progression of prolactin levels by group.
Early and late relapses were observed in 9 (11.4%) and 15 patients (19.4%), respectively. Remission was observed in 10 patients (13%). It was more common in patients treated with CB (50%), those who were female (80%), and those with microprolactinomas (40%) (Table 7). Resistance was found in 10 patients (13%). It was more frequent in patients treated with BC (100%), those who were female (60%), and those with macroprolactinomas (70%) and giant prolactinomas (30%) (Table 7).

Table 7.
Distribution of remission and resistance.
Regarding the predictive factors of remission, the reduction in tumor size by more than 50% depended, on the one hand, on the initial tumor size (p = 0.001) and, on the other hand, on the duration of treatment (p = 0.000) (Table 8).

Table 8.
Remission parameters as a function of tumor size, dose, and duration of treatment.
4. Discussion
A statistically significant positive correlation was revealed between the age of discovery and the size of the prolactinoma. This is also confirmed by a large study carried out in Iceland [4,5,6,7] where the age difference between macroprolactinomas and microprolactinomas was 10 years (42 years versus 32.5 years, respectively).
In our study, the frequency of the male sex gradually increased with the size of the adenoma; it increased from 11.2% in G1 to 77.7% in G3. This is explained in the literature by the fact that the adenoma has higher proliferative indexes (ki67 and PANCA) in men than in women [8,9,10,11]. Other studies also show that the activity and expression of transforming growth factor B1 (TGFB1) is elevated in men, leading to increased size and aggressiveness of the prolactinoma [8,12,13].
In our study, the mean initial prolactinemia was 2745.9 ng/mL (26–81,940 ng/mL). It was 933.5 ng/mL (32–21,200 ng/mL) in a Brazilian multicenter study including 250 macroprolactinomas and 444 microprolactinomas [14]. There is a close relationship between the secretory level and the size of the adenoma [11,14,15]. In our population, a statistically significant positive correlation was observed between the size of the prolactinoma and the prolactinemia (p < 0.001). This result was also confirmed in the work of Chambeh et al. with 37 prolactinomas included in their study (p < 0.001) [16]. A positive correlation was also found between prolactinemia and tumor size within the three groups. This correlation is well established in the literature for giant prolactinomas (p < 0.001) [17,18,19,20].
The prognosis of prolactin adenoma also depends on their hormonal and metabolic impact. In this context, a statistically significant difference was found between the three groups concerning the frequency of each anterior pituitary deficit which was more frequent in G3. Another positive and significant correlation was demonstrated between the size of the prolactinoma and the number of anterior pituitary deficits that affected our patients (p < 0.01). Hormonal assays also demonstrated that the mean FSH and LH levels were significantly lower in G3 versus G1 and G2. Studies by Amit Tirosh and Sibal L [21,22,23,24] confirmed the same results.
On the metabolic level, in our study, obese and/or overweight patients were statistically more frequent in G3 versus G1 and G2. Likewise, waist size means were greater in G3 in both sexes. According to two studies in the literature [10,25], patients with a higher prolactin level have a higher BMI and WS. The correlation between these parameters is not significant. However, the decrease in BMI and WS after 2 to 6 months of treatment is significant in men [25,26,27]. In the literature, hyperprolactinemia is frequently associated with a carbohydrate disorder [10,25]. Several physiopathological phenomena have been suggested concerning this subject. Indeed, prolactin causes a state of insulin resistance (IR) and hyperinsulinism secondary to the increase in the cell mass of the islets of Langerhans [28]. Overall, metabolic syndrome (MS) was more frequently observed in G3 (58.8%) versus G1 (22.2%) and G2 (37.5%) patients with a statistically significant difference. The studies by Auriemma and Dos Santos Silva [25,27] confirm our findings.
The follow-up data of our patients showed that the success of surgery for microprolactinomas is estimated at 75% [15] and can reach 90% with trained surgeons [29,30,31,32]. For macroprolactinomas, the results of two prospective studies [33,34] show the normalization of prolactinemia under CB in 77% of cases and a significant reduction in tumor size in 92% of patients. The maximum effectiveness of CB is reported after 6 months of treatment. These results are not close to our findings since the majority of our patients were treated with first-line BC. The better efficacy of CB compared to BC is confirmed by statistically significant results according to Vroonen et al. [35].
Regarding giant prolactinomas in our study, medical treatment was weakly effective, unlike the studies by Maiter et al. [18] and Espinosa et al. [17] which showed that the giant tumor responds well to medical treatment. In fact, they noted a reduction of more than 25% in tumor size on average in 74% to 98% of cases and the normalization of prolactinemia in 55% to 60% of cases. This discrepancy with our results could be explained by the large number of patients lost to follow-up in G3. The disappearance of giant prolactinoma after medical treatment is described in the literature [36,37,38]. Indeed, some authors recommend a high dose of cabergoline (3.5 to 4.5 mg/week). Others offer long-term treatment for up to 20 years. In most of the studies [19,31,39,40], surgery for giant prolactinomas is generally associated with the persistence of hyperprolactinemia and a tumor residue requiring the use of ADs postoperatively. Early recurrence is reported in 45.9% of patients in the study of Sala E et al. [25]. This does not depend on tumor size or gender, but it is correlated (p = 0.03) with prolactinemia at the time of diagnosis and upon discontinuation of treatment. In a large meta-analysis including 19 studies [41], stable normoprolactinemia was demonstrated in 21% of microprolactinomas and 16% of macroprolactinomas after discontinuation of AD [4,5]. This assumes that the majority of patients suffer from a rebound effect of hyperprolactinemia upon discontinuation of medical treatment.
In the case of resistance to treatment, data in the literature suggest that this phenomenon is rather observed in men, macroprolactinomas, and patients treated with BC (25% versus 10% for CB) [22,35]. The mechanism of this resistance is explained by the decrease in the number of dopaminergic (D2) receptors in resistant prolactinomas [42,43,44]. A molecular alteration downstream of D2 or a difference in the expression of the isoforms of D2 (D2415/D2444) could also explain this phenomenon [43,45,46,47]. According to various studies [29,48,49,50], remission is significantly more frequent in microprolactinomas (between 65% and 78%) compared to macroprolactinomas (between 57% and 36%). According to Teixeira et al. [49], remission is influenced only by the initial tumor size. Other studies [48,51,52,53] confirm that female sex and the use of CB are considered to be factors promoting remission, which is consistent with our results. Concerning the predictive factors of remission, Hofstetter and Amar [54,55,56] demonstrated a statistically significant negative correlation between the rate of prolactinemia at day 1 postoperatively and the rate of remission. In a study by Catarina Araújo et al. [28], which included 67 patients, the normalization of prolactinemia and the reduction in tumor volume by more than 50% only depended on the total duration of treatment (p = 0.001) and the maximum dose of ADs (p = 0.019). Our results confirmed the role of the duration of treatment (p = 0.027) and the initial size of the adenoma (p = 0.001) in remission. This was not influenced by the maximum dose of ADs. In fact, the latter was not achieved in many of our patients mainly due to the lack of means.
5. Conclusions
Our study made it possible to highlight the metabolic and hormonal repercussions of prolactinomas. The latter were significantly associated with the occurrence of metabolic syndrome, abnormalities in glucose tolerance, and antehypophyseal deficits. These abnormalities were all the more important for larger tumor sizes. This work also made it possible to identify the predictive factors of remission of prolactinomas, namely the duration of treatment and a smaller initial size of the adenoma. These results should guide the initial assessment and therapeutic management of prolactin adenomas.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines13051125/s1, Figure S1: Distribution of prolactin level by group; Table S1: Epidemiological characteristics of study’s population; Table S2: Frequency of anterior pituitary deficits; Table S3: Frequency of used treatment by group; Table S4: Evolution of prolactin levels by group; Table S5: Distribution of remission and resistance; Table S6: Remission parameters as a function of tumor size, dose and duration of treatment.
Author Contributions
Conceptualization, M.E. and F.L.; methodology, H.F.; software, H.F.; validation, N.M.R., D.B.S. and M.E.; formal analysis, K.B.; investigation, M.E.; resources, F.L.; data curation, D.B.S.; writing—original draft preparation, M.E.; writing—review and editing, H.F.; visualization, K.B.; supervision, N.M.R.; project administration, M.E.; funding acquisition, F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Hedi Chaker Hospital (protocol code 213/2l).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Glezer, A.; Bronstein, M.D. Prolactinoma. Arq. Bras. Endocrinol. Metabol. 2014, 58, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Gruppetta, M.; Vassallo, J. Epidemiology and radiological geometric assessment of pituitary macroadenomas: Population-based study. Clin. Endocrinol. 2016, 85, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, J.-F. Magnetic Resonance Imaging of Pituitary Tumors. Front. Horm. Res. 2016, 45, 97–120. [Google Scholar] [PubMed]
- Hu, J.; Zheng, X.; Zhang, W.; Yang, H. Current drug withdrawal strategy in prolactinoma patients treated with cabergoline: A systematic review and meta-analysis. Pituitary 2015, 18, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Sala, E.; Bellaviti Buttoni, P.; Malchiodi, E.; Verrua, E.; Carosi, G.; Profka, E.; Rodari, G.; Filopanti, M.; Ferrante, E.; Spada, A.; et al. Recurrence of hyperprolactinemia following dopamine agonist withdrawal and possible predictive factors of recurrence in prolactinomas. J. Endocrinol. Investig. 2016, 39, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Casanueva, F.F.; Hoffman, A.R.; Kleinberg, D.L.; Montori, V.M.; Schlechte, J.A.; Wass, J.A.H. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Agustsson, T.T.; Baldvinsdottir, T.; Jonasson, J.G.; Olafsdottir, E.; Steinthorsdottir, V.; Sigurdsson, G.; Thorsson, A.V.; Carroll, P.V.; Korbonits, M.; Benediktsson, R. The epidemiology of pituitary adenomas in Iceland, 1955–2012: A nationwide population-based study. Eur. J. Endocrinol. 2015, 173, 655–664. [Google Scholar] [CrossRef]
- Larouche, V.; Correa, J.A.; Cassidy, P.; Beauregard, C.; Garfield, N.; Rivera, J. Prevalence of autoimmune disease in patients with prolactinomas and non-functioning pituitary adenomas. Pituitary 2016, 19, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Delgrange, E.; Trouillas, J.; Maiter, D.; Donckier, J.; Tourniaire, J. Sex-related difference in the growth of prolactinomas: A clinical and proliferation marker study. J. Clin. Endocrinol. Metab. 1997, 82, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Delemer, B. Adénomes à prolactine: Diagnostic et prise en charge. Presse Médicale 2009, 38, 117–124. [Google Scholar] [CrossRef]
- Recouvreux, M.V.; Faraoni, E.Y.; Camilletti, M.A.; Ratner, L.; Abeledo-Machado, A.; Rulli, S.B.; Díaz-Torga, G. Sex differences in the pituitary TGFβ1 system: The role of TGFβ1 in prolactinoma development. Front. Neuroendocrinol. 2018, 50, 118–122. [Google Scholar] [CrossRef]
- Kuroda, S.; Yonekawa, Y.; Kawano, T. Pituitary prolactinoma associated with polycystic ovary—case report. Neurol. Med. Chir. 1991, 31, 736–739. [Google Scholar] [CrossRef]
- De, A.; Morgan, T.E.; Speth, R.C.; Boyadjieva, N.; Sarkar, D.K. Pituitary lactotrope expresses transforming growth factor beta (TGF beta) type II receptor mRNA and protein and contains 125I-TGF beta 1 binding sites. J. Endocrinol. 1996, 149, 19–27. [Google Scholar] [CrossRef]
- Vilar, L.; Abucham, J.; Albuquerque, J.L.; Araujo, L.A.; Azevedo, M.F.; Boguszewski, C.L.; Casulari, L.A.; Cunha, M.B.C.; Czepielewski, M.A.; Duarte, F.H.G. Controversial issues in the management of hyperprolactinemia and prolactinomas—An overview by the Neuroendocrinology Department of the Brazilian Society of Endocrinology and Metabolism. Arch. Endocrinol. Metab. 2018, 62, 236–263. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, F.F.; Molitch, M.E.; Schlechte, J.A.; Abs, R.; Bonert, V.; Bronstein, M.D.; Brue, T.; Cappabianca, P.; Colao, A.; Fahlbusch, R. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin. Endocrinol. 2006, 65, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Chambeh, W. les Prolactinomes Modalités de Diagnostic, de Suivi et de Prise en Charge: À Propos de 37 Cas; Faculté de Medecine de Sousse: Sousse, Tunisia, 2009. [Google Scholar]
- Espinosa, E.; Sosa, E.; Mendoza, V.; Ramírez, C.; Melgar, V.; Mercado, M. Giant prolactinomas: Are they really different from ordinary macroprolactinomas? Endocrine 2016, 52, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Maiter, D.; Delgrange, E. Therapy of endocrine disease: The challenges in managing giant prolactinomas. Eur. J. Endocrinol. 2014, 170, R213–R227. [Google Scholar] [CrossRef] [PubMed]
- Shimon, I.; Sosa, E.; Mendoza, V.; Greenman, Y.; Tirosh, A.; Espinosa, E.; Popovic, V.; Glezer, A.; Bronstein, M.D.; Mercado, M. Giant prolactinomas larger than 60 mm in size: A cohort of massive and aggressive prolactin-secreting pituitary adenomas. Pituitary 2016, 19, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Arafah, B.M.; Nasrallah, M.P. Pituitary tumors: Pathophysiology, clinical manifestations and management. Endocr. Relat. Cancer 2001, 8, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Sibal, L.; Ugwu, P.; Kendall-Taylor, P.; Ball, S.G.; James, R.A.; Pearce, S.H.S.; Hall, K.; Quinton, R. Medical therapy of macroprolactinomas in males:, I. Prevalence of hypopituitarism at diagnosis. II. Proportion of cases exhibiting recovery of pituitary function. Pituitary 2002, 5, 243–246. [Google Scholar] [CrossRef]
- Tirosh, A.; Benbassat, C.; Lifshitz, A.; Shimon, I. Hypopituitarism patterns and prevalence among men with macroprolactinomas. Pituitary 2015, 18, 108–115. [Google Scholar] [CrossRef]
- Behan, L.A.; Moyles, P.; Cuesta, M.; Rogers, B.; Crowley, R.K.; Ryan, J.; Brennan, P.; Smith, D.; Tormey, W.; Sherlock, M.; et al. The incidence of anterior pituitary hormone deficiencies in patients with microprolactinoma and idiopathic hyperprolactinaemia. Clin. Endocrinol. 2017, 87, 257–263. [Google Scholar] [CrossRef]
- Mychka, V.B.; Chazova, I.E.; Dmitriev, V.V.; Masenko, V.P. Role of hypothalamo-hypophyseal-adrenal axis in the pathogenesis of arterial hypertension in patients with prolactinoma of the anterior lobe of the hypophysis. Ter. Arkh. 2000, 72, 10–13. [Google Scholar] [PubMed]
- Arafah, B.M.; Prunty, D.; Ybarra, J.; Hlavin, M.L.; Selman, W.R. The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J. Clin. Endocrinol. Metab. 2000, 85, 1789–1793. [Google Scholar]
- Berinder, K.; Nyström, T.; Höybye, C.; Hall, K.; Hulting, A.-L. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary 2011, 14, 199–207. [Google Scholar] [CrossRef]
- dos Santos Silva, C.M.; Barbosa, F.R.P.; Lima, G.A.B.; Warszawski, L.; Fontes, R.; Domingues, R.C.; Gadelha, M.R. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity 2011, 19, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.; Marques, O.; Almeida, R.; Santos, M.J. Macroprolactinomas: Longitudinal assessment of biochemical and imaging therapeutic responses. Endocrine 2018, 62, 470–476. [Google Scholar] [CrossRef]
- Jethwa, P.R.; Patel, T.D.; Hajart, A.F.; Eloy, J.A.; Couldwell, W.T.; Liu, J.K. Cost-Effectiveness Analysis of Microscopic and Endoscopic Transsphenoidal Surgery Versus Medical Therapy in the Management of Microprolactinoma in the United States. World Neurosurg. 2016, 87, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Rodríguez Berrocal, V.; Díez, J.J. Giant pituitary adenoma: Histological types, clinical features and therapeutic approaches. Endocrine 2018, 61, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Buchfelder, M.; Schlaffer, S. Surgical treatment of pituitary tumours. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 677–692. [Google Scholar] [CrossRef]
- Ikeda, H.; Watanabe, K.; Tominaga, T.; Yoshimoto, T. Transsphenoidal microsurgical results of female patients with prolactinomas. Clin. Neurol. Neurosurg. 2013, 115, 1621–1625. [Google Scholar] [CrossRef]
- Colao, A.; Vitale, G.; Cappabianca, P.; Briganti, F.; Ciccarelli, A.; De Rosa, M.; Zarrilli, S.; Lombardi, G. Outcome of cabergoline treatment in men with prolactinoma: Effects of a 24-month treatment on prolactin levels, tumor mass, recovery of pituitary function, and semen analysis. J. Clin. Endocrinol. Metab. 2004, 89, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Bhansali, A.; Dutta, P.; Singh, P.; Vijaivergiya, R.; Gupta, V.; Sachdeva, N.; Bhadada, S.K.; Walia, R. A comparison between intensive and conventional cabergoline treatment of newly diagnosed patients with macroprolactinoma. Clin. Endocrinol. 2013, 79, 409–415. [Google Scholar] [CrossRef]
- Vroonen, L.; Jaffrain-Rea, M.-L.; Petrossians, P.; Tamagno, G.; Chanson, P.; Vilar, L.; Borson-Chazot, F.; Naves, L.A.; Brue, T.; Gatta, B.; et al. Prolactinomas resistant to standard doses of cabergoline: A multicenter study of 92 patients. Eur. J. Endocrinol. 2012, 167, 651–662. [Google Scholar] [CrossRef]
- Wu, Z.B.; Yu, C.J.; Su, Z.P.; Zhuge, Q.C.; Wu, J.S.; Zheng, W.M. Bromocriptine treatment of invasive giant prolactinomas involving the cavernous sinus: Results of a long-term follow up. J. Neurosurg. 2006, 104, 54–61. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.F.; Magalhães Gonzaga, M.d.F.; Naves, L.A.; Luiz Mendonça, J.; Oton de Lima, B.; Casulari, L.A. Beneficial Effects of High Doses of Cabergoline in the Treatment of Giant Prolactinoma Resistant to Dopamine Agonists: A Case Report with a 21-Year Follow-Up. Horm. Res. Paediatr. 2018, 89, 63–70. [Google Scholar] [CrossRef]
- Berwaerts, J.; Verhelst, J.; Abs, R.; Appel, B.; Mahler, C. A giant prolactinoma presenting with unilateral exophthalmos: Effect of cabergoline and review of the literature. J. Endocrinol. Investig. 2000, 23, 393–398. [Google Scholar] [CrossRef]
- Davis, J.R.; Sheppard, M.C.; Heath, D.A. Giant invasive prolactinoma: A case report and review of nine further cases. QJM Int. J. Med. 1990, 74, 227–238. [Google Scholar]
- Andujar-Plata, P.; Villar-Taibo, R.; Ballesteros-Pomar, M.D.; Vidal-Casariego, A.; Pérez-Corral, B.; Cabezas-Agrícola, J.M.; Álvarez-Vázquez, P.; Serramito, R.; Bernabeu, I. Long-term outcome of multimodal therapy for giant prolactinomas. Endocrine 2017, 55, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, O.M.; Lagro, J.; Burman, P.; Jørgensen, J.O.; Romijn, J.A.; Pereira, A.M. Recurrence of Hyperprolactinemia after Withdrawal of Dopamine Agonists: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2010, 95, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Delgrange, E.; Daems, T.; Verhelst, J.; Abs, R.; Maiter, D. Characterization of resistance to the prolactin-lowering effects of cabergoline in macroprolactinomas: A study in 122 patients. Eur. J. Endocrinol. 2009, 160, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Kukstas, L.A.; Domec, C.; Bascles, L.; Bonnet, J.; Verrier, D.; Israel, J.M.; Vincent, J.-D. Different expression of the two dopaminergic D2 receptors, D2415 and D2444, in two types of lactotroph each characterised by their response to dopamine, and modification of expression by sex steroids. Endocrinology 1991, 129, 1101–1103. [Google Scholar] [CrossRef]
- Kovacs, K.; Stefaneanu, L.; Horvath, E.; Buchfelder, M.; Fahlbusch, R.; Becker, W. Prolactin-producing pituitary tumor: Resistance to dopamine agonist therapy. J. Neurosurg. 1995, 82, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Barlier, A.; Pellegrini-Bouiller, I.; Caccavelli, L.; Gunz, G.; Morange-Ramos, I.; Jaquet, P.; Enjalbert, A. Abnormal transduction mechanisms in pituitary adenomas. Horm. Res. 1997, 47, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.C.; Cox, T.A.; Fairholm, D.; Kostashuk, E.; Nugent, R. Testosterone-related exacerbation of a prolactin-producing macroadenoma: Possible role for estrogen. J. Clin. Endocrinol. Metab. 1987, 64, 391–394. [Google Scholar] [CrossRef]
- Hu, B.; Mao, Z.; Jiang, X.; He, D.; Wang, Z.; Wang, X.; Zhu, Y.; Wang, H. Role of TGF-β1/Smad3-mediated fibrosis in drug resistance mechanism of prolactinoma. Brain Res. 2018, 1698, 204–212. [Google Scholar] [CrossRef]
- Dogansen, S.C.; Selcukbiricik, O.S.; Tanrikulu, S.; Yarman, S. Withdrawal of dopamine agonist therapy in prolactinomas: In which patients and when? Pituitary 2016, 19, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Souteiro, P.; Carvalho, D. Prolactinoma management: Predictors of remission and recurrence after dopamine agonists withdrawal. Pituitary 2017, 20, 464–470. [Google Scholar] [CrossRef]
- Colao, A.; Di Sarno, A.; Guerra, E.; Pivonello, R.; Cappabianca, P.; Caranci, F.; Elefante, A.; Cavallo, L.M.; Briganti, F.; Cirillo, S.; et al. Predictors of remission of hyperprolactinaemia after long-term withdrawal of cabergoline therapy. Clin. Endocrinol. 2007, 67, 426–433. [Google Scholar] [CrossRef]
- Romijn, J.A. Hyperprolactinemia and prolactinoma. Handb. Clin. Neurol. 2014, 124, 185–195. [Google Scholar] [PubMed]
- Colao, A.; Lombardi, G.; Annunziato, L. Cabergoline. Expert. Opin. Pharmacother. 2000, 1, 555–574. [Google Scholar] [CrossRef]
- Webster, J.; Piscitelli, G.; Polli, A.; Ferrari, C.I.; Ismail, I.; Scanlon, M.F. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N. Engl. J. Med. 1994, 331, 904–909. [Google Scholar] [CrossRef]
- Rutkowski, M.J.; Aghi, M.K. Medical versus surgical treatment of prolactinomas: An analysis of treatment outcomes. Expert. Rev. Endocrinol. Metab. 2018, 13, 25–33. [Google Scholar] [CrossRef]
- Hofstetter, C.P.; Shin, B.J.; Mubita, L.; Huang, C.; Anand, V.K.; Boockvar, J.A.; Schwartz, T.H. Endoscopic endonasal transsphenoidal surgery for functional pituitary adenomas. Neurosurg. Focus. 2011, 30, E10. [Google Scholar] [CrossRef] [PubMed]
- Amar, A.P.; Couldwell, W.T.; Chen, J.C.T.; Weiss, M.H. Predictive value of serum prolactin levels measured immediately after transsphenoidal surgery. J. Neurosurg. 2002, 97, 307–314. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).