The IL-6/JAK/STAT3 Axis in Cholangiocarcinoma and Primary Sclerosing Cholangitis: Unlocking Therapeutic Strategies Through Patient-Derived Organoids
Abstract
1. Introduction
2. Materials and Methods
2.1. FFPE Samples from Surgically Resected Tumors
2.2. Immunohistochemistry
2.3. Analysis of Immunoreactivity
2.4. Fresh Tissue Samples from PSC Patients and from Patients with Liver Malignancies
2.5. Culture of Extrahepatic Cholangiocyte Organoid from PSC Patients and Tumor Organoids Derived from Primary or Metastatic Tumors of the Liver
2.6. Organoid Treatment with a JAK Inhibitor and Measurement of Cytokines in the Organoid Supernatant
2.7. DNA Sequencing
2.8. Chemotherapy Testing
2.9. Statistical Analysis
3. Results
3.1. Establishment of a Patient-Derived Organoid Biobank for PSC
3.2. Establishment of a Patient-Derived Organoid Biobank from Liver Tumors
3.3. A Patient-Derived Organoid Model Can Be Used to Test Chemotherapy Regimens in Different Liver Malignancies
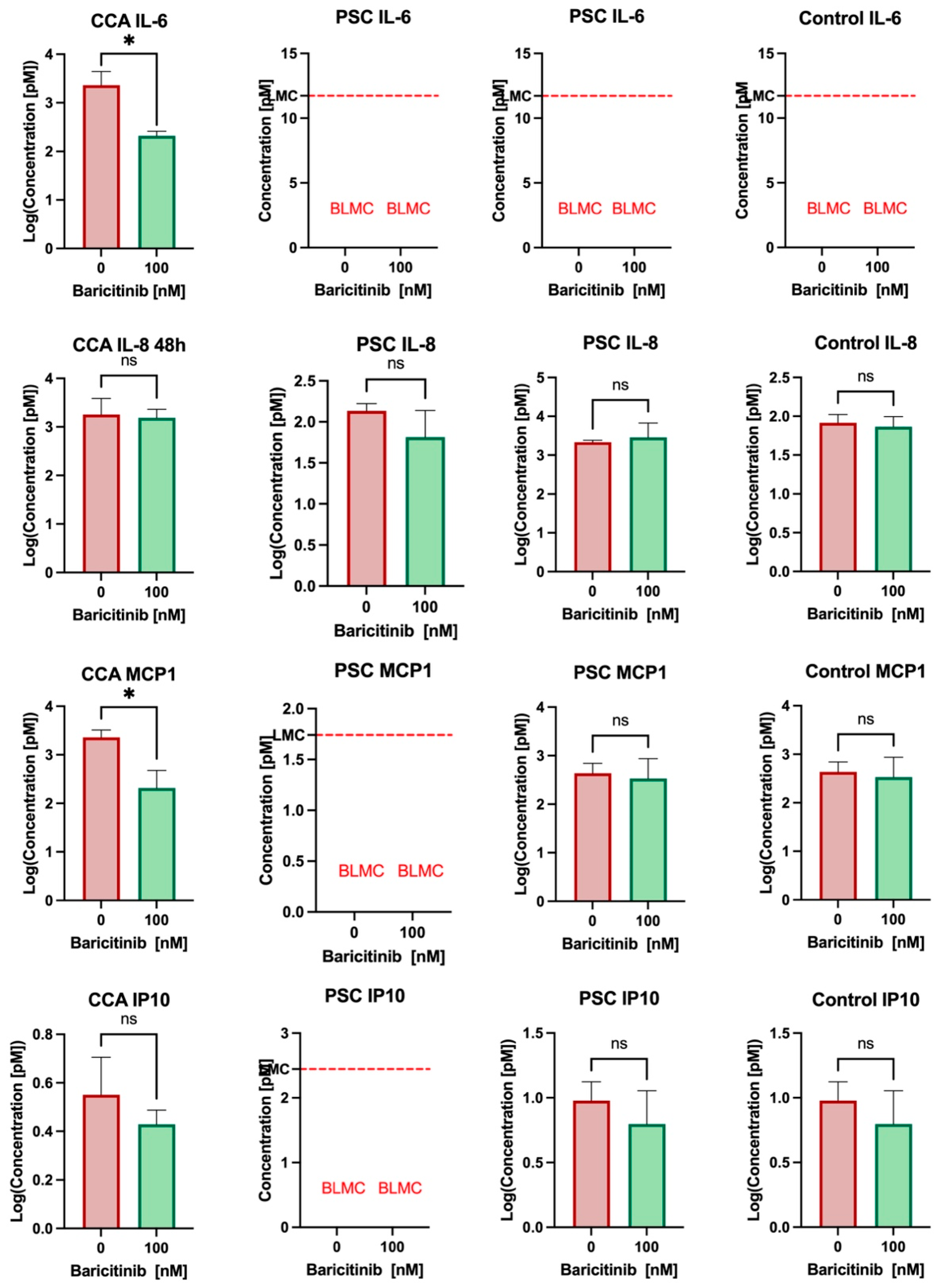
3.4. Baricitinib Reduces IL-6 and MCP1 Secretion in Cholangiocarcinoma and May Have an Effect on PSC Cholangiocytes’ Secretion
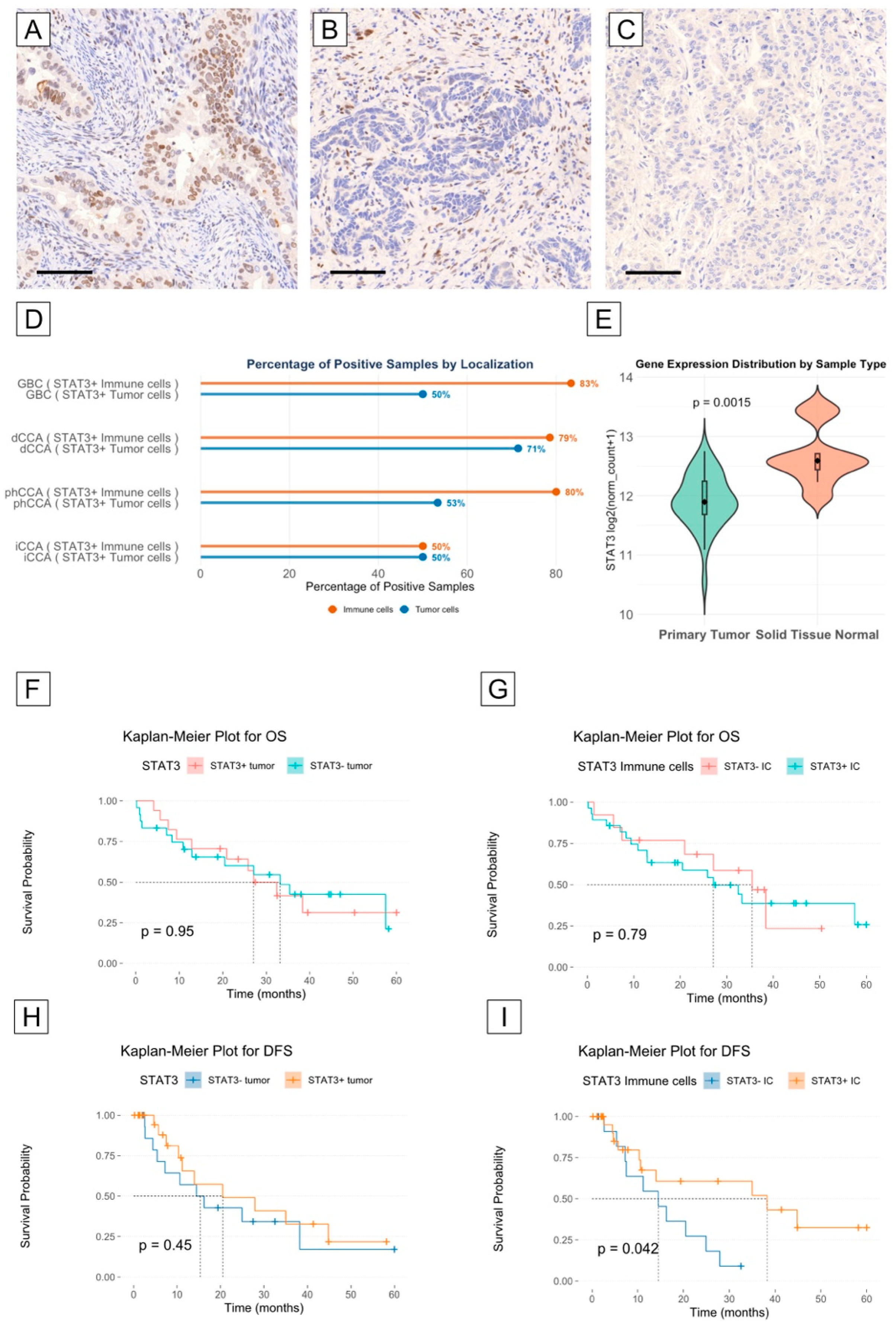
3.5. STAT3 Is Highly Expressed in the Tumor and Immune Microenvironment of Cholangiocarcinoma
3.6. STAT3 Expression May Correlate with Longer Disease-Free Survival in Cholangiocarcinoma Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PSC | Primary sclerosing cholangitis |
| BTC | Biliary tract cancer |
| CCA | Cholangiocarcinoma |
| iCCA | Intrahepatic cholangiocarcinoma |
| phCCA | Perihilar cholangiocarcinoma |
| dCCA | Distal cholangiocarcinoma |
| GBC | Gallbladder cancer |
| ERC | Endoscopic retrograde cholangiography |
| EMT | Epithelial-to-mesenchymal transition |
| FFPE | Formalin-fixed paraffin-embedded |
| ECO | extrahepatic cholangiocyte organoid |
| JAK | Janus kinase |
| IL-x | Interleukin |
| MCP-1 | Monocyte chemoattractant protein 1 |
| TNM | Tumor, node, metastasis |
| TMA | Tissue microarray |
| H&E | Hematoxylin–eosin |
| ECO | Extrahepatic cholangiocyte organoid |
| HCC | Hepatocellular carcinoma |
| CRCm | Colorectal metastasis |
| TGCA | The Cancer Genome Atlas |
| IDH1 | Isocitrate dehydrogenase 1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| IBD | Inflammatory bowel disease |
| OS | Overall survival |
| DFS | Disease-free survival |
References
- Lazaridis, K.N.; LaRusso, N.F. Primary Sclerosing Cholangitis. N. Engl. J. Med. 2016, 375, 1161–1170. [Google Scholar] [CrossRef]
- Dean, G.; Hanauer, S.; Levitsky, J. The Role of the Intestine in the Pathogenesis of Primary Sclerosing Cholangitis: Evidence and Therapeutic Implications. Hepatology 2020, 72, 1127–1138. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Bowlus, C.L.; Yimam, K.K.; Razavi, H.; Estes, C. Epidemiology, Natural History, and Outcomes of Primary Sclerosing Cholangitis: A Systematic Review of Population-Based Studies. Clin. Gastroenterol. Hepatol. 2022, 20, 1687–1700.e4. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Kareemi, H.; Parab, R.; Barkema, H.W.; Quan, H.; Myers, R.P.; Kaplan, G.G. Incidence of Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis. Hepatology 2011, 53, 1590–1599. [Google Scholar] [CrossRef]
- Takakura, W.R.; Tabibian, J.H.; Bowlus, C.L. The Evolution of Natural History of Primary Sclerosing Cholangitis. Curr. Opin. Gastroenterol. 2017, 33, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Lunder, A.K.; Hov, J.R.; Borthne, A.; Gleditsch, J.; Johannesen, G.; Tveit, K.; Viktil, E.; Henriksen, M.; Hovde, Ø.; Huppertz-Hauss, G.; et al. Prevalence of Sclerosing Cholangitis Detected by Magnetic Resonance Cholangiography in Patients with Long-Term Inflammatory Bowel Disease. Gastroenterology 2016, 151, 660–669.e4. [Google Scholar] [CrossRef]
- Folseraas, T.; Boberg, K.M. Cancer Risk and Surveillance in Primary Sclerosing Cholangitis. Clin. Liver Dis. 2016, 20, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, A.; Ekbom, A.; Olsson, R.; Kornfeldt, D.; Lööf, L.; Danielsson, Å.; Hultcrantz, R.; Lindgren, S.; Prytz, H.; Sandberg-Gertzén, H.; et al. Hepatic and Extrahepatic Malignancies in Primary Sclerosing Cholangitis. J. Hepatol. 2002, 36, 321–327. [Google Scholar] [CrossRef]
- Claessen, M.M.H.; Vleggaar, F.P.; Tytgat, K.M.A.J.; Siersema, P.D.; van Buuren, H.R. High Lifetime Risk of Cancer in Primary Sclerosing Cholangitis. J. Hepatol. 2009, 50, 158–164. [Google Scholar] [CrossRef]
- Hrad, V.; Abebe, Y.; Ali, S.H.; Velgersdyk, J.; Al Hallak, M.; Imam, M. Risk and Surveillance of Cancers in Primary Biliary Tract Disease. Gastroenterol Res. Pract. 2016, 2016, 3432640. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.M.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-Based Epidemiology, Malignancy Risk, and Outcome of Primary Sclerosing Cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Albert, J.; Arvanitakis, M.; Chazouilleres, O.; Dumonceau, J.-M.; Färkkilä, M.; Fickert, P.; Hirschfield, G.M.; Laghi, A.; Marzioni, M.; Fernandez, M.; et al. Role of Endoscopy in Primary Sclerosing Cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) Clinical Guideline. J. Hepatol. 2017, 66, 1265–1281. [Google Scholar] [CrossRef]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary Tract Cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Forner, A.; Vidili, G.; Rengo, M.; Bujanda, L.; Ponz-Sarvisé, M.; Lamarca, A. Clinical Presentation, Diagnosis and Staging of Cholangiocarcinoma. Liver Int. 2019, 39, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary Tract Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and Risk Factors. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of Liver Fluke Infection as Risk Factor for Cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert Consensus Document: Cholangiocarcinoma: Current Knowledge and Future Perspectives Consensus Statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk Factors for Intrahepatic and Extrahepatic Cholangiocarcinoma: A Systematic Review and Meta-Analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Nikolopoulos, G.K.; Aghemo, A.; Lleo, A.; Alqahtani, S.A.; Hassan, C.; Repici, A.; Bonovas, S. Environmental Risk Factors for Gallbladder Cancer: Field-Wide Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2024. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, Z.; Liu, K. Unraveling the Complexity of STAT3 in Cancer: Molecular Understanding and Drug Discovery. J. Exp. Clin. Cancer Res. 2024, 43, 23. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Li, S.Y.; Gong, H.Z.; Wang, L.X.; Lu, J.; Zhao, Y.X.; Gu, N. Clinicopathological and Prognostic Roles of STAT3 and Its Phosphorylation in Glioma. Dis. Markers 2020, 2020, 8833885. [Google Scholar] [CrossRef]
- Denley, S.M.; Jamieson, N.B.; McCall, P.; Oien, K.A.; Morton, J.P.; Carter, C.R.; Edwards, J.; McKay, C.J. Activation of the IL-6R/Jak/Stat Pathway Is Associated with a Poor Outcome in Resected Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Surg. 2013, 17, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Wang, J.; Jiang, N.; Pan, H.; Li, D. Correlation between P-STAT3 Overexpression and Prognosis in Lung Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0182282. [Google Scholar] [CrossRef]
- Sayed, D.; Badrawy, H.; Gaber, N.; Khalaf, M.R. P-Stat3 and Bcr/Abl Gene Expression in Chronic Myeloid Leukemia and Their Relation to Imatinib Therapy. Leuk. Res. 2014, 38, 243–250. [Google Scholar] [CrossRef]
- Chang, Q.; Bournazou, E.; Sansone, P.; Berishaj, M.; Gao, S.P.; Daly, L.; Wels, J.; Theilen, T.; Granitto, S.; Zhang, X.; et al. The IL-6/JAK/Stat3 Feed-Forward Loop Drives Tumorigenesis and Metastasis. Neoplasia 2013, 15, 848–862. [Google Scholar] [CrossRef]
- Zhang, H.X.; Yang, P.L.; Li, E.M.; Xu, L.Y. STAT3beta, a Distinct Isoform from STAT3. Int. J. Biochem. Cell Biol. 2019, 110, 130–139. [Google Scholar] [CrossRef]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically Exploiting STAT3 Activity in Cancer—Using Tissue Repair as a Road Map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef]
- Luo, Z.; Jegga, A.G.; Bezerra, J.A. Gene-Disease Associations Identify a Connectome with Shared Molecular Pathways in Human Cholangiopathies. Hepatology 2018, 67, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Xin-Wei, Y.; Liang, L.; Guo-Jun, H.; Xin-Zhou, Y.; Qin-Guo, X.; Lei, C.; Bao-Hua, Z.; Feng, S. STAT3 Overexpression Promotes Metastasis in Intrahepatic Cholangiocarcinoma and Correlates Negatively with Surgical Outcome. Oncotarget 2016, 8, 7710. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, H.; Zhou, Z.; Tian, Y.; Cao, X.; Cheng, G.; Liu, Q. Blocking of the EGFR-STAT3 Signaling Pathway through Afatinib Treatment Inhibited the Intrahepatic Cholangiocarcinoma. Exp. Ther. Med. 2018, 15, 4995–5000. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.A.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human Primary Liver Cancer–Derived Organoid Cultures for Disease Modeling and Drug Screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef]
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using “Ggplot2”. 2020. Available online: https://cran.r-project.org/web/packages/survminer/survminer.pdf (accessed on 15 January 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Therneau, T. A Package for Survival Analysis in R. R Package Version 3.5-5. 2023. Available online: https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf (accessed on 15 January 2025).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000; ISBN 0-387-98784-3. [Google Scholar]
- Sjoberg, D.D.; Whiting, K.; Curry, M.; Lavery, J.A.; Larmarange, J. Reproducible Summary Tables with the Gtsummary Package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- Iannone, R.; Cheng, J.; Schloerke, B.; Hughes, E.; Lauer, A.; Seo, J.; Brevoort, K.; Roy, O. Gt: Easily Create Presentation-Ready Display Tables. 2024. Available online: https://gt.rstudio.com/ (accessed on 15 January 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis. J. Stat. Softw. 2009, 35, 160–167. [Google Scholar]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and Interpreting Cancer Genomics Data via the Xena Platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in Vitro Model of Human Development and Disease. Nat. Cell Biol. 2016, 18, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef]
- Soroka, C.J.; Assis, D.N.; Alrabadi, L.S.; Roberts, S.; Cusack, L.; Jaffe, A.B.; Boyer, J.L. Bile-Derived Organoids From Patients With Primary Sclerosing Cholangitis Recapitulate Their Inflammatory Immune Profile. Hepatology 2019, 70, 871–882. [Google Scholar] [CrossRef]
- Nuciforo, S.; Fofana, I.; Matter, M.S.; Blumer, T.; Calabrese, D.; Boldanova, T.; Piscuoglio, S.; Wieland, S.; Ringnalda, F.; Schwank, G.; et al. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep. 2018, 24, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Muramatsu, T.; Kanai, Y.; Ojima, H.; Sukeda, A.; Hiraoka, N.; Arai, E.; Sugiyama, Y.; Matsuzaki, J.; Uchida, R.; et al. Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma. Cell Rep. 2019, 27, 1265–1276.e4. [Google Scholar] [CrossRef] [PubMed]
- Van De Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; Van Houdt, W.; Van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Trussoni, C.E.; O’Hara, S.P.; Splinter, P.L.; Heimbach, J.K.; LaRusso, N.F. Characterization of Cultured Cholangiocytes Isolated from Livers of Patients with Primary Sclerosing Cholangitis. Lab. Investig. 2014, 94, 1126–1133. [Google Scholar] [CrossRef]
- Verstegen, M.M.A.; Roos, F.J.M.; Burka, K.; Gehart, H.; Jager, M.; de Wolf, M.; Bijvelds, M.J.C.; de Jonge, H.R.; Ardisasmita, A.I.; van Huizen, N.A.; et al. Human Extrahepatic and Intrahepatic Cholangiocyte Organoids Show Region-Specific Differentiation Potential and Model Cystic Fibrosis-Related Bile Duct Disease. Sci. Rep. 2020, 10, 21900. [Google Scholar] [CrossRef]
- Roos, F.J.M.; Verstegen, M.M.A.; Muñoz Albarinos, L.; Roest, H.P.; Poley, J.W.; Tetteroo, G.W.M.; IJzermans, J.N.M.; van der Laan, L.J.W. Human Bile Contains Cholangiocyte Organoid-Initiating Cells Which Expand as Functional Cholangiocytes in Non-Canonical Wnt Stimulating Conditions. Front. Cell Dev. Biol. 2021, 8, 630492. [Google Scholar] [CrossRef]
- Moreno, A.S.G.; Guicciardi, M.E.; Wixom, A.Q.; Jessen, E.; Yang, J.; Ilyas, S.I.; Bianchi, J.K.; Pinto E Vairo, F.; Lazaridis, K.N.; Gores, G.J. IL-17 Signaling in Primary Sclerosing Cholangitis Patient-Derived Organoids. Hepatol. Commun. 2024, 8, e0454. [Google Scholar] [CrossRef]
- Chapman, R.W.; Williamson, K.D. Are Dominant Strictures in Primary Sclerosing Cholangitis a Risk Factor for Cholangiocarcinoma? Curr. Hepatol Rep. 2017, 16, 124–129. [Google Scholar] [CrossRef]
- Chapman, M.H.; Webster, G.J.M.; Bannoo, S.; Johnson, G.J.; Wittmann, J.; Pereira, S.P. Cholangiocarcinoma and Dominant Strictures in Patients with Primary Sclerosing Cholangitis; a 25 Year Single Centre Experience. Eur. J. Gastroenterol. Hepatol. 2012, 24, 1051. [Google Scholar] [CrossRef]
- Chikhoune, L.; Poggi, C.; Moreau, J.; Dubucquoi, S.; Hachulla, E.; Collet, A.; Launay, D. JAK Inhibitors (JAKi): Mechanisms of Action and Perspectives in Systemic and Autoimmune Diseases. Rev. Med. Interne 2025, 46, 89–106. [Google Scholar] [CrossRef]
- Asuri, S.; McIntosh, S.; Taylor, V.; Rokeby, A.; Kelly, J.; Shumansky, K.; Field, L.L.; Yoshida, E.M.; Arbour, L. Primary Biliary Cholangitis in British Columbia First Nations: Clinical Features and Discovery of Novel Genetic Susceptibility Loci. Liver Int. 2018, 38, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Dold, L.; Frank, L.; Lutz, P.; Kaczmarek, D.J.; Krämer, B.; Nattermann, J.; Weismüller, T.J.; Branchi, V.; Toma, M.; Gonzalez-Carmon, M.; et al. IL-6–Dependent STAT3 Activation and Induction of Proinflammatory Cytokines in Primary Sclerosing Cholangitis. Clin. Transl. Gastroenterol. 2023, 14, e00603. [Google Scholar] [CrossRef] [PubMed]
- Poch, T.; Krause, J.; Casar, C.; Liwinski, T.; Glau, L.; Kaufmann, M.; Ahrenstorf, A.E.; Hess, L.U.; Ziegler, A.E.; Martrus, G.; et al. Single-Cell Atlas of Hepatic T Cells Reveals Expansion of Liver-Resident Naive-like CD4+ T Cells in Primary Sclerosing Cholangitis. J. Hepatol. 2021, 75, 414–423. [Google Scholar] [CrossRef]
- Dokduang, H.; Techasen, A.; Namwat, N.; Khuntikeo, N.; Pairojkul, C.; Murakami, Y.; Loilome, W.; Yongvanit, P. STATs Profiling Reveals Predominantly-Activated STAT3 in Cholangiocarcinoma Genesis and Progression. J. Hepatobiliary Pancreat. Sci. 2014, 21, 767–776. [Google Scholar] [CrossRef]
- Cadamuro, M.; Strazzabosco, M. Inflammatory Pathways and Cholangiocarcinoma Risk Mechanisms and Prevention. Adv. Cancer Res. 2022, 156, 39–73. [Google Scholar] [CrossRef] [PubMed]
- Shriki, A.; Lanton, T.; Sonnenblick, A.; Levkovitch-Siany, O.; Eidelshtein, D.; Abramovitch, R.; Rosenberg, N.; Pappo, O.; Elgavish, S.; Nevo, Y.; et al. Multiple Roles of Il6 in Hepatic Injury, Steatosis, and Senescence Aggregate to Suppress Tumorigenesis. Cancer Res. 2021, 81, 4766–4777. [Google Scholar] [CrossRef]
- Cheon, Y.K.; Cho, Y.D.; Moon, J.H.; Jang, J.Y.; Kim, Y.S.; Kim, Y.S.; Lee, M.S.; Lee, J.S.; Shim, C.S. Diagnostic Utility of Interleukin-6 (IL-6) for Primary Bile Duct Cancer and Changes in Serum IL-6 Levels Following Photodynamic Therapy. Am. J. Gastroenterol. 2007, 102, 2164–2170. [Google Scholar] [CrossRef]
- Kleinegger, F.; Hofer, E.; Wodlej, C.; Golob-Schwarzl, N.; Birkl-Toeglhofer, A.M.; Stallinger, A.; Petzold, J.; Orlova, A.; Krassnig, S.; Reihs, R.; et al. Pharmacologic IL-6Rα Inhibition in Cholangiocarcinoma Promotes Cancer Cell Growth and Survival. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 308–321. [Google Scholar] [CrossRef]
- Nguyen, M.L.T.; Bui, K.C.; Scholta, T.; Xing, J.; Bhuria, V.; Sipos, B.; Wilkens, L.; Nguyen Linh, T.; Velavan, T.P.; Bozko, P.; et al. Targeting Interleukin 6 Signaling by Monoclonal Antibody Siltuximab on Cholangiocarcinoma. J. Gastroenterol. Hepatol. 2021, 36, 1334–1345. [Google Scholar] [CrossRef]
- Hao, Q.; Vadgama, J.V.; Wang, P. CCL2/CCR2 Signaling in Cancer Pathogenesis. Cell Commun. Signal. 2020, 18, 82. [Google Scholar] [CrossRef]
- Chen, W.; Gao, Q.; Han, S.; Pan, F.; Fan, W. The CCL2/CCR2 Axis Enhances IL-6-Induced Epithelial-Mesenchymal Transition by Cooperatively Activating STAT3-Twist Signaling. Tumor Biol. 2015, 36, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Zabransky, D.J.; Kartalia, E.; Lee, J.W.; Leatherman, J.M.; Charmsaz, S.; Young, S.E.; Chhabra, Y.; Franch-Expósito, S.; Kang, M.; Maru, S.; et al. Tumor-Derived CCL2 Drives Tumor Growth and Immunosuppression in IDH1-Mutant Cholangiocarcinoma. Hepatology 2024. [Google Scholar] [CrossRef] [PubMed]


| Sample Number | Age at ERCP | Sex | Age at First Diagnosis | Sampling | Amsterdam Score | Dominant Stricture | Dysplasia | Cancer | Diagnosis | Pathology Notes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | 49 | HDB | 3 | HDB | 0 | 0 | PSC | / |
| 2 | 49 | M | 17 | LHD | 3 | LHD | 0 | 0 | PSC | increased focal IG4 Plasma cells |
| 3 | 49 | M | 48 | LHD | 2 | LHD | 0 | 0 | PSC | / |
| 4 | 34 | F | 34 | HDB | 2 | HDB | 0 | 0 | PSC | / |
| 5 | 47 | F | 33 | LHD | 3 | LHD | 0 | 0 | PSC | / |
| 6 | 27 | M | 21 | LHD | 3 | LHD | 0 | 0 | PSC | / |
| 7 | 42 | M | 42 | HDB | 3 | HDB | 0 | 0 | PSC | / |
| 8 | 41 | M | 23 | LHD | 3 | LHD | 0 | 0 | PSC | / |
| 9 | 49 | F | 47 | HDB | / | HDB | 0 | 0 | Benign stricture | / |
| checked10 | 39 | F | 39 | CBD | 2 | CBD | 0 | 0 | PSC | / |
| Age | Sex | Tumor Type | Sampling Site | T | N | M | G | L | V | Pn | R |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 61 | M | phCCA | Segment III | pT2b | pN1 | M0 | G2 | L1 | V0 | Pn0 | R0 |
| 72 | F | iCCA | Segment V | pT3 | pN0 | M0 | G2 | L0 | V1 | Pn1 | R1 |
| 68 | M | phCCA | Segment IVa | pT2b | pN0 | M0 | G2 | L0 | V0 | Pn1 | R1 |
| 82 | F | HCC | Segment III | pT2 | pNx | M0 | G3 | L0 | V0 | Pn0 | R0 |
| 73 | F | iCCA | Segment IVa | 1b | pN0 | M0 | G2 | L0 | V0 | Pn0 | R1 |
| 76 | M | CRC Met | Segment III | pT4a | pN0 | M1 | G2 | L0 | V0 | Pn0 | R0 |
| 78 | M | HCC | Segment VIII | pT2 | pN0 | M0 | G3 | L0 | V1 | Pn0 | R0 |
| 75 | M | dCCA | CBD | pT3 | pN0 | M0 | G2 | L0 | V0 | Pn0 | R0 |
| 75 | M | HCC | Segment II | pT2 | pNx | M0 | G2 | L0 | V1 | Pn0 | R0 |
| 42 | F | CRC Met | Segment V | ypT2 | ypN0 | M2 | NA | L0 | V0 | Pn0 | R0 |
| 80 | F | phCCA | Segment III | pT1a | pN0 | M0 | G1 | L0 | V0 | Pn0 | R0 |
| 73 | M | CRC Met | Segment V | pT2 | pN0 | M2 | NA | L0 | V0 | Pn0 | R0 |
| 60 | M | HCC | Segment IVb | pT3 | pN0 | M0 | G2 | L0 | V1 | Pn0 | R0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boden, C.; Esser, L.K.; Dold, L.; Langhans, B.; Zhou, T.; Kaczmarek, D.J.; Gonzalez-Carmona, M.A.; Weismüller, T.J.; Kristiansen, G.; Kalff, J.C.; et al. The IL-6/JAK/STAT3 Axis in Cholangiocarcinoma and Primary Sclerosing Cholangitis: Unlocking Therapeutic Strategies Through Patient-Derived Organoids. Biomedicines 2025, 13, 1083. https://doi.org/10.3390/biomedicines13051083
Boden C, Esser LK, Dold L, Langhans B, Zhou T, Kaczmarek DJ, Gonzalez-Carmona MA, Weismüller TJ, Kristiansen G, Kalff JC, et al. The IL-6/JAK/STAT3 Axis in Cholangiocarcinoma and Primary Sclerosing Cholangitis: Unlocking Therapeutic Strategies Through Patient-Derived Organoids. Biomedicines. 2025; 13(5):1083. https://doi.org/10.3390/biomedicines13051083
Chicago/Turabian StyleBoden, Corinna, Laura K. Esser, Leona Dold, Bettina Langhans, Taotao Zhou, Dominik J. Kaczmarek, Maria A. Gonzalez-Carmona, Tobias J. Weismüller, Glen Kristiansen, Jörg C. Kalff, and et al. 2025. "The IL-6/JAK/STAT3 Axis in Cholangiocarcinoma and Primary Sclerosing Cholangitis: Unlocking Therapeutic Strategies Through Patient-Derived Organoids" Biomedicines 13, no. 5: 1083. https://doi.org/10.3390/biomedicines13051083
APA StyleBoden, C., Esser, L. K., Dold, L., Langhans, B., Zhou, T., Kaczmarek, D. J., Gonzalez-Carmona, M. A., Weismüller, T. J., Kristiansen, G., Kalff, J. C., Hölzel, M., Matthaei, H., Toma, M. I., & Branchi, V. (2025). The IL-6/JAK/STAT3 Axis in Cholangiocarcinoma and Primary Sclerosing Cholangitis: Unlocking Therapeutic Strategies Through Patient-Derived Organoids. Biomedicines, 13(5), 1083. https://doi.org/10.3390/biomedicines13051083







