Modulation of Neurturin Expression by Lumbosacral Spinal Stenosis, Lifestyle Factors, and Glycemic Dysregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Study Group
2.2. Pain Assessment
2.3. Surgical Procedure
2.4. Control Group
2.5. Sample Collection and Molecular Analysis
2.6. Ribonucleic Acid (RNA) Isolation and Quality Control
2.7. NRTN mRNA Analysis by Real-Time Polymerase Chain Reaction Technique Preceded by Reverse Transcription (RTqPCR)
2.8. NTRT Protein Concentration Analysis via Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Western Blot
2.10. Immunohistochemical (IHC) Detection of NRTN
2.11. Statistical Analysis
3. Results
3.1. Expression Profile of NRTN at the mRNA Level
3.2. Expression Profile of NRTN at the Protein Level in Test and Control Samples
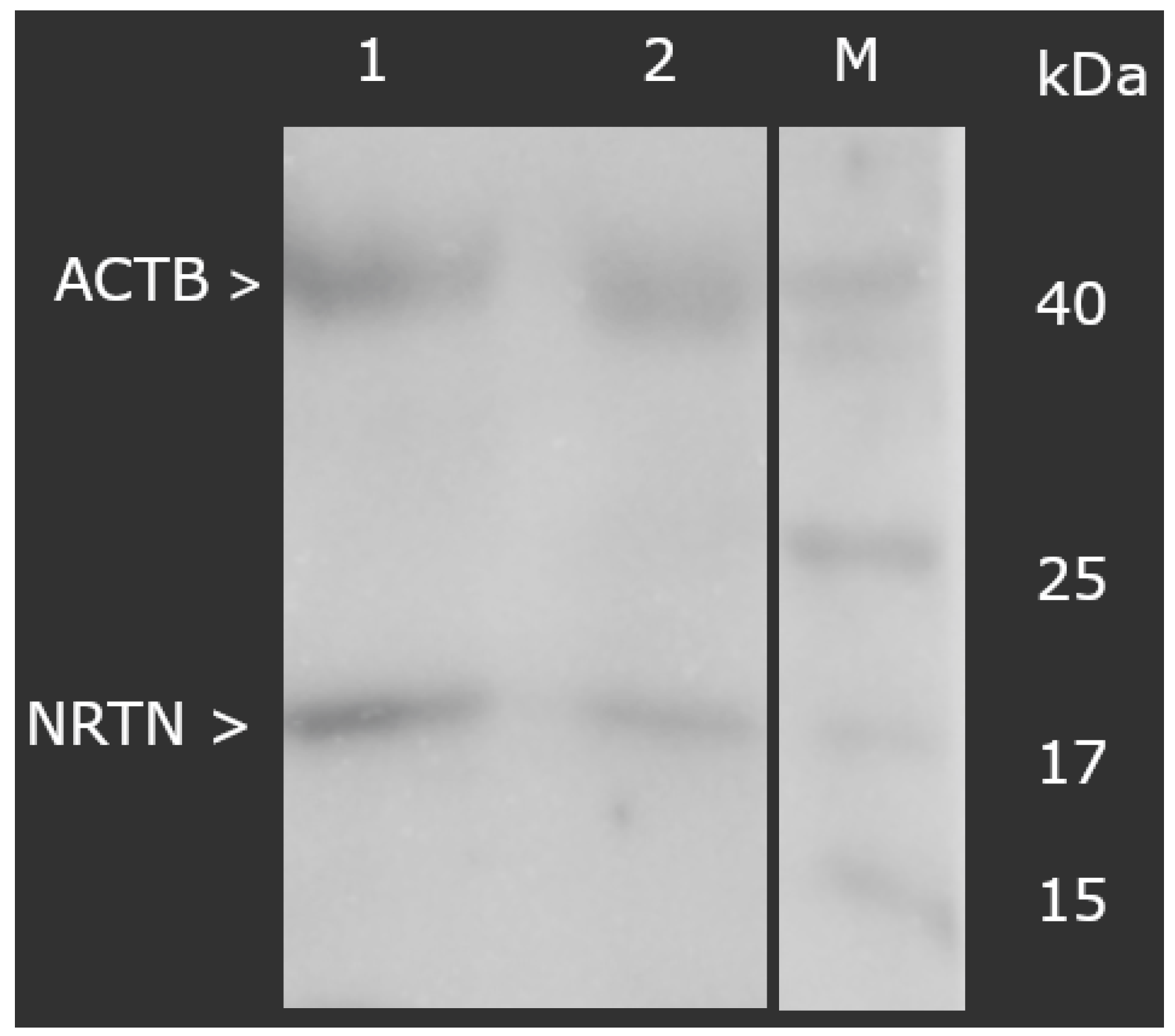
3.2.1. Concentration of NRTN Obtained via ELISA Assay and Western Blot Analysis
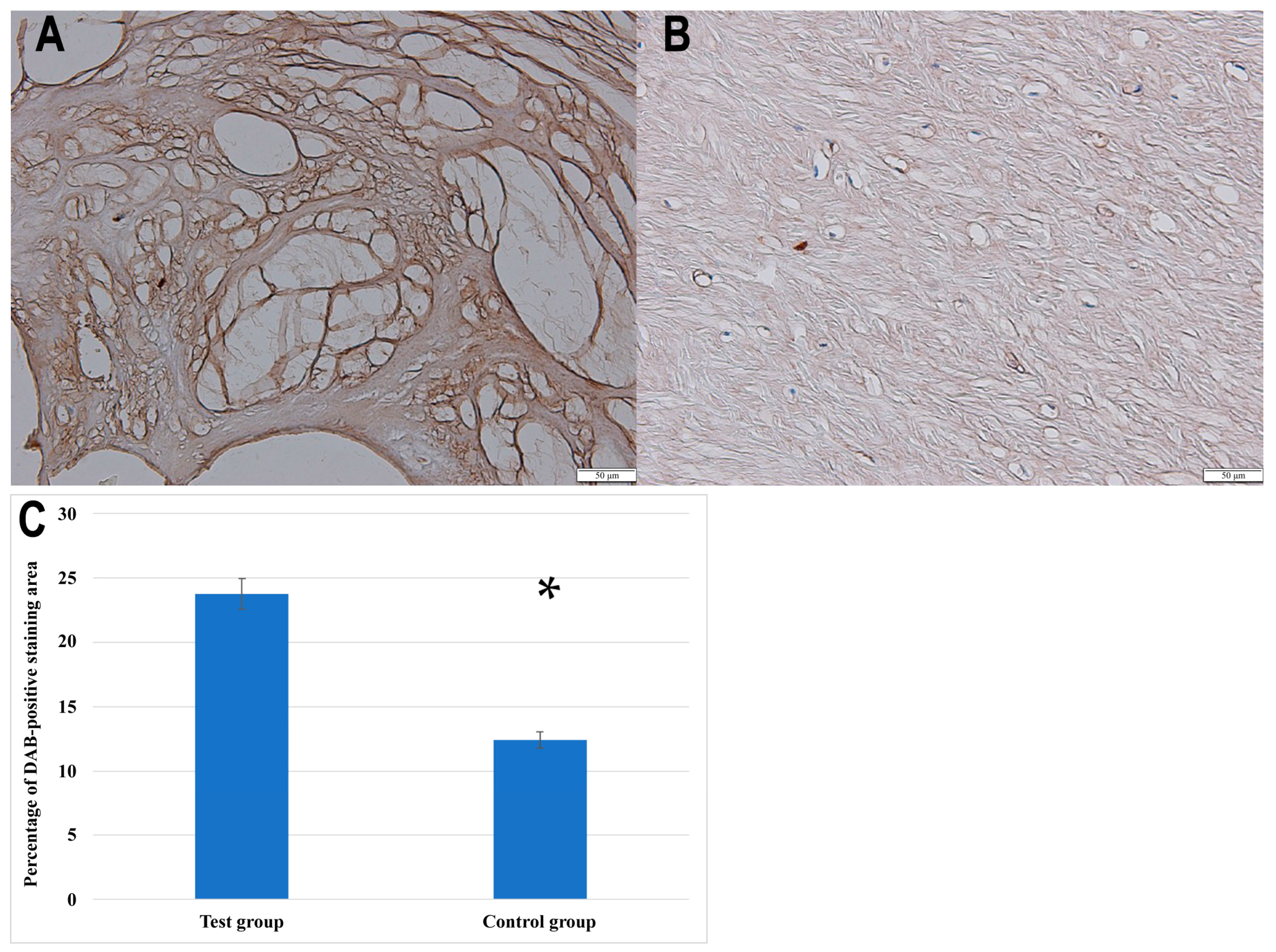
3.2.2. IHC Analysis
3.3. The Concentrations of mRNA and Protein of NRTN in the Tested Samples Depending on the Pain Degree Measured with the VAS
3.4. Variances in the Expression Profiles of NRTN at the mRNA and Protein Levels in Ligamentum Flavum Samples Obtained from the Study and Control Groups
3.5. Regression Analysis of Variables Potentially Associated with NRTN Levels in Ligamentum Flavum Samples from the Study Groups
3.5.1. Univariate Regression Analyses
3.5.2. Multivariate Regression Analyses
3.5.3. Multivariate Regression Analyses of Variables Associated with NRTN Levels in Control and Study Group Ligamentum Flavum
3.5.4. Multivariate NRTNT Protein Levels: Impact of Pain Severity and Lifestyle Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahere, M.; Hlongwa, M.; Ginindza, T.G. A Scoping Review on the Epidemiology of Chronic Low Back Pain among Adults in Sub-Saharan Africa. Int. J. Environ. Res. Public Health 2022, 19, 2964. [Google Scholar] [CrossRef]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.; Zundert, J.V.; Cohen, S.P. Low Back Pain: Epidemiology, Mechanisms, and Treatment. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Carregaro, R.L.; Tottoli, C.R.; da Silva Rodrigues, D.; Bosmans, J.E.; da Silva, E.N.; van Tulder, M. Low Back Pain Should Be Considered a Health and Research Priority in Brazil: Lost Productivity and Healthcare Costs between 2012 to 2016. PLoS ONE 2020, 15, e0230902. [Google Scholar] [CrossRef]
- Covaro, A.; Vilà-Canet, G.; De Frutos, A.G.; Ubierna, M.T.; Ciccolo, F.; Caceres, E. Management of Degenerative Lumbar Spinal Stenosis: An Evidence-Based Review. EFORT Open Rev. 2016, 1, 267–274. [Google Scholar] [CrossRef]
- Deer, T.; Sayed, D.; Michels, J.; Josephson, Y.; Li, S.; Calodney, A.K. A Review of Lumbar Spinal Stenosis with Intermittent Neurogenic Claudication: Disease and Diagnosis. Pain Med. 2019, 20, S32–S44. [Google Scholar] [CrossRef]
- Burgstaller, J.M.; Porchet, F.; Steurer, J.; Wertli, M.M. Arguments for the Choice of Surgical Treatments in Patients with Lumbar Spinal Stenosis—A Systematic Appraisal of Randomized Controlled Trials. BMC Musculoskelet. Disord. 2015, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Moon, S.-H.; Suk, K.-S.; Kim, H.-S.; Yang, J.-H.; Lee, H.-M. Lumbar Spinal Stenosis: Pathophysiology and Treatment Principle: A Narrative Review. Asian Spine J. 2020, 14, 682. [Google Scholar] [CrossRef] [PubMed]
- Byvaltsev, V.A.; Kalinin, A.A.; Hernandez, P.A.; Shepelev, V.V.; Pestryakov, Y.Y.; Aliyev, M.A.; Giers, M.B. Molecular and Genetic Mechanisms of Spinal Stenosis Formation: Systematic Review. Int. J. Mol. Sci. 2022, 23, 13479. [Google Scholar] [CrossRef]
- Ribeiro, F.F.; Xapelli, S. Intervention of Brain-Derived Neurotrophic Factor and Other Neurotrophins in Adult Neurogenesis. In Recent Advances in NGF and Related Molecules; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 1331, pp. 95–115. [Google Scholar] [CrossRef]
- Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Laurà, R.; Cometa, M.; Levanti, M.; Abbate, F.; Cobo, T.; Capitelli, G.; et al. The BDNF/TrkB Neurotrophin System in the Sensory Organs of Zebrafish. Int. J. Mol. Sci. 2022, 23, 2621. [Google Scholar] [CrossRef]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Mahato, A.K.; Saarma, M. Neurotrophic Factors in Parkinson’s Disease: Clinical Trials. In Regenerative Medicine and Brain Repair; Peplow, P.V., Martinez, B., Gennarelli, T.A., Eds.; Stem Cell Biology and Regenerative Medicine; Springer International Publishing: Cham, Switzerland, 2024; Volume 75, pp. 109–137. ISBN 978-3-031-49743-8. [Google Scholar]
- Correia, J.C.; Kelahmetoglu, Y.; Jannig, P.R.; Schweingruber, C.; Shvaikovskaya, D.; Zhengye, L.; Cervenka, I.; Khan, N.; Stec, M.; Oliveira, M. Muscle-Secreted Neurturin Couples Myofiber Oxidative Metabolism and Slow Motor Neuron Identity. Cell Metab. 2021, 33, 2215–2230. [Google Scholar] [CrossRef]
- Tenenbaum, L.; Humbert-Claude, M. Glial Cell Line-Derived Neurotrophic Factor Gene Delivery in Parkinson’s Disease: A Delicate Balance between Neuroprotection, Trophic Effects, and Unwanted Compensatory Mechanisms. Front. Neuroanat. 2017, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, X.; Shen, H.; Zhang, C. Molecular Mechanisms of Cell Death in Intervertebral Disc Degeneration (Review). Int. J. Mol. Med. 2016, 37, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Meeker, R.B.; Williams, K.S. The P75 Neurotrophin Receptor: At the Crossroad of Neural Repair and Death. Neural Regen. Res. 2015, 10, 721. [Google Scholar] [CrossRef]
- Mitre, M.; Mariga, A.; Chao, M.V. Neurotrophin Signalling: Novel Insights into Mechanisms and Pathophysiology. Clin. Sci. 2016, 131, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Sobański, D.; Staszkiewicz, R.; Sobańska, M.; Strojny, D.; Grabarek, B.O. Effects of Pain in Lumbosacral Stenosis and Lifestyle-Related Factors on Brain-Derived Neurotrophic Factor Expression Profiles. Mol. Pain 2025, 21, 17448069241309001. [Google Scholar] [CrossRef]
- Sobański, D.; Bogdał, P.; Staszkiewicz, R.; Sobańska, M.; Filipowicz, M.; Czepko, R.A.; Strojny, D.; Grabarek, B.O. Evaluation of Differences in Expression Pattern of Three Isoforms of the Transforming Growth Factor Beta in Patients with Lumbosacral Stenosis. Cell Cycle 2024, 23, 555–572. [Google Scholar] [CrossRef]
- Staszkiewicz, R.; Sobański, D.; Bryś, K.; Och, W.; Garczarek, M.; Ulasavets, U.; Stasiowski, M.; Dammermann, W.; Strojny, D.; Grabarek, B.O. Effect of Glycemic Disorders and Habits on the Concentration of Selected Neurotrophic Factors in Patients with Lumbosacral Intervertebral Disc Degeneration. Curr. Pharm. Biotechnol. 2023, 25, 908–923. [Google Scholar] [CrossRef]
- Kulczyńska-Przybik, A.; Mroczko, P.; Dulewicz, M.; Mroczko, B. The Implication of Reticulons (RTNs) in Neurodegenerative Diseases: From Molecular Mechanisms to Potential Diagnostic and Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4630. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman Jr, R.A.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory Biomarkers of Low Back Pain and Disc Degeneration: A Review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Pradhan, L.K.; Das, S.K. The Regulatory Role of Reticulons in Neurodegeneration: Insights Underpinning Therapeutic Potential for Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2021, 41, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.; Ji, R.-R.; Chen, G. Neuroinflammation, Bone Marrow Stem Cells, and Chronic Pain. Front. Immunol. 2017, 8, 1014. [Google Scholar] [CrossRef]
- Phillips, C. Lifestyle Modulators of Neuroplasticity: How Physical Activity, Mental Engagement, and Diet Promote Cognitive Health during Aging. Neural Plast. 2017, 2017, 3589271. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, L.I.; Routtenberg, A. GAP-43: An Intrinsic Determinant of Neuronal Development and Plasticity. Trends Neurosci. 1997, 20, 84–91. [Google Scholar] [CrossRef]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Overview of Oxidative Stress and Inflammation in Diabetes. J. Diabetes 2024, 16, e70014. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Potrykus, M.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Pro-Inflammatory Profile of Adipokines in Obesity Contributes to Pathogenesis, Nutritional Disorders, and Cardiovascular Risk in Chronic Kidney Disease. Nutrients 2022, 14, 1457. [Google Scholar] [CrossRef] [PubMed]
- Casoli, T.; Di Stefano, G.; Gracciotti, N.; Fattoretti, P.; Solazzi, M.; Bertoni-Freddari, C. Age-Related Effects of Moderate Alcohol Consumption on GAP-43 Levels in Rat Hippocampus. Mech. Ageing Dev. 2001, 122, 1723–1738. [Google Scholar] [CrossRef]
- Sobański, D.; Sobańska, M.; Staszkiewicz, R.; Strojny, D.; Grabarek, B.O. Changes in the Expression Profile of Growth-Associated Protein 43 in Degenerative Lumbosacral Stenosis. J. Clin. Med. 2025, 14, 1223. [Google Scholar] [CrossRef]
- Jones, M.R.; Jones, J.; Pandu, P.; Liu, C.; Carey, C.D.; Falo, L.D.; Albers, K.M. Neurturin GF Enhances the Acute Cytokine Response of Inflamed Skin. J. Investig. Dermatol. 2025, 145, 583–592. [Google Scholar] [CrossRef]
- Iwasaki, T.; Akeda, K.; Kawaguchi, K.; Yamada, J.; Hasegawa, T.; Takegami, N.; Fujiwara, T.; Sudo, A. Expression of Glial-Cell-Line-Derived Neurotrophic Factor Family Ligands in Human Intervertebral Discs. Int. J. Mol. Sci. 2023, 24, 15874. [Google Scholar] [CrossRef]
- Malfait, A.-M.; Miller, R.E.; Block, J.A. Targeting Neurotrophic Factors: Novel Approaches to Musculoskeletal Pain. Pharmacol. Ther. 2020, 211, 107553. [Google Scholar] [CrossRef] [PubMed]
- Xiaogang, M.; Quanshan, H.; Liping, Z.; Kaken, H. The Expression of Cytokine and Its Significance for the Intervertebral Disks of Kazakhs. J. Clin. Lab. Anal. 2017, 31, e22087. [Google Scholar] [CrossRef]
- Nakazawa, K.R.; Walter, B.A.; Laudier, D.M.; Krishnamoorthy, D.; Mosley, G.E.; Spiller, K.L.; Iatridis, J.C. Accumulation and Localization of Macrophage Phenotypes with Human Intervertebral Disc Degeneration. Spine J. 2018, 18, 343–356. [Google Scholar] [CrossRef]
- Nicol, V.; Verdaguer, C.; Daste, C.; Bisseriex, H.; Lapeyre, É.; Lefèvre-Colau, M.-M.; Rannou, F.; Rören, A.; Facione, J.; Nguyen, C. Chronic Low Back Pain: A Narrative Review of Recent International Guidelines for Diagnosis and Conservative Treatment. J. Clin. Med. 2023, 12, 1685. [Google Scholar] [CrossRef]
- Franzmeier, N.; Dehsarvi, A.; Steward, A.; Biel, D.; Dewenter, A.; Roemer, S.N.; Wagner, F.; Groß, M.; Brendel, M.; Moscoso, A.; et al. Elevated CSF GAP-43 Is Associated with Accelerated Tau Accumulation and Spread in Alzheimer’s Disease. Nat. Commun. 2024, 15, 202. [Google Scholar] [CrossRef]
- Elmasry, S.; Asfour, S.; de Rivero Vaccari, J.P.; Travascio, F. Effects of Tobacco Smoking on the Degeneration of the Intervertebral Disc: A Finite Element Study. PLoS ONE 2015, 10, e0136137. [Google Scholar] [CrossRef] [PubMed]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Kopp, W. Pathogenesis of (Smoking-Related) Non-Communicable Diseases—Evidence for a Common Underlying Pathophysiological Pattern. Front. Physiol. 2022, 13, 1037750. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among Smoking, Oxidative Stress, Inflammation, Macromolecular Damage, and Cancer. Mutat. Res./Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Xiao, S.; Wei, T.; Xiao, M.; An, Z.; Shan, M.; Luo, Z.; Zhou, J.; Li, N.; Lu, X. Negative Air Ions Alleviate Nicotine-Induced Renal Damage of Spontaneously Hypertensive Rats via Inhibiting Oxidative Stress and TGF-β/Smad Pathway. Ecotoxicol. Environ. Saf. 2025, 291, 117882. [Google Scholar] [CrossRef]
- Ustawa z Dnia 1 Lipca 2005 r. o Pobieraniu, Przechowywaniu i Przeszczepianiu Komórek, Tkanek i Narządów. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20051691411 (accessed on 13 February 2024).
| mRNA | Oligonucleotide Sequence |
|---|---|
| NRTN | Forward: 5′-CTGCCTGTGATGCCATTCTC-3′ Reverse 5′-GCCTTTGACTTTGAACGCCT-3′ |
| GAPDH | Forward: 5′-GGTGAAGGTCGGAGTCAACGGA-3′ Reverse 5′-GAGGGATCTCGCTCCTGGAAGA-3′ |
| VAS Pain Intensity | NRTN mRNA Expression (Fold Change ± SD) | NRTN Protein Concentration (ng/mL ± SD) | ANOVA (p) |
|---|---|---|---|
| 2 | 27.84 ± 0.48 | 9.91 ± 0.34 | 0.032 a 0.041 b |
| 3 | 29.93 ± 0.39 | 10.57 ± 0.31 | |
| 4 | 31.18 ± 0.52 | 11.23 ± 0.36 | |
| 5 | 32.42 ± 0.47 | 11.77 ± 0.44 | |
| 6 | 32.76 ± 0.40 | 12.19 ± 0.42 | |
| 7 | 33.64 ± 0.38 | 12.49 ± 0.39 | |
| 8 | 34.47 ± 0.33 | 12.86 ± 0.49 | |
| 9 | 35.73 ± 0.42 | 13.17 ± 0.51 | |
| 10 | 36.94 ± 0.58 | 13.56 ± 0.60 |
| Comparison | mRNA | Student’s t-Test 1 or ANOVA 2 (Study Group) | Protein | Student’s t-Test 1 or ANOVA 2 (Control Group) | |
|---|---|---|---|---|---|
| Gender | Female (n = 43) | 31.87 ± 1.67 | 0.761 1 | 11.97 ± 1.03 | 0.651 1 |
| Male (n = 38) | 33.47 ± 1.84 | 12.71 ± 0.77 | |||
| BMI (kg/m2) | Normal (n = 54) | 1.00 | 0.013 2 | 8.31 ± 0.76 | < 0.0001 2 |
| Overweight (n = 42) | 26.12 ± 2.34 | 11.97 ± 0.66 | |||
| Obesity (n = 17) | 39.19 ± 3.18 | 16.73 ± 0.57 | |||
| Diabetes | No (n = 91) | 27.54 ± 2.52 | 0.024 1 | 6.52 ± 0.87 | < 0.0001 1 |
| Yes (n = 22) | 37.39 ± 1.87 | 18.15 ± 1.23 | |||
| Smoking | No (n = 77) | 32.37 ± 1.34 | 0.819 1 | 12.29 ± 0.54 | 0.742 1 |
| Yes (n =36) | 32.29 ± 2.19 | 12.39 ± 0.81 | |||
| Drinking alcohol | No (n = 8) | 31.17 ± 2.11 | 0.438 1 | 11.08 ± 0.44 | 0.046 1 |
| Yes (n = 105) | 34.16 ± 1.48 | 13.59 ± 1.13 | |||
| Variable | Expression Type | Univariate β | Univariate p-Value |
|---|---|---|---|
| Gender | mRNA | 0.200 | 0.010 |
| Protein | 0.170 | 0.010 | |
| BMI | mRNA | 0.800 | 0.860 |
| Protein | 0.770 | 0.800 | |
| Diabetes | mRNA | 0.550 | 0.380 |
| Protein | 0.550 | 0.370 | |
| Drinking alcohol | mRNA | 0.520 | 0.200 |
| Protein | 0.400 | 0.220 | |
| Smoking | mRNA | 0.790 | 0.440 |
| Protein | 0.800 | 0.530 |
| Variable | Expression Type | Multivariate β | Multivariate p-Value |
|---|---|---|---|
| Gender | mRNA | 0.430 | 0.410 |
| Protein | 0.480 | 0.410 | |
| BMI | mRNA | 0.000 | 0.019 |
| Protein | 0.000 | 0.022 | |
| Diabetes | mRNA | 0.000 | 0.018 |
| Protein | 0.000 | 0.022 | |
| Drinking alcohol | mRNA | 0.032 | 0.018 |
| Protein | 0.030 | 0.021 | |
| Smoking | mRNA | 0.010 | 0.023 |
| Protein | 0.004 | 0.030 |
| Characteristic | Expression Level | Control Group | Study Group | ||
|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | ||
| Gender | mRNA | - | - | - | - |
| Protein | - | - | - | - | |
| BMI (kg/m2) | mRNA | 0.11 | 0.062 | 0.47 | 0.013 |
| Protein | 0.13 | 0.049 | 0.50 | 0.015 | |
| Diabetes | mRNA | 0.09 | 0.071 | 0.36 | 0.014 |
| Protein | 0.11 | 0.056 | 0.39 | 0.017 | |
| Smoking | mRNA | 0.13 | 0.065 | 0.34 | 0.019 |
| Protein | 0.15 | 0.051 | 0.38 | 0.020 | |
| Drinking Alcohol | mRNA | 0.07 | 0.081 | 0.18 | 0.017 |
| Protein | 0.09 | 0.074 | 0.21 | 0.018 | |
| Factor | Association with NRTNT (Univariate) | p-Value (Univariate) | Coefficient in Multivariate Model | p-Value (Multivariate) |
|---|---|---|---|---|
| VAS Pain Score | Positive | 0.032 | 0.31 | 0.017 |
| BMI | Positive | <0.0001 | 0.50 | 0.015 |
| Smoking | Positive | 0.004 | 0.38 | 0.020 |
| Alcohol Consumption | Positive | 0.03 | 0.21 | 0.018 |
| Diabetes | Positive | <0.0001 | 0.39 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobańska, M.; Sobański, D.; Staszkiewicz, R.; Gogol, P.; Strojny, D.; Pawłaszek, T.; Dammerman, W.; Grabarek, B.O. Modulation of Neurturin Expression by Lumbosacral Spinal Stenosis, Lifestyle Factors, and Glycemic Dysregulation. Biomedicines 2025, 13, 1102. https://doi.org/10.3390/biomedicines13051102
Sobańska M, Sobański D, Staszkiewicz R, Gogol P, Strojny D, Pawłaszek T, Dammerman W, Grabarek BO. Modulation of Neurturin Expression by Lumbosacral Spinal Stenosis, Lifestyle Factors, and Glycemic Dysregulation. Biomedicines. 2025; 13(5):1102. https://doi.org/10.3390/biomedicines13051102
Chicago/Turabian StyleSobańska, Małgorzata, Dawid Sobański, Rafał Staszkiewicz, Paweł Gogol, Damian Strojny, Tomasz Pawłaszek, Werner Dammerman, and Beniamin Oskar Grabarek. 2025. "Modulation of Neurturin Expression by Lumbosacral Spinal Stenosis, Lifestyle Factors, and Glycemic Dysregulation" Biomedicines 13, no. 5: 1102. https://doi.org/10.3390/biomedicines13051102
APA StyleSobańska, M., Sobański, D., Staszkiewicz, R., Gogol, P., Strojny, D., Pawłaszek, T., Dammerman, W., & Grabarek, B. O. (2025). Modulation of Neurturin Expression by Lumbosacral Spinal Stenosis, Lifestyle Factors, and Glycemic Dysregulation. Biomedicines, 13(5), 1102. https://doi.org/10.3390/biomedicines13051102





