The Power of Movement: How Exercise Influences Chemotherapy-Induced Peripheral Neuropathy
Abstract
1. Introduction
2. CIPN: Etiology, Prevalence, Agents, and Impact
3. Mechanisms of CIPN
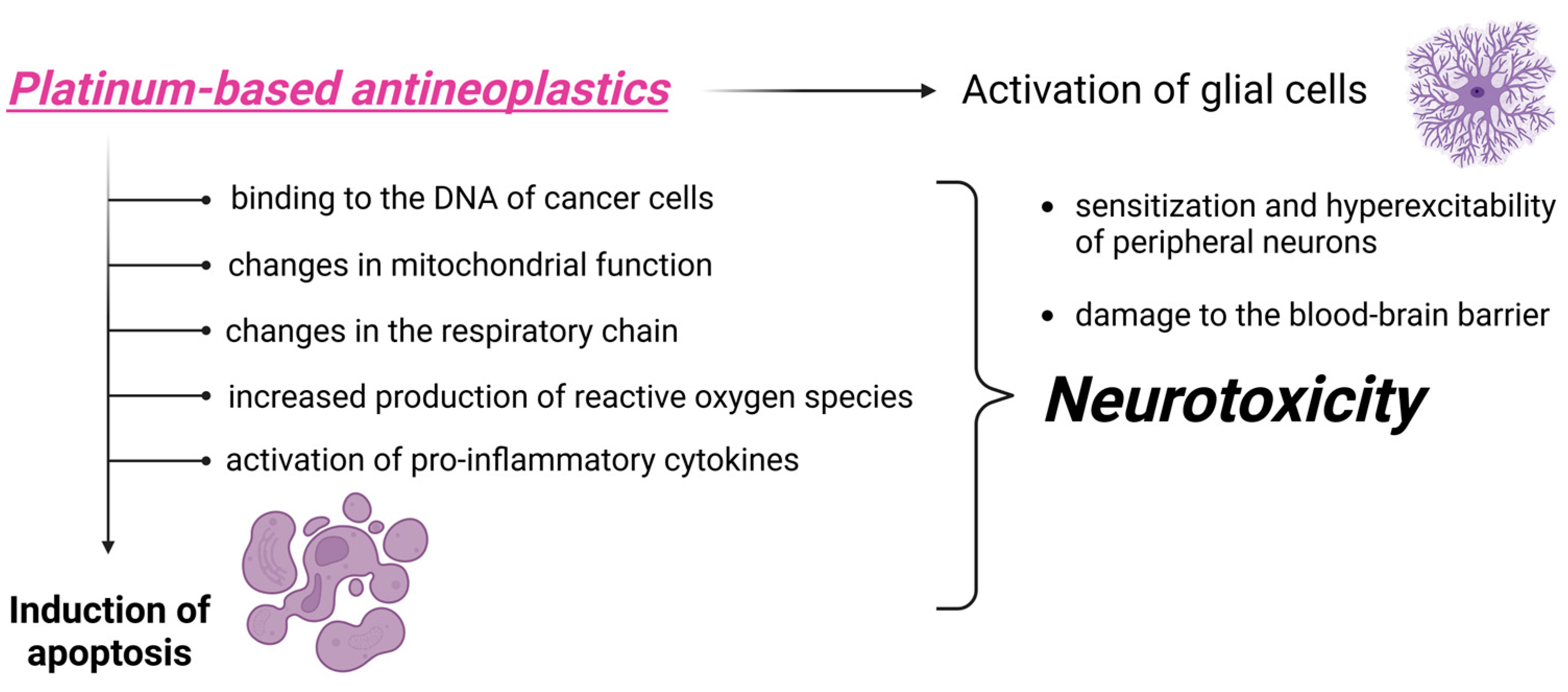
3.1. Toxicity Induced by Platinum-Derived Compounds
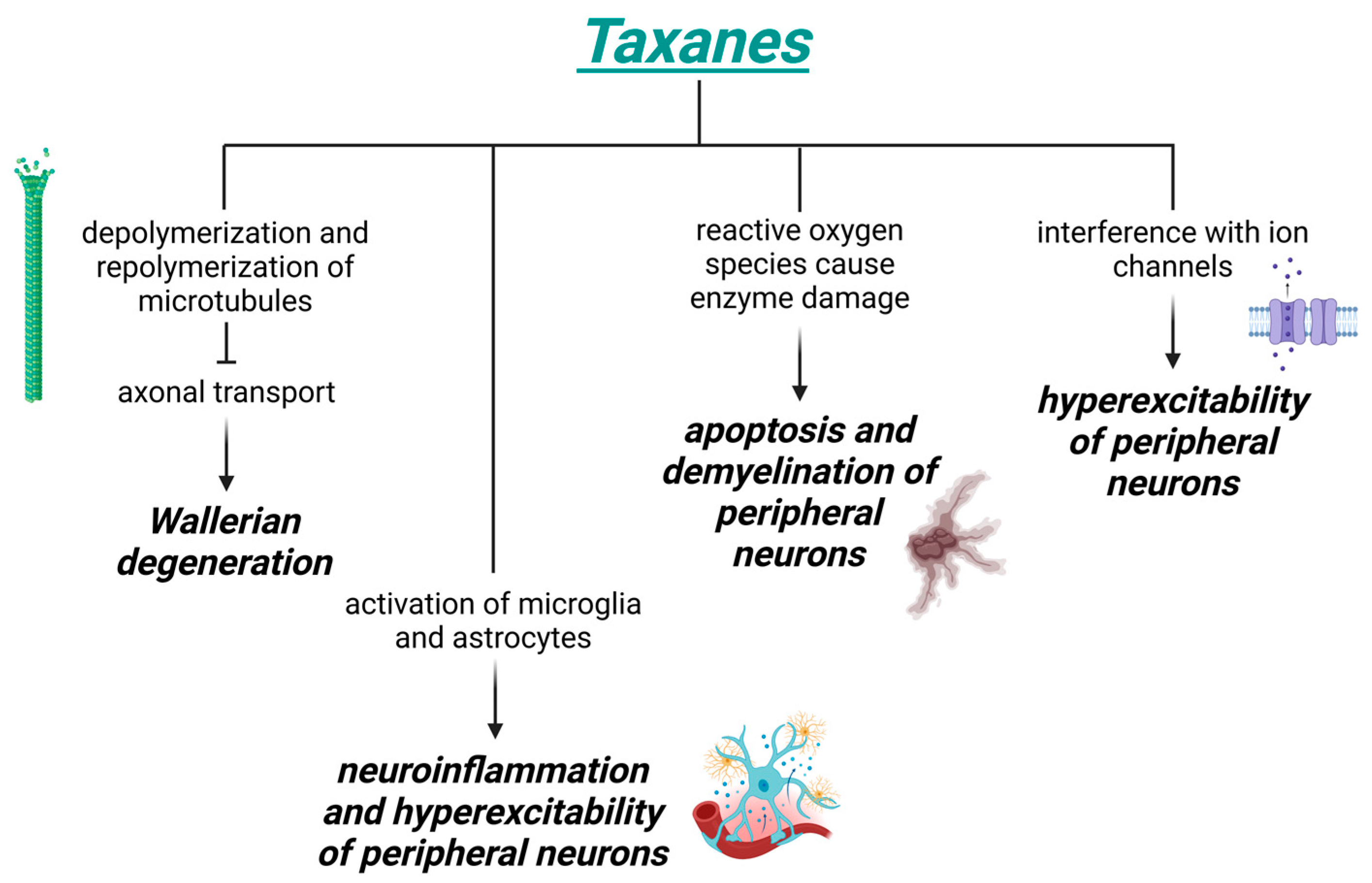
3.2. Toxicity Induced by Taxanes
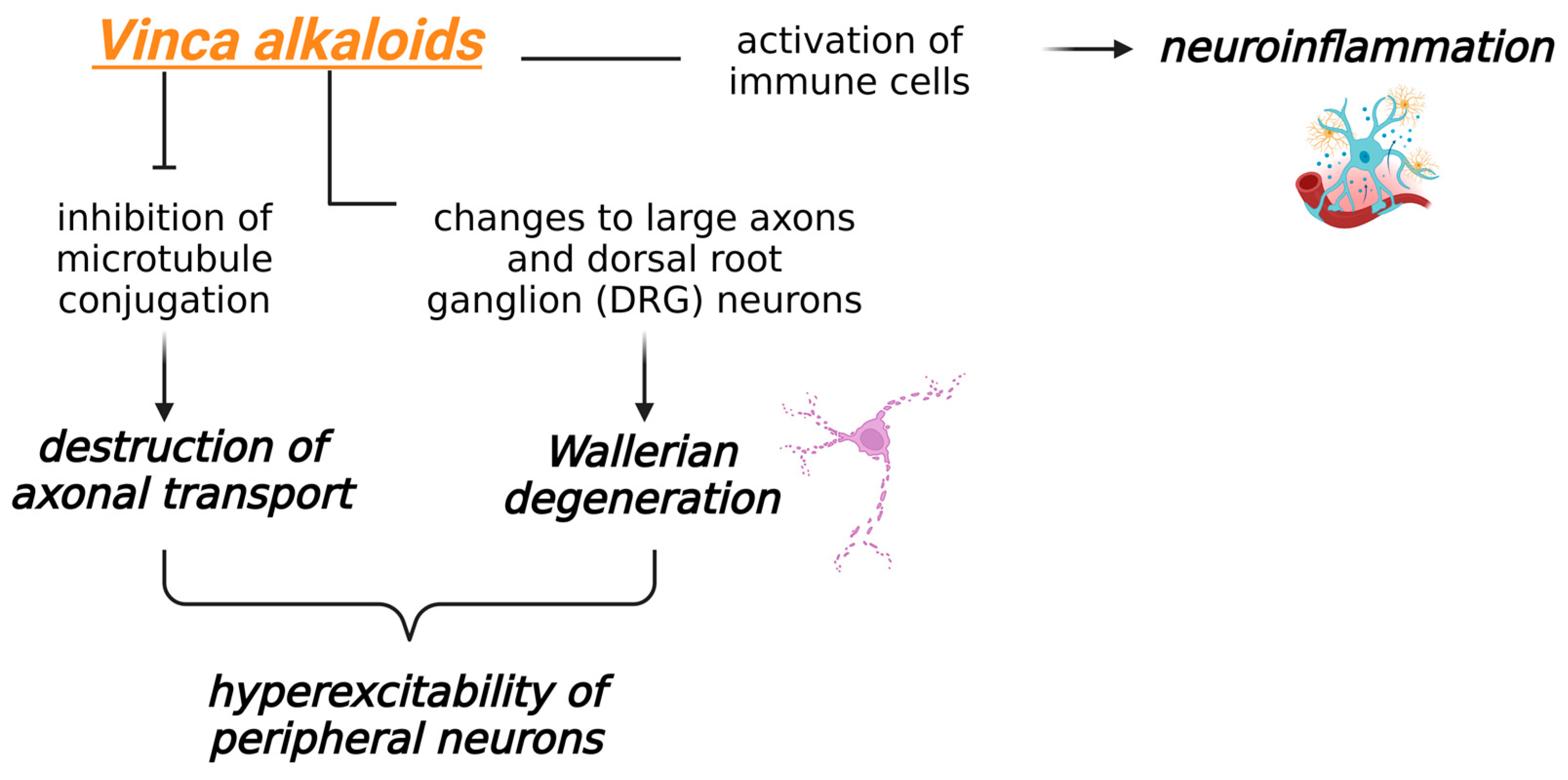
3.3. Toxicity Induced by Vinca Alkaloids
4. Central Effects of Chemotherapy-Induced Peripheral Neuropathy
5. Exercise as a Complementary Approach to the Prevention and Treatment of CIPN
6. Mechanisms for Exercise Improvement in CIPN
6.1. Effects on the Somatosensory System
6.2. Well-Being and Psychological Effects
6.3. Emerging Fields: The Gut Microbiome and Sex Differences
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIPN | Chemotherapy-induced peripheral neuropathy |
| RCT | Randomized controlled trial |
| SNPs | Single-nucleotide polymorphisms |
| CNS | Central nervous system |
| PNS | Peripheral nervous system |
| TRP | Transient receptor potential |
| DRG | Dorsal root ganglion |
| GABA | Gamma-aminobutyric acid |
| GPCR/MAPK | G protein-coupled receptor/mitogen-activated protein kinase |
| PAG | Periaqueductal gray matter |
| IL | Interleukin |
| TNF-alpha | Tumor necrosis factor alpha |
| 5-HT/NA | Serotonin and noradrenaline |
| RVM | Rostral ventromedial medulla |
| EORTC QLQ C-30/LC-13 | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30 |
| mCRC | Metastasized colorectal cancer |
| EXCAP | Exercise for Cancer Patients © ® |
| SMT | Sensorimotor training |
| WBV | Whole-body vibration training |
| CI | Confidence interval |
| fMRI | Functional magnetic resonance imaging |
| GDNF | Glial cell line-derived neurotrophic factor |
| BDNF | Brain-derived neurotrophic factor |
| IGF-1 | Insulin-like growth factor |
| MOR | Mu opioid receptor |
| DOR | Delta opioid receptor |
| KOR | Kappa opioid receptor |
| CB1 | Cannabinoid receptor type 1 |
| CB2 | Cannabinoid receptor type 2 |
| NO | Nitric oxide |
| SCFAs | Short-chain fatty acids |
References
- Zajaczkowska, R.; Kocot-Kepska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef]
- Bower, J.E. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Newton, R.U.; Spence, R.R.; Galvão, D.A. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J. Sci. Med. Sport 2019, 22, 1175–1199. [Google Scholar] [CrossRef] [PubMed]
- Cormie, P.; Zopf, E.M.; Zhang, X.; Schmitz, K.H. The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiol. Rev. 2017, 39, 71–92. [Google Scholar] [CrossRef]
- Squires, R.W.; Shultz, A.M.; Herrmann, J. Exercise Training and Cardiovascular Health in Cancer Patients. Curr. Oncol. Rep. 2018, 20, 1–20. [Google Scholar] [CrossRef]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef]
- Sweegers, M.G.; Altenburg, T.M.; Brug, J.; May, A.M.; Van Vulpen, J.K.; Aaronson, N.K.; Arbane, G.; Bohus, M.; Courneya, K.S.; Daley, A.J.; et al. Effects and moderators of exercise on muscle strength, muscle function and aerobic fitness in patients with cancer: A meta-analysis of individual patient data. Br. J. Sports Med. 2019, 53, 812. [Google Scholar] [CrossRef]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvão, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef]
- Streckmann, F.; Zopf, E.M.; Lehmann, H.C.; May, K.; Rizza, J.; Zimmer, P.; Gollhofer, A.; Bloch, W.; Baumann, F.T. Exercise intervention studies in patients with peripheral neuropathy: A systematic review. Sports Med. 2014, 44, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M.; Walsh, D.; Furlong, B.; Loughney, L.; McCaffrey, N.; Moyna, N.; Woods, C. Physical Activity Across the Cancer Journey: Experiences and Recommendations From People Living With and Beyond Cancer. Phys. Ther. 2020, 100, 575–585. [Google Scholar] [CrossRef]
- Crichton, M.; Yates, P.M.; Agbejule, O.A.; Spooner, A.; Chan, R.J.; Hart, N.H. Non-Pharmacological Self-Management Strategies for Chemotherapy-Induced Peripheral Neuropathy in People with Advanced Cancer: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2403. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Park, S.B.; Streckmann, F.; Wiskemann, J.; Mohile, N.; Kleckner, A.S.; Colloca, L.; Dorsey, S.G.; Kleckner, I.R. Mechanisms, Mediators, and Moderators of the Effects of Exercise on Chemotherapy-Induced Peripheral Neuropathy. Cancers 2022, 14, 1224. [Google Scholar] [CrossRef]
- Bulls, H.W.; Hoogland, A.I.; Small, B.J.; Kennedy, B.; James, B.W.; Arboleda, B.L.; Shahzad, M.M.K.; Gonzalez, B.D.; Jim, H.S.L. Lagged Relationships Among Chemotherapy-Induced Peripheral Neuropathy, Sleep Quality, and Physical Activity During and After Chemotherapy. Ann. Behav. Med. 2021, 55, 844–852. [Google Scholar] [CrossRef]
- Brami, C.; Bao, T.; Deng, G. Natural products and complementary therapies for chemotherapy-induced peripheral neuropathy: A systematic review. Crit. Rev. Oncol. Hematol. 2016, 98, 325–334. [Google Scholar] [CrossRef]
- Kannarkat, G.; Lasher, E.E.; Schiff, D. Neurologic complications of chemotherapy agents. Curr. Opin. Neurol. 2007, 20, 719–725. [Google Scholar] [CrossRef]
- Quasthoff, S.; Hartung, H.P. Chemotherapy-induced peripheral neuropathy. J. Neurol. 2002, 249, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Weiler, M.; Schneeweiss, A.; Haag, G.M.; Steindorf, K.; Wick, W.; Wiskemann, J. Preventive effect of sensorimotor exercise and resistance training on chemotherapy-induced peripheral neuropathy: A randomised-controlled trial. Br. J. Cancer 2021, 125, 955–965. [Google Scholar] [CrossRef]
- Kleckner, I.R.; Kamen, C.; Gewandter, J.S.; Mohile, N.A.; Heckler, C.E.; Culakova, E.; Fung, C.; Janelsins, M.C.; Asare, M.; Lin, P.J.; et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: A multicenter, randomized controlled trial. Support. Care Cancer 2018, 26, 1019–1028. [Google Scholar] [CrossRef]
- Schwenk, M.; Grewal, G.S.; Holloway, D.; Muchna, A.; Garland, L.; Najafi, B. Interactive Sensor-Based Balance Training in Older Cancer Patients with Chemotherapy-Induced Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology 2016, 62, 553–563. [Google Scholar] [CrossRef]
- Adamek, P.; Heles, M.; Bhattacharyya, A.; Pontearso, M.; Slepicka, J.; Palecek, J. Dual PI3Kdelta/gamma Inhibitor Duvelisib Prevents Development of Neuropathic Pain in Model of Paclitaxel-Induced Peripheral Neuropathy. J. Neurosci. 2022, 42, 1864–1881. [Google Scholar] [CrossRef] [PubMed]
- Akin, E.J.; Alsaloum, M.; Higerd, G.P.; Liu, S.; Zhao, P.; Dib-Hajj, F.B.; Waxman, S.G.; Dib-Hajj, S.D. Paclitaxel increases axonal localization and vesicular trafficking of Nav1.7. Brain 2021, 144, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; Wang, Z.J. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience 2011, 193, 440–451. [Google Scholar] [CrossRef]
- Li, Y.; North, R.Y.; Rhines, L.D.; Tatsui, C.E.; Rao, G.; Edwards, D.D.; Cassidy, R.M.; Harrison, D.S.; Johansson, C.A.; Zhang, H.; et al. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J. Neurosci. 2018, 38, 1124–1136. [Google Scholar] [CrossRef]
- Mao, Q.; Wu, S.; Gu, X.; Du, S.; Mo, K.; Sun, L.; Cao, J.; Bekker, A.; Chen, L.; Tao, Y.X. DNMT3a-triggered downregulation of K(2p) 1.1 gene in primary sensory neurons contributes to paclitaxel-induced neuropathic pain. Int. J. Cancer 2019, 145, 2122–2134. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, S.; Wu, I.; Mata, M.; Fink, D.J. Activation of TLR-4 to produce tumour necrosis factor-alpha in neuropathic pain caused by paclitaxel. Eur. J. Pain 2015, 19, 889–898. [Google Scholar] [CrossRef]
- Kawakami, K.; Chiba, T.; Katagiri, N.; Saduka, M.; Abe, K.; Utsunomiya, I.; Hama, T.; Taguchi, K. Paclitaxel increases high voltage-dependent calcium channel current in dorsal root ganglion neurons of the rat. J. Pharmacol. Sci. 2012, 120, 187–195. [Google Scholar] [CrossRef]
- Flatters, S. The contribution of mitochondria to sensory processing and pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 119–146. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Wang, L.; Liu, P.; Zhao, S.; Li, H.; Jiang, Y.; Guo, Y.; Wang, X. Resveratrol inhibits paclitaxel-induced neuropathic pain by the activation of PI3K/Akt and SIRT1/PGC1alpha pathway. J. Pain Res. 2019, 12, 879–890. [Google Scholar] [CrossRef]
- Wu, P.; Chen, Y. Evodiamine ameliorates paclitaxel-induced neuropathic pain by inhibiting inflammation and maintaining mitochondrial anti-oxidant functions. Hum. Cell 2019, 32, 251–259. [Google Scholar] [CrossRef]
- Goel, Y.; Fouda, R.; Gupta, K. Endoplasmic Reticulum Stress in Chemotherapy-Induced Peripheral Neuropathy: Emerging Role of Phytochemicals. Antioxidants 2022, 11, 265. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Hwang, S.H.; Lee, S.O.; Kim, S.H.; Abdi, S. Pentoxifylline Ameliorates Mechanical Hyperalgesia in a Rat Model of Chemotherapy-Induced Neuropathic Pain. Pain Physician 2016, 19, E589–E600. [Google Scholar] [PubMed]
- Chen, L.H.; Yeh, Y.M.; Chen, Y.F.; Hsu, Y.H.; Wang, H.H.; Lin, P.C.; Chang, L.Y.; Lin, C.K.; Chang, M.S.; Shen, M.R. Targeting interleukin-20 alleviates paclitaxel-induced peripheral neuropathy. Pain 2020, 161, 1237–1254. [Google Scholar] [CrossRef]
- Kalynovska, N.; Diallo, M.; Sotakova-Kasparova, D.; Palecek, J. Losartan attenuates neuroinflammation and neuropathic pain in paclitaxel-induced peripheral neuropathy. J. Cell Mol. Med. 2020, 24, 7949–7958. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Dias-Carvalho, A.; Ferreira, M.; Ferreira, R.; Bastos, M.D.L.; Sá, S.I.; Capela, J.P.; Carvalho, F.; Costa, V.M. Four decades of chemotherapy-induced cognitive dysfunction: Comprehensive review of clinical, animal and in vitro studies, and insights of key initiating events. Arch. Toxicol. 2022, 96, 11–78. [Google Scholar] [CrossRef]
- Zippo, A.G.; Rodriguez-Menendez, V.; Pozzi, E.; Canta, A.; Chiorazzi, A.; Ballarini, E.; Monza, L.; Alberti, P.; Meregalli, C.; Bravin, A.; et al. Paclitaxel alters the microvascular network in the central and peripheral nervous system of rats with chemotherapy-induced painful peripheral neuropathy. J. Peripher. Nerv. Syst. 2024, 29, 537–554. [Google Scholar] [CrossRef]
- Heinricher, M.M.; Tavares, I.; Leith, J.L.; Lumb, B.M. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009, 60, 214–225. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Invest. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Bannister, K.; Dickenson, A.H. What do monoamines do in pain modulation? Curr. Opin. Support. Palliat. Care 2016, 10, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Tavares, I.; Costa-Pereira, J.T.; Martins, I. Monoaminergic and Opioidergic Modulation of Brainstem Circuits: New Insights Into the Clinical Challenges of Pain Treatment? Front. Pain Res. 2021, 2, 696515. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy. Front. Immunol. 2020, 11, 626687. [Google Scholar] [CrossRef]
- Costa-Pereira, J.T.; Oliveira, R.; Guadilla, I.; Guillen, M.J.; Tavares, I.; Lopez-Larrubia, P. Neuroimaging uncovers neuronal and metabolic changes in pain modulatory brain areas in a rat model of chemotherapy-induced neuropathy—MEMRI and ex vivo spectroscopy studies. Brain Res. Bull. 2023, 192, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhao, H.; Gao, H.; Zhao, H.; Liu, D.; Li, J. Participation of pro-inflammatory cytokines in neuropathic pain evoked by chemotherapeutic oxaliplatin via central GABAergic pathway. Mol. Pain. 2018, 14, 1744806918783535. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, H.Z.; Zhang, W.W.; Matsuda, M.; Lv, N.; Chen, G.; Xu, Z.Z.; Zhang, Y.Q. Interleukin-17 Regulates Neuron-Glial Communications, Synaptic Transmission, and Neuropathic Pain after Chemotherapy. Cell Rep. 2019, 29, 2384–2397.e5. [Google Scholar] [CrossRef]
- El-Sawaf, E.S.; El Maraghy, N.N.; El-Abhar, H.S.; Zaki, H.F.; Zordoky, B.N.; Ahmed, K.A.; Abouquerin, N.; Mohamed, A.F. Melatonin mitigates vincristine-induced peripheral neuropathy by inhibiting TNF-alpha/astrocytes/microglial cells activation in the spinal cord of rats, while preserving vincristine’s chemotherapeutic efficacy in lymphoma cells. Toxicol. Appl. Pharmacol. 2024, 492, 117134. [Google Scholar] [CrossRef]
- Ruivo, J.; Tavares, I.; Pozza, D. Molecular targets in bone cancer pain: A systematic review of inflammatory cytokines. J. Mol. Med. 2024, 102, 1063–1088. [Google Scholar] [CrossRef]
- Janes, K.; Wahlman, C.; Little, J.W.; Doyle, T.; Tosh, D.K.; Jacobson, K.A.; Salvemini, D. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav. Immun. 2015, 44, 91–99. [Google Scholar] [CrossRef]
- Makker, P.G.S.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PLoS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Cervetto, C.; Toti, A.; Lucarini, E.; Parisio, C.; Marcoli, M.; Ghelardini, C. Neuronal alarmin IL-1alpha evokes astrocyte-mediated protective signals: Effectiveness in chemotherapy-induced neuropathic pain. Neurobiol. Dis. 2022, 168, 105716. [Google Scholar] [CrossRef] [PubMed]
- Odling-Smee, L. Chronic pain can be treated—So why are millions still suffering? Nature 2023, 615, 782–786. [Google Scholar] [CrossRef]
- Stein, C.; Gaveriaux-Ruff, C. Opioids and Pain. In The Oxford Handbook of the Neurobiology of Pain; Oxford University Press: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Medrano-Escalada, Y.; Plaza-Manzano, G.; Fernandez-de-Las-Penas, C.; Valera-Calero, J.A. Structural, Functional and Neurochemical Cortical Brain Changes Associated with Chronic Low Back Pain. Tomography 2022, 8, 2153–2163. [Google Scholar] [CrossRef]
- Chao, C.C.; Tseng, M.T.; Hsieh, P.C.; Lin, C.J.; Huang, S.L.; Hsieh, S.T.; Chiang, M.C. Brain Mechanisms of Pain and Dysautonomia in Diabetic Neuropathy: Connectivity Changes in Thalamus and Hypothalamus. J. Clin. Endocrinol. Metab. 2022, 107, e1167–e1180. [Google Scholar] [CrossRef]
- Fischer, T.Z.; Waxman, S.G. Neuropathic pain in diabetes—Evidence for a central mechanism. Nat. Rev. Neurol. 2010, 6, 462–466. [Google Scholar] [CrossRef]
- Nudelman, K.N.; McDonald, B.C.; Wang, Y.; Smith, D.J.; West, J.D.; O’Neill, D.P.; Zanville, N.R.; Champion, V.L.; Schneider, B.P.; Saykin, A.J. Cerebral Perfusion and Gray Matter Changes Associated With Chemotherapy-Induced Peripheral Neuropathy. J. Clin. Oncol. 2016, 34, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.; Tavares, I.; Costa-Pereira, J.T. Centralizing the Knowledge and Interpretation of Pain in Chemotherapy-Induced Peripheral Neuropathy: A Paradigm Shift towards Brain-Centric Approaches. Brain. Sci. 2024, 14, 659. [Google Scholar] [CrossRef]
- Boland, E.G.; Selvarajah, D.; Hunter, M.; Ezaydi, Y.; Tesfaye, S.; Ahmedzai, S.H.; Snowden, J.A.; Wilkinson, I.D. Central Pain Processing in Chronic Chemotherapy-Induced Peripheral Neuropathy: A Functional Magnetic Resonance Imaging Study. PLoS ONE 2014, 9, e96474. [Google Scholar] [CrossRef]
- Seretny, M.; Romaniuk, L.; Whalley, H.; Sladdin, K.; Lawrie, S.; Warnaby, C.E.; Roberts, N.; Colvin, L.; Tracey, I.; Fallon, M. Neuroimaging reveals a potential brain-based pre-existing mechanism that confers vulnerability to development of chronic painful chemotherapy-induced peripheral neuropathy. Br. J. Anaesth. 2023, 130, 83–93. [Google Scholar] [CrossRef]
- Rizvi, T.A.; Ennis, M.; Behbehani, M.M.; Shipley, M.T. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. J. Comp. Neurol. 1991, 303, 121–131. [Google Scholar] [CrossRef]
- Bandler, R.; Keay, K.A. Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog. Brain Res. 1996, 107, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Floyd, N.S.; Price, J.L.; Ferry, A.T.; Keay, K.A.; Bandler, R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J. Comp. Neurol. 2000, 422, 556–578. [Google Scholar] [CrossRef] [PubMed]
- Ennis, M.; Behbehani, M.; Shipley, M.T.; Van Bockstaele, E.J.; Aston-Jones, G. Projections from the periaqueductal gray to the rostromedial pericoerulear region and nucleus locus coeruleus: Anatomic and physiologic studies. J. Comp. Neurol. 1991, 306, 480–494. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Fields, H.L. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984, 7, 309–338. [Google Scholar] [CrossRef]
- Almeida, A.; Cobos, A.; Tavares, I.; Lima, D. Brain afferents to the medullary dorsal reticular nucleus: A retrograde and anterograde tracing study in the rat. Eur. J. Neurosci. 2002, 16, 81–95. [Google Scholar] [CrossRef]
- Cobos, A.; Lima, D.; Almeida, A.; Tavares, I. Brain afferents to the lateral caudal ventrolateral medulla: A retrograde and anterograde tracing study in the rat. Neuroscience 2003, 120, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, G.; Ai, G.; Xu, X.; Niu, X.; Zhang, M. Selective Ablation of Descending Serotonin from the Rostral Ventromedial Medulla Unmasks Its Pro-Nociceptive Role in Chemotherapy-Induced Painful Neuropathy. J. Pain Res. 2020, 13, 3081–3094. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Xin, W.J.; He, S.; Deng, J.; Ruan, X. Somatostatin Neurons from Periaqueductal Gray to Medulla Facilitate Neuropathic Pain in Male Mice. J. Pain 2023, 24, 1020–1029. [Google Scholar] [CrossRef]
- Costa-Pereira, J.T.; Serrao, P.; Martins, I.; Tavares, I. Serotoninergic pain modulation from the rostral ventromedial medulla (RVM) in chemotherapy-induced neuropathy: The role of spinal 5-HT3 receptors. Eur. J. Neurosci. 2020, 51, 1756–1769. [Google Scholar] [CrossRef]
- Gang, J.; Park, K.T.; Kim, S.; Kim, W. Involvement of the Spinal Serotonergic System in the Analgesic Effect of [6]-Shogaol in Oxaliplatin-Induced Neuropathic Pain in Mice. Pharmaceuticals 2023, 16, 1465. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Song, J.A.; Kim, W.M.; Yoon, M.H. Antiallodynic Effect of Intrathecal Korean Red Ginseng in Cisplatin-Induced Neuropathic Pain Rats. Pharmacology 2020, 105, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Viisanen, H.; Pertovaara, A. Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience 2007, 146, 1785–1794. [Google Scholar] [CrossRef]
- Wei, H.; Pertovaara, A. Regulation of neuropathic hypersensitivity by alpha(2)-adrenoceptors in the pontine A7 cell group. Basic Clin. Pharmacol. Toxicol. 2013, 112, 90–95. [Google Scholar] [CrossRef]
- Pertovaara, A. The noradrenergic pain regulation system: A potential target for pain therapy. Eur. J. Pharmacol. 2013, 716, 2–7. [Google Scholar] [CrossRef]
- Costa-Pereira, J.T.; Ribeiro, J.; Martins, I.; Tavares, I. Role of Spinal Cord alpha(2)-Adrenoreceptors in Noradrenergic Inhibition of Nociceptive Transmission During Chemotherapy-Induced Peripheral Neuropathy. Front. Neurosci. 2019, 13, 1413. [Google Scholar] [CrossRef]
- Bonhof, C.S.; van de Poll-Franse, L.V.; Vissers, P.A.J.; Wasowicz, D.K.; Wegdam, J.A.; Revesz, D.; Vreugdenhil, G.; Mols, F. Anxiety and depression mediate the association between chemotherapy-induced peripheral neuropathy and fatigue: Results from the population-based PROFILES registry. Psychooncology 2019, 28, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Tian, J.; Wu, L.H. The influence of chemotherapy-induced neurotoxicity on psychological distress and sleep disturbance in cancer patients. Curr. Oncol. 2014, 21, 174–180. [Google Scholar] [CrossRef]
- Ferini-Strambi, L. Neuropathic Pain and Sleep: A Review. Pain Ther. 2017, 6, 19–23. [Google Scholar] [CrossRef]
- Wirtz, P.; Baumann, F.T. Physical Activity, Exercise and Breast Cancer—What Is the Evidence for Rehabilitation, Aftercare, and Survival? A Review. Breast Care 2018, 13, 93–101. [Google Scholar] [CrossRef]
- Tajerian, M.; Clark, J.D. Nonpharmacological Interventions in Targeting Pain-Related Brain Plasticity. Neural Plast. 2017, 2017, 2038573. [Google Scholar] [CrossRef]
- Brett Whalen, L.; Zachary Wright, W.; Kundur, P.; Angadi, S.; Modesitt, S.C. Beneficial effects of exercise on chemotherapy-induced peripheral neuropathy and sleep disturbance: A review of literature and proposed mechanisms. Gynecol. Oncol. Rep. 2022, 39, 100927. [Google Scholar] [CrossRef]
- Leitzelar, B.N.; Koltyn, K.F. Exercise and Neuropathic Pain: A General Overview of Preclinical and Clinical Research. Sports Med. Open 2021, 7, 21. [Google Scholar] [CrossRef]
- Wilcoxon, A.; Kober, K.M.; Viele, C.; Topp, K.; Smoot, B.; Abrams, G.; Chesney, M.; Paul, S.M.; Conley, Y.P.; Levine, J.D.; et al. Association Between Physical Activity Levels and Chemotherapy-Induced Peripheral Neuropathy Severity in Cancer Survivors. Oncol. Nurs. Forum 2020, 47, 703–719. [Google Scholar] [CrossRef]
- Zimmer, P.; Trebing, S.; Timmers-Trebing, U.; Schenk, A.; Paust, R.; Bloch, W.; Rudolph, R.; Streckmann, F.; Baumann, F.T. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: A randomized controlled trial. Support. Care Cancer 2018, 26, 615–624. [Google Scholar] [CrossRef]
- Bobinski, F.; Martins, D.F.; Bratti, T.; Mazzardo-Martins, L.; Winkelmann-Duarte, E.C.; Guglielmo, L.G.; Santos, A.R. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neuroscience 2011, 194, 337–348. [Google Scholar] [CrossRef]
- Wakaizumi, K.; Kondo, T.; Hamada, Y.; Narita, M.; Kawabe, R.; Narita, H.; Watanabe, M.; Kato, S.; Senba, E.; Kobayashi, K.; et al. Involvement of mesolimbic dopaminergic network in neuropathic pain relief by treadmill exercise: A study for specific neural control with Gi-DREADD in mice. Mol. Pain 2016, 12, 1744806916681567. [Google Scholar] [CrossRef]
- Kami, K.; Taguchi Ms, S.; Tajima, F.; Senba, E. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol. Pain 2016, 12, 1744806916629059. [Google Scholar] [CrossRef]
- Zheng, Y.N.; Zheng, Y.L.; Wang, X.Q.; Chen, P.J. Role of Exercise on Inflammation Cytokines of Neuropathic Pain in Animal Models. Mol. Neurobiol. 2024, 61, 10288–10301. [Google Scholar] [CrossRef]
- Lee, T.H.; Devaki, M.; Formolo, D.A.; Rosa, J.M.; Cheng, A.S.K.; Yau, S.Y. Effects of Voluntary Wheel Running Exercise on Chemotherapy-Impaired Cognitive and Motor Performance in Mice. Int. J. Environ. Res. Public Health 2023, 20, 5371. [Google Scholar] [CrossRef]
- Slivicki, R.A.; Mali, S.S.; Hohmann, A.G. Voluntary exercise reduces both chemotherapy-induced neuropathic nociception and deficits in hippocampal cellular proliferation in a mouse model of paclitaxel-induced peripheral neuropathy. Neurobiol. Pain 2019, 6, 100035. [Google Scholar] [CrossRef]
- Duregon, F.; Vendramin, B.; Bullo, V.; Gobbo, S.; Cugusi, L.; Di Blasio, A.; Neunhaeuserer, D.; Zaccaria, M.; Bergamin, M.; Ermolao, A. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: A systematic review. Crit. Rev. Oncol. Hematol. 2018, 121, 90–100. [Google Scholar] [CrossRef]
- Henke, C.C.; Cabri, J.; Fricke, L.; Pankow, W.; Kandilakis, G.; Feyer, P.C.; de Wit, M. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support. Care Cancer 2014, 22, 95–101. [Google Scholar] [CrossRef]
- Streckmann, F.; Elter, T.; Lehmann, H.C.; Baurecht, H.; Nazarenus, T.; Oschwald, V.; Koliamitra, C.; Otten, S.; Draube, A.; Heinen, P.; et al. Preventive Effect of Neuromuscular Training on Chemotherapy-Induced Neuropathy: A Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 1046–1053. [Google Scholar] [CrossRef]
- Kleckner, I.R.; Manuweera, T.; Lin, P.-J.; Chung, K.H.; Kleckner, A.S.; Gewandter, J.S.; Culakova, E.; Tivarus, M.E.; Dunne, R.F.; Loh, K.P.; et al. Pilot trial testing the effects of exercise on chemotherapy-induced peripheral neurotoxicity (CIPN) and the interoceptive brain system. Support. Care Cancer 2024, 32, 677. [Google Scholar] [CrossRef]
- Brandolini, L.; d’Angelo, M.; Antonosante, A.; Allegretti, M.; Cimini, A. Chemokine Signaling in Chemotherapy-Induced Neuropathic Pain. Int. J. Mol. Sci. 2019, 20, 2904. [Google Scholar] [CrossRef]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef]
- Park, J.S.; Hoke, A. Treadmill exercise induced functional recovery after peripheral nerve repair is associated with increased levels of neurotrophic factors. PLoS ONE 2014, 9, e90245. [Google Scholar] [CrossRef]
- Cobianchi, S.; Arbat-Plana, A.; Lopez-Alvarez, V.M.; Navarro, X. Neuroprotective Effects of Exercise Treatments After Injury: The Dual Role of Neurotrophic Factors. Curr. Neuropharmacol. 2017, 15, 495–518. [Google Scholar] [CrossRef]
- Koltyn, K.F. Analgesia following exercise: A review. Sports Med. 2000, 29, 85–98. [Google Scholar] [CrossRef]
- Lemley, K.J.; Drewek, B.; Hunter, S.K.; Hoeger Bement, M.K. Pain relief after isometric exercise is not task-dependent in older men and women. Med. Sci. Sports Exerc. 2014, 46, 185–191. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar] [CrossRef]
- Simantov, R.; Snyder, S.H. Morphine-like peptides in mammalian brain: Isolation, structure elucidation, and interactions with the opiate receptor. Proc. Natl. Acad. Sci. USA 1976, 73, 2515–2519. [Google Scholar] [CrossRef]
- Connor, M.; Christie, M.D. Opioid receptor signalling mechanisms. Clin. Exp. Pharmacol. Physiol. 1999, 26, 493–499. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Fields, H.L. The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: Further studies on the anatomy of pain modulation. J. Comp. Neurol. 1979, 187, 513–531. [Google Scholar] [CrossRef]
- Colt, E.W.; Wardlaw, S.L.; Frantz, A.G. The effect of running on plasma beta-endorphin. Life Sci. 1981, 28, 1637–1640. [Google Scholar] [CrossRef]
- Pierce, E.F.; Eastman, N.W.; Tripathi, H.T.; Olson, K.G.; Dewey, W.L. Plasma beta-endorphin immunoreactivity: Response to resistance exercise. J. Sports Sci. 1993, 11, 499–502. [Google Scholar] [CrossRef]
- Olausson, B.; Eriksson, E.; Ellmarker, L.; Rydenhag, B.; Shyu, B.C.; Andersson, S.A. Effects of naloxone on dental pain threshold following muscle exercise and low frequency transcutaneous nerve stimulation: A comparative study in man. Acta Physiol. Scand. 1986, 126, 299–305. [Google Scholar] [CrossRef]
- Droste, C.; Meyer-Blankenburg, H.; Greenlee, M.W.; Roskamm, H. Effect of physical exercise on pain thresholds and plasma beta-endorphins in patients with silent and symptomatic myocardial ischaemia. Eur. Heart J. 1988, 9, 25–33. [Google Scholar] [CrossRef]
- Janal, M.N.; Colt, E.W.D.; Clark, C.W.; Glusman, M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: Effects of naloxone. Pain 1984, 19, 13–25. [Google Scholar] [CrossRef]
- Lundberg, A.; Vyklicky, L. Inhibitory interaction between spinal relexes to primary afferents. Experientia 1963, 19, 247–248. [Google Scholar] [CrossRef]
- MacDonald, E.; Kobilka, B.K.; Scheinin, M. Gene targeting—Homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol. Sci. 1997, 18, 211–219. [Google Scholar] [CrossRef]
- Nicholas, A.P.; Hokfelt, T.; Pieribone, V.A. The distribution and significance of CNS adrenoceptors examined with in situ hybridization. Trends Pharmacol. Sci. 1996, 17, 245–255. [Google Scholar] [CrossRef]
- Romero, T.R.; Duarte, I.D. Alpha(2)-Adrenoceptor agonist xylazine induces peripheral antinociceptive effect by activation of the L-arginine/nitric oxide/cyclic GMP pathway in rat. Eur. J. Pharmacol. 2009, 613, 64–67. [Google Scholar] [CrossRef]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Brown, B.S.; Payne, T.; Kim, C.; Moore, G.; Krebs, P.; Martin, W. Chronic response of rat brain norepinephrine and serotonin levels to endurance training. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 46, 19–23. [Google Scholar] [CrossRef]
- Matsunaga, D.; Nakagawa, H.; Ishiwata, T. Comparison of forced and voluntary exercise types on male rat brain monoamine levels, anxiety-like behaviour, and physiological indexes under light and dark phases. Behav. Brain. Res. 2025, 479, 115321. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid receptors and pain. Prog. Neurobiol. 2001, 63, 569–611. [Google Scholar] [CrossRef]
- Fan, Y.; Hooker, B.A.; Garrison, T.R.; El-Kouhen, O.F.; Idler, K.B.; Holley-Shanks, R.R.; Meyer, M.D.; Yao, B.B. Pharmacological and molecular characterization of a dorsal root ganglion cell line expressing cannabinoid CB(1) and CB(2) receptors. Eur. J. Pharmacol. 2011, 659, 161–168. [Google Scholar] [CrossRef]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise activates the endocannabinoid system. Neuroreport 2003, 14, 2209–2211. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Carpio, O.; Ishiguro, H.; Schanz, N.; Uhl, G.R.; Benno, R. Behavioral effects of CB2 cannabinoid receptor activation and its influence on food and alcohol consumption. Ann. N. Y. Acad. Sci. 2008, 1139, 426–433. [Google Scholar] [CrossRef]
- Rawls, S.M.; Benamar, K. Effects of opioids, cannabinoids, and vanilloids on body temperature. Front. Biosci. 2011, 3, 822–845. [Google Scholar] [CrossRef]
- Keeney, B.K.; Raichlen, D.A.; Meek, T.H.; Wijeratne, R.S.; Middleton, K.M.; Gerdeman, G.L.; Garland, T., Jr. Differential response to a selective cannabinoid receptor antagonist (SR141716: Rimonabant) in female mice from lines selectively bred for high voluntary wheel-running behaviour. Behav. Pharmacol. 2008, 19, 812–820. [Google Scholar] [CrossRef]
- Galdino, G.; Romero, T.; Pinho da Silva, J.F.; Aguiar, D.; de Paula, A.M.; Cruz, J.; Parrella, C.; Piscitelli, F.; Duarte, I.; Di Marzo, V.; et al. Acute resistance exercise induces antinociception by activation of the endocannabinoid system in rats. Anesth. Analg. 2014, 119, 702–715. [Google Scholar] [CrossRef]
- Galdino, G.; Romero, T.R.; Silva, J.F.; Aguiar, D.C.; de Paula, A.M.; Cruz, J.S.; Parrella, C.; Piscitelli, F.; Duarte, I.D.; Di Marzo, V.; et al. The endocannabinoid system mediates aerobic exercise-induced antinociception in rats. Neuropharmacology 2014, 77, 313–324. [Google Scholar] [CrossRef]
- Reis, G.M.; Pacheco, D.; Perez, A.C.; Klein, A.; Ramos, M.A.; Duarte, I.D. Opioid receptor and NO/cGMP pathway as a mechanism of peripheral antinociceptive action of the cannabinoid receptor agonist anandamide. Life Sci. 2009, 85, 351–356. [Google Scholar] [CrossRef]
- Negrete, R.; Hervera, A.; Leanez, S.; Martin-Campos, J.M.; Pol, O. The antinociceptive effects of JWH-015 in chronic inflammatory pain are produced by nitric oxide-cGMP-PKG-KATP pathway activation mediated by opioids. PLoS ONE 2011, 6, e26688. [Google Scholar] [CrossRef]
- Lichtman, A.H.; Martin, B.R. Cannabinoid-induced antinociception is mediated by a spinal alpha 2-noradrenergic mechanism. Brain Res. 1991, 559, 309–314. [Google Scholar] [CrossRef]
- Narin, S.O.; Pinar, L.; Erbas, D.; Ozturk, V.; Idiman, F. The effects of exercise and exercise-related changes in blood nitric oxide level on migraine headache. Clin. Rehabil. 2003, 17, 624–630. [Google Scholar] [CrossRef]
- Lovick, T.A. Role of nitric oxide in medullary raphe-evoked inhibition of neuronal activity in the periaqueductal gray matter. Neuroscience 1996, 75, 1203–1209. [Google Scholar] [CrossRef]
- Hamalainen, M.M.; Lovick, T.A. Involvement of nitric oxide and serotonin in modulation of antinociception and pressor responses evoked by stimulation in the dorsolateral region of the periaqueductal gray matter in the rat. Neuroscience 1997, 80, 821–827. [Google Scholar] [CrossRef]
- Smith, P.J.; Merwin, R.M. The Role of Exercise in Management of Mental Health Disorders: An Integrative Review. Annu. Rev. Med. 2021, 72, 45–62. [Google Scholar] [CrossRef]
- Weng, T.B.; Pierce, G.L.; Darling, W.G.; Falk, D.; Magnotta, V.A.; Voss, M.W. The Acute Effects of Aerobic Exercise on the Functional Connectivity of Human Brain Networks. Brain Plast. 2017, 2, 171–190. [Google Scholar] [CrossRef]
- Andreescu, C.; Ajilore, O.; Aizenstein, H.J.; Albert, K.; Butters, M.A.; Landman, B.A.; Karim, H.T.; Krafty, R.; Taylor, W.D. Disruption of Neural Homeostasis as a Model of Relapse and Recurrence in Late-Life Depression. Am. J. Geriatr. Psychiatry 2019, 27, 1316–1330. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Hoptman, M.J.; Kanellopoulos, D.; Murphy, C.F.; Lim, K.O.; Gunning, F.M. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord 2012, 139, 56–65. [Google Scholar] [CrossRef]
- Kolesar, T.A.; Bilevicius, E.; Wilson, A.D.; Kornelsen, J. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. Neuroimage Clin. 2019, 24, 102016. [Google Scholar] [CrossRef]
- Scult, M.A.; Fresco, D.M.; Gunning, F.M.; Liston, C.; Seeley, S.H.; Garcia, E.; Mennin, D.S. Changes in Functional Connectivity Following Treatment With Emotion Regulation Therapy. Front. Behav. Neurosci. 2019, 13, 10. [Google Scholar] [CrossRef]
- Gallen, C.L.; D’Esposito, M. Brain Modularity: A Biomarker of Intervention-related Plasticity. Trends Cogn. Sci. 2019, 23, 293–304. [Google Scholar] [CrossRef]
- Baniqued, P.L.; Gallen, C.L.; Voss, M.W.; Burzynska, A.Z.; Wong, C.N.; Cooke, G.E.; Duffy, K.; Fanning, J.; Ehlers, D.K.; Salerno, E.A.; et al. Brain Network Modularity Predicts Exercise-Related Executive Function Gains in Older Adults. Front. Aging Neurosci. 2017, 9, 426. [Google Scholar] [CrossRef]
- Sheps, D.S.; Koch, G.; Bragdon, E.E.; Ballenger, M.N.; McMurray, R.G. The reproducibility of resting and post exercise plasma beta-endorphins. Life Sci. 1988, 43, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain regulation by gut microbiota: Molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010, 16, 1267–1276. [Google Scholar] [CrossRef]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut microbiota regulates neuropathic pain: Potential mechanisms and therapeutic strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Shatunova, S.; Aktar, R.; Peiris, M.; Lee, J.Y.P.; Vetter, I.; Starobova, H. The role of the gut microbiome in neuroinflammation and chemotherapy-induced peripheral neuropathy. Eur. J. Pharmacol. 2024, 979, 176818. [Google Scholar] [CrossRef]
- Ma, P.; Mo, R.; Liao, H.; Qiu, C.; Wu, G.; Yang, C.; Zhang, Y.; Zhao, Y.; Song, X.J. Gut microbiota depletion by antibiotics ameliorates somatic neuropathic pain induced by nerve injury, chemotherapy, and diabetes in mice. J. Neuroinflamm. 2022, 19, 169. [Google Scholar] [CrossRef]
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017, 20, 1213–1216. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Corleto, J.; Ruegger, P.M.; Logan, G.D.; Peacock, B.B.; Mendonca, S.; Yamaki, S.; Adamson, T.; Ermel, R.; McKemy, D.; et al. Dominant Role of the Gut Microbiota in Chemotherapy Induced Neuropathic Pain. Sci. Rep. 2019, 9, 20324. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Park, S.H.; Jeong, H.T.; Shin, M.S.; Kim, M.K.; Kim, B.K.; Yoon, H.S.; Kim, S.H.; Kim, T.W. The effect of treadmill exercise on memory function and gut microbiota composition in old rats. J. Exerc. Rehabil. 2024, 20, 205–212. [Google Scholar] [CrossRef]
- Souza, P.B.; de Araujo Borba, L.; Castro de Jesus, L.; Valverde, A.P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Major Depressive Disorder and Gut Microbiota: Role of Physical Exercise. Int. J. Mol. Sci. 2023, 24, 16870. [Google Scholar] [CrossRef] [PubMed]
- Kubrak, C.; Martin, L.; Grossberg, A.J.; Olson, B.; Ottery, F.; Findlay, M.; Bauer, J.D.; Jha, N.; Scrimger, R.; Debenham, B.; et al. Quantifying the severity of sarcopenia in patients with cancer of the head and neck. Clin. Nutr. 2024, 43, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Koti, M.; Ingersoll, M.A.; Gupta, S.; Lam, C.M.; Li, X.; Kamat, A.M.; Black, P.C.; Siemens, D.R. Sex Differences in Bladder Cancer Immunobiology and Outcomes: A Collaborative Review with Implications for Treatment. Eur. Urol. Oncol. 2020, 3, 622–630. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvao, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef]
| Reference | Intervention | Methods | Outcomes | Statements from Authors |
|---|---|---|---|---|
| [94] | Conventional physiotherapy + supervised endurance (walking exercise in the hallway—6 min and stair walking exercise—2 min, 5 days a week) and strength training (abdominal exercise, a biceps curl exercise, and a triceps extension exercise for 3 sets, with patient-dependent number of repetitions, the other days) while the patient received three cycles of chemotherapy (breathing techniques or manual therapy) | (1) RCT; 46 lung cancer patients in stages IIIA/IIIB/IV who received palliative platinum-based chemotherapy were randomized into two groups: additional strength and endurance training program under the supervision of a licensed physiotherapist or only conventional physiotherapy; (2) After the intervention, they applied scales such as EORTC QLQ C-30/LC-13 questionnaire and Barthel Index | (1) Significant differences were detectable in the Barthel Index (independence in carrying out activities of daily living) in single scores of the EORTCQLQ C-30/LC-13 questionnaire (physical functioning, hemoptysis, pain in arms or shoulder, peripheral neuropathy, cognitive functioning), in the 6 min walk test, stair walking, strength capacity, and in the patient’s dyspnea perception during submaximal walking activities (Intervention Group > Control Group) | (1) “The training program has a positive impact on the patient’s independence in carrying out activities of daily living”; (2) “The training has a positive effect on the patient’s endurance and strength capacity”; (3) “This study demonstrated that even lung cancer patients receiving a palliative chemotherapy treatment should have enhanced physical activity intervention” |
| [86] | Eight-week supervised exercise program, including endurance, resistance and balance training (2×/week for 60 min) | (1) Two-armed, monocentric design. Metastasized colorectal cancer patients were allocated to an intervention group (n = 17) attending an exercise program or a waitlist control group (n = 13) which received written standard recommendations to obtain physical fitness; (2) All patients were assessed at baseline (t0) and after the intervention (t1) + follow up 4 weeks (t2) | (1) Neuropathic symptoms remained stable in the intervention group over time, while CIPN significantly worsened in the control group from t0 to t1 and t0 to t2; (2) The intervention group significantly improved in strength and balance function; (3) Changes in CIPN correlated with changes in balance | (1) “This study provides first evidence that a multimodal exercise program counteracts a worsening of CIPN and further improves balance and strength in a palliative setting with patients suffering from mCRC” |
| [20] | Six-week home-based exercise program (EXCAP: walking prescription, increasing the total number of steps walked daily by 5–20% each week + therapeutic band prescription, with varying levels of resistance) | (1) Secondary analysis of an RCT designed to assess the effects of exercise on fatigue; (2) It included all 456 patients receiving neurotoxic chemotherapy regimens (taxane-, platinum-, or vinca alkaloid-based chemotherapy) from the RCT. From the 420 patients who completed baseline assessments, 355 patients (85%) also completed post-intervention assessments (170 exercisers, 185 controls); (3) Patients reported CIPN symptoms of numbness and tingling and hot/coldness in hands/feet (0–10 scales) pre- and post-intervention. It was explored baseline neuropathy, sex, age, body mass index, cancer stage, and cancer type as possible factors associated with CIPN symptoms and exercise effectiveness | (1) Exercise reduced CIPN symptoms of hot/coldness in hands/feet and numbness and tingling compared to the control; (2) Exercise reduced CIPN symptoms more for patients who were older | (1) “Our results are consistent with cross-sectional evidence that more physical activity and larger muscle volume is associated with less severe CIPN symptoms”; (2) “Our results suggesting that exercise treats CIPN better for older patients are consistent with results that older patients require less exercise to treat CIPN”; (3) “Exercise shows promise in the treatment of CIPN and so this research should be continued, especially given the dearth of available treatments for CIPN” |
| [95] | Supervised SMT or WBV sessions twice a week, each lasting approximately 15 to 30 min, concomitant to medical therapy | (1) Prospective multicenter randomized clinical trial with 158 patients with cancer receiving chemotherapy (oxaliplatin or vinca alkaloids); (2) Patients were assigned to SMT, WBV, or treatment as usual in a 1:1:1 ratio; (3) All patients were assessed at baseline prior to initial chemotherapy and reassessed after 12 weeks + follow up 12 weeks after completion of chemotherapy | (1) The incidence of CIPN was significantly different across groups (treatment as usual: 70.6%, 95% CI, 58.0–83.2%; WBV: 41.2%, 95% CI, 27.9–54.5%; SMT: 30.0%, 95% CI, 17.9–42.1%); (2) SMT can decrease CIPN, maintain/improve vibration sensitivity, sense of touch, lower leg strength, pain, burning sensation, and balance control; patients needed fewer dose reductions and had less mortality, better quality of life, and higher physical activity levels; (3) WBV reduced incidence of CIPN and improved balance in a bipedal stance; (4) Patients receiving vinca alkaloids benefited most from SMT and WBV interventions, showing the lowest incidence of CIPN | (1) “The human neuromuscular system, if exposed to regular use and trained at maximum progression, seems to be able to maintain relevant neural functions even throughout chemotherapy”; (2) “Peripheral nerve regeneration is possible, and exercise plays a decisive role in maintaining and restoring neuromuscular function” |
| [96] | 12-week EXCAP intervention (home-based, individually tailored, moderate-intensity walking and resistance exercise program) | (1) 90 patients (65 ± 11 years old, 52% women; cancer type: breast, gastrointestinal, multiple myeloma) starting neurotoxic chemotherapy were randomized to 12 weeks of exercise or active control (nutrition education). CIPN symptoms were assessed pre-, mid-, and post-intervention; (2) At pre- and post-intervention, it was used task-free (“resting”) fMRI to assess functional connectivity in the interoceptive brain system, involving the salience and default mode networks | (1) Moderate/large beneficial effects of exercise on CIPN symptoms, CIPN signs and physical function were observed; (2) Patients with worse CIPN had lower functional connectivity within the default mode network and higher functional connectivity within the salience network; (3) Exercise tended to increase hypoconnectivity and decrease hyperconnectivity seen in CIPN | (1) “Our small data set also tentatively suggests that exercise during neurotoxic chemotherapy can partially protect against CIPN with clinically meaningful benefits and that the interoceptive brain system plays a role in CIPN and its treatment by exercise” |
| Mechanism | Effect of Exercise | Impact on CIPN | References |
|---|---|---|---|
| Increased expression of GDNF, BDNF and IGF-1 | Promotes axonal regeneration, cellular survival, and neuroprotection | [14,99,100] |
| Release of anti-inflammatory cytokines (IL-10 and IL-1RA (…)); reduction of oxidative stress markers | Ameliorates CIPN via anti-inflammatory cascades | [14] |
| Increases activation of MOR, KOR, and DOR by endorphins, enkephalins and dynorphins | Analgesia; pain modulation | [103,104,105] |
| Activates noradrenergic system; increases 5-HT in pain control areas of the brain | Pain modulation | [112,116,117,118] |
| Increases endocannabinoid levels; activates CB1 and CB2 receptors | Analgesia, decreases mechanical allodynia and thermal hyperalgesia, euphoria | [122,124,125,126,127,128,129] |
| Increases connectivity in important neurocircuits (salience network (including the amygdala and the anterior cingulate cortex), executive control network, and default mode network (including the hippocampus)) | Psychological well-being | [135,136,137,138,139,140,141] |
| Release of endorphins, neurotrophic factors and neurotransmitters, involvement of the hypothalamic-pituitary-adrenal axis reducing stress and improving mood | Psychological well-being | [142] |
| Influences gut microbiota composition and diversity | Decreased inflammatory signaling, increased neuroprotection; antidepressant effects | [143,144,145,146,153,154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loureiro, J.; Costa-Pereira, J.T.; Pozza, D.H.; Tavares, I. The Power of Movement: How Exercise Influences Chemotherapy-Induced Peripheral Neuropathy. Biomedicines 2025, 13, 1103. https://doi.org/10.3390/biomedicines13051103
Loureiro J, Costa-Pereira JT, Pozza DH, Tavares I. The Power of Movement: How Exercise Influences Chemotherapy-Induced Peripheral Neuropathy. Biomedicines. 2025; 13(5):1103. https://doi.org/10.3390/biomedicines13051103
Chicago/Turabian StyleLoureiro, Joana, José Tiago Costa-Pereira, Daniel H. Pozza, and Isaura Tavares. 2025. "The Power of Movement: How Exercise Influences Chemotherapy-Induced Peripheral Neuropathy" Biomedicines 13, no. 5: 1103. https://doi.org/10.3390/biomedicines13051103
APA StyleLoureiro, J., Costa-Pereira, J. T., Pozza, D. H., & Tavares, I. (2025). The Power of Movement: How Exercise Influences Chemotherapy-Induced Peripheral Neuropathy. Biomedicines, 13(5), 1103. https://doi.org/10.3390/biomedicines13051103








