Aerobic Exercise and Neuropathic Pain: Insights from Animal Models and Implications for Human Therapy
Abstract
:1. Introduction
1.1. Definition of Pain
1.2. Insight into Neuropathic Pain
1.3. Assessment of Neuropathic Pain
1.4. Epidemiology of Neuropathic Pain
1.5. Mechanisms Involved in the Experience of Neuropathic Pain
1.6. Physical Exercise and Health
1.7. Physical Exercise and Pain
1.8. Physical Exercise and Neuropathic Pain
1.9. Justification
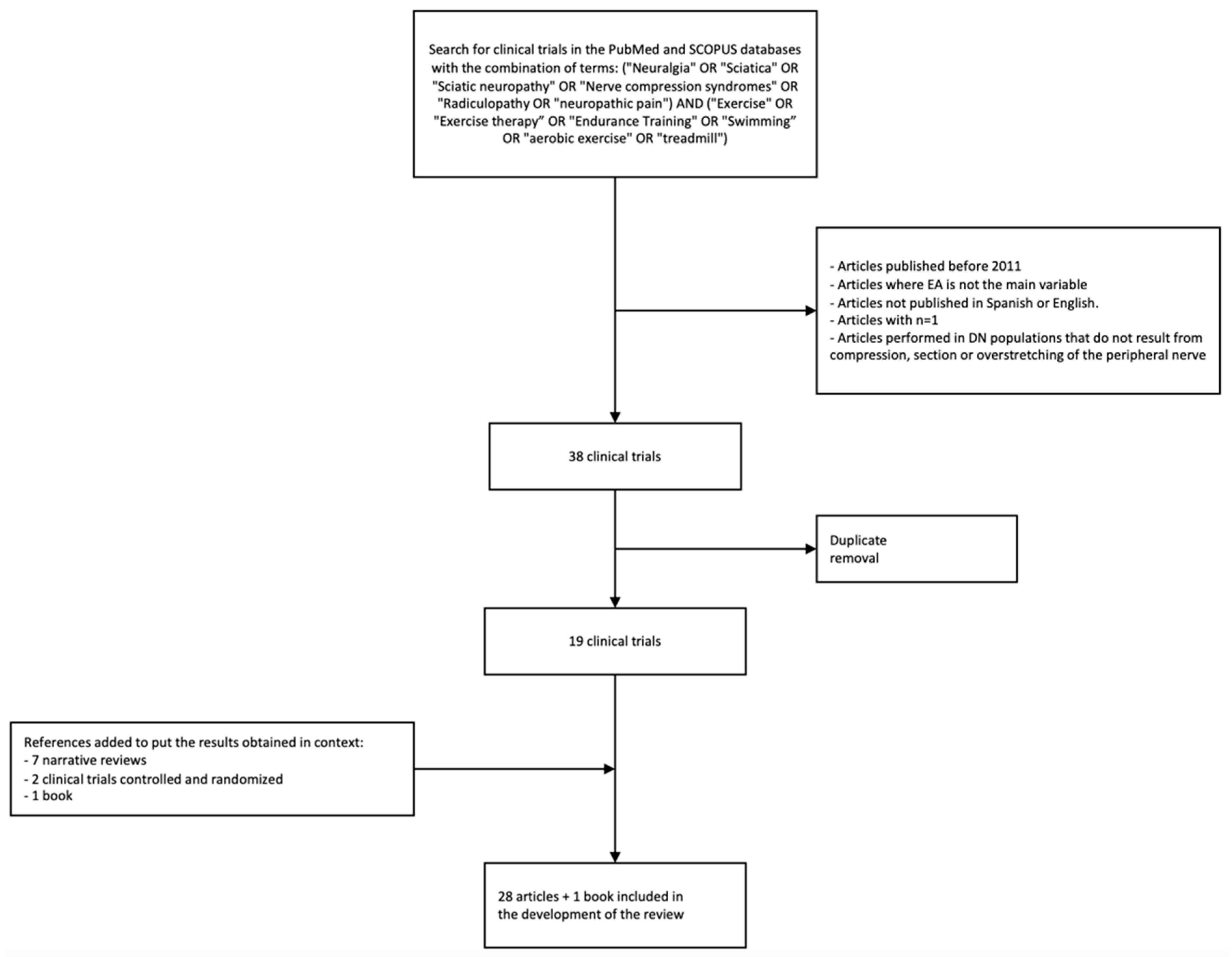
2. Methodology
3. Evidence in Animal Models and Responsible Mechanisms
- Mechanical allodynia, mainly quantified by Von Frey filaments, a punctate stimulus that is applied to the skin in the territory innervated by a nerve, whether it is the one itself injured (common in models of partial ligation [30] and chronic constriction [31]) or others that are not injured (common in nerve transection models in the initial stages due to the denervation that occurs in the injured nerve), generally in the saphenous nerve in sciatic injury models [32,33,34]. The punctate stimulus is applied through increasing intensity [29] or through temporal summation at the same intensity [35]. Another method used is a pressure algometer of increasing intensity [36].
- Allodynia or thermal hypersensitivity, generally quantified by measuring the latency time after the emission of radiant heat at a controlled intensity, usually for a maximum of 20 s to avoid damage [36,37]. Some studies also measure hypersensitivity to cold, using cold surfaces (measuring the withdrawal latency time) [28] or acetone injections [36].
3.1. Potential of Aerobic Exercise in Relation to Mechanical Allodynia
3.2. Potential of Aerobic Exercise in Relation to Thermal Hypersensitivity
3.3. Immunological and Neuroplastic Changes in the Peripheral Nervous System Involved in Exercise-Induced Hypoalgesia
3.3.1. Role of Proinflammatory Cytokines and Chemokines
3.3.2. Role of Macrophages and Anti-Inflammatory Cytokines
3.3.3. Role of Neurotrophins
3.4. Immunological and Neuroplastic Changes in the Spinal Cord Involved in Exercise-Induced Hypoalgesia
Role of Central Glial Cells
3.5. Changes in Descending Pain Modulating Systems in Neuropathic Pain Models and the Influence of Exercise
4. Evidence in Human Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; De Baets, L.; Hodges, P. Phenotyping nociceptive, neuropathic, and nociplastic pain: Who, how, & why? Braz. J. Phys. Ther. 2023, 27, 100537. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D.; et al. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, G.; Di Lionardo, A.; Di Pietro, G.; Truini, A. Neuropathic Pain Related to Peripheral Neuropathies According to the IASP Grading System Criteria. Brain Sci. 2020, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D. Neuropathic pain: Definition, assessment and epidemiology. Rev. Neurol. 2018, 175, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.C.; Böttger, K.; Slater, H.; Cook, C.; Farrell, S.F.; Hailey, L.; Tampin, B.; Schmid, A.B. Concurrent validity of a low-cost and time-efficient clinical sensory test battery to evaluate somatosensory dysfunction. Eur. J. Pain 2019, 23, 1826–1838. [Google Scholar] [CrossRef]
- Truini, A. A Review of Neuropathic Pain: From Diagnostic Tests to Mechanisms. Pain Ther. 2017, 6, 5–9. [Google Scholar] [CrossRef]
- La Cesa, S.; Tamburin, S.; Tugnoli, V.; Sandrini, G.; Paolucci, S.; Lacerenza, M.; Marchettini, P.; Cruccu, G.; Truini, A. How to diagnose neuropathic pain? The contribution from clinical examination, pain questionnaires and diagnostic tests. Neurol. Sci. 2015, 36, 2169–2175. [Google Scholar] [CrossRef]
- Szewczyk, A.K.; Jamroz-Wiśniewska, A.; Haratym, N.; Rejdak, K. Neuropathic pain and chronic pain as an underestimated interdisciplinary problem. Int. J. Occup. Med. Environ. Heal. 2022, 35, 249–264. [Google Scholar] [CrossRef]
- Truini, A.; Cruccu, G. How diagnostic tests help to disentangle the mechanisms underlying neuropathic pain symptoms in painful neuropathies. Pain 2016, 157, S53–S59. [Google Scholar] [CrossRef]
- Boadas-Vaello, P.; Castany, S.; Homs, J.; Álvarez-Pérez, B.; Deulofeu, M.; Verdú, E. Neuroplasticity of ascending and descending pathways after somatosensory system injury: Reviewing knowledge to identify neuropathic pain therapeutic targets. Spinal Cord 2016, 54, 330–340. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; Koltzenburg, M.; Tracey, I.; Turk, D.C. Wall & Melzack’s Textbook of Pain; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharmacal Res. 2018, 41, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2017, 8, a029694. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Physical Exercise for Human Health; Springer: Singapore, 2020. [Google Scholar]
- Swenson, S.; Blum, K.; McLaughlin, T.; Gold, M.S.; Thanos, P.K. The therapeutic potential of exercise for neuropsychiatric diseases: A review. J. Neurol. Sci. 2020, 412, 116763. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, B.; Maurya, N.; Lee, S.-D.; Kumar, V.B. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5895. [Google Scholar] [CrossRef] [PubMed]
- Childs, E.; de Wit, H. Regular exercise is associated with emotional resilience to acute stress in healthy adults. Front. Physiol. 2014, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; English, A.W. Strategies to promote peripheral nerve regeneration: Electrical stimulation and/or exercise. Eur. J. Neurosci. 2015, 43, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Agata, V.; Trovato, B.; Roggio, F.; Castorina, A.; Vecchio, M.; Di Rosa, M.; Musumeci, G. The role of exercise on peripheral nerve regeneration: From animal model to clinical application. Heliyon 2021, 7, e08281. [Google Scholar] [CrossRef]
- Nestler, E.J.; Waxman, S.G. Resilience to Stress and Resilience to Pain: Lessons from Molecular Neurobiology and Genetics. Trends Mol. Med. 2020, 26, 924–935. [Google Scholar] [CrossRef]
- Lima, L.V.; Abner, T.S.S.; Sluka, K.A. Does exercise increase or decrease pain? Central mechanisms underlying these two phenomena. J. Physiol. 2017, 595, 4141–4150. [Google Scholar] [CrossRef]
- Sluka, K.A.; Frey-Law, L.; Bement, M.H. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain 2018, 159, S91–S97. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, J.; Nijs, J.; Ickmans, K.; Godderis, L.; Ghosh, M.; Polli, A. The Interplay between Oxidative Stress, Exercise, and Pain in Health and Disease: Potential Role of Autonomic Regulation and Epigenetic Mechanisms. Antioxidants 2020, 9, 1166. [Google Scholar] [CrossRef] [PubMed]
- Swinkels-Meewisse, I.E.; Roelofs, J.; Oostendorp, R.A.; Verbeek, A.L.; Vlaeyen, J.W. Acute low back pain: Pain-related fear and pain catastrophizing influence physical performance and perceived disability. Pain 2006, 120, 36–43. [Google Scholar] [CrossRef]
- Da Silva Santos, R.; Galdino, G. Endogenous systems involved in exercise-induced analgesia. J. Physiol. Pharmacol. 2018, 69, 3–13. [Google Scholar] [PubMed]
- Chen, Y.-M.; Wang, X.-Q. Bibliometric Analysis of Exercise and Neuropathic Pain Research. J. Pain Res. 2020, 13, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, F.; Martins, D.; Bratti, T.; Mazzardo-Martins, L.; Winkelmann-Duarte, E.; Guglielmo, L.; Santos, A. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neuroscience 2011, 194, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Rostami, Z.; Ghasemi, S.; Farzadmanesh, H.; Safari, M.; Ghanbari, A. Sex Difference in Trigeminal Neuropathic Pain Response to Exercise: Role of Oxidative Stress. Pain Res. Manag. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Kami, K.; Taguchi, S.; Tajima, F.; Senba, E. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol. Pain 2016, 12, 1744806916629059. [Google Scholar] [CrossRef]
- Grace, P.M.; Fabisiak, T.J.; Green-Fulgham, S.M.; Anderson, N.D.; Strand, K.A.; Kwilasz, A.J.; Galer, E.L.; Walker, F.R.; Greenwood, B.N.; Maier, S.F.; et al. Prior voluntary wheel running attenuates neuropathic pain. Pain 2016, 157, 2012–2023. [Google Scholar] [CrossRef]
- Lopez-Alvarez, V.M.; Puigdomenech, M.; Navarro, X.; Cobianchi, S. Monoaminergic descending pathways contribute to modulation of neuropathic pain by increasing-intensity treadmill exercise after peripheral nerve injury. Exp. Neurol. 2018, 299, 42–55. [Google Scholar] [CrossRef]
- Cobianchi, S.; Casals-Diaz, L.; Jaramillo, J.; Navarro, X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp. Neurol. 2013, 240, 157–167. [Google Scholar] [CrossRef] [PubMed]
- López-Álvarez, V.M.; Modol, L.; Navarro, X.; Cobianchi, S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain 2015, 156, 1812–1825. [Google Scholar] [CrossRef]
- Tian, J.; Yu, T.; Xu, Y.; Pu, S.; Lv, Y.; Zhang, X.; DU, D. Swimming Training Reduces Neuroma Pain by Regulating Neurotrophins. Med. Sci. Sports Exerc. 2018, 50, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Farzad, B.; Rajabi, H.; Gharakhanlou, R.; Allison, D.J.; Hayat, P.; Jameie, S.B. Swimming Training Attenuates Allodynia and Hyperalgesia Induced by Peripheral Nerve Injury in an Adult Male Rat Neuropathic Model: Effects on Irisin and GAD. Pain Med. 2018, 19, 2236–2245. [Google Scholar] [CrossRef]
- Kami, K.; Taguchi, S.; Tajima, F.; Senba, E. Histone Acetylation in Microglia Contributes to Exercise-Induced Hypoalgesia in Neuropathic Pain Model Mice. J. Pain 2016, 17, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, F.; Ferreira, T.A.; Córdova, M.M.; Dombrowski, P.A.; da Cunha, C.; Santo, C.C.D.E.; Poli, A.; Pires, R.G.; Martins-Silva, C.; Sluka, K.A.; et al. Role of brainstem serotonin in analgesia produced by low-intensity exercise on neuropathic pain after sciatic nerve injury in mice. Pain 2015, 156, 2595–2606. [Google Scholar] [CrossRef]
- Cho, Y.-H.; Kim, J.-E.; Seo, T.-B. Effect of treadmill exercise on pain-related Wnt/β-catenin signaling pathway in dorsal root ganglion neurons at the early phase regeneration of the injured sciatic nerve. J. Exerc. Rehabil. 2021, 17, 96–102. [Google Scholar] [CrossRef]
- Bobinski, F.; Teixeira, J.M.; Sluka, K.A.; Santos, A.R.S. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 2017, 159, 437–450. [Google Scholar] [CrossRef]
- Huang, P.-C.; Tsai, K.-L.; Chen, Y.-W.; Lin, H.-T.; Hung, C.-H. Exercise Combined with Ultrasound Attenuates Neuropathic Pain in Rats Associated With Downregulation of IL-6 and TNF-α, but With Upregulation of IL-Obstet. Anesth. Dig. 2017, 124, 2038–2044. [Google Scholar] [CrossRef]
- Hung, C.-H.; Huang, P.-C.; Tzeng, J.-I.; Wang, J.-J.; Chen, Y.-W. Therapeutic Ultrasound and Treadmill Training Suppress Peripheral Nerve Injury–Induced Pain in Rats. Phys. Ther. 2016, 96, 1545–1553. [Google Scholar] [CrossRef]
- Stagg, N.J.; Mata, H.P.; Ibrahim, M.M.; Henriksen, E.J.; Porreca, F.; Vanderah, T.W.; Malan, T.P. Regular Exercise Reverses Sensory Hypersensitivity in a Rat Neuropathic Pain Model. Anesthesiology 2011, 114, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; DeMaman, A.; Kusuda, R.; Cadetti, F.; Ravanelli, M.I.; Queiroz, A.L.; Sousa, T.A.; Zanon, S.; Silveira, L.R.; Lucas, G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain 2015, 156, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Li, Y.-T.; Li, Z.-Y.; Hung, C.-H. Exercise Training Attenuates Neuropathic Pain and Cytokine Expression After Chronic Constriction Injury of Rat Sciatic Nerve. Obstet. Anesth. Dig. 2012, 114, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-L.; Huang, P.-C.; Wang, L.-K.; Hung, C.-H.; Chen, Y.-W. Incline treadmill exercise suppresses pain hypersensitivity associated with the modulation of pro-inflammatory cytokines and anti-inflammatory cytokine in rats with peripheral nerve injury. Neurosci. Lett. 2017, 643, 27–31. [Google Scholar] [CrossRef]
- Kuffler, D.P. Injury-Induced Effectors of Neuropathic Pain. Mol. Neurobiol. 2019, 57, 51–66. [Google Scholar] [CrossRef]
- Domoto, R.; Sekiguchi, F.; Tsubota, M.; Kawabata, A. Macrophage as a Peripheral Pain Regulator. Cells 2021, 10, 1881. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Liu, R.; Li, W.; Hua, B.; Bao, Y. Wnt Signaling Pathways: A Role in Pain Processing. Neuro. Mol. Med. 2022, 24, 233–249. [Google Scholar] [CrossRef]
- Khan, N.; Smith, M.T. Neurotrophins and Neuropathic Pain: Role in Pathobiology. Molecules 2015, 20, 10657–10688. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef]
- Feng, N.; Huke, S.; Zhu, G.; Tocchetti, C.G.; Shi, S.; Aiba, T.; Kaludercic, N.; Hoover, D.B.; Beck, S.E.; Mankowski, J.L.; et al. Constitutive BDNF/TrkB signaling is required for normal cardiac contraction and relaxation. Proc. Natl. Acad. Sci. USA 2015, 112, 1880–1885. [Google Scholar] [CrossRef]
- Yadav, A.; Matson, K.J.; Li, L.; Hua, I.; Petrescu, J.; Kang, K.; Alkaslasi, M.R.; Lee, D.I.; Hasan, S.; Galuta, A.; et al. A cellular taxonomy of the adult human spinal cord. Neuron 2023, 111, 328–344.e7. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, T. The Role of Astrocytes in the Modulation ofK+-Cl−-Cotransporter-2 Function. Int. J. Mol. Sci. 2020, 21, 9539. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.; Llorca-Torralba, M.; Berrocoso, E.; Micó, J.A. Monoamines as Drug Targets in Chronic Pain: Focusing on Neuropathic Pain. Front. Neurosci. 2019, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, I.M.; Diab, A.A. The Effect of Adding Forward Head Posture Corrective Exercises in the Management of Lumbosacral Radiculopathy: A Randomized Controlled Study. J. Manip. Physiol. Ther. 2015, 38, 167–178. [Google Scholar] [CrossRef]
- Toth, C.; Brady, S.; Gagnon, F.; Wigglesworth, K. A Randomized, Single-Blind, Controlled, Parallel Assignment Study of Exercise Versus Education as Adjuvant in the Treatment of Peripheral Neuropathic Pain. Clin. J. Pain 2014, 30, 111–118. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruimonte-Crespo, J.; Plaza-Manzano, G.; Díaz-Arribas, M.J.; Navarro-Santana, M.J.; López-Marcos, J.J.; Fabero-Garrido, R.; Seijas-Fernández, T.; Valera-Calero, J.A. Aerobic Exercise and Neuropathic Pain: Insights from Animal Models and Implications for Human Therapy. Biomedicines 2023, 11, 3174. https://doi.org/10.3390/biomedicines11123174
Ruimonte-Crespo J, Plaza-Manzano G, Díaz-Arribas MJ, Navarro-Santana MJ, López-Marcos JJ, Fabero-Garrido R, Seijas-Fernández T, Valera-Calero JA. Aerobic Exercise and Neuropathic Pain: Insights from Animal Models and Implications for Human Therapy. Biomedicines. 2023; 11(12):3174. https://doi.org/10.3390/biomedicines11123174
Chicago/Turabian StyleRuimonte-Crespo, Jorge, Gustavo Plaza-Manzano, María José Díaz-Arribas, Marcos José Navarro-Santana, José Javier López-Marcos, Raúl Fabero-Garrido, Tamara Seijas-Fernández, and Juan Antonio Valera-Calero. 2023. "Aerobic Exercise and Neuropathic Pain: Insights from Animal Models and Implications for Human Therapy" Biomedicines 11, no. 12: 3174. https://doi.org/10.3390/biomedicines11123174
APA StyleRuimonte-Crespo, J., Plaza-Manzano, G., Díaz-Arribas, M. J., Navarro-Santana, M. J., López-Marcos, J. J., Fabero-Garrido, R., Seijas-Fernández, T., & Valera-Calero, J. A. (2023). Aerobic Exercise and Neuropathic Pain: Insights from Animal Models and Implications for Human Therapy. Biomedicines, 11(12), 3174. https://doi.org/10.3390/biomedicines11123174




