Differential Effects of Canonical Androgens and 11-Ketotestosterone on Reproductive Phenotypes and Folliculogenesis in Mouse Model of PCOS
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Assessment of the Estrous Cycle
2.3. Mouse Ovary Collection and Follicle Classification
2.4. Hormone Assays
2.5. Mouse Follicle Isolation and In Vitro Culture
2.6. Isolation of Mouse Primary Granulosa Cells and Cell Culture
2.7. Western Blotting and RT-qPCR Analysis
2.8. Luciferase Assay
2.9. ELISAs for 17β-Estradiol Quantification
2.10. Statistical Analysis
3. Results
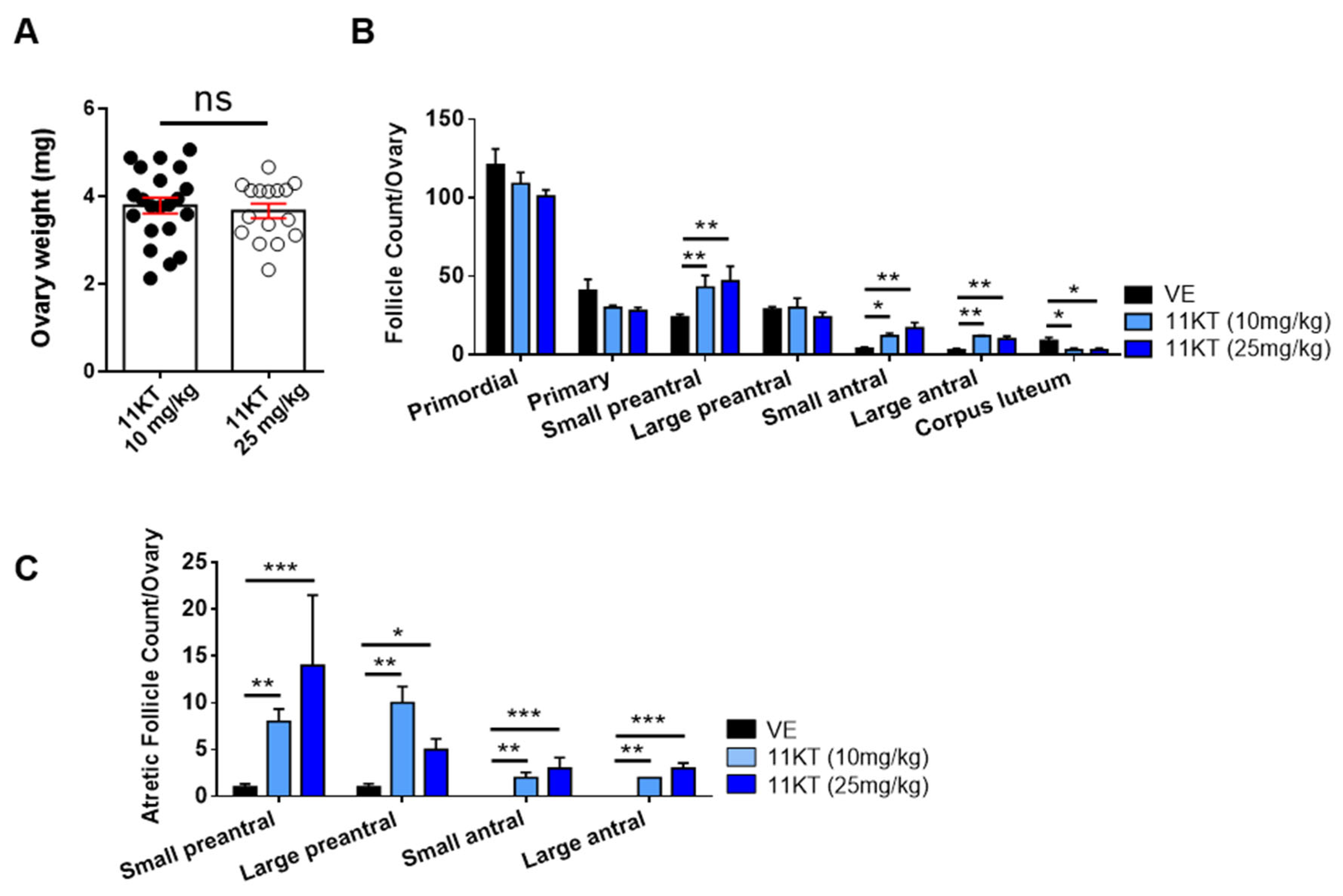
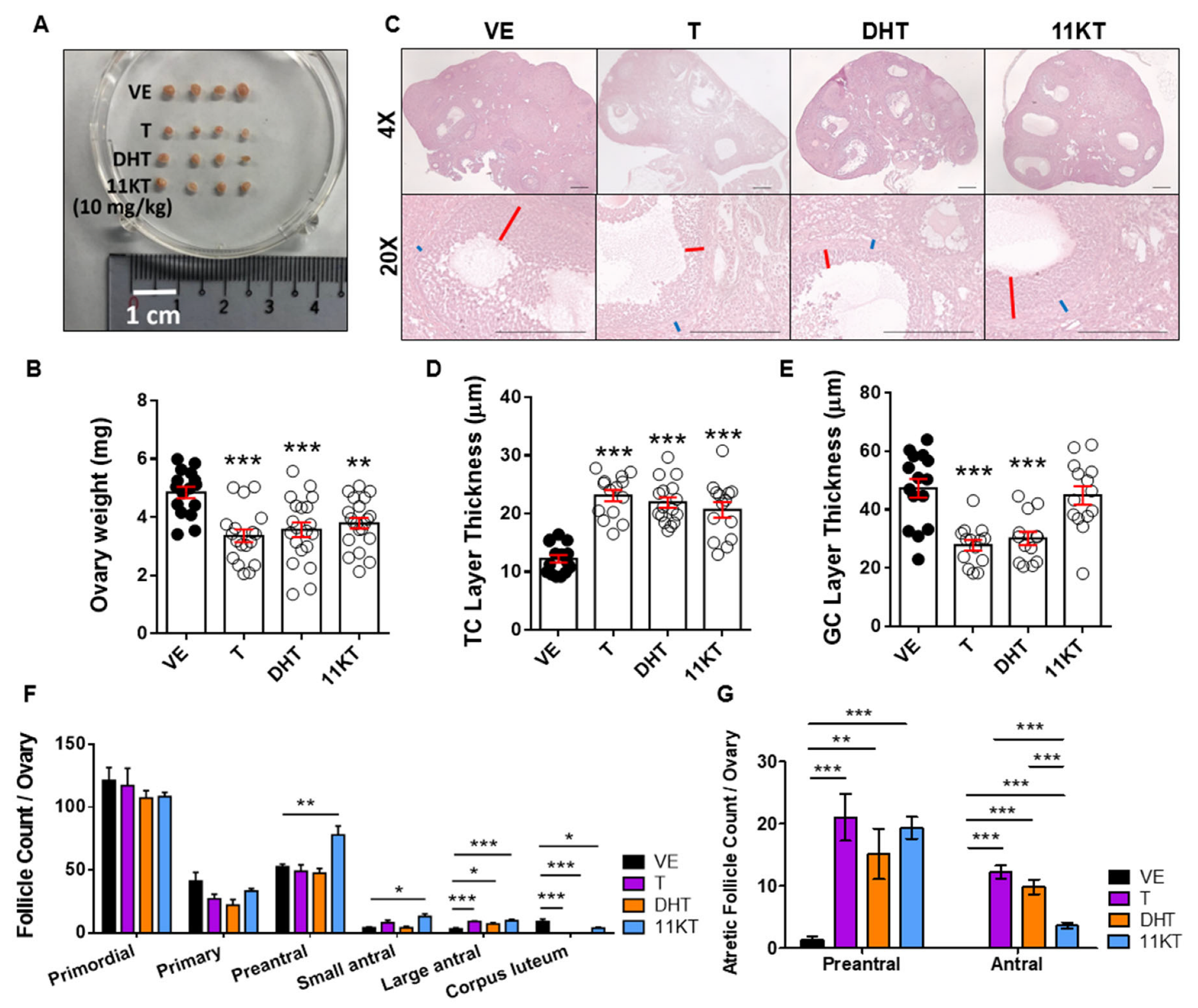
3.1. Establishment and Comparison of Hyperandrogenized Mouse Models for Different Traits of PCOS
3.2. Promotion of Follicle Growth Caused by High Androgen Level and Its Negative Impact on Survival Rate and the Function of Ovulation in In-Vitro Follicle Culture System
3.3. Distinct Effects of 11KT on AR Expression and Downstream Signaling in PCOS Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- McCartney, C.R.; Marshall, J.C. Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 1398–1399. [Google Scholar] [CrossRef] [PubMed]
- Lizneva, D.; Suturina, L.; Walker, W.; Brakta, S.; Gavrilova-Jordan, L.; Azziz, R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Taieb, A.; Feryel, A. Deciphering the Role of Androgen in the Dermatologic Manifestations of Polycystic Ovary Syndrome Patients: A State-of-the-Art Review. Diagnostics 2024, 14, 2578. [Google Scholar] [CrossRef]
- Wang, K.; Li, Y.; Chen, Y. Androgen excess: A hallmark of polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1273542. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, bnaa010. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Eid, S.; Kay, C.R.; Jimenez, M.; McMahon, A.C.; Desai, R.; Allan, C.M.; Smith, J.T.; Handelsman, D.J.; Walters, K.A. Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology 2015, 156, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.S.L.; Edwards, M.C.; Desai, R.; Jimenez, M.; Gilchrist, R.B.; Handelsman, D.J.; Walters, K.A. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E3334–E3343. [Google Scholar] [CrossRef]
- Aflatounian, A.; Edwards, M.C.; Rodriguez Paris, V.; Bertoldo, M.J.; Desai, R.; Gilchrist, R.B.; Ledger, W.L.; Handelsman, D.J.; Walters, K.A. Androgen signaling pathways driving reproductive and metabolic phenotypes in a PCOS mouse model. J. Endocrinol. 2020, 245, 381–395. [Google Scholar] [CrossRef]
- van Houten, E.L.; Kramer, P.; McLuskey, A.; Karels, B.; Themmen, A.P.; Visser, J.A. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology 2012, 153, 2861–2869. [Google Scholar] [CrossRef]
- Caldwell, A.S.; Middleton, L.J.; Jimenez, M.; Desai, R.; McMahon, A.C.; Allan, C.M.; Handelsman, D.J.; Walters, K.A. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014, 155, 3146–3159. [Google Scholar] [CrossRef]
- Ryan, G.E.; Malik, S.; Mellon, P.L. Antiandrogen Treatment Ameliorates Reproductive and Metabolic Phenotypes in the Letrozole-Induced Mouse Model of PCOS. Endocrinology 2018, 159, 1734–1747. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, R.; Fabbri, R.; Battaglia, C.; Venturoli, S. Ovulatory effects of flutamide in the polycystic ovary syndrome. Gynecol. Endocrinol. 2013, 29, 391–395. [Google Scholar] [CrossRef]
- Vassiliadi, D.A.; Barber, T.M.; Hughes, B.A.; McCarthy, M.I.; Wass, J.A.; Franks, S.; Nightingale, P.; Tomlinson, J.W.; Arlt, W.; Stewart, P.M. Increased 5 alpha-reductase activity and adrenocortical drive in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 3558–3566. [Google Scholar] [CrossRef]
- O’Reilly, M.W.; Taylor, A.E.; Crabtree, N.J.; Hughes, B.A.; Capper, F.; Crowley, R.K.; Stewart, P.M.; Tomlinson, J.W.; Arlt, W. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: The utility of serum androstenedione. J. Clin. Endocrinol. Metab. 2014, 99, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; Kempegowda, P.; Jenkinson, C.; Taylor, A.E.; Quanson, J.L.; Storbeck, K.H.; Arlt, W. 11-Oxygenated C19 Steroids Are the Predominant Androgens in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Villani, M.; Garofalo, S.; Faccin, G.; Bonora, E.; Fiers, T.; Kaufman, J.M.; Moghetti, P. Clinical Value of Serum Levels of 11-Oxygenated Metabolites of Testosterone in Women With Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, e2047–e2055. [Google Scholar] [CrossRef]
- Mody, A.P.; Lodish, M.B.; Auchus, R.J.; Turcu, A.F.; Jiang, F.; Huddleston, H.G. Exploring the Predictive Role of 11-Oxyandrogens in Diagnosing Polycystic Ovary Syndrome. Endocrinol. Diabetes Metab. 2025, 8, e70022. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsuzaki, T.; Miyado, M.; Saito, K.; Iwasa, T.; Matsubara, Y.; Ogata, T.; Irahara, M.; Fukami, M. 11-oxygenated C19 steroids as circulating androgens in women with polycystic ovary syndrome. Endocr. J. 2018, 65, 979–990. [Google Scholar] [CrossRef]
- Imamichi, Y.; Yuhki, K.I.; Orisaka, M.; Kitano, T.; Mukai, K.; Ushikubi, F.; Taniguchi, T.; Umezawa, A.; Miyamoto, K.; Yazawa, T. 11-Ketotestosterone Is a Major Androgen Produced in Human Gonads. J. Clin. Endocrinol. Metab. 2016, 101, 3582–3591. [Google Scholar] [CrossRef]
- Rege, J.; Nakamura, Y.; Satoh, F.; Morimoto, R.; Kennedy, M.R.; Layman, L.C.; Honma, S.; Sasano, H.; Rainey, W.E. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J. Clin. Endocrinol. Metab. 2013, 98, 1182–1188. [Google Scholar] [CrossRef]
- Swart, A.C.; Schloms, L.; Storbeck, K.H.; Bloem, L.M.; Toit, T.; Quanson, J.L.; Rainey, W.E.; Swart, P. 11beta-hydroxyandrostenedione, the product of androstenedione metabolism in the adrenal, is metabolized in LNCaP cells by 5alpha-reductase yielding 11beta-hydroxy-5alpha-androstanedione. J. Steroid Biochem. Mol. Biol. 2013, 138, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, T.; Uesaka, M.; Inaoka, Y.; Mizutani, T.; Sekiguchi, T.; Kajitani, T.; Kitano, T.; Umezawa, A.; Miyamoto, K. Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: Conservation and evolution of the androgen metabolic pathway. Endocrinology 2008, 149, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.F.; Rege, J.; Auchus, R.J.; Rainey, W.E. 11-Oxygenated androgens in health and disease. Nat. Rev. Endocrinol. 2020, 16, 284–296. [Google Scholar] [CrossRef]
- Kalhori, Z.; Azadbakht, M.; Soleimani Mehranjani, M.; Shariatzadeh, M.A. Improvement of the folliculogenesis by transplantation of bone marrow mesenchymal stromal cells in mice with induced polycystic ovary syndrome. Cytotherapy 2018, 20, 1445–1458. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef]
- Hardy, K.; Fenwick, M.; Mora, J.; Laird, M.; Thomson, K.; Franks, S. Onset and Heterogeneity of Responsiveness to FSH in Mouse Preantral Follicles in Culture. Endocrinology 2017, 158, 134–147. [Google Scholar] [CrossRef]
- Lan, K.C.; Cheng, Y.H.; Chang, Y.C.; Wei, K.T.; Weng, P.L.; Kang, H.Y. Interaction between Chromodomain Y-like Protein and Androgen Receptor Signaling in Sertoli Cells Accounts for Spermatogenesis. Cells 2024, 13, 851. [Google Scholar] [CrossRef]
- Liu, H.; Tu, M.; Yin, Z.; Zhang, D.; Ma, J.; He, F. Unraveling the complexity of polycystic ovary syndrome with animal models. J. Genet. Genom. 2024, 51, 144–158. [Google Scholar] [CrossRef]
- Walters, K.A.; Gilchrist, R.B.; Ledger, W.L.; Teede, H.J.; Handelsman, D.J.; Campbell, R.E. New Perspectives on the Pathogenesis of PCOS: Neuroendocrine Origins. Trends Endocrinol. Metab. 2018, 29, 841–852. [Google Scholar] [CrossRef]
- Kar, S. Clomiphene citrate or letrozole as first-line ovulation induction drug in infertile PCOS women: A prospective randomized trial. J. Hum. Reprod. Sci. 2012, 5, 262–265. [Google Scholar] [CrossRef]
- Legro, R.S.; Brzyski, R.G.; Diamond, M.P.; Coutifaris, C.; Schlaff, W.D.; Casson, P.; Christman, G.M.; Huang, H.; Yan, Q.; Alvero, R.; et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N. Engl. J. Med. 2014, 371, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International, P.N. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Arao, Y.; Hamilton, K.J.; Ray, M.K.; Scott, G.; Mishina, Y.; Korach, K.S. Estrogen receptor alpha AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc. Natl. Acad. Sci. USA 2011, 108, 14986–14991. [Google Scholar] [CrossRef]
- Silva, M.S.; Prescott, M.; Campbell, R.E. Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI Insight 2018, 3, e99405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Zhu, F.F.; Xiong, J.; Shi, X.B.; Fu, S.X. Characteristics of different phenotypes of polycystic ovary syndrome based on the Rotterdam criteria in a large-scale Chinese population. BJOG 2009, 116, 1633–1639. [Google Scholar] [CrossRef]
- van Houten, E.L.; Visser, J.A. Mouse models to study polycystic ovary syndrome: A possible link between metabolism and ovarian function? Reprod. Biol. 2014, 14, 32–43. [Google Scholar] [CrossRef]
- Manneras, L.; Cajander, S.; Holmang, A.; Seleskovic, Z.; Lystig, T.; Lonn, M.; Stener-Victorin, E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 2007, 148, 3781–3791. [Google Scholar] [CrossRef]



| Vehicle Group | Androgenized Mouse Models | F Value | η2 | ||||
|---|---|---|---|---|---|---|---|
| T | DHT | 11KT (10 mg/kg) | 11KT (25 mg/kg) | ||||
| LH (pg/mL) | 60.40 ± 3.41 | 98.57 ± 13.23 *** | 87.40 ± 13.87 ** | 90.73 ± 13.13 *** | 95.07 ± 6.77 *** | 12.0 | 0.64 |
| T (pg/mL) | 40.52 ± 24.46 | 244.09 ± 51.14 *** | 31.96 ± 16.33 | 29.75 ± 2.56 | 15.11 ± 1.88 | 59.2 | 0.88 |
| DHT (pg/mL) | 38.15 ± 7.94 | 48.95 ± 17.05 | 492.24 ± 230.42 *** | 58.92 ± 19.20 | 62.81 ± 18.68 | 21.5 | 0.77 |
| 11KT (pg/mL) | 18.69 ± 1.99 | 30.02 ± 9.95 | 39.07 ± 3.61 | 3112.19 ± 684.94 *** | 3167.67 ± 758.24 *** | 104.6 | 0.93 |
| E1(pg/mL) | 1.29 ± 0.16 | 1.60 ± 0.18 * | 1.59 ± 0.37 * | 1.28 ± 0.19 | 1.12 ± 0.08 | 8.1 | 0.52 |
| E2 (pg/mL) | 39.17 ± 4.42 | 48.79 ± 8.45 * | 49.77 ± 5.26 ** | 43.34 ± 4.59 | 44.27 ± 5.57 | 3.8 | 0.35 |
| Human PCOS Traits | Mouse Models | ||
|---|---|---|---|
| T | DHT | 11KT | |
| Irregular Cycle (IC)/Acyclicity (A) | A | A | IC |
| Oligo (O)/Anovulation (A) | A | A | O |
| Hyperandrogenism | ✓ | ✓ | ✓ |
| ↑ LH | ✓ | ✓ | ✓ |
| ↑ Body weight * | ✗ | ✓ | ✗ |
| ↑ Large antral follicles | ✓ | ✓ | ✓ |
| ↑ Small antral follicles | ✗ | ✗ | ✓ |
| ↑ Preantral follicles | ✗ | ✗ | ✓ |
| ↑ Atretic antral Follicles | ✓ | ✓ | ✓ ** |
| ↓ Granulosa cell layer thickness | ✓ | ✓ | ✗ |
| ↑ Theca cell layer thickness | ✓ | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, Y.-R.; Liao, Y.-N.; Tsai, C.-J.; Lee, Y.-A.; Hsia, S.-M.; Lan, K.-C.; Kang, H.-Y. Differential Effects of Canonical Androgens and 11-Ketotestosterone on Reproductive Phenotypes and Folliculogenesis in Mouse Model of PCOS. Biomedicines 2025, 13, 1077. https://doi.org/10.3390/biomedicines13051077
Tsai Y-R, Liao Y-N, Tsai C-J, Lee Y-A, Hsia S-M, Lan K-C, Kang H-Y. Differential Effects of Canonical Androgens and 11-Ketotestosterone on Reproductive Phenotypes and Folliculogenesis in Mouse Model of PCOS. Biomedicines. 2025; 13(5):1077. https://doi.org/10.3390/biomedicines13051077
Chicago/Turabian StyleTsai, Yi-Ru, Yen-Nung Liao, Cheng-Ju Tsai, Yu-Ang Lee, Shih-Min Hsia, Kuo-Chung Lan, and Hong-Yo Kang. 2025. "Differential Effects of Canonical Androgens and 11-Ketotestosterone on Reproductive Phenotypes and Folliculogenesis in Mouse Model of PCOS" Biomedicines 13, no. 5: 1077. https://doi.org/10.3390/biomedicines13051077
APA StyleTsai, Y.-R., Liao, Y.-N., Tsai, C.-J., Lee, Y.-A., Hsia, S.-M., Lan, K.-C., & Kang, H.-Y. (2025). Differential Effects of Canonical Androgens and 11-Ketotestosterone on Reproductive Phenotypes and Folliculogenesis in Mouse Model of PCOS. Biomedicines, 13(5), 1077. https://doi.org/10.3390/biomedicines13051077







