1. Introduction
The chicken embryo (CE) is considered an important model that over time has contributed to the development of valuable concepts in immunology, genetics, virology, cancer, and cell biology [
1]. Historically, the CE attracted the interest of the ancient Egyptians and Aristotle, who opened eggs at different stages of incubation to examine their developmental progression [
2]. Over the years, a few pioneers (Rawles, Fell, Wetzel, etc.), through studies of embryonic development, perfected embryo culture and microsurgery methods, making the CE model approach accessible [
1]. The first comprehensive atlas of chick morphology was published in 1889 [
3], and in 1951, Hamburger and Hamilton studied the embryonic development of the CE, describing 46 distinct morphological phases, divided according to the number of stages rather than the incubation time [
4].
This model is relatively simple, rapid, and low-cost, and it offers unique opportunities to study embryonic development, genetic regulation, disease mechanisms, and the effects of various substances in a controlled environment.
The model can be manipulated and cultured in most laboratories as, in the early stages, it can be considered a 3D tissue culture model that does not require ethical approval. Because of its short development period, results can be quickly obtained, significantly reducing the time and cost of research. The easy handling of the CE facilitates direct visualization of stability, biocompatibility, circulation, release, degradation, and therapeutic efficacy, key factors that predict in vivo performance in rodents and higher-order mammals. In addition, CEs are accessible for manipulation and injections under a stereomicroscope, followed by in vivo imaging in ovo and non-invasive monitoring during later development [
5]. It is an established model for tissue/cell transplantation, and due to the lack of an immune system in the early stages of development, the CE is increasingly recognized as a model of choice for mammalian biology, with new applications for stem cell and cancer research. Due to their operability and intrinsic immunotolerance, CEs have been used in a series of studies to evaluate the proliferation, differentiation, and migration capacity of rodent and human stem cells [
6]. This model has been used to monitor the growth and metastatic properties of various cancer cell lines grafted on the chorioallantoic membrane (CAM), which is an excellent method to study tumor metastasis [
7,
8]. In addition, transplantation of cancer cell lines into the CE, which can be coupled with new non-invasive live monitoring methods, represents a powerful new tool to identify signaling pathways controlling tumorigenesis and to validate new targets for therapy [
5].
Accordingly, this model represents a promising alternative to traditional mouse models, in line with the ethical principles of the 3Rs (Replacement, Reduction, and Refinement), as extensively reported in ancient studies. However, its full potential in applications using molecular imaging methods remains underexplored and requires further optimization.
In the light of this, the aim of our review was not only to highlight the fundamental and established features of the CE model but also to analyze its current applications. Indeed, molecular imaging has the potential to transform research in various areas, such as drug development, biomarker discovery, and regenerative medicine, advancing more ethical and innovative practices in biomedical science. Here, by integrating the CE model with molecular imaging methods, we aim to illustrate how these technologies can open new paths for the advancement of preclinical research.
2. The Chicken Genome
The chicken genome is a landmark in genomics and evolutionary biology, representing the first avian genome to be sequenced and serving as a model system for research in agriculture, developmental biology, and comparative genomics. It has become a keystone for understanding vertebrate evolution, bridging the gap between mammalian and other non-mammalian genomes. The study of the chicken genome dates back to 1936, when the first genetic linkage map was constructed, making the chicken one of the earliest species to have its genetic architecture systematically explored [
9]. The chicken genome is relatively compact, with an approximate size of 1.2 billion base pairs distributed across 39 pairs of chromosomes, including all 10 autosomal macrochromosomes, both the
Z and
W sex chromosomes, and two-thirds of the 28 microchromosomes [
10]. In 2004, the chicken genome became the first avian genome to be sequenced. The sequencing was accompanied by the development of essential resources, such as Bacterial Artificial Chromosome (BAC) libraries and a BAC-based physical map [
11], which allowed researchers to organize and analyze the genome systematically. The identification of 2.8 million single nucleotide polymorphisms (SNPs) further enhanced the genetic setting, enabling studies on genetic diversity and its association with traits of interest [
12]. Despite its size being smaller than most mammalian genomes, the chicken genome contains a lot of information that has illuminated key aspects of vertebrate biology. The chicken genome in most cases has a 1:1 correspondence between homologous genes in mammals and birds, which includes a high level of sequence conservation in intronic and non-coding regions that are likely to contain important regulatory elements, allowing for extensive genetic analysis and comparison with humans, thus enabling the expansion of transgenic techniques in the CE model [
1]. The main advantages of using CEs for experimental embryology are the ease of transplanting cell lines and tissues and their similarity to mammalian systems [
13].
3. Development Stages
The development of CEs is a well-defined process that occurs in several stages [
14]. Here is a developmental timeline with a comprehensive view of how a CE progresses from fertilization to hatching, highlighting key milestones in embryonic development.
Fertilization occurs in the oviduct of the hen after mating. The sperm fertilizes the egg, resulting in a zygote. The fertilized egg then begins its journey through the oviduct, where it accumulates layers of albumen (egg white), membranes, and the shell.
Cleavage and early development: the fertilized egg transforms into a blastoderm approximately 0–3 h after fertilization. The fertilized egg undergoes a series of rapid mitotic divisions, known as cleavage, resulting in a cluster of cells. These cells form a small disc on the surface of the yolk called the blastoderm or the blastodisc.
Gastrulation: the formation of germ layers occurs approximately 6–12 h after fertilization. The blastoderm undergoes gastrulation, forming three primary germ layers: the ectoderm (develops into the skin and the nervous system), the mesoderm (forms muscles, bones, and the circulatory system), and the endoderm (becomes the digestive tract and the internal organs).
Neurulation: the formation of the neural tube occurs around 24–48 h after fertilization. The ectoderm forms the neural plate, which folds to create the neural tube. This tube will eventually develop into the central nervous system (the brain and the spinal cord).
Organogenesis: the development of organs and tissues occurs from about 48 h to several days. The germ layers differentiate into various organs and tissues.
Morphogenesis: this occurs over 3–5 days until hatching. The embryo’s body undergoes changes to take on the recognizable form of a chick. Features, such as feathers, the beak, and talons, start to develop. The embryo’s organs become more defined and functional.
Hatching: preparation for birth occurs approximately 20–21 days after fertilization. The embryo becomes fully developed and ready for hatching. It absorbs the remaining yolk sac, which provides the nutrients needed to survive outside of the egg. The chick uses an egg tooth (a small, temporary structure on its beak) to break through the shell.
It is also possible to differentiate the stages according to phenotypic changes. In fact, in the early stages, the embryo is transparent and simple structures are visible, whereas in later stages more complex structures, like organs and limbs, become visible [
15]. All of the steps are well-differentiated based on the time and days of development. We summarized all of the changes in the following table.
Developmental stages can also be observed in vitro when CE is used as an experimental model. To this aim, the fertilized egg must be placed in a specialized incubator. This incubation system is designed to automatically regulate the temperature and humidity. The most critical component of incubation is maintaining a constant temperature of 37.7 °C, which is essential for embryo development over a specified period. Additionally, maintaining a consistent humidity level of 47% is crucial; if the surrounding air is too dry, the egg can lose excessive water, thus hindering or preventing development [
16].
The artificial incubation process offers several advantages:
It allows for planned development and hatching;
It prevents the spread of diseases and parasites;
The likelihood of egg spoilage is minimized because all eggs are kept at optimal hatching temperatures;
It eliminates the risk of hens damaging the eggs by pecking, which is common in natural incubation [
17].
It has been reported that development begins the moment the fertilized eggs are placed in the incubator, that is, day 0 [
18]. From this point on, development begins with the introduction of the eggs into the incubator. From this time, the blastoderm is formed, and gastrulation begins (
Table 1). During incubation, it is essential to turn the eggs regularly. Turning prevents the embryo from floating and adhering to the shell, which would complicate further work with the embryo. This practice also prevents premature adhesion of embryo membranes, helps the embryo move into the correct developmental position (thereby reducing abnormalities and malposition), stimulates membrane growth, and increases the heart rate. The increased heart rate and membrane growth enhance nutrient uptake and improve the exchange of oxygen and carbon dioxide within the egg [
18] (
Figure 1).
The Hamburger–Hamilton (HH) Stages
The Hamburger–Hamilton (HH) stages are a widely accepted system for describing the development of CEs. Developed by Viktor Hamburger and Howard L. Hamilton in 1951, this staging system provides a detailed and standardized framework to describe embryonic development from the earliest stages to hatching [
4]. The HH staging system divides chicken embryonic development into 40 stages, each representing specific developmental milestones and features (
Table S1).
4. Chorioallantoic Membrane (CAM)
The CAM of the CE is a vital structure that supports embryonic development by enabling efficient gas exchange, nutrient absorption, and waste elimination [
19]. It is formed through the fusion of two membranes: the chorion, which is the outermost layer that develops from the trophoblast and initially forms around the embryo, and the allantois, which is an outgrowth of the embryonic posterior intestine that extends into the extraembryonic coeloma and finally fuses with the chorion to form the CAM. On the other hand, the mature stage of the CAM comprises three distinct layers. At the surface, there is an epithelial layer composed of epithelial cells. Below that, the mesoderm layer contains blood vessels and connective tissue, and deep down, there is a dense network of blood vessels that supplies oxygen and nutrients to the developing embryo and is critical for the study of angiogenesis (
Figure 2) [
19].
Due to its high vascularity, the CAM facilitates the exchange of oxygen and carbon dioxide between the embryo and the external environment through the eggshell. It is also involved in the transfer of calcium from the eggshell to the developing embryo, which is essential for the formation of bones and other structures, and in the removal of waste due to the allantoic portion acting as a reservoir for waste products, particularly uric acid, produced by the embryo. This helps to keep the embryonic environment clean and suitable for development [
20]. The developmental timeline of the CAM begins in the early stages of incubation. Around HH Stage 8, which corresponds to approximately 72–84 h of incubation, the allantois starts to form. By HH Stage 15, approximately 156–168 h into incubation, the allantois has extended and fused with the chorion, resulting in the formation of the CAM. During the late stages of development, the CAM continues to expand and becomes fully functional, playing a crucial role in gas exchange and nutrient transfer until the chick is ready to hatch. Experimental techniques involving the CAM begin with setting up the CAM assay. To expose the CAM without disturbing the embryo, a small window is created in the eggshell, allowing for direct observation and manipulation. Tumor cells, drugs, or other substances can be grafted onto or into the CAM to study its effects.
For observation and analysis, various microscopy techniques, such as bright field, fluorescence, and confocal microscopy, are used to visualize blood vessels and other structures within the CAM [
21]. In addition, vessel density, branching, and other parameters are measured to evaluate the effects of experimental treatments. The CAM is used in a variety of research applications. In angiogenesis studies, it serves as a valuable model to observe and quantify the formation of new blood vessels in response to various stimuli or treatments. It is also used in drug testing to assess the impact of anti-angiogenic drugs or other compounds on blood vessel growth. In cancer research, implanting tumor cells on the CAM allows for studying tumor growth and vascularization, which is critical for understanding tumor progression and spread [
22]. In developmental biology, the CAM is used to explore vascular development and the formation and regulation of blood vessels during embryonic growth. It also provides information on the effects of genetic changes on vascular development and embryogenesis [
23].
In this scenario, the relevance of the CAM to human health is significant because the mechanisms of angiogenesis observed in the CAM are like those in human tissues [
24]. This similarity allows researchers to explore the fundamental processes of blood vessel formation and function, which are relevant to various human conditions, including cancer and cardiovascular disease. Ethically, the CAM model presents fewer problems than vertebrate or higher mammalian models. The rapid development of the CAM also allows experiments of relatively short duration to be conducted, reducing the time and potential inconvenience associated with prolonged studies in more complex animal models.
5. Legislative and Ethical Issues
Within the framework of the 3Rs principles, the use of CEs in biomedical research is an excellent example of partial replacement [
25]. Until a specific stage of their embryonic life, CEs are not deemed capable of experiencing pain [
26]. Indeed, the European Directive 2010/63/EU exempts the use of avian embryos at the earliest stages of development from its scope [
27]. Specifically, avian embryos are not subject to the same regulations as live animals until they are capable of independent life and experiencing pain, which typically corresponds to the last third of the incubation period [
26,
28,
29]. However, determining the exact developmental stage at which embryos start pain perception represents a complex subject that intersects with developmental biology, neuroscience, and ethics [
30]. Although the nervous system begins to develop at the end of embryonic day 1 (ED1), the anatomical structures involved in pain perception are not fully formed before ED13, coinciding with the development of a functional brain [
4,
31,
32,
33].
However, it is not guaranteed that the CE perceives pain only due to the presence of these structures, because the associated functional pathways must also be considered [
34]. Electroencephalogram (EEG) onset has been identified at ED13, and recent studies have monitored pain responses in CEs at various developmental stages [
28,
29]. When exposed to noxious stimuli, alterations in arterial pressure and heart rate, as well as behavioral responses (such as movements of the beak and eyes), began around ED16 [
29,
35]. These physiological features must be considered during the design of experimental protocols involving CEs. Anesthesia should be administered during procedures for embryos at developmental stages above ED13 [
36,
37]. Anesthetic protocols for younger embryos are reported to induce immobilization and avoid motion artifacts during imaging studies [
38,
39].
Different euthanasia methods are recommended based on the developmental stage of the embryo. While hypothermia represents a suitable method for embryos before ED13, embryos from ED14 onwards could already be capable of experiencing suffering and should consequently be euthanized with humane methods [
36]. Therefore, the use of CEs in biomedical research offers a valuable alternative to more sentient animal models by exploiting the developmental stages of embryos before pain perception is possible.
6. Manipulation of the CEs In Ovo and Ex Ovo
In studies involving CEs, both “in ovo” and “ex ovo” techniques are employed. The in ovo technique involves manipulating the embryo within the egg, while the ex ovo technique entails extracting and culturing the embryo outside of the egg [
40]. The primary method for in ovo handling of CEs includes incubating and eggs’ fenestration. During incubation, fertilized chicken eggs are maintained at 37.7 °C with 47% humidity. Fenestration, which consists of creating a small window in the eggshell to access the embryo, is usually performed with a dental drill or small scissors. For ex ovo manipulations, the embryo is removed from the egg and cultured externally in new culture environments, including an eggshell surrogate, Petri dishes, and artificial eggshell-like vessels (
Figure 3).
In ovo manipulation maintains the embryo within its natural egg environment, providing a more realistic developmental context but with limited accessibility. Ex ovo manipulation, on the other hand, allows for greater experimental control and accessibility but involves culturing the embryo in an artificial environment. Each method has its own advantages and limitations, making them suitable for different types of studies in developmental biology (
Table S2). The ex ovo method involves explanting and culturing the CE in vitro by immersing the egg contents in a salt solution, separating the embryo from the yolk while preserving as much of the vitelline membrane as possible, and then transferring the embryo into culture until it reaches HH18. Due to the high skill level required, the lengthy process, and the difficulty of adapting the method for time-lapse imaging, Chapman et al. and Rupp et al. modified Denis New’s technique to simplify and speed up the process [
41,
42]. Their modifications addressed the complexities of the original technique, reduced the required skill level, eliminated the need for glass rings, and made the method easily adaptable for microscope imaging and other applications [
43]. These advances have made possible the manipulation and observation of CEs for various research purposes, improving the understanding of embryological development and facilitating the advancement of medical research. Ex ovo techniques are extensively used in developmental biology to study the mechanisms of embryogenesis, organ development, and differentiation.
It is possible to observe the direct effects of genetic and environmental manipulations on developing embryos. The in ovo model is primarily used to study tumor cell extravasation and metastasis, while the ex ovo model is more suited to angiogenesis studies due to easier observation of the CAM [
44].
Several in ovo and ex ovo techniques are used, including microinjection, electroporation, surgical manipulations, and molecular imaging.
Microinjection involves introducing substances, such as DNA, RNA, proteins, and dyes, directly into the embryo or yolk.
Electroporation uses electric fields to introduce DNA or other molecules into cells to study gene function in CEs, and it has demonstrated valuable application in developmental biology and disease modeling.
Molecular imaging, combined with in ovo and ex ovo techniques, allows for real-time visualization and manipulation of biological processes within living organisms. The ex ovo method is compatible with time-lapse imaging using light or confocal microscopy, as the embryo develops in an optically clear environment that does not scatter light. However, it has the disadvantage of short and low embryo survival and a high mortality rate of approximately 50%. In contrast, the in ovo technique offers better survival rates (65–80% depending on the stage of development) and allows for longer culture periods, although it is less suitable for in vivo imaging [
43].
7. The Use of CEs in Preclinical Research
The first study of CEs dates back to 1911 when Rous et al. and Murphy et al. demonstrated how it was possible to transplant the chicken sarcoma onto the CAM and observe its growth [
45]. In the following years, several researchers demonstrated the potential use of CEs as an animal model for implantation of tumor cells and tissues [
46,
47,
48]. Nowadays, the CAM model not only provides a unique three-dimensional environment to observe and study tumor growth and metastatic invasion in real time, but it can also be used to evaluate angiogenesis, as different vascular patterns can be observed during embryonic development, allowing researchers to test the efficacy of new drugs and compounds.
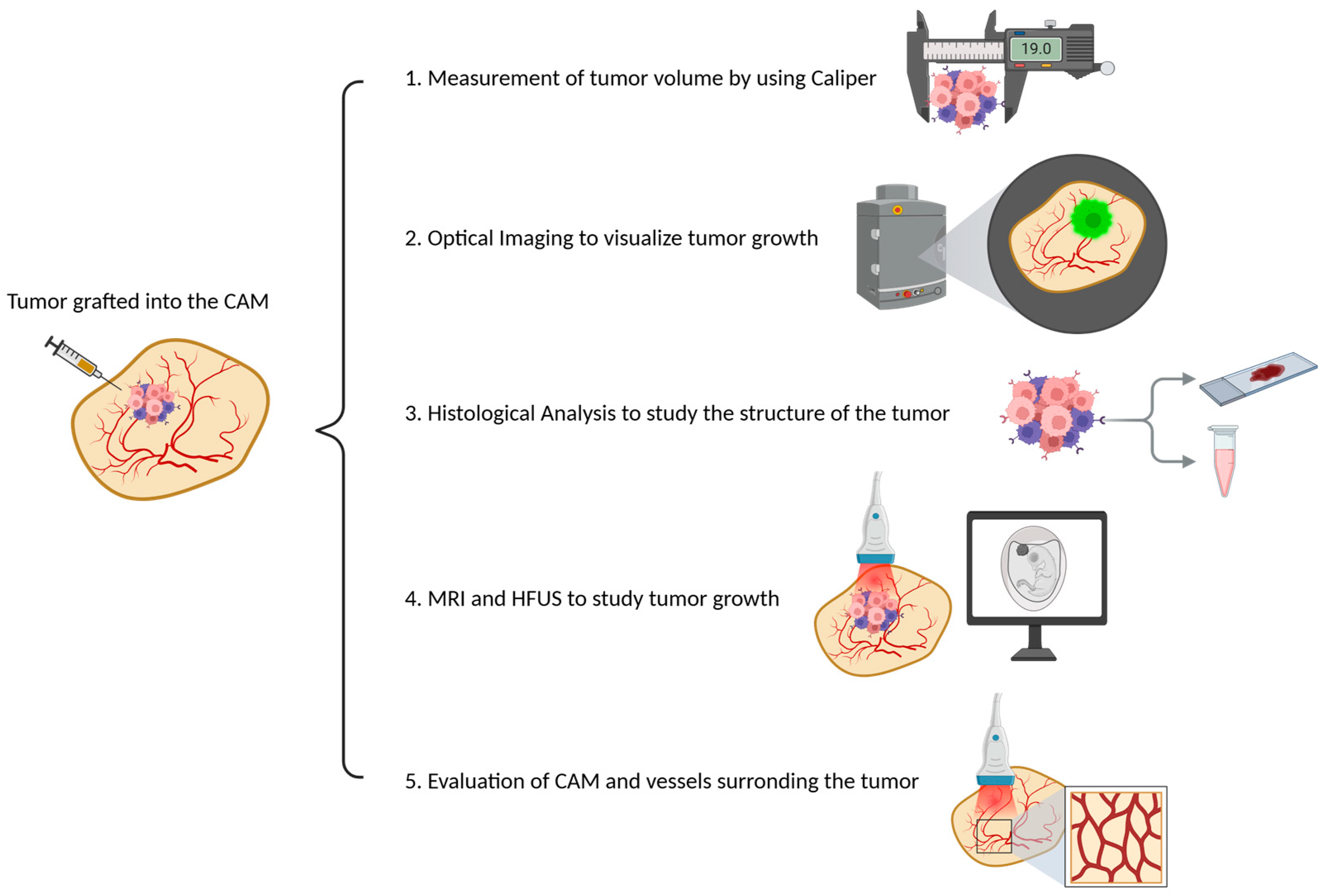
Tumor mass in CE models can be measured using the following methods:
Direct measurement of tumor volume using calipers;
Optical imaging to visualize tumor growth in real time;
Histological analysis to study the structure of the tumor at the cellular level;
Magnetic resonance imaging (MRI) and high-frequency ultrasound (HFUS) to monitor tumor growth and detailed anatomical information (
Figure 4).
In addition, the angiogenesis in CAM models can be quantitatively and qualitatively assessed using the following methods:
Microvascular density analysis that allows researchers to visualize blood vessels and calculate the density of microvessels in both the tumor and the surrounding tissue;
Fluorescent labeling and imaging through fluorescent labeling of tumor cells and blood vessels that allow for real-time tracking of vessel growth using confocal microscopy, providing insight into how tumor cells modify their microenvironment to promote angiogenesis;
Repetitive color-duplex-ultrasonography, which was used for the analysis of tumor growth and tumor vascularization, allowing for the quantification of tumor size and the monitoring of angiogenesis [
49] (
Figure 4).
CEs could be used as a drug delivery system (DDS) to deliver therapeutic agents to specific sites of injury or disease. The transition from cultured cells in vitro to preclinical animal models in vivo is complicated and has a low success rate compared with expected results. In this scenario, the CAM has proven to be an excellent alternative to test a DDS in a model considered intermediate between in vitro analysis and preclinical in vivo validation in animal models [
50]. The CAM model can be used to test pro-angiogenic drugs in the context of tissue repair after an ischemic insult by controlling angiogenesis directed at the damaged site. These drugs, which are mainly pro-angiogenic factors or their analogue, can be administered in different modalities as drug conjugates using various carriers, such as micro or nanoparticles. A major advantage is the possibility of the administering candidate compound directly into the membrane with a localized application that minimizes systemic effects in the embryo [
23,
51,
52,
53] (
Figure 5). The importance of the CAM assay for angiogenic drug development lies in its translational value; the molecular pathways that drive angiogenesis in CEs, such as Vascular Endothelial Growth Factor (VEGF) and Fibroblast Growth Factor (FGF), are the same as those underlying human neo-angiogenesis. Drugs with anti-angiogenic action can be studied using the CAM model as the optimal platform for direct observation of the response in terms of inhibition of neo-vascularization or with regard to their efficacy in inhibiting tumor neo-angiogenesis, as previously described [
54].
In the context of angiogenesis studies, for example, Ribatti et al. described the use of the CE CAM model as an in vivo platform for studying human neuroblastoma [
55]. The grafted tumor cells interact with the dense network of blood vessels and stimulate the formation of new vessels infiltrating the tumor, a process identical to what occurs in human tumors. The authors after 7 days of incubation excised the tumor surrounding the CAM tissue and fixed it for histological analysis to assess tumor-induced angiogenesis [
55].
Lokman et al. demonstrated the application of the CAM model for studying ovarian cancer growth, angiogenesis, and drug testing, highlighting its utility for screening new anti-cancer therapies [
56]. The authors measured the tumor size with a caliper or imaging software over time, while angiogenesis was assessed by visually examining and quantifying the number of new blood vessels forming around the tumor. The authors used anti-angiogenic therapeutic agents directly on the CAM and evaluated their effects on ovarian tumor growth and angiogenesis. Treated tumors showed a significant reduction in size compared with untreated controls, reduced cell proliferation within the tumor, as evidenced by decreased Ki-67 staining, along with a reduced number of blood vessels forming around the tumor, as demonstrated by decreased microvascular density (MVD) in histologic analysis. Dagg et al. demonstrated that the human epidermoid carcinoma cell line implanted on the CAM spontaneously metastasizes to various organs, including the eye, brain, liver, and myocardium [
57], and that chicks hatched from eggs inoculated with tumor cells died within a few weeks due to the development of tumors in the brain, liver, heart, and kidney. Easty et al. made it possible to detect and quantify micro-metastases by measuring the distribution of cells from several 3H-labeled murine tumor lines [
58]. Endo et al. standardized a sensitive method for the specific detection of tumor cells present at secondary sites using Polymerase Chain Reaction (PCR) with primers specific for the β-globin gene [
59]. Next, the migration of tumor cells in different organs of the CE was studied using intravital imaging [
60,
61]. Deryugina et al. [
60,
62,
63], used fluoresceinated HT-1080 human fibrosarcoma cell variants along with vasculature labeling with Lens Culinaris Agglutinin (LCA) to follow with real-time imaging the intravasation, dissemination, and extravasation of tumor cells with different dissemination potentials. Ronca et al. used histological methods to visualize mock cells and previously transduced human pentraxin 3 (hPTX3-B16-F10) cells and quantify their invasive capacity through the CAM to the underlying mesoderm. For this purpose, melanoma cells possibly present in the CAM mesenchyme were counted 4 days after grafting, and the results showed a significantly reduced number of hPTX3-overexpressing cells compared with mock cells [
64]. These data confirmed using the CAM model that PTX3 inhibits Epithelial–Mesenchymal Transition (EMT) in melanoma cells, reducing their metastatic potential. Lugassi et al. used the CAM to explore the ability of angiotropic melanoma cells to metastasize by migrating to secondary sites [
65]. In particular, the authors described the implantation of C8161 human melanoma cells labeled with Green Fluorescent Protein (GFP) on the CAM and their invasion into distant organs of the CE by using fluorescence microscopy. In parallel experiments, they performed PCR analysis to detect human cancer cells in the organs of chicks.
In the field of DDSs, Guedes et al. used a breast CAM model obtained by engrafting MDA-MB-231 cells to test the effect of different anti-tumor heterobimetallic Ru(II)/Fe(II) complexes including two anti-angiogenic ones. They demonstrated that the CAM model is an optimal platform for evaluating their antiangiogenic complexes because of the ease with which the vasculature density can be observed to thus quantify the inhibitory effect in terms of its decrease in response to administration [
66].
8. In Ovo and Ex Ovo Applications in Molecular Preclinical Imaging
Non-invasive preclinical imaging represents an essential tool to develop modern and translational biomedical research. Recently, imaging studies have focused on mice and rats due to their analogy to human systems, which can also be applied to other animal models, such as CEs, which show genetic similarity with humans up to 80%, compared to 75% between humans and zebrafish and 60% between humans and melanogaster [
67]. In detail, imaging of CEs and the CAM has emerged as a preclinical in vivo model that bridges the gap between in vitro and in vivo studies [
68]. Consequently, it is not a replacement for the mouse model, but it could be considered as a preliminary step to in vivo studies using more neurologically developed animals compared to cells, with lower management costs compared to mouse models. This provides the best conditions for translating the in vivo studies performed and reducing the number of animals to be used, according to the 3Rs principle. The combination of imaging with the study of CEs is characterized by several advantages. This model is cheaper than other commonly used animal species, there are no housing costs, and the space required to house the eggs consists of small incubators. Furthermore, physical access to the egg is easy because it has no barriers beyond the shell itself, allowing for small operations, such as the implantation of tumor cells and the injection of fluorescent probes/microbubbles/contrast agents [
69].
In ovo and ex ovo techniques are used in combination with advanced molecular imaging both to gain information about the development of the CE by studying embryonic development, gene function, and molecular interactions and to study human diseases and treatment responses. In the following sections, some examples where the various imaging methods are used for in ovo and ex ovo studies of CEs are shown.
8.1. Preclinical HFUS in CE Studies
HFUS is a method widely used in the preclinical field. Indeed, most research in the oncology, metabolic, and embryological development fields has been developed using small animal models thanks to the use of HFUS. In particular, HFUS is a diffused method to study tumor biology, novel anti-cancer treatments, and angiogenesis in CE, so the peak frequencies exceeding 40 MHz of this imaging technique enable a higher resolution of micro vessels, which is essential for the in ovo visualization of CAM vasculature [
70] (
Figure 6).
Furthermore, in the cardiovascular field, the heart of the CE is the first organ that starts to work. Indeed, it beats at 33 h post-incubation, and the time of heart development is longer than in other species, allowing researchers to perform deeper longitudinal studies [
71]. Adapting preclinical US systems and parameters, Hegemann et al. were the first to characterize the CE from ED 8 to ED 13 through B-mode and M-mode imaging, obtaining a four-chamber view as in clinical and pre-clinical practice. This study demonstrated that HFUSs on CEs can be a useful tool for evaluating the effect of drugs and innovative therapies on cardiopulmonary development while avoiding the sacrifice of further animal species with advanced neurological development [
67].
In order to observe angiogenesis development during cystic tissue growth, Schueler et al. established human renal cystic tissue onto the CAM between the 7th and 10th days of embryonic development. HFUS allows for revealing the engraftment, increase of vascularization, and changes in lesion dimensions in real time and in non-invasive ways [
70]. In vivo models of cystic tissue, in combination with imaging of anastomosis and angiogenesis development using HFUS, offers promising instruments to study personalized therapy [
70].
The microvascular study of tumors in a preclinical setting requires high sensitivity in the detection of small vessel dimensions and blood flow. For this purpose, ultrafast Ultrasound Microvessel Imaging (UMI) was developed, which is essentially capable of increasing the doppler sensitivity at low flow speeds coming from micro vessels characteristic of the CAM. In the CAM model of a renal cell tumor, the HFUS system Vevo 3100 supporting UMI software was able to distinguish between the untreated tumor and the sunitinib-treated tumors, which were characterized by a more heterogeneous blood supply distribution, with a perfusion gradient starting from the center of the upper tumor surface (where the drug was administrated) to the periphery of the tumor. The main advantage of UMI is the ability to analyze the angiogenesis and vascularization in a tumor engrafted into the CAM of CEs while avoiding the technical difficulty of injection of microbubble contrast agents [
72].
8.2. Preclinical MRI in CE Studies
In the last years, MRI, with the diffusion of high field scanners, has also gained importance in the field of preclinical research. MRI provides detailed morphological images conjugating excellent soft tissue contrast with high spatial resolution (up to 50 μm in animal studies) and temporal resolution. It also allows for the collection of information about tissue composition, perfusion, oxygenation levels, tissue elasticity, and metabolism, and, recently, it further evolved toward imaging of molecular events, which represents the true challenge for imaging techniques [
73,
74]. Thanks to its flexibility in contrasts, MRI already raised interest in imaging CEs in the 1980s [
75] (
Figure 6).
Several authors in the past years have focused their activity on the development of specific procedures and tools to optimize MRI of the CE, providing useful indications for successive studies. For example, to obtain good quality images, motion-induced image artifacts should be avoided, and this is usually obtained by placing the egg in ice chips between 10 and 90 min. Waschkies et al. [
39] further explored the immobilization of the CE, comparing three anesthetic regimes consisting of dropping onto the surface of the CAM medetomidine at a dosage of 0.3 mg/kg, thiopental at 100 mg/kg, and ketamine/midazolam at 50 mg/kg and 1 mg/kg. The study resulted in better performances for medetomidine in terms of reduced motion, time of onset of anesthesia, and anesthesia duration. Another technical aspect to consider when performing CE MRI is the choice of the coil to optimize SNR and B1 homogeneity. Standard rat body birdcage coil is commonly employed, but it is not fully tailored to CEs in terms of size and length, and because the position of the CE changes over time, the organ of interest may not always be placed within the sensitive region of the coil. To avoid this issue, Choi et al. [
76] introduced a new curved approach to coil design specifically tailored for CE MR scans, showing practical potential for in ovo studies. Holmes et al. [
77] investigated the feasibility of a self-gated cine MRI protocol that incorporates a navigator-based retrospective gating technique to study CE hearts, overcoming the difficulty of monitoring chick ECG and respiration signals.
As for the methodological applications, thanks to its ability to differentiate soft tissues, MRI was used to monitor the development during embryogenesis of specific organs of interest. Zhou et al. [
78] explored brain development using T2-Weighted Imaging (T2WI) for volume estimation and Diffusion Tensor Imaging (DTI) to reflect the evolution of neural bundle structures. Lindner et al. [
79] studied chick eyes through T2WI, concluding that MRI has the ability to depict embryonic ocular development in a noninvasive and truly longitudinal manner. Finally, Chen et al. [
80] evaluated the morphologic evolution of the CE, the allantois, and the CAM throughout ED1 until ED20, using T2WI and T1WI and semi-automated segmentation algorithm for volume computation.
In the field of human disease studies, thanks to the refinement of CE xenograft models, several applications using MRI have been described in oncology, for the development of nanoparticle-based carriers and imaging agents, for treatment evaluation, and for the optimization of imaging techniques. After the optimization of an acquisition protocol for tumor visualization composed by T2WI, DWI and T2 mapping sequences [
81], Zuo et al. used MRI to compare the biodistribution of Gd-DOTA conjugated micelles to standard Gd-DOTA in xenografts of human breast cancer cells on the CAM, showing an improved signal-to-noise ratio with the micelles [
82]. Hafner et al. [
83] used MRI to investigate the localization of a nanocarrier composed of albumin and PEG loaded with doxorubicin and labeled with DOTA-Gd in triple-negative breast cancer, showing that the particles, approximately 100 nm in size, preferentially accumulated in xenograft tumors over time. Another intriguing application of MRI is the tracking of cells labeled with MRI-visible nano- and microparticles over time. SPION-labeled melanoma cells were injected into the neural tube of a CE, and their migration along the neural crest pathways was monitored for 16 days post-injection [
84]. This method was also used for mesenchymal stem cells labeled with iron oxide nanoparticles implanted in the avian embryo’s brain. Notably, these mesenchymal stem cells were also tagged with a fluorescent protein enabling the co-registration of live cell locations via optical imaging, confirming particle retention within the cells [
85]. Herman et al. [
86] evaluated the advantages and limitations of MRI to study metastatic dissemination of neuroblastoma in the chick starting from MPIO-labeled neuroblastoma cells implantation. Buschmann et al. and Waschkies et al. tried to characterize different tumors in living CEs in terms of their reaction to gas challenges. They used T1 and T2* mapping to enable differential characterization of tumor grafts with respect to their vascular and oxygenation status [
87,
88].
An interesting utilization of MRI was developed by Kivrak Pfiffner et al. [
89]. The authors used the CAM as an environment for 3D biomaterial scaffold proliferation and presented a novel in vivo method for analyzing their perfusion capacity, thus enabling the evaluation of bioengineered material properties through this imaging modality.
With the development of simultaneous the Positron Emission Tomography (PET)/MRI scanner, the combination of the multi-contrast capabilities of MRI with the outstanding sensitivity of PET appears promising for in ovo imaging. A very recent study [
90] used a simultaneous scanner to study a patient-derived xenograft model generated from the liver metastasis of a colorectal cancer with [
68Ga]Ga-Pentixafor.
8.3. Other Imaging Applications in CE Studies
To assess the metabolic pattern of tumor cells grown on CE models, Smith et al. performed dynamic PET imaging in ovo, producing a high tumor-background signal for both
18F-2-fluoro-2-deoxy-D-glucose (
18F-FDG) and (4S)-4-(3-
18F-fluoropropyl)-L-glutamate (
18F-FSPG) to assess glucose metabolism and uptake. It was possible to delineate radiotracer uptake in both the tumor and the CE [
91]. Zlatopolskiy et al. reported an efficient method for the preparation and biological evaluation of fluoro-tryptophan (Trp) labeled at different positions with
18F. They analyzed the bone metabolism with
18F-fluoride and the Trp metabolic pathway with 7-
18F-fluorotriptophan (7-[
18F] FTrp) on the CAM membrane, where the uptake mechanisms and dehalogenation levels were comparable to those in mice. Therefore, 7-[
18F] FTrp was identified as a very promising PET probe for imaging of Trp metabolism [
92]. Warnock et al. combined the in ovo CAM glioblastoma model with in vivo PET/CT imaging, offering an innovative, cost-effective, and ethically beneficial study. In particular, they successfully demonstrated PET imaging of glucose metabolism and protein synthesis in CAM glioblastoma U87 cell lines. By capturing the PET tracer
18F-FDG over time in individual tumors, they obtained information about glucose metabolism, while through CT imaging they improved the accuracy of tumor volume measurements [
93]. Optical methods, such as Optical Coherence Tomography (OCT) and doppler techniques, have been successfully applied to derive functional and physiological properties of the embryo. Li et al. used an ultrafast 1310 nm dual-camera Spectral-Domain Optical Coherence Tomography (SDOCT) system to characterize in parallel the dynamic radial deformation rate of the myocardial wall and the doppler velocity of the underlying blood within a beating CE in vivo. Through this system, they obtained simultaneous characterization of tissue motion and blood flow [
94]. Li et al. also demonstrated the possibility of revealing complex myocardial activity in the live chick heart using OCT. They performed a measurement of in vivo deformation and the myocardial strain rate by analyzing the periodic variation of myocardial wall thickness calculated from real-time serial OCT images [
95]. Other studies performed on the CE have allowed for the evaluation of useful bone regeneration for tissue engineering purposes using the CAM as a bioreactor to culture and study live human bone regeneration.
Microcomputed tomography (μCT) was used to quantify the extent and location of bone volume changes, followed by histological analysis to assess bone repair. This human–avian system described above provides a simple refinement model for animal research and a step toward an in vivo humanized model for tissue engineering [
96]. New methods for nuclear imaging were also transferred for in ovo studies, especially for the initial testing of new labeling strategies [
91].
9. Conclusions
The CE model holds promise as a versatile and cost-effective alternative for preclinical molecular imaging and research, especially when compared with traditional mammalian models (
Figure 7).
Its unique features, such as external development, ease of genetic manipulation, and ethical advantages, pose it as an ideal tool for studying developmental processes and real-time observation and imaging of complex biological processes, including tumor growth, angiogenesis, metastasis, and drug delivery. On the other hand, despite these advantages, the use of this model has several limiting disadvantages. The lack of a fully developed immune system in the CE model limits its ability to simulate the full complexity of human biology, particularly in terms of immune–tumor interactions. For early development stages studies, which do not require ethical approval, the time to conduct experimental procedures is very short, precluding long-term observations, which are essential for studying, e.g., tumor relapse and remission phenomena or response to therapies. Another challenge is the administration of molecules. Because oral administration is not an option, the CAM model requires the use of solubilized molecules to be injected, strongly limiting the types of compounds that can be tested using this model.
Despite these limitations and the difficulty of directly translating the obtained results to human biology, given the diversity between the two biological systems, the model’s detailed cellular dynamics, together with the possibility to monitor them through real-time imaging, provide insights that are useful in preclinical oncology, regenerative medicine, and neuroscience.
Author Contributions
Conceptualization, A.S., Y.F., C.T., S.A., S.M. and L.L.; investigation, A.S., Y.F., C.T., S.A., S.M. and L.L.; data curation, A.S., Y.F., C.T., S.A., S.M. and L.L.; writing—original draft preparation, A.S., Y.F., C.T., S.A., S.M. and L.L.; writing—review and editing, M.M.; visualization, A.S., Y.F., C.T., S.A., S.M. and L.L.; supervision, M.M.; project administration, M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
A.S., Y.F., C.T. and L.L. were supported by “SEE LIFE—StrEngthEning the ItaLIan InFrastructure of Euro-bioimaging”, area ESFRI “Health and Food”–IR0000023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors thank Euro-BioImaging and the Multi-Modal Molecular Imaging Italian Node Facility at the Institute of Biostructures and Bioimaging (CNR), Naples for the support with preclinical research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stern, C.D. The chick; a great model system becomes even greater. Dev. Cell 2005, 8, 9–17. [Google Scholar] [PubMed]
- Needham, J. A History of Embryology, 2nd ed.; Cambridge University Press: Cambridge, UK, 1934. [Google Scholar]
- Duval, M. Atlas d’Embryologie; Kessinger Publishing: Whitefish, MT, USA, 1889. [Google Scholar]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef]
- Rashidi, H.; Sottile, V. The chick embryo: Hatching a model for contemporary biomedical research. Bioessays 2009, 31, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Beauvais-Jouneau, A.; Pla, P.; Bernex, F.; Dufour, S.; Salamero, J.; Fässler, R.; Panthier, J.-J.; Thiery, J.P.; Larue, L. A novel model to study the dorsolateral migration of melanoblasts. Mech. Dev. 1999, 89, 3–14. [Google Scholar] [CrossRef]
- Ribatti, D. The CAM assay in the study of the metastatic process. Exp. Cell Res. 2021, 400, 112510. [Google Scholar] [CrossRef]
- Pawlikowska, P.; Tayoun, T.; Oulhen, M.; Faugeroux, V.; Rouffiac, V.; Aberlenc, A.; Pommier, A.L.; Honore, A.; Marty, V.; Bawa, O.; et al. Exploitation of the chick embryo chorioallantoic membrane (CAM) as a platform for anti-metastatic drug testing. Sci. Rep. 2020, 10, 16876. [Google Scholar] [CrossRef]
- Groenen, M.A.; Cheng, H.H.; Bumstead, N.; Benkel, B.F.; Briles, W.E.; Burke, T.; Burt, D.W.; Crittenden, L.B.; Dodgson, J.; Hillel, J.; et al. A consensus linkage map of the chicken genome. Genome Res. 2000, 10, 137–147. [Google Scholar]
- Gunter, C. Pecking away at evolution. Nat. Rev. Genet. 2005, 6, 4–5. [Google Scholar] [CrossRef]
- Wallis, J.W.; Aerts, J.; Groenen, M.A.; Crooijmans, R.P.; Layman, D.; Graves, T.A.; Scheer, D.E.; Kremitzki, C.; Fedele, M.J.; Mudd, N.K.; et al. A physical map of the chicken genome. Nature 2004, 432, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.K.; Liu, B.; Wang, J.; Zhang, Y.; Yang, X.; Zhang, Z.; Meng, Q.; Zhou, J.; Li, D.; Zhang, J.; et al. International Chicken Polymorphism Map Consortium. A genetic variation map for chicken with 2.8 million single-nucleotide polymorphisms. Nature 2004, 432, 717–722. [Google Scholar]
- Ribatti, D.; Annese, T. Chick embryo in experimental embryology and more. Pathol. Res. Pract. 2023, 245, 154478. [Google Scholar] [CrossRef]
- Gilbert, S.F. Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Eyal-Giladi, H.; Kochav, S. From cleavage to primitive streak formation: A complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev. Biol. 1976, 49, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.; Özkan, S.; Shah, T. Incubation Temperature and Lighting: Effect on Embryonic Development, Post-Hatch Growth, and Adaptive Response. Front. Physiol. 2022, 13, 899977. [Google Scholar] [CrossRef]
- Bello, M.I.; Bala, A.; Adamu, A.I.; Bari, A.S.; Baballe, M.A. Construction of the artificial egg incubator. Far East J. Electron. Commun. 2023, 27, 51–60. [Google Scholar] [CrossRef]
- Wageningen, N.V.; Meinderts, J.; Bonnier, P.; Kasper, H. Hatching Eggs by Hens or in an Incubator; Digigrafi Publisher: Veenendaal, The Netherlands, 2004; p. 80. [Google Scholar]
- Chen, L.; Wang, S.; Feng, Y.; Zhang, J.; Du, Y.; Zhang, J.; Ongeval, C.V.; Ni, Y.; Li, Y. Utilisation of Chick Embryo Chorioallantoic Membrane as a Model Platform for Imaging-Navigated Biomedical Research. Cells 2021, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Halgrain, M.; Georgeault, S.; Bernardet, N.; Hincke, M.T.; Réhault-Godbert, S. Concomitant Morphological Modifications of the Avian Eggshell, Eggshell Membranes and the Chorioallantoic Membrane During Embryonic Development. Front. Physiol. 2022, 13, 838013. [Google Scholar] [CrossRef]
- Patiño-Morales, C.C.; Jaime-Cruz, R.; Ramírez-Fuentes, T.C.; Villavicencio-Guzmán, L.; Salazar-García, M. Technical Implications of the Chicken Embryo Chorioallantoic Membrane Assay to Elucidate Neuroblastoma Biology. Int. J. Mol. Sci. 2023, 24, 14744. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.Y.; Koh, A.P.; Antony, J.; Huang, R.Y. Applications of the Chick Chorioallantoic Membrane as an Alternative Model for Cancer Studies. Cells Tissues Organs 2022, 211, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.C.; Coen, B.; Wheatley, A.M.; McCullagh, K.J.A. Microvascular Experimentation in the Chick Chorioallantoic Membrane as a Model for Screening Angiogenic Agents including from Gene-Modified Cells. Int. J. Mol. Sci. 2021, 23, 452. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co.: London, UK, 1959; Volume 1. [Google Scholar]
- Rosenbruch, M. The sensitivity of chicken embryos in incubated eggs. Altex 1997, 14, 111–113. [Google Scholar] [PubMed]
- Official Journal of the European Union. Directive 2010/63/EU of the European Parlament and of the Council of 22 September 2010. Available online: https://eur-lex.europa.eu/ (accessed on 11 September 2024).
- Kollmansperger, S.; Anders, M.; Werner, J.; Saller, A.M.; Weiss, L.; Süß, S.C.; Reiser, J.; Schneider, G.; Schusser, B.; Baumgartner, C.; et al. Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception. Animals 2023, 13, 2839. [Google Scholar] [CrossRef] [PubMed]
- Süß, S.C.; Werner, J.; Saller, A.M.; Weiss, L.; Reiser, J.; Ondracek, J.M.; Zablotski, Y.; Kollmansperger, S.; Anders, M.; Potschka, H.; et al. Nociception in Chicken Embryos, Part III: Analysis of Movements before and after Application of a Noxious Stimulus. Animals 2023, 13, 2859. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Recognition and Alleviation of Pain in Laboratory Animals. Recognition and Alleviation of Pain in Laboratory Animals; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Peters, J.; Vonderahe, A.; Schmid, D. Onset of cerebral electrical activity associated with behavioral sleep and attention in the developing chick. J. Exp. Zool. 1965, 160, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Katori, M. The development of the spontaneous electrical activity in the brain of a chick embryo and the effects of several drugs on it. Jpn. J. Pharmacol. 1962, 12, 9–25. [Google Scholar] [CrossRef]
- Garcia-Austt, E. Development of electrical activity in cerebral hemispheres of the chick embryo. Proc. Soc. Exp. Biol. Med. 1954, 86, 348–352. [Google Scholar] [CrossRef]
- Erickson, H.H.; Kitchell, R.L. Pain perception and alleviation in animals. Fed. Proc. 1984, 43, 1307–1312. [Google Scholar] [PubMed]
- Weiss, L.; Saller, A.M.; Werner, J.; Süß, S.C.; Reiser, J.; Kollmansperger, S.; Anders, M.; Potschka, H.; Fenzl, T.; Schusser, B.; et al. Nociception in Chicken Embryos, Part I: Analysis of Cardiovascular Responses to a Mechanical Noxious Stimulus. Animals 2023, 13, 2710. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz, E.; Herr, I. Ethical euthanasia and short-term anesthesia of the chick embryo. Altex 2015, 32, 143–147. [Google Scholar]
- Horr, M.; Sommerfeld, S.; Silva, M.V.; Fonseca, B.B. A fast and simple protocol to anaesthesia in chicken embryos. Exp. Anim. 2023, 72, 294–301. [Google Scholar] [CrossRef]
- Heidrich, A.; Würbach, L.; Opfermann, T.; Saluz, H.P. Motion-artifact-free in vivo imaging utilizing narcotized avian embryos in ovo. Mol. Imaging Biol. 2011, 13, 208–214. [Google Scholar] [CrossRef]
- Waschkies, C.; Nicholls, F.; Buschmann, J. Comparison of medetomidine, thiopental and ketamine/midazolam anesthesia in chick embryos for in ovo Magnetic Resonance Imaging free of motion artifacts. Sci. Rep. 2015, 5, 15536. [Google Scholar] [CrossRef] [PubMed]
- Sukparangsi, W.; Thongphakdee, A.; Intarapat, S. Avian Embryonic Culture: A Perspective of In Ovo to Ex Ovo and In Vitro Studies. Front. Physiol. 2022, 13, 903491. [Google Scholar] [CrossRef]
- Chapman, S.C.; Collignon, J.; Schoenwolf, G.C.; Lumsden, A. Improved method for chick whole-embryo culture using a filter paper carrier. Dev. Dyn. 2001, 220, 284–289. [Google Scholar] [CrossRef]
- Rupp, P.A.; Rongish, B.J.; Czirok, A.; Little, C.D. Culturing of avian embryos for time-lapse imaging. Biotechniques 2003, 34, 274–278. [Google Scholar] [CrossRef]
- El-Ghali, N.; Rabadi, M.; Ezin, A.M.; De Bellard, M.E. New methods for chicken embryo manipulations. Microsc. Res. Tech. 2010, 73, 58–66. [Google Scholar] [CrossRef]
- Mesas, C.; Chico, M.A.; Doello, K.; Lara, P.; Moreno, J.; Melguizo, C.; Perazzoli, G.; Prados, J. Experimental Tumor Induction and Evaluation of Its Treatment in the Chicken Embryo Chorioallantoic Membrane Model: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 837. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.B.; Rous, P. The behavior of chicken sarcoma implanted in the developing embryo. J. Exp. Med. 1912, 15, 119–132. [Google Scholar] [CrossRef]
- Murphy, J.B. Transplantability of tissues to the embryo of foreign species: Its bearing on questions of tissue specificity and tumor immunity. J. Exp. Med. 1913, 17, 482–493. [Google Scholar] [CrossRef]
- Murphy, J.B. Factors of resistance to heteroplastic tissue-grafting: Studies in tissue specificity. III. J. Exp. Med. 1914, 19, 513–522. [Google Scholar] [CrossRef]
- Murphy, J.B. Studies in tissue specificity: II. The ultimate fate of mammalian tissue implanted in the chick embryo. J. Exp. Med. 1914, 19, 181–186. [Google Scholar] [CrossRef]
- Eckrich, J.; Kugler, P.; Buhr, C.R.; Ernst, B.P.; Mendler, S.; Baumgart, J.; Brieger, J.; Wiesmann, N. Monitoring of tumor growth and vascularization with repetitive ultrasonography in the chicken chorioallantoic-membrane-assay. Sci. Rep. 2020, 10, 18585. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Pund, S.; Borade, G.; Rasve, G. Improvement of anti-inflammatory and anti-angiogenic activity of berberine by novel rapid dissolving nanoemulsifying technique. Phytomedicine 2014, 21, 307–314. [Google Scholar] [CrossRef]
- Ribatti, D. The chick embryo chorioallantoic membrane as a model for tumor biology. Exp. Cell Res. 2014, 328, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; van Beijnum, J.R.; van Berkel, M.; van den Bergh, H.; Griffioen, A.W. Vascular regrowth following photodynamic therapy in the chicken embryo chorioallantoic membrane. Angiogenesis 2010, 1, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Vacca, A.; Roncali, L.; Dammacco, F. The chick embryo chorioallantoic membrane as a model for in vivo research on anti-angiogenesis. Curr. Pharm. Biotechnol. 2000, 1, 73–82. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R. The chick embryo chorioallantoic membrane as an in vivo experimental model to study human neuroblastoma. J. Cell Physiol. 2018, 234, 152–157. [Google Scholar] [CrossRef]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick chorioallantoic membrane (CAM) assay as an in vivo model to study the effect of newly identified molecules on ovarian cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Dagg, C.P.; Karnofsky, D.A.; Roddy, J. Growth of transplantable human tumors in the chick embryo and hatched chick. Cancer Res. 1956, 16, 589–594. [Google Scholar]
- Easty, G.C.; Easty, D.M.; Tchao, R. The growth of heterologous tumour cells in chick embryos. Eur. J. Cancer 1965, 5, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Sasaki, T.; Harada, F.; Noguchi, M. Specific detection of metastasized human tumor cells in embryonic chicks by the polymerase chain reaction. Jpn. J. Cancer Res. 1990, 81, 723–726. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Chick embryo chorioallantoic membrane model systems to study and visualize human tumor cell metastasis. Histochem. Cell Biol. 2008, 130, 1119–1130. [Google Scholar] [CrossRef]
- Zijlstra, A.; Lewis, J.; Degryse, B.; Stuhlmann, H.; Quigley, J.P. The inhibition of tumor cell intravasation and subsequent metastasis via regulation of in vivo tumor cell motility by the tetraspanin CD151. Cancer Cell 2008, 13, 221–234. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Zijlstra, A.; Partridge, J.J.; Kupriyanova, T.A.; Madsen, M.A.; Papagiannakopoulos, T.; Quigley, J.P. Unexpected effect of matrix metalloproteinase down-regulation on vascular intravasation and metastasis of human fibrosarcoma cells selected in vivo for high rates of dissemination. Cancer Res. 2005, 65, 10959–10969. [Google Scholar] [CrossRef]
- Deryugina, E.I. Chorioallantoic Membrane Microtumor Model to Study the Mechanisms of Tumor Angiogenesis, Vascular Permeability, and Tumor Cell Intravasation. Methods Mol. Biol. 2016, 1430, 283–298. [Google Scholar]
- Ronca, R.; Di Salle, E.; Giacomini, A.; Leali, D.; Alessi, P.; Coltrini, D.; Ravelli, C.; Matarazzo, S.; Ribatti, D.; Vermi, W.; et al. Long pentraxin-3 inhibits epithelial-mesenchymal transition in melanoma cells. Mol. Cancer Ther. 2013, 12, 2760–2771. [Google Scholar] [CrossRef]
- Lugassy, C.; Torres-Muñoz, J.E.; Kleinman, H.K.; Ghanem, G.; Vernon, S.; Barnhill, R.L. Overexpression of malignancy-associated laminins and laminin receptors by angiotropic human melanoma cells in a chick chorioallantoic membrane model. J. Cutan. Pathol. 2009, 36, 1237–1243. [Google Scholar] [CrossRef]
- Guedes, A.P.M.; Mello-Andrade, F.; Pires, W.C.; de Sousa, M.A.M.; da Silva, P.F.F.; Gemeiner, H.; Amauri, M.A.; Gomes Cardoso, C.; de Melo Reis, P.R.; Silveira-Lacerda, E.P.; et al. Heterobimetallic Ru(ii)/Fe(ii) complexes as potent anticancer agents against breast cancer cells, inducing apoptosis through multiple targets. Metallomics 2020, 12, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, N.; Bintig, W.; Perret, P.L.; Rees, J.; Viperino, A.; Eickholt, B.; Kuebler, W.M.; Höpfner, M.; Nitzsche, B.; Grune, J. In-ovo echocardiography for application in cardiovascular research. Basic. Res. Cardiol. 2023, 118, 19. [Google Scholar] [CrossRef]
- Butler, K.S.; Brinker, C.J.; Leong, H.S. Bridging the In Vitro to In Vivo gap: Using the Chick Embryo Model to Accelerate Nanoparticle Validation and Qualification for In Vivo studies. ACS Nano 2022, 16, 19626–19650. [Google Scholar] [CrossRef]
- Jefferies, B.; Lenze, F.; Sathe, A.; Truong, N.; Anton, M.; von Eisenhart-Rothe, R.; Nawroth, R.; Mayer-Kuckuk, P. Non-invasive imaging of engineered human tumors in the living chicken embryo. Sci. Rep. 2017, 7, 4991. [Google Scholar] [CrossRef] [PubMed]
- Schueler, J.; Kuenzel, J.; Thuesing, A.; Pion, E.; Behncke, R.Y.; Haegerling, R.; Fuchs, D.; Kraus, A.; Buchholz, B.; Huang, B.; et al. Ultra-high frequency ultrasound enables real-time visualization of blood supply from chorioallantoic membrane to human autosomal dominant polycystic kidney tissue. Sci. Rep. 2024, 14, 10063. [Google Scholar] [CrossRef]
- Benslimane, F.M.; Alser, M.; Zakaria, Z.Z.; Sharma, A.; Abdelrahman, H.A.; Yalcin, H.C. Adaptation of a Mice Doppler Echocardiography Platform to Measure Cardiac Flow Velocities for Embryonic Chicken and Adult Zebrafish. Front. Bioeng. Biotechnol. 2019, 7, 96. [Google Scholar] [CrossRef]
- Huang, C.; Lowerison, M.R.; Lucien, F.; Gong, P.; Wang, D.; Song, P.; Chen, S. Noninvasive Contrast-Free 3D Evaluation of Tumor Angiogenesis with Ultrasensitive Ultrasound Microvessel Imaging. Sci. Rep. 2019, 9, 4907. [Google Scholar] [CrossRef]
- Marzola, P.; Osculati, F.; Sbarbati, A. High field MRI in preclinical research. Eur. J. Radiol. 2003, 48, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Truppa, M.E.; Albanese, S.; Mancini, M. Radiomics and Radiogenomics in Preclinical Imaging on Murine Models: A Narrative Review. J. Pers. Med. 2023, 13, 1204. [Google Scholar] [CrossRef]
- Winter, G.; Koch Andrea, B.F.; Löffler, J.; Jelezko, F.; Mika, L.; Li, H.; Abaei, A.; Zuo, Z.; Beer, A.J.; Rasche, V. In vivo PET/MRI Imaging of the Chorioallantoic Membrane. Front. Phys. 2020, 8, 151. [Google Scholar] [CrossRef]
- Choi, C.H.; Bruch, M.; Hong, S.M.; Krause, S.; Stegmayr, C.; Schwan, S.; Worthoff, W.A.; Felder, J.; Shah, N.J. A Modified Quadrature Birdcage Coil Incorporated With a Curved Feature for In Ovo MR Imaging. IEEE Open J. Eng. Med. Biol. 2024, 5, 534–541. [Google Scholar] [CrossRef]
- Holmes, W.M.; McCabe, C.; Mullin, J.M.; Condon, B.; Bain, M.M. Images in cardiovascular medicine. Noninvasive self-gated magnetic resonance cardiac imaging of developing chick embryos in ovo. Circulation 2008, 117, e346–e347. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Z.; Shan, J.; Ma, W.; Li, L.; Zu, J.; Xu, J. Monitoring brain development of chick embryos in vivo using 3.0 T MRI: Subdivision volume change and preliminary structural quantification using DTI. BMC Dev. Biol. 2015, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Klose, R.; Streckenbach, F.; Stahnke, T.; Hadlich, S.; Kühn, J.P.; Guthoff, R.F.; Wree, A.; Neumann, A.M.; Frank, M.; et al. Morphologic and biometric evaluation of chick embryo eyes in ovo using 7 Tesla MRI. Sci. Rep. 2017, 7, 2647. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Fu, X.; Wang, S.; Feng, Y.; Coudyzer, W.; Wu, S.; Zhang, H.; Chai, Z.; Li, Y.; et al. Dynamic 3D morphology of chick embryos and allantois depicted nondestructively by 3.0T clinical magnetic resonance imaging. Poult. Sci. 2023, 102, 102902. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Syrovets, T.; Genze, F.; Abaei, A.; Ma, G.; Simmet, T.; Rasche, V. High-resolution MRI analysis of breast cancer xenograft on the chick chorioallantoic membrane. NMR Biomed. 2015, 2, 440–447. [Google Scholar] [CrossRef]
- Zuo, Z.; Syrovets, T.; Wu, Y.; Hafner, S.; Vernikouskaya, I.; Liu, W.; Ma, G.; Weil, T.; Simmet, T.; Rasche, V. The CAM cancer xenograft as a model for initial evaluation of MR labelled compounds. Sci. Rep. 2017, 7, 46690. [Google Scholar] [CrossRef] [PubMed]
- Hafner, S.; Raabe, M.; Wu, Y.; Wang, T.; Zuo, Z.; Rasche, V.; Syrovets, T.; Weil, T.; Simmet, T. High-Contrast Magnetic Resonance Imaging and Efficient Delivery of an Albumin Nanotheranostic in Triple-Negative Breast Cancer Xenografts. Adv. Ther. 2019, 2, 1900084. [Google Scholar] [CrossRef]
- Oppitz, M.; Pintaske, J.; Kehlbach, R.; Schick, F.; Schriek, G.; Busch, C. Magnetic resonance imaging of iron-oxide labeled SK-Mel 28 human melanoma cells in the chick embryo using a clinical whole body MRI scanner. Magma 2007, 20, 1–9. [Google Scholar] [CrossRef]
- Taylor, A.; Herrmann, A.; Moss, D.; Sée, V.; Davies, K.; Williams, S.R.; Murray, P. Assessing the efficacy of nano- and micro-sized magnetic particles as contrast agents for MRI cell tracking. PLoS ONE 2014, 9, e100259. [Google Scholar] [CrossRef]
- Herrmann, A.; Taylor, A.; Murray, P.; Poptani, H.; Sée, V. Magnetic Resonance Imaging for Characterization of a Chick Embryo Model of Cancer Cell Metastases. Mol. Imaging 2018, 17, 1536012118809585. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, J.; Heuberger, D.M.; Kivrak Pfiffner, F.; Wolint, P.; Jang, J.H.; Jungraithmayr, W.; Giovanoli, P.; Calcagni, M.; Waschkies, C.F. Probing Vasoreactivity and Hypoxic Phenotype in Different Tumor Grafts Grown on the Chorioallantoic Membrane of the Chicken Embryo In Ovo Using MRI. Cancers 2022, 14, 3114. [Google Scholar] [CrossRef]
- Waschkies, C.F.; Pfiffner, F.K.; Heuberger, D.M.; Calcagni, M.; Giovanoli, P.; Buschmann, J. Tumor grafts grown on the chicken chorioallantoic membrane are distinctively characterized by MRI under functional gas challenge. Sci. Rep. 2020, 10, 7505. [Google Scholar] [CrossRef]
- Kivrak Pfiffner, F.; Waschkies, C.; Tian, Y.; Woloszyk, A.; Calcagni, M.; Giovanoli, P.; Rudin, M.; Buschmann, J. A new in vivo magnetic resonance imaging method to noninvasively monitor and quantify the perfusion capacity of three-dimensional biomaterials grown on the chorioallantoic membrane of chick embryos. Tissue Eng. Part. C Methods 2015, 21, 339–346. [Google Scholar] [CrossRef]
- Benčurová, K.; Tran, L.; Friske, J.; Bevc, K.; Helbich, T.H.; Hacker, M.; Bergmann, M.; Zeitlinger, M.; Haug, A.; Mitterhauser, M.; et al. An in vivo tumour organoid model based on the chick embryonic chorioallantoic membrane mimics key characteristics of the patient tissue: A proof-of-concept study. EJNMMI Res. 2024, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Greenwood, H.E.; Tyrrell, W.E.; Edwards, R.S.; de Santis, V.; Baark, F.; Firth, G.; Tanc, M.; Terry, S.Y.A.; Herrmann, A.; et al. The chicken chorioallantoic membrane as a low-cost, high-throughput model for cancer imaging. npj Imaging 2023, 1, 1. [Google Scholar] [CrossRef]
- Zlatopolskiy, B.D.; Zischler, J.; Schäfer, D.; Urusova, E.A.; Guliyev, M.; Bannykh, O.; Endepols, H.; Neumaier, B. Discovery of 7-[18F]Fluorotryptophan as a Novel Positron Emission Tomography (PET) Probe for the Visualization of Tryptophan Metabolism In Vivo. J. Med. Chem. 2018, 61, 189–206. [Google Scholar] [CrossRef]
- Warnock, G.; Turtoi, A.; Blomme, A.; Bretin, F.; Bahri, M.A.; Lemaire, C.; Libert, L.C.; Seret, A.E.; Luxen, A.; Castronovo, V.; et al. In vivo PET/CT in a human glioblastoma chicken chorioallantoic membrane model: A new tool for oncology and radiotracer development. J. Nucl. Med. 2013, 54, 1782–1788. [Google Scholar] [CrossRef]
- Li, P.; Yin, X.; Shi, L.; Rugonyi, S.; Wang, R.K. In vivo functional imaging of blood flow and wall strain rate in outflow tract of embryonic chick heart using ultrafast spectral domain optical coherence tomography. J. Biomed. Opt. 2012, 17, 96006-1. [Google Scholar] [CrossRef]
- Li, P.; Yin, X.; Shi, L.; Liu, A.; Rugonyi, S.; Wang, R. Measurement of strain and strain rate in embryonic chick heart in vivo using spectral domain optical coherence tomography. IEEE Trans. Biomed. Eng. 2011, 58, 2333–2338. [Google Scholar]
- Moreno-Jiménez, I.; Hulsart-Billstrom, G.; Lanham, S.; Evans, N.D.; Oreffo, R.O. The chorioallantoic membrane (CAM) assay for the study of human bone regeneration: A refinement animal model for tissue engineering. Sci. Rep. 2016, 6, 32168. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).