Assessing the Antioxidant, Hepatoprotective, and Iron-Chelating Potential of Perilla frutescens Seed
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Rosmarinic Acid-Enriched Fraction from Perilla frutescens Seeds
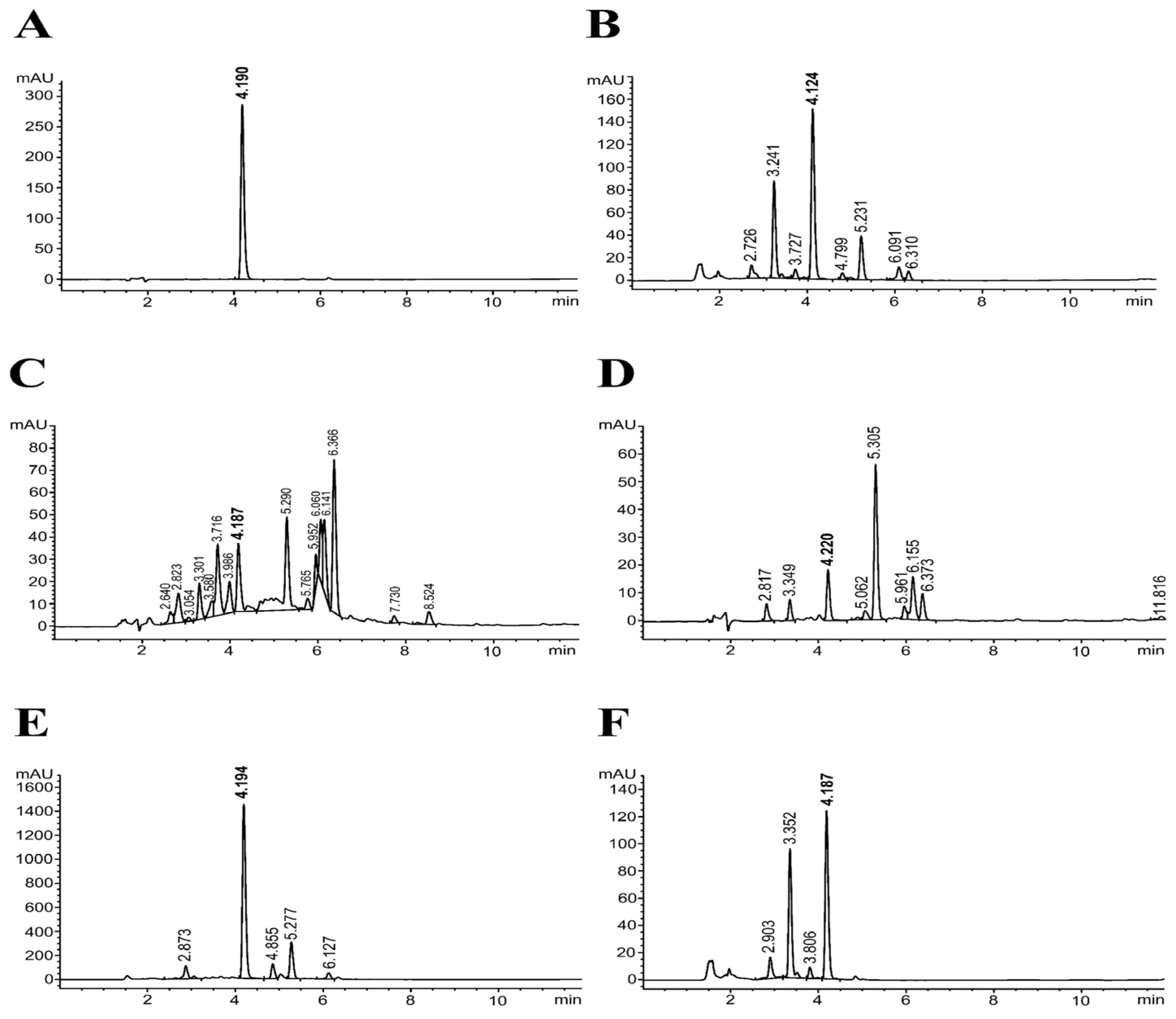
2.2. Determination of Rosmarinic Acid Content in Perilla Seed Extract
2.3. Determination of Total Phenolic Content
2.4. Determination of Total Flavonoid Content
2.5. DPPH Radical Scavenging Assay
2.6. ABTS Radical Scavenging Assay
2.7. Ferric Reducing Antioxidant Power Assay
2.8. Cell Culture
2.9. Cell Viability Assay
2.10. Intracellular ROS Production
2.11. Determination of Lipid Peroxidation in HepG2 Cells
2.12. Inhibition of Lipid Peroxidation in Egg Yolk
2.13. Nitric Oxide Assay
2.14. Ferric (Fe3+) Ion Chelation Assay
2.15. Intracellular Iron Chelation Assay
2.16. Transferrin Receptor (TfR) Expression Assessment
2.17. Statistical Analysis
3. Results
3.1. Extraction Yields, TPC, TFC, and RAC in PS Extract and Its Fractions
3.2. Antioxidant Abilities and Reducing Power Property of PS Extract and Its Fractions
3.3. Cytotoxic Effect of EtOAc Fraction
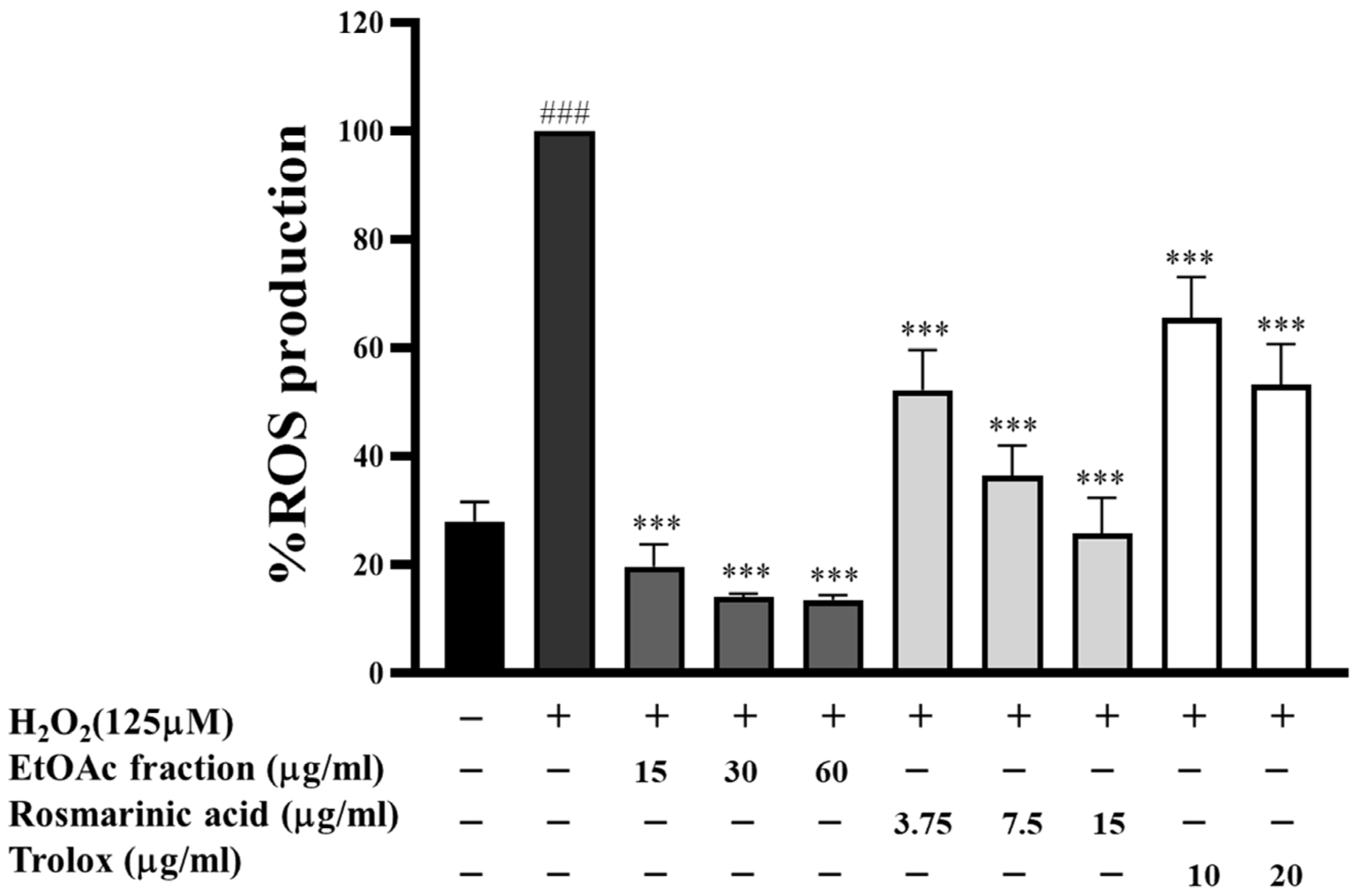
3.4. Effect of EtOAc Fraction on Intracellular ROS Generation in HepG2 Cells
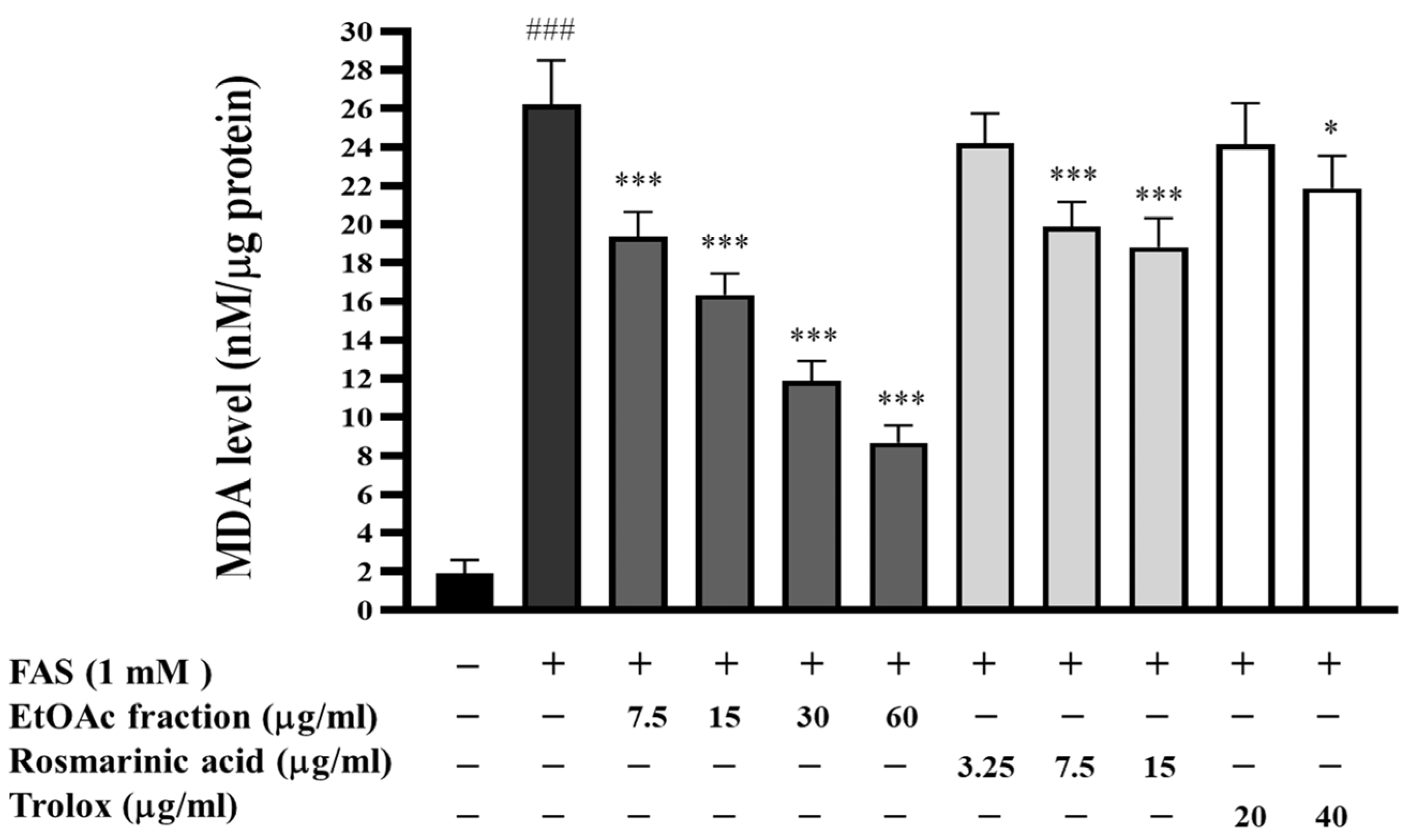
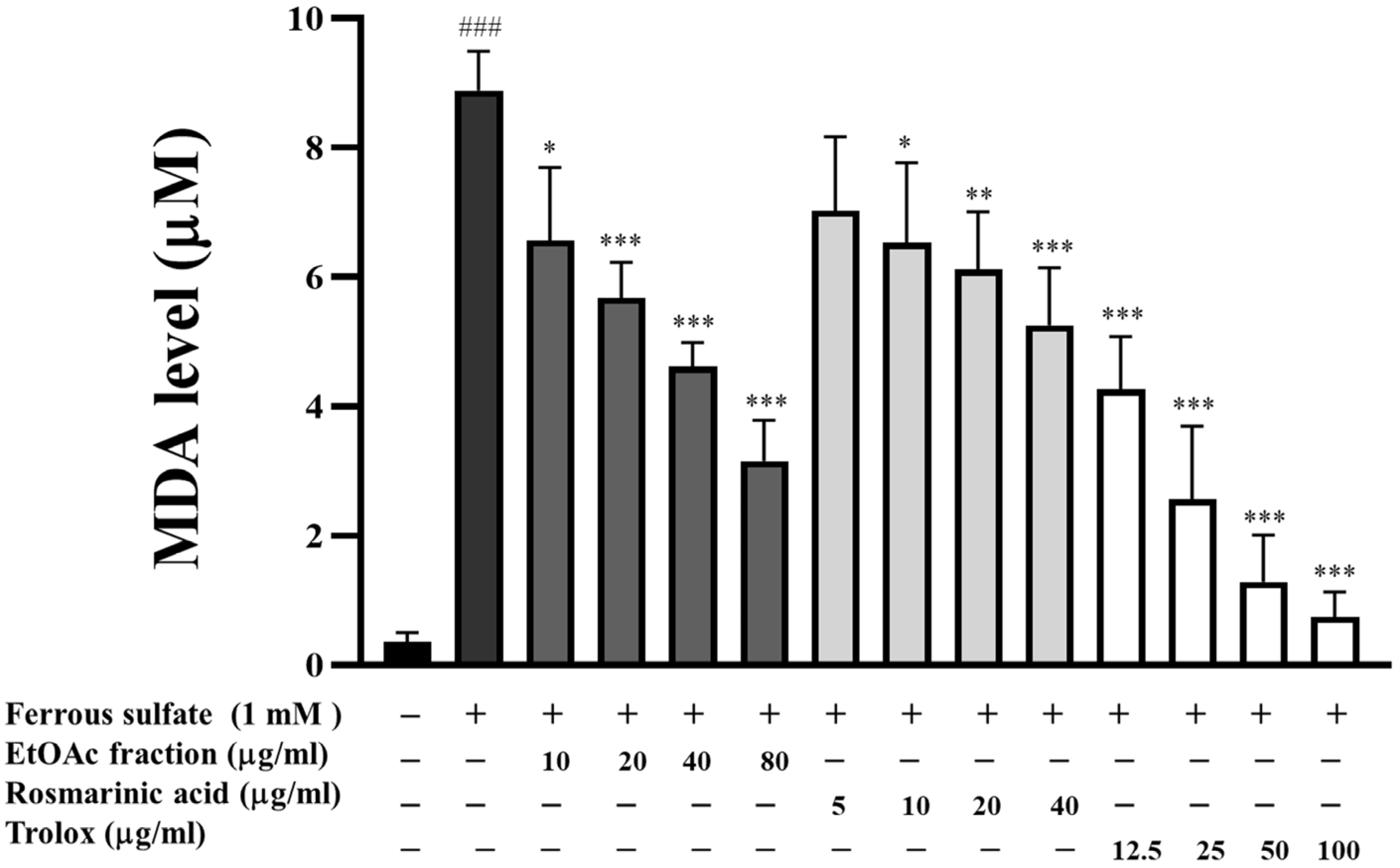
3.5. Inhibitory Effect of EtOAc Fraction on Lipid Peroxidation
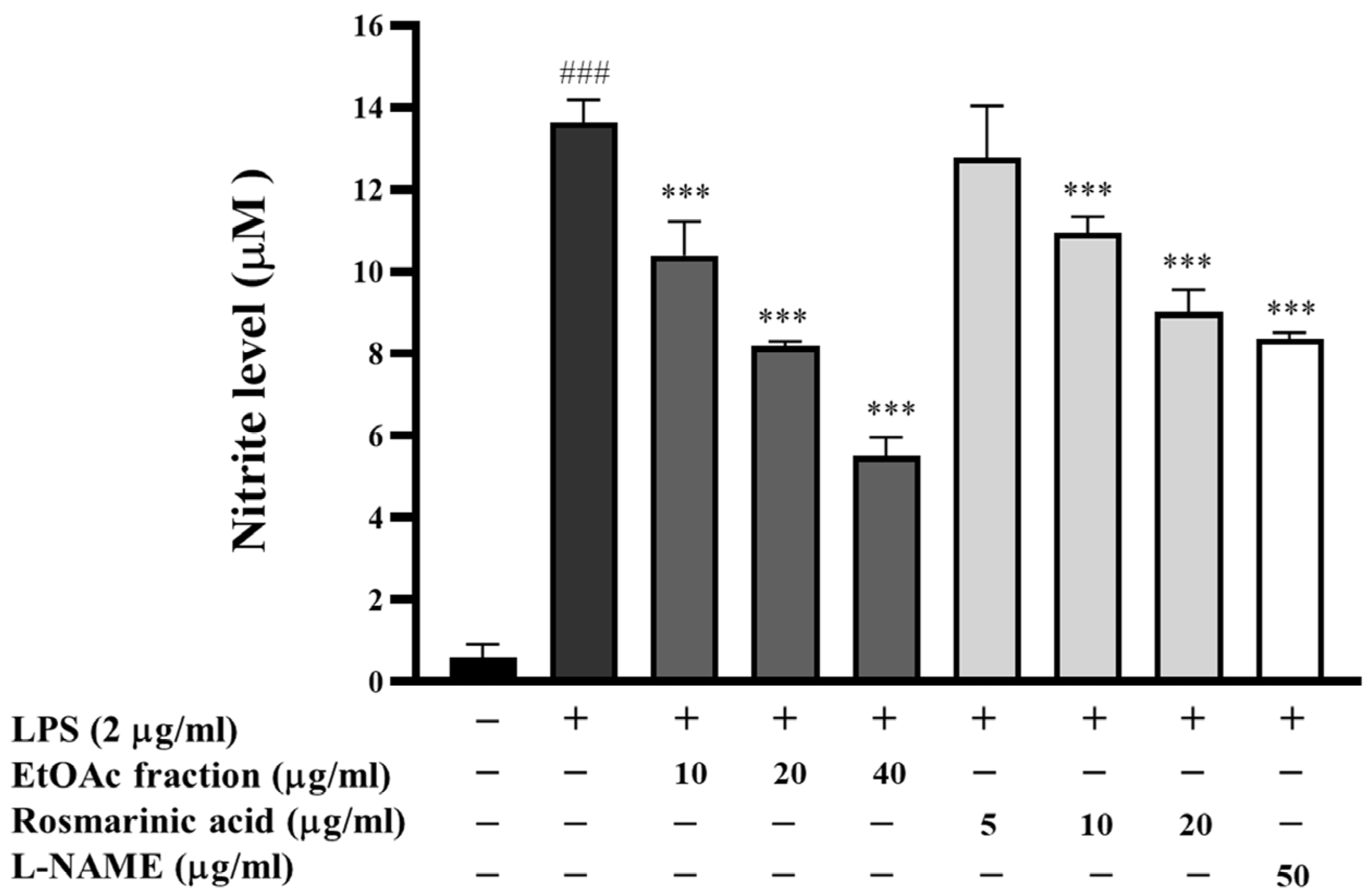
3.6. Effect of EtOAc Fraction on LPS-Induced Nitric Oxide Production in RAW 264.7 Cells
3.7. Effect of EtOAc Fraction on Iron Chelation Activity
3.8. Effect of EtOAc Fraction on Iron Chelation in HepG2 Cells
3.9. Effect of EtOAc Fraction on Transferrin Receptor (TfR) Expression in HepG2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milto, I.V.; Suhodolo, I.V.; Prokopieva, V.D.; Klimenteva, T.K. Molecular and Cellular Bases of Iron Metabolism in Humans. Biochemistry 2016, 81, 549–564. [Google Scholar] [CrossRef]
- Dutt, S.; Hamza, I.; Bartnikas, T.B. Molecular Mechanisms of Iron and Heme Metabolism. Annu. Rev. Nutr. 2022, 42, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.; Pujol Gimenez, J.; Cabra, R.S.; Hofstetter, W.; Hediger, M.A.; Albrecht, C. New Insights into the Physiology of Iron Transport: An Interdisciplinary Approach. Chimia 2022, 76, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Li, J.; Zhang, Y.; Chang, Y.Z. Cellular Iron Metabolism and Regulation. Adv. Exp. Med. Biol. 2019, 1173, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.S.; Arsiwala, T.; Mohsen, M.; Vogel, M.; Manolova, V.; Bachmann, M.F. On Iron Metabolism and Its Regulation. Int. J. Mol. Sci. 2021, 22, 4591. [Google Scholar] [CrossRef]
- Kawabata, T. Iron-Induced Oxidative Stress in Human Diseases. Cells 2022, 11, 2152. [Google Scholar] [CrossRef]
- Venkataramani, V. Iron Homeostasis and Metabolism: Two Sides of a Coin. Adv. Exp. Med. Biol. 2021, 1301, 25–40. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2023, 24, 449. [Google Scholar]
- Molina-Sánchez, P.; Lujambio, A. Iron overload and liver cancer. J. Exp. Med. 2019, 216, 723–724. [Google Scholar] [CrossRef]
- Franke, G.-N.; Kubasch, A.S.; Cross, M.; Vucinic, V.; Platzbecker, U. Iron overload and its impact on outcome of patients with hematological diseases. Mol. Asp. Med. 2020, 75, 100868. [Google Scholar] [CrossRef]
- Camarena, V.; Huff, T.C.; Wang, G. Epigenomic regulation by labile iron. Free Radic. Biol. Med. 2021, 170, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, J.L. Clinical Challenges with Iron Chelation in Beta Thalassemia. Hematol. Oncol. Clin. N. Am. 2023, 37, 379–391. [Google Scholar] [CrossRef]
- Valgimigli, L. Lipid Peroxidation and Antioxidant Protection. Biomolecules 2023, 13, 1291. [Google Scholar] [CrossRef]
- Petrovic, S.; Arsic, A.; Ristic-Medic, D.; Cvetkovic, Z.; Vucic, V. Lipid Peroxidation and Antioxidant Supplementation in Neurodegenerative Diseases: A Review of Human Studies. Antioxidants 2020, 9, 1128. [Google Scholar] [CrossRef] [PubMed]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Pangjit, K.; Udomsuk, L.; Upanan, S.; Pongjanta, A.; Chansiw, N.; Srichairatanakool, S. Iron-Chelating and Anti-Hemolytic Properties of Ethanolic Extract of Lotus (Nelumbonucifera Gaertn) Leaves. J. Med. Assoc. Thail. 2016, 99 (Suppl. S1), S58–S66. [Google Scholar]
- Wu, X.; Dong, S.; Chen, H.; Guo, M.; Sun, Z.; Luo, H. Perilla frutescens: A traditional medicine and food homologous plant. Chin. Herb. Med. 2023, 15, 369–375. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules 2019, 24, 102. [Google Scholar] [CrossRef]
- Adam, G.; Robu, S.; Flutur, M.-M.; Cioanca, O.; Vasilache, I.-A.; Adam, A.-M.; Mircea, C.; Nechita, A.; Harabor, V.; Harabor, A.; et al. Applications of Perilla frutescens Extracts in Clinical Practice. Antioxidants 2023, 12, 727. [Google Scholar] [CrossRef]
- Kangwan, N.; Pintha, K.; Lekawanvijit, S.; Suttajit, M. Rosmarinic Acid Enriched Fraction from Perilla frutescens Leaves Strongly Protects Indomethacin-Induced Gastric Ulcer in Rats. Biomed Res. Int. 2019, 2019, 9514703. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Chaiwangyen, W.; Suttajit, M.; Kangwan, N.; Kaowinn, S.; Khanaree, C.; Punfa, W.; Pintha, K. Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells. Molecules 2021, 26, 6757. [Google Scholar] [CrossRef] [PubMed]
- Tipsuwan, W.; Chaiwangyen, W. Preventive effects of polyphenol-rich perilla leaves on oxidative stress and haemolysis. ScienceAsia 2018, 44, 162. [Google Scholar] [CrossRef]
- Stalikas, C.D. Phenolic acids and flavonoids: Occurrence and analytical methods. Methods Mol. Biol. 2010, 610, 65–90. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Pintha, K.; Tantipaiboonwong, P.; Yodkeeree, S.; Chaiwangyen, W.; Chumphukam, O.; Khantamat, O.; Khanaree, C.; Kangwan, N.; Thongchuai, B.; Suttajit, M. Thai perilla (Perilla frutescens) leaf extract inhibits human breast cancer invasion and migration. Maejo Int. J. Sci. Technol. 2018, 12, 112–123. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. [Google Scholar]
- Perez-de-Arce, K.; Foncea, R.; Leighton, F. Reactive oxygen species mediates homocysteine-induced mitochondrial biogenesis in human endothelial cells: Modulation by antioxidants. Biochem. Biophys. Res. Commun. 2005, 338, 1103–1109. [Google Scholar] [CrossRef]
- Pangjit, K.; Banjerdpongchai, R.; Phisalaphong, C.; Fucharoen, S.; Srichairatanakool, S. Efficacy of 1-(N-acetyl-6-aminohexyl)-3-hydroxypyridin-4-one (CM1) in treatment of iron-loaded hepatocyte cultures. Adv. Biosci. Biotechnol. 2012, 3, 1060–1067. [Google Scholar] [CrossRef][Green Version]
- Sabir, S.M.; Abbas, S.R.; Shahida, S.; Khan, M.F. In-Vitro antioxidant, anti-lipid peroxidative activities and In-Silico study of Terminalia chebula bioactive compounds. Clin. Phytosci. 2020, 6, 83. [Google Scholar] [CrossRef]
- Zhou, X.J.; Yan, L.L.; Yin, P.P.; Shi, L.L.; Zhang, J.H.; Liu, Y.J.; Ma, C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef]
- Hou, T.; Netala, V.R.; Zhang, H.; Xing, Y.; Li, H.; Zhang, Z. Perilla frutescens: A Rich Source of Pharmacological Active Compounds. Molecules 2022, 27, 3578. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Pintha, K.; Chaiwangyen, W.; Suttajit, M.; Khanaree, C.; Khantamat, O. Bioefficacy of Nga-Mon (Perilla frutescens) Fresh and Dry Leaf: Assessment of Antioxidant, Antimutagenicity, and Anti-Inflammatory Potential. Plants 2023, 12, 2210. [Google Scholar] [CrossRef] [PubMed]
- Chumphukam, O.; Pintha, K.; Khanaree, C.; Chewonarin, T.; Chaiwangyen, W.; Tantipaiboonwong, P.; Suttajit, M.; Khantamat, O. Potential anti-mutagenicity, antioxidant, and anti-inflammatory capacities of the extract from perilla seed meal. J. Food Biochem. 2018, 42, e12556. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Pintha, K.; Chaiwangyen, W.; Yodkeeree, S.; Suttajit, M.; Tantipaiboonwong, P. Suppressive Effects of Rosmarinic Acid Rich Fraction from Perilla on Oxidative Stress, Inflammation and Metastasis Ability in A549 Cells Exposed to PM via C-Jun, P-65-Nf-Κb and Akt Signaling Pathways. Biomolecules 2021, 11, 1090. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Z.; Xie, X.; Cui, H.; Kong, K.W.; Zhang, L. Perilla frutescens Leaf Extract and Fractions: Polyphenol Composition, Antioxidant, Enzymes (α-Glucosidase, Acetylcholinesterase, and Tyrosinase) Inhibitory, Anticancer, and Antidiabetic Activities. Foods 2021, 10, 315. [Google Scholar] [CrossRef]
- Phromnoi, K.; Suttajit, M.; Saenjum, C.; Limtrakul Dejkriengkraikul, P. Inhibitory Effect of a Rosmarinic Acid-Enriched Fraction Prepared from Nga-Mon (Perilla frutescens) Seed Meal on Osteoclastogenesis through the RANK Signaling Pathway. Antioxidants 2021, 10, 307. [Google Scholar] [CrossRef]
- Kanokkarn, P.; Maitree, S.; Chalermpong, S. Polyphenols and Rosmarinic acid Contents, Antioxidant and Anti-Inflammatory Activities of Different Solvent Fractions from Nga- Mon (Perilla frutescens) Leaf. J. Pharm. Nutr. Sci. 2019, 9, 239–246. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Chen, C.; Song, Z.; Chang, M.; Wang, X.; Wang, X. Effect of infrared roasting of perilla seeds on the content of bioactive components and antioxidant capacity in oil. J. Am. Oil Chem. Soc. 2024, 101, 513–522. [Google Scholar] [CrossRef]
- Serrano, C.A.; Villena, G.K.; Rodríguez, E.F. Phytochemical profile and rosmarinic acid purification from two Peruvian Lepechinia Willd. species (Salviinae, Mentheae, Lamiaceae). Sci. Rep. 2021, 11, 7260. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Vasyliev, G.S.; Vorobyova, V.I.; Linyucheva, O.V. Evaluation of Reducing Ability and Antioxidant Activity of Fruit Pomace Extracts by Spectrophotometric and Electrochemical Methods. J. Anal. Methods Chem. 2020, 2020, 8869436. [Google Scholar] [CrossRef]
- Georgi, A.; Velasco Polo, M.; Crincoli, K.; Mackenzie, K.; Kopinke, F.D. Accelerated Catalytic Fenton Reaction with Traces of Iron: An Fe-Pd-Multicatalysis Approach. Environ. Sci. Technol. 2016, 50, 5882–5891. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules 2015, 20, 20841–20872. [Google Scholar] [CrossRef]
- Topal, M.; Gulcin, I. Evaluation of the in vitro antioxidant, antidiabetic and anticholinergic properties of rosmarinic acid from rosemary (Rosmarinus officinalis L.). Biocatal. Agric. Biotechnol. 2022, 43, 102417. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Fernandez, A.M.; Banfi, C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019, 23, 101119. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Yamamoto, Y.; Yoshinaka, N.; Namba, M.; Matsuo, H.; Okuyama, T.; Yoshigai, E.; Okumura, T.; Nishizawa, M.; Ikeya, Y. A new flavanone and other flavonoids from green perilla leaf extract inhibit nitric oxide production in interleukin 1β-treated hepatocytes. Biosci. Biotechnol. Biochem. 2015, 79, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-H.; Ja Son, H.; Hyun Lee, S.; Hwan Sohn, D. Two neolignans from Perilla frutescens and Their inhibition of nitric oxide synthase and tumor necrosis factor-α expression in murine macrophage cell line RAW 264.7. Bioorg. Med. Chem. Lett. 2002, 12, 649–651. [Google Scholar] [CrossRef]
- Chutvanichkul, B.; Vattanaviboon, P.; Mas-Oodi, S.; U-Pratya, Y.; Wanachiwanawin, W. Labile iron pool as a parameter to monitor iron overload and oxidative stress status in beta-thalassemic erythrocytes. Cytom. Part B Clin. Cytom. 2018, 94, 631–636. [Google Scholar] [CrossRef]
- Zanninelli, G.; Loreal, O.; Brissot, P.; Konijn, A.M.; Slotki, I.N.; Hider, R.C.; Ioav Cabantchik, Z. The labile iron pool of hepatocytes in chronic and acute iron overload and chelator-induced iron deprivation. J. Hepatol. 2002, 36, 39–46. [Google Scholar] [CrossRef]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of mammalian iron homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]




| Samples | %Yield | TPC | TFC | RAC | IC50 (µg/mL) | FRAP Value | |
|---|---|---|---|---|---|---|---|
| DPPH Assay | ABTS Assay | ||||||
| EtOH | 18.68 | 180.56 ± 4.47 | 72.27 ± 3.58 | 41.77 ± 0.02 | 100.75 ± 3.91 | 35.92 ± 0.70 | 486.23 ± 27.22 |
| Hex | 5.79 | 80.60 ± 6.62 | 17.96 ± 0.73 | 4.74 ± 0.20 | 244.66 ± 3.38 | 100.97 ± 2.41 | 169.44 ± 13.38 |
| DCM | 2.69 | 192.70 ± 5.65 | 35.26 ± 1.35 | 10.42 ± 0.07 | 130.35 ± 4.91 | 24.32 ± 0.50 | 341.16 ± 14.95 |
| EtOAc | 2.24 | 1314.05 ± 23.04 | 329.30 ± 10.37 | 393.81 ± 0.05 | 13.35 ± 0.81 | 3.98 ± 0.13 | 5062.95 ± 354.87 |
| Water | 70.40 | 136.12 ± 1.69 | 62.66 ± 2.47 | 32.92 ± 0.01 | 123.77 ± 3.19 | 31.38 ± 0.79 | 403.91 ± 24.93 |
| Rosmarinic acid | - | - | - | - | 8.69 ± 0.37 | 5.45 ± 0.04 | 7775.37 ± 439.56 |
| Vitamin C | - | - | - | - | 5.47 ± 0.11 | 5.18 ± 0.06 | 4347.10 ± 57.09 |
| Trolox | - | - | - | - | 5.20 ± 0.02 | 5.17 ± 0.04 | 4885.00 ± 95.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakdeepromma, S.; Pintha, K.; Tantipaiboonwong, P.; Thephinlap, C.; Suttajit, M.; Kaowinn, S.; Kangwan, N.; Suwannaloet, W.; Pangjit, K. Assessing the Antioxidant, Hepatoprotective, and Iron-Chelating Potential of Perilla frutescens Seed. Biomedicines 2025, 13, 851. https://doi.org/10.3390/biomedicines13040851
Pakdeepromma S, Pintha K, Tantipaiboonwong P, Thephinlap C, Suttajit M, Kaowinn S, Kangwan N, Suwannaloet W, Pangjit K. Assessing the Antioxidant, Hepatoprotective, and Iron-Chelating Potential of Perilla frutescens Seed. Biomedicines. 2025; 13(4):851. https://doi.org/10.3390/biomedicines13040851
Chicago/Turabian StylePakdeepromma, Sirichatnach, Komsak Pintha, Payungsak Tantipaiboonwong, Chonthida Thephinlap, Maitree Suttajit, Sawaruj Kaowinn, Napapan Kangwan, Wanwisa Suwannaloet, and Kanjana Pangjit. 2025. "Assessing the Antioxidant, Hepatoprotective, and Iron-Chelating Potential of Perilla frutescens Seed" Biomedicines 13, no. 4: 851. https://doi.org/10.3390/biomedicines13040851
APA StylePakdeepromma, S., Pintha, K., Tantipaiboonwong, P., Thephinlap, C., Suttajit, M., Kaowinn, S., Kangwan, N., Suwannaloet, W., & Pangjit, K. (2025). Assessing the Antioxidant, Hepatoprotective, and Iron-Chelating Potential of Perilla frutescens Seed. Biomedicines, 13(4), 851. https://doi.org/10.3390/biomedicines13040851







