Circulating Soluble EPCR Levels Are Reduced in Patients with Ischemic Peripheral Artery Disease and Associated with Markers of Endothelial and Vascular Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Perfusion Tests
2.3. Blood Sampling, Serum, and Plasma Preparation
2.4. Routine Laboratory Methods
2.5. Enzyme-Linked Immunoassays
2.6. Statistical Analysis
3. Results
3.1. Soluble EPCR Levels in Plasma Are Reduced in Patients with Peripheral Artery Disease
3.2. Elevated Biomarkers of Endothelial Dysfunction in Patients with Peripheral Artery Disease
3.3. Increased Inflammatory Biomarkers in Patients with Cardiovascular Risk Factors
3.4. Biomarkers of Angiogenesis and Anti-Angiogenesis in Peripheral Artery Disease Are Elevated, but Not Associated with Biomarkers of Endothelial or Vascular Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stearns-Kurosawa, D.J.; Kurosawa, S.; Mollica, J.S.; Ferrell, G.L.; Esmon, C.T. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc. Natl. Acad. Sci. USA 1996, 93, 10212–10216. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.; Aleksic, N.; Ballantyne, C.M.; Ahn, C.; Juneja, H.; Boerwinkle, E. Interaction between soluble thrombomodulin and intercellular adhesion molecule-1 in predicting risk of coronary heart disease. Circulation 2003, 107, 1729–1732. [Google Scholar] [CrossRef] [PubMed]
- Freestone, B.; Chong, A.Y.; Nuttall, S.; Blann, A.D.; Lip, G.Y. Soluble E-selectin, von Willebrand factor, soluble thrombomodulin, and total body nitrate/nitrite product as indices of endothelial damage/dysfunction in paroxysmal, persistent, and permanent atrial fibrillation. Chest 2007, 132, 1253–1258. [Google Scholar] [CrossRef]
- Wei, H.J.; Li, Y.H.; Shi, G.Y.; Liu, S.L.; Chang, P.C.; Kuo, C.H.; Wu, H.L. Thrombomodulin domains attenuate atherosclerosis by inhibiting thrombin-induced endothelial cell activation. Cardiovasc. Res. 2011, 92, 317–327. [Google Scholar] [CrossRef]
- Budzyń, M.; Gryszczyńska, B.; Boruczkowski, M.; Kaczmarek, M.; Begier-Krasińska, B.; Osińska, A.; Bukowska, A.; Iskra, M.; Kasprzak, M.P. The endothelial status reflected by circulating endothelial cells, circulating endothelial progenitor cells and soluble thrombomodulin in patients with mild and resistant hypertension. Vascul. Pharmacol. 2019, 113, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Seigneur, M.; Dufourcq, P.; Conri, C.; Constans, J.; Mercié, P.; Pruvost, A.; Amiral, J.; Midy, D.; Baste, J.-C.; Boisseau, M.R. Levels of plasma thrombomodulin are increased in atheromatous arterial disease. Thromb. Res. 1993, 71, 423–431. [Google Scholar] [CrossRef]
- Blann, A.D.; Amiral, J.; McCollum, C.N. Prognostic value of increased soluble thrombomodulin and increased soluble E-selectin in ischaemic heart disease. Eur. J. Haematol. 1997, 59, 115–120. [Google Scholar] [CrossRef]
- Gu, J.M.; Katsuura, Y.; Ferrell, G.L.; Grammas, P.; Esmon, C.T. Endotoxin and thrombin elevate rodent endothelial cell protein C receptor mRNA levels and increase receptor shedding in vivo. Blood 2000, 95, 1687–1693. [Google Scholar] [CrossRef]
- Qu, D.; Wang, Y.; Esmon, N.L.; Esmon, C.T. Regulated endothelial protein C receptor shedding is mediated by tumor necrosis factor-alpha converting enzyme/ADAM17. J. Thromb. Haemost. 2007, 5, 395–402. [Google Scholar] [CrossRef]
- Tanalp, A.C.; Oduncu, V.; Erkol, A.; Gözübüyük, G.; Ozveren, O.; Dündar, C.; Canbay, A.; Kirma, C. Soluble endothelial protein C receptor levels and protein C activity in patients with acute ST-segment elevation myocardial infarction. Coron. Artery Dis. 2013, 24, 209–216. [Google Scholar] [CrossRef]
- Ku, S.K.; Han, M.S.; Park, E.J.; Na, D.H.; Bae, J.S. Exendin-4 inhibits endothelial protein C receptor shedding in vitro and in vivo. Pharmacol. Res. 2014, 84, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Timmers, L.; Henriques, J.P.; de Kleijn, D.P.; DeVries, J.H.; Kemperman, H.; Steendijk, P.; Verlaan, C.W.; Kerver, M.; Piek, J.J.; Doevendans, P.A.; et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J. Am. Coll. Cardiol. 2009, 53, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Lønborg, J.; Vejlstrup, N.; Kelbæk, H.; Bøtker, H.E.; Kim, W.Y.; Mathiasen, A.B.; Jørgensen, E.; Helqvist, S.; Saunamäki, K.; Clemmensen, P.; et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur. Hear. J. 2011, 33, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Bochenek, M.L.; Gogiraju, R.; Großmann, S.; Krug, J.; Orth, J.; Reyda, S.; Georgiadis, G.S.; Spronk, H.M.; Konstantinides, S.; Münzel, T.; et al. EPCR-PAR1 biased signaling regulates perfusion recovery and neovascularization in peripheral ischemia. JCI Insight. 2022, 7, e157701. [Google Scholar] [CrossRef]
- Fontaine, R.; Kim, M.; Kieny, R. Surgical treatment of peripheral circulation disorders. Helv. Chir. Acta 1954, 21, 499–533. [Google Scholar]
- Hardman, R.L.; Jazaeri, O.; Yi, J.; Smith, M.; Gupta, R. Overview of classification systems in peripheral artery disease. Semin. Interv. Radiol. 2014, 31, 378–388. [Google Scholar] [CrossRef]
- Wild, P.S.; Sinning, C.R.; Roth, A.; Wilde, S.; Schnabel, R.B.; Lubos, E.; Zeller, T.; Keller, T.; Lackner, K.J.; Blettner, M.; et al. Distribution and categorization of left ventricular measurements in the general population: Results from the population-based Gutenberg Heart Study. Circ. Cardiovasc. Imaging 2010, 3, 604–613. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Wild, P.S.; Schulz, A.; Zeller, T.; Sinning, C.R.; Wilde, S.; Kunde, J.; Lubos, E.; Lackner, K.J.; Warnholtz, A.; et al. Multiple endothelial biomarkers and noninvasive vascular function in the general population: The Gutenberg Health Study. Hypertension 2012, 60, 288–295. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar]
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Espinola-Klein, C.; Rupprecht, H.J.; Bickel, C.; Lackner, K.; Savvidis, S.; Messow, C.M.; Munzel, T.; Blankenberg, S.; AtheroGene Investigators. Different calculations of ankle-brachial index and their impact on cardiovascular risk prediction. Circulation 2008, 118, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Kieback, A.G.; Espinola-Klein, C.; Lamina, C.; Moebus, S.; Tiller, D.; Lorbeer, R.; Schulz, A.; Meisinger, C.; Medenwald, D.; Erbel, R.; et al. One simple claudication question as first step in Peripheral Arterial Disease (PAD) screening: A meta-analysis of the association with reduced Ankle Brachial Index (ABI) in 27,945 subjects. PLoS ONE 2019, 14, e0224608. [Google Scholar] [CrossRef]
- Uchiba, M.; Okajima, K.; Oike, Y.; Ito, Y.; Fukudome, K.; Isobe, H.; Suda, T. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ. Res. 2004, 95, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.C.; Song, W.; Wang, D.; Zeng, Y.A. Identification of blood vascular endothelial stem cells by the expression of protein C receptor. Cell Res. 2016, 26, 1079–1098. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Minhas, N.; Xue, M.; Jackson, C.J. Activated protein C binds directly to Tie2: Possible beneficial effects on endothelial barrier function. Cell. Mol. Life Sci. 2017, 74, 1895–1906. [Google Scholar] [CrossRef]
- Hughes, D.P.; Marron, M.B.; Brindle, N.P.J. The Antiinflammatory Endothelial Tyrosine Kinase Tie2 Interacts With a Novel Nuclear Factor-B Inhibitor ABIN-2. Circ. Res. 2003, 92, 630–636. [Google Scholar] [CrossRef]
- Feistritzer, C.; Schuepbach, R.A.; Mosnier, L.O.; Bush, L.A.; Di Cera, E.; Griffin, J.H.; Riewald, M. Protective signaling by activated protein C is mechanistically linked to protein C activation on endothelial cells. J. Biol. Chem. 2006, 281, 20077–20084. [Google Scholar] [CrossRef]
- Riewald, M.; Schuepbach, R.A. Protective signaling pathways of activated protein C in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1–3. [Google Scholar] [CrossRef]
- Dopheide, J.F.; Obst, V.; Doppler, C.; Radmacher, M.C.; Scheer, M.; Radsak, M.P.; Gori, T.; Warnholtz, A.; Fottner, C.; Daiber, A.; et al. Phenotypic characterisation of pro-inflammatory monocytes and dendritic cells in peripheral arterial disease. Thromb. Haemost. 2012, 108, 1198–1207. [Google Scholar]
- Ziegler, L.; Hedin, U.; Gottsäter, A. Circulating Biomarkers in Lower Extremity Artery Disease. Eur. Cardiol. 2022, 17, e09. [Google Scholar] [CrossRef]
- Fadini, G.P.; Sartore, S.; Albiero, M.; Baesso, I.; Murphy, E.; Menegolo, M.; Grego, F.; de Kreutzenberg, S.V.; Tiengo, A.; Agostini, C.; et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2140–2146. [Google Scholar] [CrossRef]
- Bitterli, L.; Afan, S.; Bühler, S.; DiSanto, S.; Zwahlen, M.; Schmidlin, K.; Yang, Z.; Baumgartner, I.; Diehm, N.; Kalka, C. Endothelial progenitor cells as a biological marker of peripheral artery disease. Vasc. Med. 2015, 21, 3–11. [Google Scholar] [CrossRef]
- Jacobs, M.; van Greevenbroek, M.M.; van der Kallen, C.J.; Ferreira, I.; Blaak, E.E.; Feskens, E.J.; Jansen, E.H.J.M.; Schalkwijk, C.G.; Stehouwer, C.D.A. The association between the metabolic syndrome and peripheral, but not coronary, artery disease is partly mediated by endothelial dysfunction: The CODAM study. Eur. J. Clin. Investig. 2010, 41, 167–175. [Google Scholar] [CrossRef]
- Leeuwenberg, J.F.; Smeets, E.F.; Neefjes, J.J.; Shaffer, M.A.; Cinek, T.; Jeunhomme, T.M.; Ahern, T.J.; Buurman, W.A. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 1992, 77, 543–549. [Google Scholar]
- Miwa, K.; Igawa, A.; Inoue, H. Soluble E-selectin, ICAM-1 and VCAM-1 levels in systemic and coronary circulation in patients with variant angina. Cardiovasc. Res. 1997, 36, 37–44. [Google Scholar] [CrossRef]
- Laszik, Z.G.; Zhou, X.J.; Ferrell, G.L.; Silva, F.G.; Esmon, C.T. Down-regulation of endothelial expression of endothelial cell protein C receptor and thrombomodulin in coronary atherosclerosis. Am. J. Pathol. 2001, 159, 797–802. [Google Scholar] [CrossRef]
- Hikita, A.; Tanaka, N.; Yamane, S.; Ikeda, Y.; Furukawa, H.; Tohma, S.; Suzuki, R.; Tanaka, S.; Mitomi, H.; Fukui, N. Involvement of a disintegrin and metalloproteinase 10 and 17 in shedding of tumor necrosis factor-alpha. Biochem. Cell Biol. 2009, 87, 581–593. [Google Scholar] [CrossRef]
- Bell, J.H.; Herrera, A.H.; Li, Y.; Walcheck, B. Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J. Leukoc. Biol. 2007, 82, 173–176. [Google Scholar] [CrossRef]
- McMahan, R.S.; Riehle, K.J.; Fausto, N.; Campbell, J.S. A disintegrin and metalloproteinase 17 regulates TNF and TNFR1 levels in inflammation and liver regeneration in mice. Am. J. Physiol. Liver Physiol. 2013, 305, G25–G34. [Google Scholar] [CrossRef]
- Bartoli-Leonard, F.; Zimmer, J.; Sonawane, A.R.; Perez, K.; Turner, M.E.; Kuraoka, S.; Pham, T.; Li, F.; Aikawa, M.; Singh, S.; et al. NLRP3 Inflammasome Activation in Peripheral Arterial Disease. J. Am. Heart Assoc. 2023, 12, e026945. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.S.; Yates, D.P.; Kramer, C.M.; Feller, A.; Mahling, P.; Colin, L.; Clough, T.; Wang, T.; LaPerna, L.; Patel, A.; et al. A randomized, placebo-controlled trial of canakinumab in patients with peripheral artery disease. Vasc. Med. 2019, 24, 414–421. [Google Scholar] [CrossRef]
- Poledniczek, M.; Neumayer, C.; Kopp, C.W.; Schlager, O.; Gremmel, T.; Jozkowicz, A.; Gschwandtner, M.E.; Koppensteiner, R.; Wadowski, P.P. Micro- and Macrovascular Effects of Inflammation in Peripheral Artery Disease-Pathophysiology and Translational Therapeutic Approaches. Biomedicines 2023, 11, 2284. [Google Scholar] [CrossRef]
- von Asmuth, E.J.; Smeets, E.F.; Ginsel, L.A.; Onderwater, J.J.; Leeuwenberg, J.F.; Buurman, W.A. Evidence for endocytosis of E-selectin in human endothelial cells. Eur. J. Immunol. 1992, 22, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Esmon, C.T.; Esmon, N.L. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood 1989, 73, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.; van Greevenbroek, M.M.; van der Kallen, C.J.; Ferreira, I.; Blaak, E.E.; Feskens, E.J.; Jansen, E.H.J.M.; Schalkwijk, C.G.; Stehouwer, C.D.A. Low-grade inflammation can partly explain the association between the metabolic syndrome and either coronary artery disease or severity of peripheral arterial disease: The CODAM study. Eur. J. Clin. Investig. 2009, 39, 437–444. [Google Scholar] [CrossRef]
- Regan, L.M.; Mollica, J.S.; Rezaie, A.R.; Esmon, C.T. The interaction between the endothelial cell protein C receptor and protein C is dictated by the gamma-carboxyglutamic acid domain of protein C. J. Biol. Chem. 1997, 272, 26279–26284. [Google Scholar] [CrossRef]
- Liaw, P.C.; Neuenschwander, P.F.; Smirnov, M.D.; Esmon, C.T. Mechanisms by which soluble endothelial cell protein C receptor modulates protein C and activated protein C function. J. Biol. Chem. 2000, 275, 5447–5452. [Google Scholar] [CrossRef]
- Mosnier, L.O.; Zlokovic, B.V.; Griffin, J.H. The cytoprotective protein C pathway. Blood 2007, 109, 3161–3172. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Mohler, E.R., 3rd; Lederman, R.J.; Mendelsohn, F.O.; Saucedo, J.F.; Goldman, C.K.; Blebea, J.; Macko, J.; Kessler, P.D.; Rasmussen, H.S.; et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: A phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 2003, 108, 1933–1938. [Google Scholar]
- Zabolotny, J.M.; Kim, Y.B.; Welsh, L.A.; Kershaw, E.E.; Neel, B.G.; Kahn, B.B. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J. Biol. Chem. 2008, 283, 14230–14241. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Anzaldi, M.; Fiore, V.; Simili, M.; Puccia, G.; Libra, M.; Malaponte, G.; Neri, S. Patients with unrecognized peripheral arterial disease (PAD) assessed by ankle-brachial index (ABI) present a defined profile of proinflammatory markers compared to healthy subjects. Cytokine 2012, 59, 294–298. [Google Scholar] [CrossRef]
 ). The results of the statistical analysis (using Mann–Whitney test) are shown within the graph. (B), Plasma sEPCR levels in age- and sex-matched healthy persons with (
). The results of the statistical analysis (using Mann–Whitney test) are shown within the graph. (B), Plasma sEPCR levels in age- and sex-matched healthy persons with ( ) and without (◯) cardiovascular risk factors and in sex-matched individuals 35 years of age or younger (□). The results of the statistical analysis (using Kruskall–Wallis, Dunn’s multiple comparisons test) are shown within the graph. (C), Comparison of plasma sEPCR levels in patients with PAD stage II (●) and stage III/IV (♦), examined within the same experiments. The results of the statistical analysis (using Mann–Whitney test) are shown within the graph. Simple linear regression analysis of the association of plasma sEPCR levels with the ankle-brachial index (D), age (E), body mass index (BMI; (F)), and plasma protein C activity (G) or ADAM17 levels (H) was performed. The r-squared and the p-value for each analysis are shown within the graphs. Note that the ankle-brachial index was available only in PAD stage II patients (●), age and BMI also in age- and sex-matched healthy persons with (
) and without (◯) cardiovascular risk factors and in sex-matched individuals 35 years of age or younger (□). The results of the statistical analysis (using Kruskall–Wallis, Dunn’s multiple comparisons test) are shown within the graph. (C), Comparison of plasma sEPCR levels in patients with PAD stage II (●) and stage III/IV (♦), examined within the same experiments. The results of the statistical analysis (using Mann–Whitney test) are shown within the graph. Simple linear regression analysis of the association of plasma sEPCR levels with the ankle-brachial index (D), age (E), body mass index (BMI; (F)), and plasma protein C activity (G) or ADAM17 levels (H) was performed. The r-squared and the p-value for each analysis are shown within the graphs. Note that the ankle-brachial index was available only in PAD stage II patients (●), age and BMI also in age- and sex-matched healthy persons with ( ) and without (◯) cardiovascular risk factors and in sex-matched individuals 35 years of age or younger (□). Significant differences are highlighted in bold.
) and without (◯) cardiovascular risk factors and in sex-matched individuals 35 years of age or younger (□). Significant differences are highlighted in bold.
 ). The results of the statistical analysis (using Mann–Whitney test) are shown within the graph. (B), Plasma sEPCR levels in age- and sex-matched healthy persons with (
). The results of the statistical analysis (using Mann–Whitney test) are shown within the graph. (B), Plasma sEPCR levels in age- and sex-matched healthy persons with ( ) and without (◯) cardiovascular risk factors and in sex-matched individuals 35 years of age or younger (□). The results of the statistical analysis (using Kruskall–Wallis, Dunn’s multiple comparisons test) are shown within the graph. (C), Comparison of plasma sEPCR levels in patients with PAD stage II (●) and stage III/IV (♦), examined within the same experiments. The results of the statistical analysis (using Mann–Whitney test) are shown within the graph. Simple linear regression analysis of the association of plasma sEPCR levels with the ankle-brachial index (D), age (E), body mass index (BMI; (F)), and plasma protein C activity (G) or ADAM17 levels (H) was performed. The r-squared and the p-value for each analysis are shown within the graphs. Note that the ankle-brachial index was available only in PAD stage II patients (●), age and BMI also in age- and sex-matched healthy persons with (
) and without (◯) cardiovascular risk factors and in sex-matched individuals 35 years of age or younger (□). The results of the statistical analysis (using Kruskall–Wallis, Dunn’s multiple comparisons test) are shown within the graph. (C), Comparison of plasma sEPCR levels in patients with PAD stage II (●) and stage III/IV (♦), examined within the same experiments. The results of the statistical analysis (using Mann–Whitney test) are shown within the graph. Simple linear regression analysis of the association of plasma sEPCR levels with the ankle-brachial index (D), age (E), body mass index (BMI; (F)), and plasma protein C activity (G) or ADAM17 levels (H) was performed. The r-squared and the p-value for each analysis are shown within the graphs. Note that the ankle-brachial index was available only in PAD stage II patients (●), age and BMI also in age- and sex-matched healthy persons with ( ) and without (◯) cardiovascular risk factors and in sex-matched individuals 35 years of age or younger (□). Significant differences are highlighted in bold.
) and without (◯) cardiovascular risk factors and in sex-matched individuals 35 years of age or younger (□). Significant differences are highlighted in bold.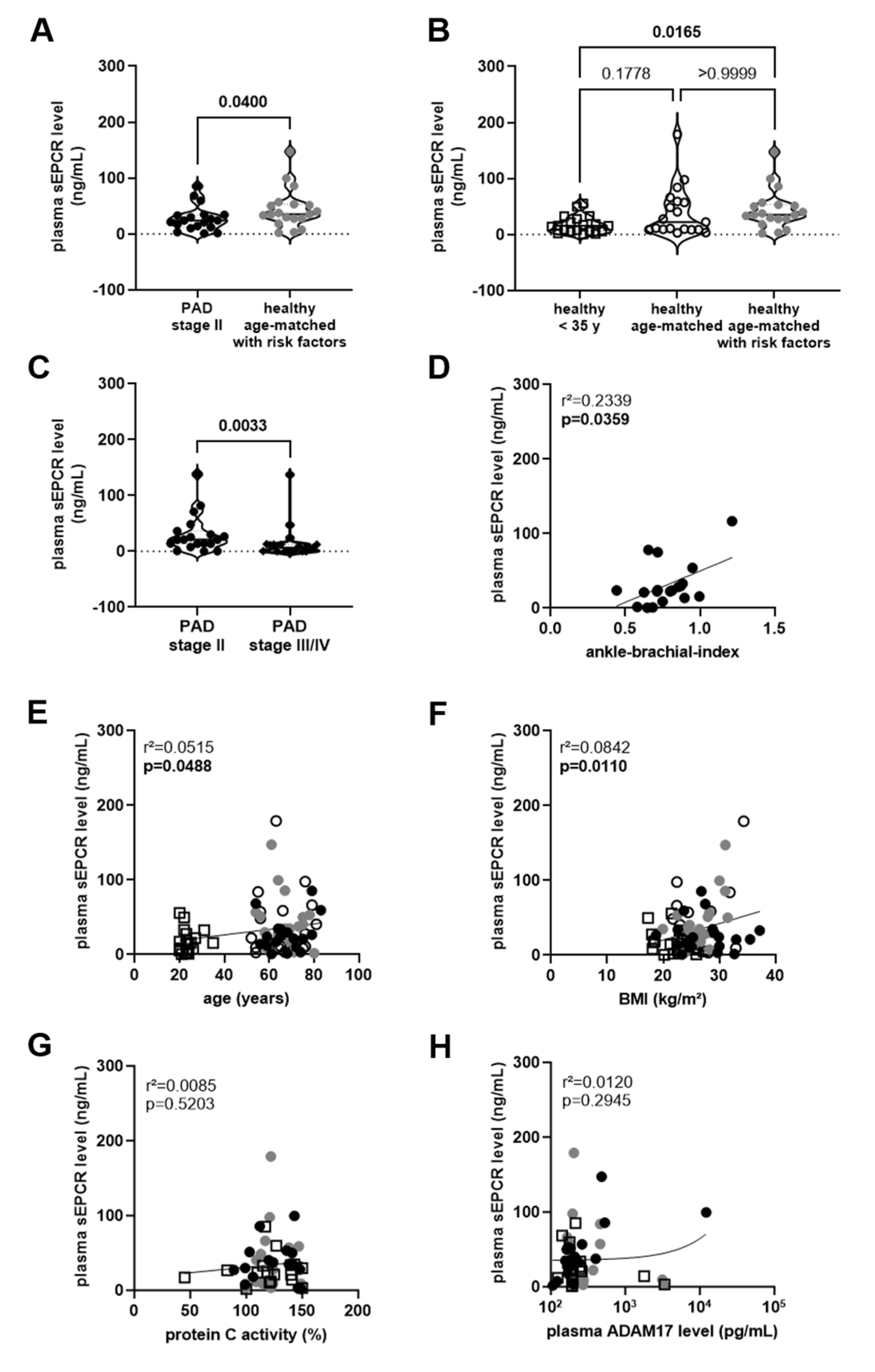
 ) and without (◯) cardiovascular risk factors. The results of the statistical analysis (using One-Way-ANOVA, Sidak’s multiple comparisons test) are shown within the graph. Simple linear regression analysis of the association of plasma sEPCR and sE-selectin levels in age-matched person with risk factors (
) and without (◯) cardiovascular risk factors. The results of the statistical analysis (using One-Way-ANOVA, Sidak’s multiple comparisons test) are shown within the graph. Simple linear regression analysis of the association of plasma sEPCR and sE-selectin levels in age-matched person with risk factors ( ; (B)) or with sTie2 levels in PAD stage II patients (●; (D)). Simple linear regression analysis of the association of plasma sE-selectin (E) or sTie2 (F) levels with the ankle-brachial index, determined in PAD stage II patients (●). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.
; (B)) or with sTie2 levels in PAD stage II patients (●; (D)). Simple linear regression analysis of the association of plasma sE-selectin (E) or sTie2 (F) levels with the ankle-brachial index, determined in PAD stage II patients (●). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.
 ) and without (◯) cardiovascular risk factors. The results of the statistical analysis (using One-Way-ANOVA, Sidak’s multiple comparisons test) are shown within the graph. Simple linear regression analysis of the association of plasma sEPCR and sE-selectin levels in age-matched person with risk factors (
) and without (◯) cardiovascular risk factors. The results of the statistical analysis (using One-Way-ANOVA, Sidak’s multiple comparisons test) are shown within the graph. Simple linear regression analysis of the association of plasma sEPCR and sE-selectin levels in age-matched person with risk factors ( ; (B)) or with sTie2 levels in PAD stage II patients (●; (D)). Simple linear regression analysis of the association of plasma sE-selectin (E) or sTie2 (F) levels with the ankle-brachial index, determined in PAD stage II patients (●). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.
; (B)) or with sTie2 levels in PAD stage II patients (●; (D)). Simple linear regression analysis of the association of plasma sE-selectin (E) or sTie2 (F) levels with the ankle-brachial index, determined in PAD stage II patients (●). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.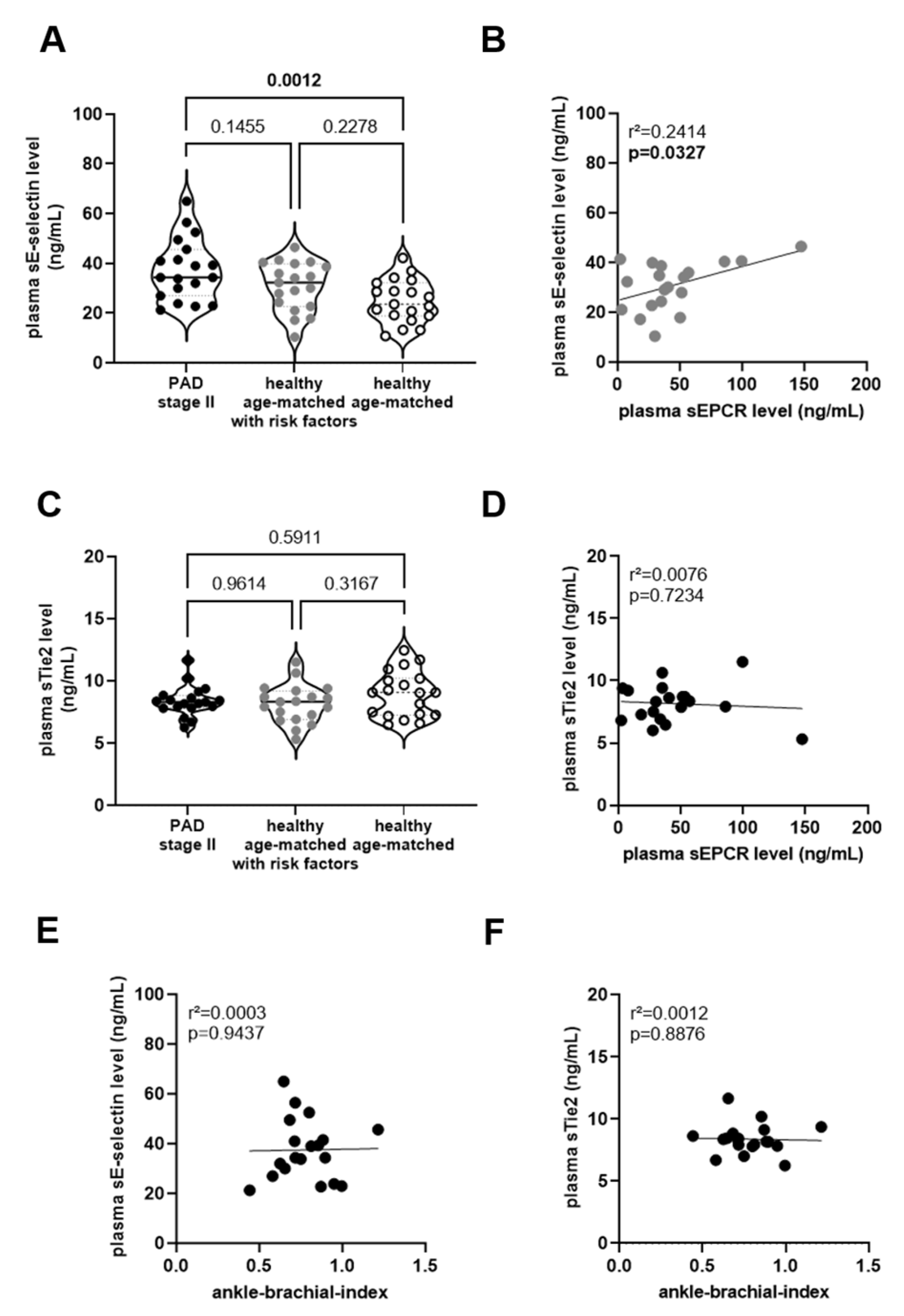
 ) and without (◯) cardiovascular risk factors. Plasma from these groups was also examined for the concentrations of high-sensitive C-reactive protein (hsCRP; (B)), tumor necrosis factor-alpha (TNFα; (C)), or interleukin-1beta (IL1β; (D)). The results of the statistical analysis ((A,C): One-Way-ANOVA, Sidak’s multiple comparisons test; (B,D): Kruskall–Wallis, Dunn’s multiple comparisons test) are shown within the graph. Simple linear regression analysis of the association of sEPCR and hsCRP levels in plasma (E) or with the white blood cell count in whole blood (F). In sex-matched individuals 35 years of age or younger (□). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.
) and without (◯) cardiovascular risk factors. Plasma from these groups was also examined for the concentrations of high-sensitive C-reactive protein (hsCRP; (B)), tumor necrosis factor-alpha (TNFα; (C)), or interleukin-1beta (IL1β; (D)). The results of the statistical analysis ((A,C): One-Way-ANOVA, Sidak’s multiple comparisons test; (B,D): Kruskall–Wallis, Dunn’s multiple comparisons test) are shown within the graph. Simple linear regression analysis of the association of sEPCR and hsCRP levels in plasma (E) or with the white blood cell count in whole blood (F). In sex-matched individuals 35 years of age or younger (□). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.
 ) and without (◯) cardiovascular risk factors. Plasma from these groups was also examined for the concentrations of high-sensitive C-reactive protein (hsCRP; (B)), tumor necrosis factor-alpha (TNFα; (C)), or interleukin-1beta (IL1β; (D)). The results of the statistical analysis ((A,C): One-Way-ANOVA, Sidak’s multiple comparisons test; (B,D): Kruskall–Wallis, Dunn’s multiple comparisons test) are shown within the graph. Simple linear regression analysis of the association of sEPCR and hsCRP levels in plasma (E) or with the white blood cell count in whole blood (F). In sex-matched individuals 35 years of age or younger (□). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.
) and without (◯) cardiovascular risk factors. Plasma from these groups was also examined for the concentrations of high-sensitive C-reactive protein (hsCRP; (B)), tumor necrosis factor-alpha (TNFα; (C)), or interleukin-1beta (IL1β; (D)). The results of the statistical analysis ((A,C): One-Way-ANOVA, Sidak’s multiple comparisons test; (B,D): Kruskall–Wallis, Dunn’s multiple comparisons test) are shown within the graph. Simple linear regression analysis of the association of sEPCR and hsCRP levels in plasma (E) or with the white blood cell count in whole blood (F). In sex-matched individuals 35 years of age or younger (□). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.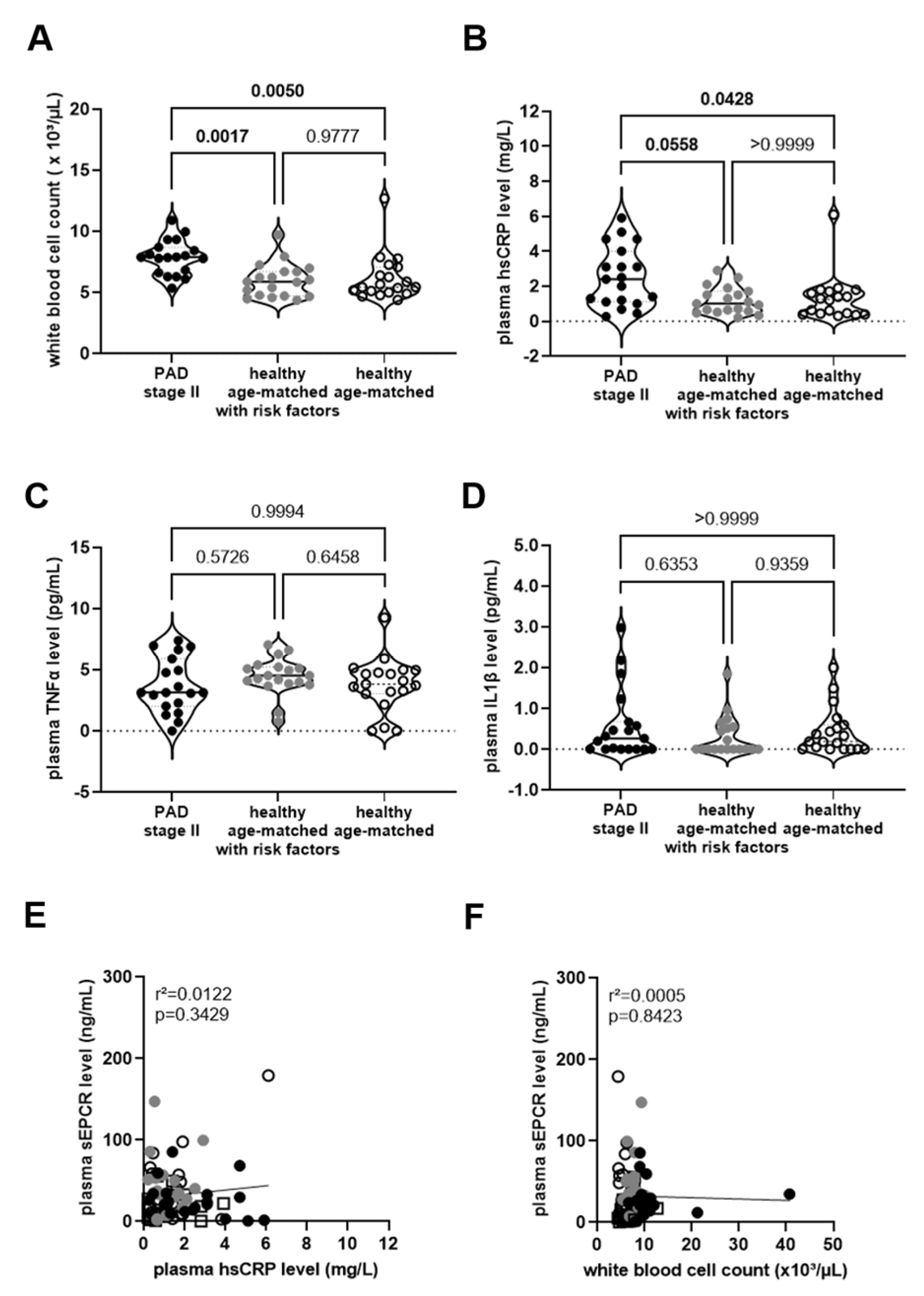
 ) and without (◯) cardiovascular risk factors. The results of the statistical analysis (A: Kruskall–Wallis, Dunn’s multiple comparisons test; B: One-Way ANOVA, Sidak’s multiple comparisons test) are shown within the graph. Simple linear regression analysis to determine the association of VEGF (C) or active TGFβ1 (D) with sE-selectin levels in plasma of PAD stage II patients (●) and in age- and sex-matched healthy persons, with (
) and without (◯) cardiovascular risk factors. The results of the statistical analysis (A: Kruskall–Wallis, Dunn’s multiple comparisons test; B: One-Way ANOVA, Sidak’s multiple comparisons test) are shown within the graph. Simple linear regression analysis to determine the association of VEGF (C) or active TGFβ1 (D) with sE-selectin levels in plasma of PAD stage II patients (●) and in age- and sex-matched healthy persons, with ( ) and without (◯) cardiovascular risk factors, and of plasma VEGF (E) or active TGFβ1 (F) and the ankle-brachial index (in PAD stage II patients only). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.
) and without (◯) cardiovascular risk factors, and of plasma VEGF (E) or active TGFβ1 (F) and the ankle-brachial index (in PAD stage II patients only). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.
 ) and without (◯) cardiovascular risk factors. The results of the statistical analysis (A: Kruskall–Wallis, Dunn’s multiple comparisons test; B: One-Way ANOVA, Sidak’s multiple comparisons test) are shown within the graph. Simple linear regression analysis to determine the association of VEGF (C) or active TGFβ1 (D) with sE-selectin levels in plasma of PAD stage II patients (●) and in age- and sex-matched healthy persons, with (
) and without (◯) cardiovascular risk factors. The results of the statistical analysis (A: Kruskall–Wallis, Dunn’s multiple comparisons test; B: One-Way ANOVA, Sidak’s multiple comparisons test) are shown within the graph. Simple linear regression analysis to determine the association of VEGF (C) or active TGFβ1 (D) with sE-selectin levels in plasma of PAD stage II patients (●) and in age- and sex-matched healthy persons, with ( ) and without (◯) cardiovascular risk factors, and of plasma VEGF (E) or active TGFβ1 (F) and the ankle-brachial index (in PAD stage II patients only). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.
) and without (◯) cardiovascular risk factors, and of plasma VEGF (E) or active TGFβ1 (F) and the ankle-brachial index (in PAD stage II patients only). The r-squared and the p-values are shown within the graphs. Significant differences are highlighted in bold.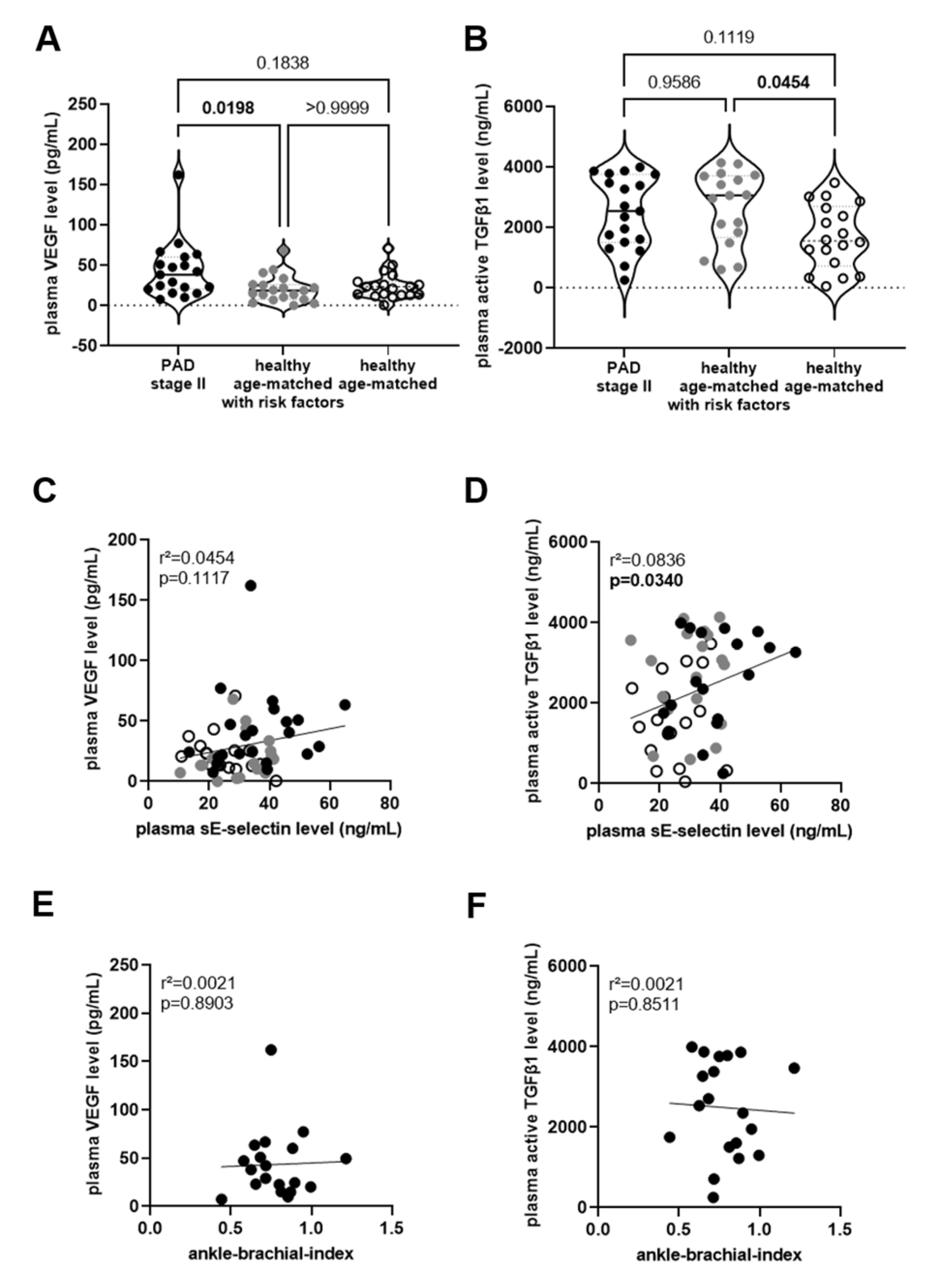
| PAD Stage II | PAD Stage III/IV | Healthy Age-Matched with Risk Factors | Healthy Age-Matched | Healthy ≤35 Years | p-Values | |
|---|---|---|---|---|---|---|
| Number | 19 | 19 | 19 | 19 | 19 | n.s. |
| Male sex (%) | 9 (47.4%) | 9 (47.4%) | 9 (47.4%) | 9 (47.4%) | 9 (47.4%) | n.s. |
| Age, years | 67.4 ± 8.1 #### | 68.9 ± 10.8 #### | 67.2 ± 7.3 #### | 66.1 ± 9.5 #### | 23.7 ± 4.0 | **** |
| BMI, kg/m2 | 27.6 ± 4.8 ### | 26.8 ± 4.3 ## | 26.7 ± 3.1 ## | 25.7 ± 3.8 # | 21.9 ± 2.9 | *** |
| Diabetes mellitus (%) | 6 (31.6%) | 8 (42.1%) | 2 (10.5%) | 0 | 0 | 0.0878 |
| Dyslipidemia (%) | 14 (73.7%) | 9 (47.3%) | 6 (31.2%) | 0 | 0 | * |
| Hypertension (%) | 16 (84.2%) | 11 (57.9%) | 14 (73.7%) | 0 | 0 | n.s. |
| Metabolic Syndrome (%) | 8 (42.1%) | 4 (21.1%) | 4 (21.1%) | 0 | 0 | n.s. |
| Smoking, active (%) | 7 (36.8%) | 1 (5.2%) # | 1 (5.3%) | 0 | 0 | ** |
| Smoking, former (%) | 10 (52.6%) | 13 (68.4%) | 8 (42.1%) | 10 (52.6%) | 0 | n.s. |
| Family history (%) | 2 (10.5%) | 4 (21.1%) | 6 (31.2%) | 2 (10.5%) | 0 | n.s. |
| ACE inhibitor/AT1 blocker (%) | 12 (63.2%) | 9 (47.4%) | 13 (68.4%) | 0 | 0 | n.s. |
| Aspirin (%) | 17 (89.5%) | 11 (57.9%) | 3 (15.8%) | 0 | 0 | **** |
| β-blocker (%) | 10 (52.6%) | 6 (31.6%) | 4 (21.1%) | 0 | 0 | n.s. |
| Calcium antagonists (%) | 11 (57.9%) | 3 (15.8%) | 5 (26.3%) | 0 | 0 | * |
| DOACs/VKAs (%) | 2 (10.5%) | 5 (26.3%) | 1 (5.3%) | 0 | 0 | n.s. |
| Fibrates (%) | 1 (5.3%) | 0 | 0 | 0 | 0 | n.s. |
| Nitrates (%) | 0 | 1 (5.3%) | 0 | 0 | 0 | n.s. |
| P2Y12 antagonists (%) | 2 (10.5%) | 8 (42.1%) | 1 (5.3%) | 0 | 0 | ** |
| Statins (%) | 15 (78.9%) | 9 (47.4%) | 4 (21.1%) | 0 | 0 | ** |
| PAD Stage II | PAD Stage III/IV | Healthy Age-Matched with Risk Factors | Healthy Age-Matched | Healthy ≤35 Years | p-Value | |
|---|---|---|---|---|---|---|
| n | 19 | 19 | 19 | 19 | 19 | |
| Glucose, mg/dL | 102 ± 14 && | 134 ± 52 #### §§§§ & | 91 ± 6.2 | 88 ± 5.5 | 86 ± 12 | ** |
| Triglycerides, mg/dL | 161 ± 70 §§ | 120 ± 46 | 118 ± 62 | 87 ± 29 | 119 ± 60 | ** |
| Total cholesterol, mg/dL | 193 ± 35 § && | 178 ± 54 §§§ &&&& | 242 ± 39 ## | 234 ± 36 # | 194 ± 24 | **** |
| LDL cholesterol, mg/dL | 101 ± 31 §§§ &&&& | 101 ± 44 §§§ &&&& | 153 ± 38 ### | 151 ± 31 ### | 105 ± 16 | **** |
| HDL cholesterol, mg/dL | 59 ± 17 | 53 ± 17 | 65 ± 18 | 65 ± 13 | 65 ± 21 | n.s. |
| C-reactive protein, mg/L | 2.6 ± 1.6 # | 19 ± 21 #### §§§ &&& | 1.2 ± 0.7 | 1.4 ± 1.4 | 1.3 ± 1.4 | **** |
| Creatinine, mg/dL | 0.87 ± 0.15 | 0.97 ± 0.37 | 0.87 ± 0.16 | 0.92 ± 0.18 | 0.87 ± 0.13 | n.s. |
| White blood cell count, ×103/μL | 7.9 ± 1.4 | 11.7 ± 7.6 #### §§§ &&&& % | 6.0 ± 1.4 | 6.2 ± 1.9 | 5.9 ± 1.0 | **** |
| Red blood cell count, ×106/μL | 4.6 ± 0.6 | 4.3 ± 0.6 # § | 4.6 ± 0.4 | 4.7 ± 0.5 | 4.7 ± 0.3 | * |
| Hematocrit % | 43 ± 4.3 | 42 ± 13 | 43 ± 2.7 | 43 ± 4.4 | 42 ± 3.1 | n.s. |
| Hemoglobin, g/dL | 14.2 ± 1.3 | 14.1 ± 6.0 | 14 ± 1.0 | 14 ± 1.5 | 14 ± 1.3 | n.s. |
| Platelets, ×103/μL | 263 ± 58 | 285 ± 114 | 264 ± 51 | 230 ± 33 | 245 ± 45 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krug, J.; Bochenek, M.L.; Gogiraju, R.; Laubert-Reh, D.; Lackner, K.J.; Münzel, T.; Wild, P.S.; Espinola-Klein, C.; Schäfer, K. Circulating Soluble EPCR Levels Are Reduced in Patients with Ischemic Peripheral Artery Disease and Associated with Markers of Endothelial and Vascular Function. Biomedicines 2023, 11, 2459. https://doi.org/10.3390/biomedicines11092459
Krug J, Bochenek ML, Gogiraju R, Laubert-Reh D, Lackner KJ, Münzel T, Wild PS, Espinola-Klein C, Schäfer K. Circulating Soluble EPCR Levels Are Reduced in Patients with Ischemic Peripheral Artery Disease and Associated with Markers of Endothelial and Vascular Function. Biomedicines. 2023; 11(9):2459. https://doi.org/10.3390/biomedicines11092459
Chicago/Turabian StyleKrug, Janina, Magdalena L. Bochenek, Rajinikanth Gogiraju, Dagmar Laubert-Reh, Karl J. Lackner, Thomas Münzel, Philipp S. Wild, Christine Espinola-Klein, and Katrin Schäfer. 2023. "Circulating Soluble EPCR Levels Are Reduced in Patients with Ischemic Peripheral Artery Disease and Associated with Markers of Endothelial and Vascular Function" Biomedicines 11, no. 9: 2459. https://doi.org/10.3390/biomedicines11092459
APA StyleKrug, J., Bochenek, M. L., Gogiraju, R., Laubert-Reh, D., Lackner, K. J., Münzel, T., Wild, P. S., Espinola-Klein, C., & Schäfer, K. (2023). Circulating Soluble EPCR Levels Are Reduced in Patients with Ischemic Peripheral Artery Disease and Associated with Markers of Endothelial and Vascular Function. Biomedicines, 11(9), 2459. https://doi.org/10.3390/biomedicines11092459







